Dissolved Organic Matter Quality and Biofilm Composition Affect Microbial Organic Matter Uptake in Stream Flumes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Analyses
2.3. Calculations
2.4. Statistics
3. Results
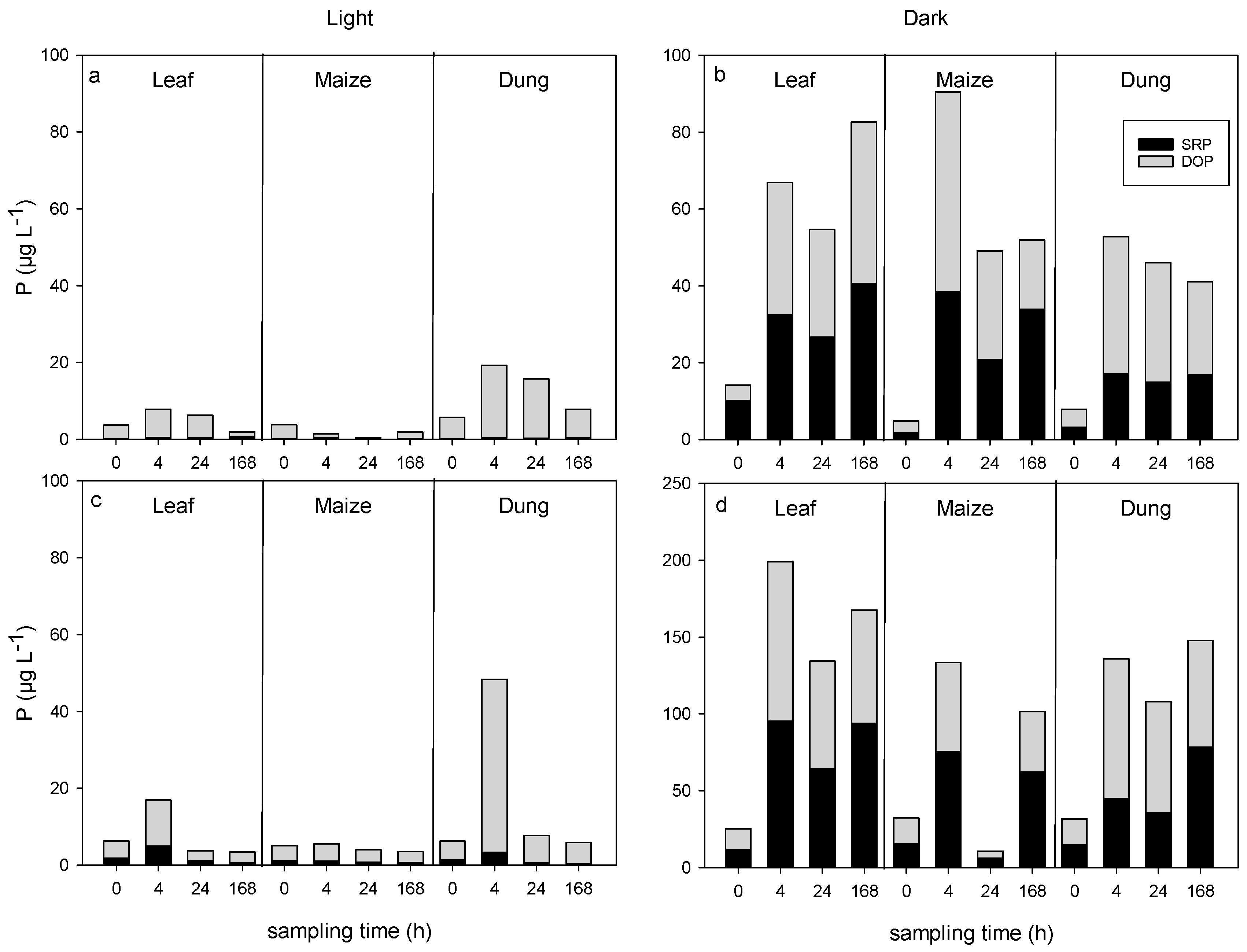
3.1. Changes in Hydrochemistry by Leachate Additions
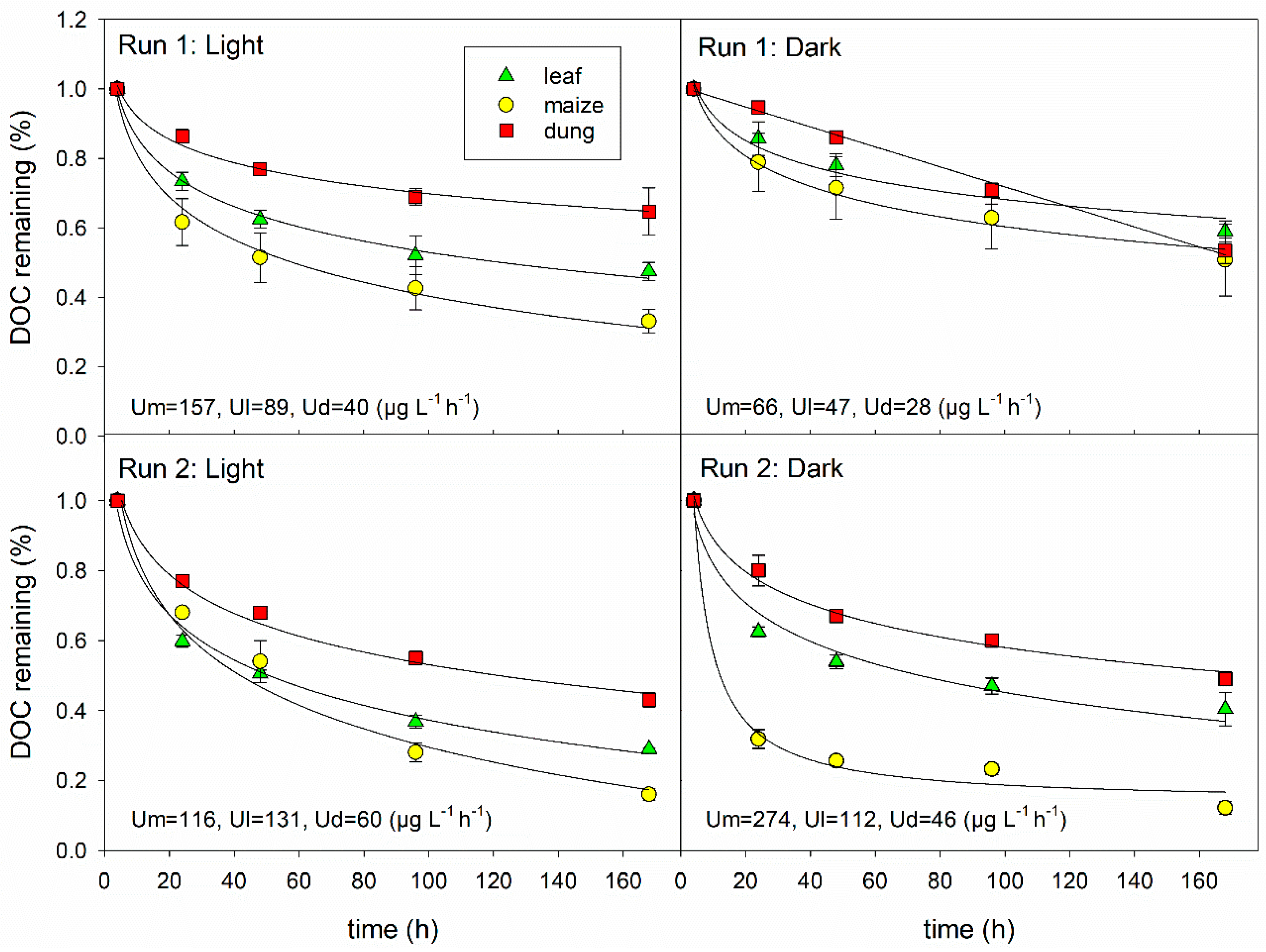
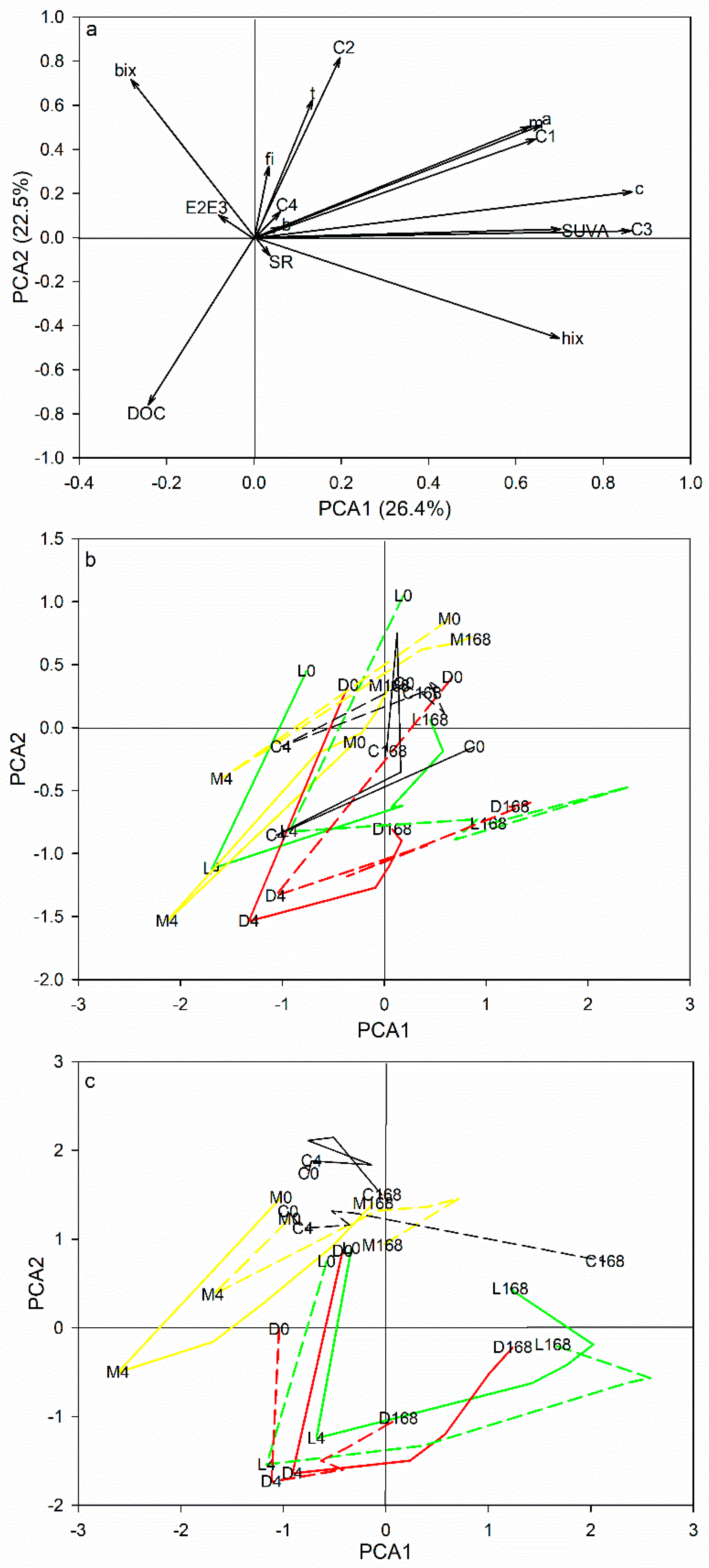
3.2. DOC Uptake and Changes in DOM Composition
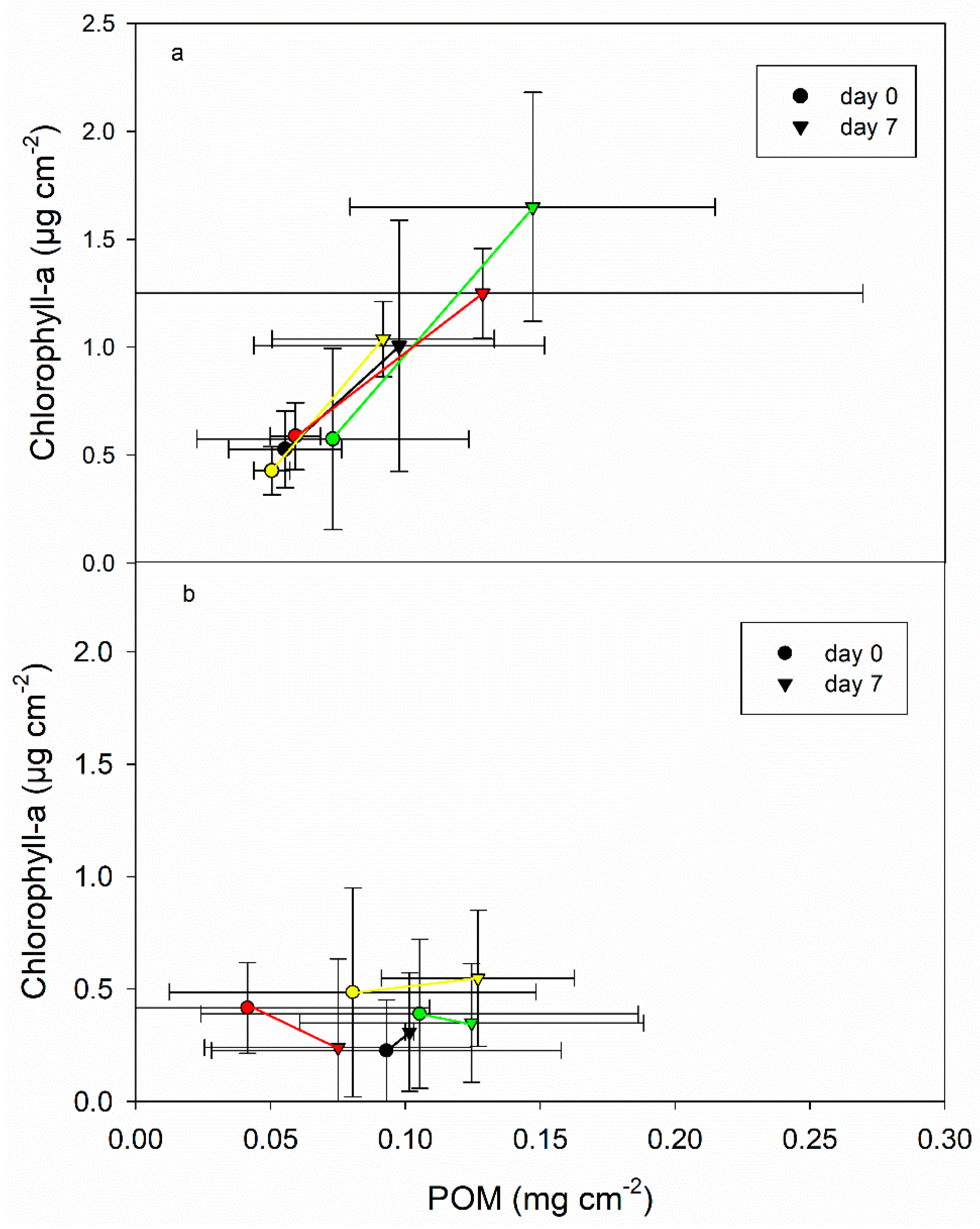
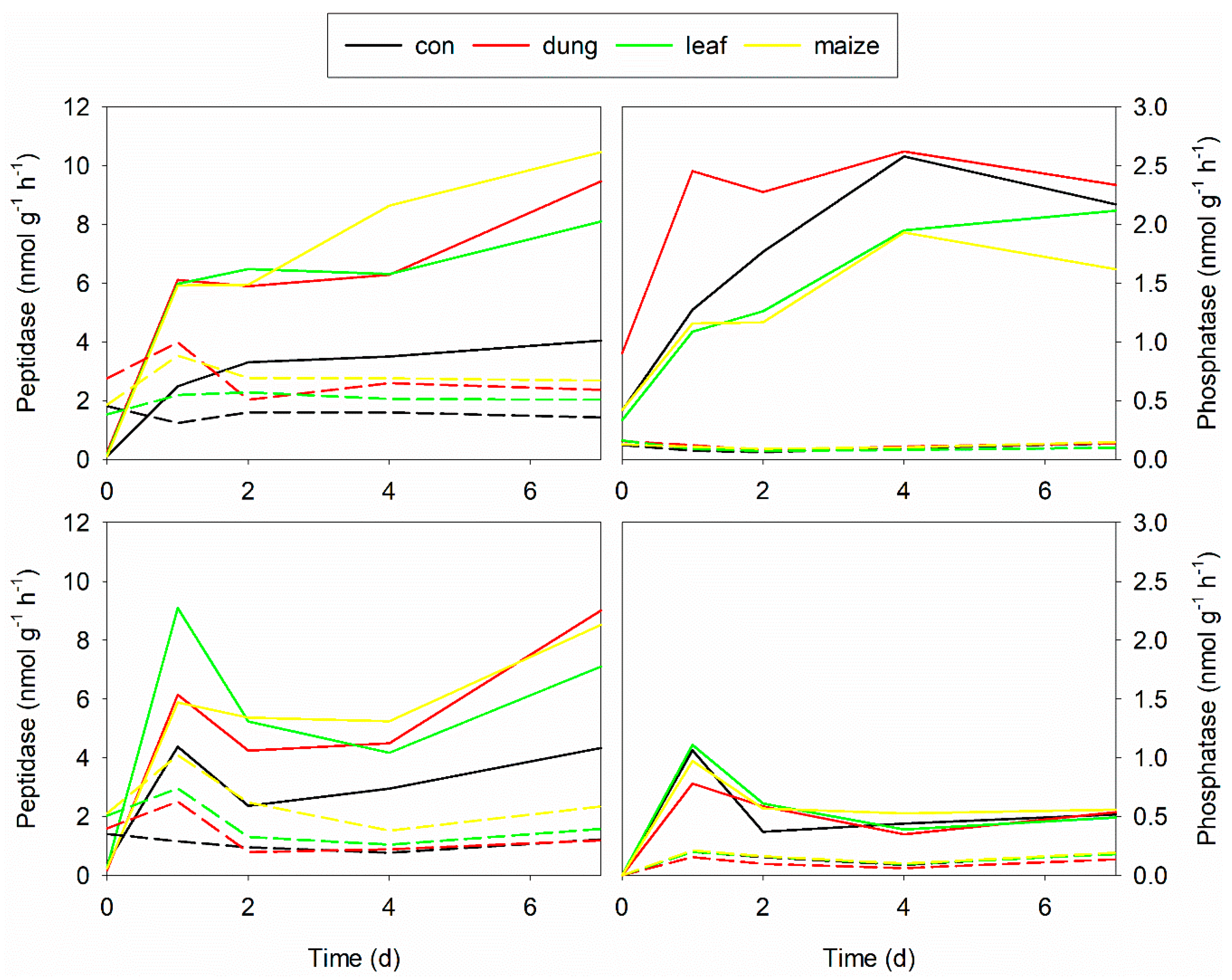
3.3. Changes in Epipsammic and Epilithic Biofilm Properties
4. Discussion
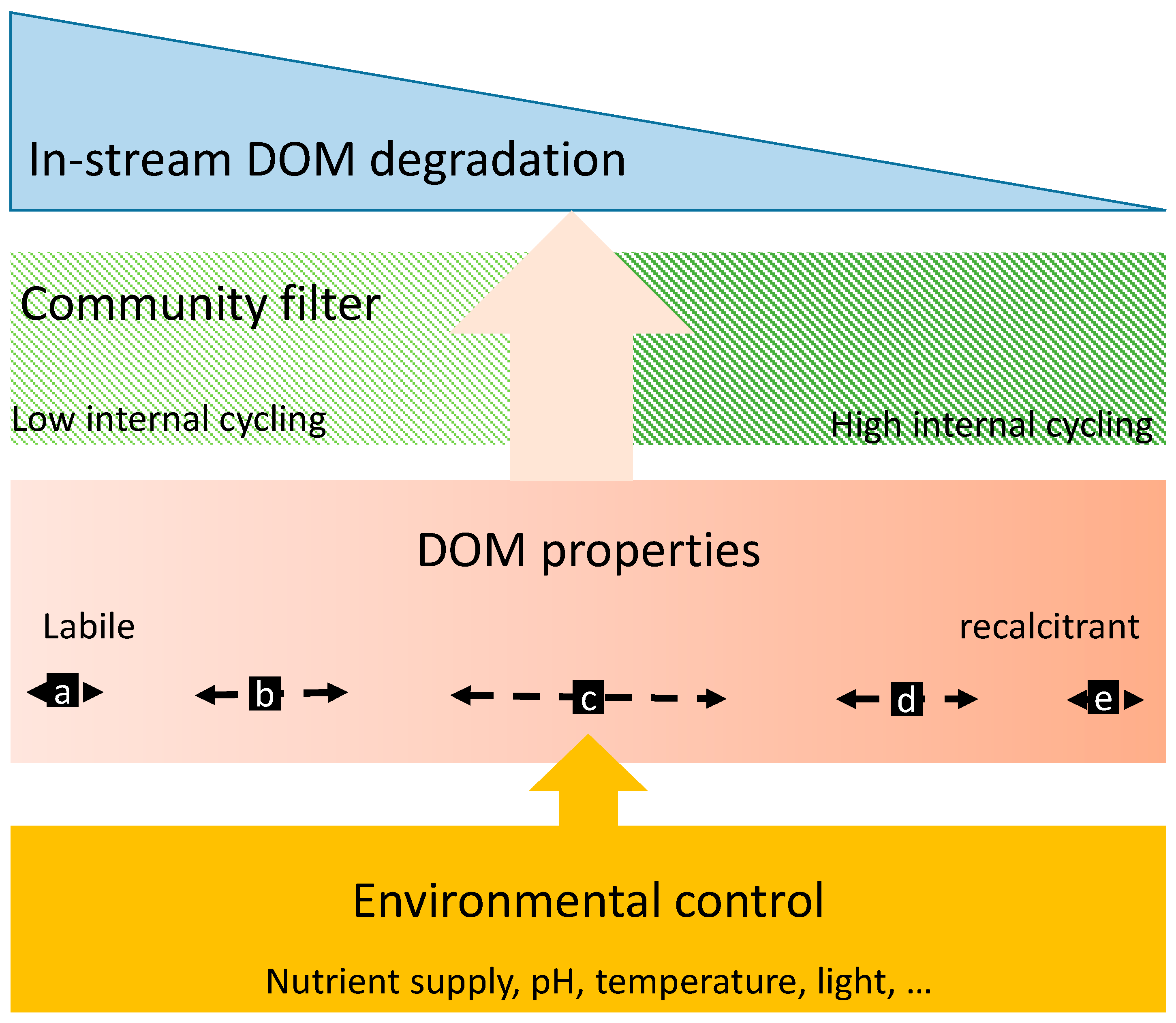
4.1. Effects of DOM Quality on DOM Degradation
4.2. Effects of Biofilm Composition on DOM Degradation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gordon, L.J.; Peterson, G.D.; Bennett, E.M. Agricultural modifications of hydrological flows create ecological surprises. Trends Ecol. Evol. 2008, 23, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.E.; Brisco, S.L. Relationships between the isotopic composition of dissolved organic carbon and its bioavailability in contrasting Ozark streams. Hydrobiologia 2004, 513, 153–169. [Google Scholar] [CrossRef]
- Bechtold, H.A.; Marcarelli, A.M.; Baxter, C.V.; Inouye, R.S. Effects of N, P, and organic carbon on stream biofilm nutrient limitation and uptake in a semi-arid watershed. Limnol. Oceanogr. 2012, 57, 1544–1554. [Google Scholar] [CrossRef]
- Graeber, D.; Gelbrecht, J.; Pusch, M.T.M.T.; Anlanger, C.; von Schiller, D.; Schiller, D. Von Agriculture has changed the amount and composition of dissolved organic matter in Central European headwater streams. Sci. Total Environ. 2012, 438, 435–446. [Google Scholar] [CrossRef]
- Stutter, M.I.; Graeber, D.; Evans, C.D.; Wade, A.J.; Withers, P.J.A. Balancing macronutrient stoichiometry to alleviate eutrophication. Sci. Total Environ. 2018, 634, 439–447. [Google Scholar] [CrossRef]
- Wilson, H.F.; Xenopoulos, M.A. Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nat. Geosci. 2009, 2, 37–41. [Google Scholar] [CrossRef]
- Tiefenbacher, A.; Weigelhofer, G.; Klik, A.; Pucher, M.; Santner, J.; Wenzel, W.; Eder, A.; Strauss, P. Short-term effects of fertilization on dissolved organic matter in soil leachate. Water 2020, 12, 1617. [Google Scholar] [CrossRef]
- Williams, C.J.; Yamashita, Y.; Wilson, H.F.; Jaffe, R.; Jaffé, R.; Xenopoulos, M. Unraveling the role of land use and microbial activity in shaping dissolved organic matter characteristics in stream ecosystems. Limnol. Oceanogr. 2010, 55, 1159–1171. [Google Scholar] [CrossRef]
- Guillemette, F.; Del Giorgio, P.A. Reconstructing the various facets of dissolved organic carbon bioavailability in freshwater ecosystems. Limnol Ocean. 2011, 56, 734–748. [Google Scholar] [CrossRef]
- Koehler, B.; Von Wachenfeldt, E.; Kothawala, D.; Tranvik, L.J. Reactivity continuum of dissolved organic carbon decomposition in lake water. J. Geophys. Res. Biogeosci. 2012, 117, 1–14. [Google Scholar] [CrossRef]
- Berggren, M.; Giorgio, P.A. Distinct patterns of microbial metabolism associated to riverine dissolved organic carbon of different source and quality. J. Geophys. Res. Biogeosci. 2015, 120, 989–999. [Google Scholar] [CrossRef]
- Hansen, A.M.; Kraus, T.E.C.; Pellerin, B.A.; Fleck, J.A.; Downing, B.D.; Bergamaschi, B.A. Optical properties of dissolved organic matter (DOM): Effects of biological and photolytic degradation. Limnol. Oceanogr. 2016, 61, 1015–1032. [Google Scholar] [CrossRef]
- Lupon, A.; Catalán, N.; Marti, E.; Bernal, S. Influence of Dissolved Organic Matter Sources on In-Stream Net Dissolved Organic Carbon Uptake in a Mediterranean Stream. Water 2020, 12, 1722. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; McDowell, W.H. Twenty years apart: Comparisons of DOM uptake during leaf leachate releases to Hubbard Brook valley streams in 1979 versus 2000. J. Geophys. Res. Biogeosci. 2008, 113, 1–8. [Google Scholar] [CrossRef]
- Berggren, M.; Laudon, H.; Haei, M.; Ström, L.; Jansson, M. Efficient aquatic bacterial metabolism of dissolved low-molecular-weight compounds from terrestrial sources. ISME J. 2010, 4, 408–416. [Google Scholar] [CrossRef]
- Lane, C.S.; Lyon, D.R.; Ziegler, E. Cycling of two carbon substrates of contrasting lability by heterotrophic biofilms across a nutrient gradient of headwater streams. Aquat. Sci. 2013, 75, 235–250. [Google Scholar] [CrossRef]
- Amon, R.M.W.; Benner, R. Bacterial utilization of different size classes of dissolved organic matter. Limnol. Oceanogr. 1996, 41, 41–51. [Google Scholar] [CrossRef]
- Casas-Ruiz, J.P.; Catalán, N.; Gómez-Gener, L.; von Schiller, D.; Obrador, B.; Kothawala, D.N.; López, P.; Sabater, S.; Marcé, R. A tale of pipes and reactors: Controls on the in-stream dynamics of dissolved organic matter in rivers. Limnol. Oceanogr. 2017, 62, S85–S94. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Bengtsson, M.M.; Wagner, K.; Burns, N.R.; Herberg, E.R.; Wanek, W.; Kaplan, L.A.; Battin, T.J. No evidence of aquatic priming effects in hyporheic zone microcosms. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Brookshire, E.N.J.; Valett, H.M.; Thomas, S.A.; Webster, J.R. Coupled Cycling of Dissolved Organic Nitrogen and Carbon in a Forest Stream. Ecology 2005, 86, 2487–2496. [Google Scholar] [CrossRef]
- Catalán, N.; Casas-Ruiz, J.P.; Arce, M.I.; Abril, M.; Bravo, A.G.; Del Campo, J.A.; Esteves, E.; Freixa, A. Behind the scenes: Mechanisms regulating climatic patterns of dissolved organic carbon uptake in headwater streams. Glob. Biogeochem. Cycles 2018, 32, 1528–1541. [Google Scholar] [CrossRef]
- Berggren, M.; Laudon, H.; Jansson, M. Landscape regulation of bacterial growth efficiency in boreal freshwaters. Glob. Biogeochem. Cycles 2007, 21, 1–9. [Google Scholar] [CrossRef]
- Rosemond, A.D.; Benstead, J.P.; Bumpers, P.M.; Gulis, V.; Kominoski, J.S.; Manning, D.W.P.; Suberkropp, K.; Wallace, J.B. Experimental nutrient additions accelerate terrestrial carbon loss from stream ecosystems. Freshw. Ecol. 2015, 347, 1142–1145. [Google Scholar] [CrossRef]
- Wickland, K.P.; Aiken, G.R.; Butler, K.; Dornblaser, M.M.; Spencer, R.G.M.; Striegl, R.G. Biodegradability of dissolved organic carbon in the Yukon River and its tributaries: Seasonality and importance of inorganic nitrogen. Glob. Biogeochem. Cycles 2012, 26, 1–14. [Google Scholar] [CrossRef]
- Graeber, D.; Boëchat, I.G.; Encina-Montoya, F.; Esse, C.; Gelbrecht, J.; Goyenola, G.; Gücker, B.; Heinz, M.; Kronvang, B.; Meerhoff, M.; et al. Global effects of agriculture on fluvial dissolved organic matter. Sci. Rep. 2015, 5, 16328. [Google Scholar] [CrossRef]
- Mutschlecner, A.E.; Guerard, J.J.; Jones, J.B.; Harms, T.K. Phosphorus Enhances Uptake of Dissolved Organic Matter in Boreal Streams. Ecosystems 2018, 21, 675–688. [Google Scholar] [CrossRef]
- Stets, E.G.; Cotner, J.B. The influence of dissolved organic carbon on bacterial phosphorus uptake and bacteria-phytoplankton dynamics in two Minnesota lakes. Limnol. Oceanogr. 2008, 53, 137–147. [Google Scholar] [CrossRef]
- Thingstad, T.F.; Bellerby, R.G.J.; Bratbak, G.; Børsheim, K.Y.; Egge, J.K.; Heldal, M.; Larsen, A.; Neill, C.; Nejstgaard, J.; Norland, S.; et al. Counterintuitive carbon-to-nutrient coupling in an Arctic pelagic ecosystem. Nature 2008, 455, 387–390. [Google Scholar] [CrossRef]
- Oviedo-Vargas, D.; Royer, T.V.; Johnson, L.T. Dissolved organic carbon manipulation reveals coupled cycling of carbon, nitrogen, and phosphorus in a nitrogen-rich stream. Limnol. Oceanogr. 2013, 58, 1196–1206. [Google Scholar] [CrossRef]
- Scott, J.T.; Back, J.A.; Taylor, J.M.; King, R.S. Does nutrient enrichment decouple algal—Bacterial production in periphyton? J. N. Am. Benthol. Soc. 2008, 27, 332–344. [Google Scholar] [CrossRef]
- Wagner, K.; Bengtsson, M.M.; Findlay, R.H.; Battin, T.J.; Ulseth, A.J. High light intensity mediates a shift from allochthonous to autochthonous carbon use in phototrophic stream biofilms. J. Geophys. Res. Biogeosci. 2017, 122, 1806–1820. [Google Scholar] [CrossRef]
- Demars, B.O.L.; Friberg, N.; Kemp, J.L.; Thornton, B. Reciprocal carbon subsidies between autotrophs and bacteria in stream food webs under stoichiometric constraints. bioRxiv 2018, 447987. [Google Scholar] [CrossRef]
- Romaní, A.M.; Guasch, H.; Muñoz, I.; Ruana, J.; Vilalta, E.; Schwartz, T.; Emtiazi, F.; Sabater, S. Biofilm structure and function and possible implications for riverine DOC dynamics. Microb. Ecol. 2004, 47, 316–328. [Google Scholar] [CrossRef]
- Rier, S.T.; Shirvinski, J.M.; Kinek, K.C. In situ light and phosphorus manipulations reveal potential role of biofilm algae in enhancing enzyme-mediated decomposition of organic matter in streams. Freshw. Biol. 2014, 59, 1039–1051. [Google Scholar] [CrossRef]
- Rier, S.T.; Stevenson, R.J. Effects of light, dissolved organic carbon, and inorganic nutrients on the relationship between algae and heterotrophic bacteria in stream periphyton. Hydrobiologia 2002, 489, 179–184. [Google Scholar] [CrossRef]
- Rier, S.T.; Kuehn, K.A.; Francoeur, S.N.; Rier, S.T.; Kuehn, K.A.; Francoeur, S.N. Algal regulation of extracellular enzyme activity in stream microbial communities associated with inert substrata and detritus Algal regulation of extracellular enzyme activity in stream microbial communities associated with inert substrata and detritus. J. N. Am. Benthol. Soc. 2007, 26, 439–449. [Google Scholar] [CrossRef]
- Sobczak, W.V. Epilithic Bacterial Responses to Variations in Algal Biomass and Labile Dissolved Organic Carbon during Biofilm Colonization. J. N. Am. Benthol. Soc. 1996, 15, 143–154. [Google Scholar] [CrossRef]
- Ziegler, S.E.; Lyon, D.R. Factors regulating epilithic biofilm carbon cycling and release with nutrient enrichment in headwater streams. Hydrobiologia 2010, 657, 71–88. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Edwards, R.T.; D’Amore, D.V. Changes in the concentration, biodegradability, and fluorescent properties of dissolved organic matter during stormflows in coastal temperate watersheds. J. Geophys. Res. 2009, 114. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Waste Water, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T.; Kulbe, E.W.; Boyer, P.K.; Westerhoff, P.T.; Doran, T.; et al. Spectroflourometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Kothawala, D.N.; Murphy, K.R.; Stedmon, C.A.; Weyhenmeyer, G.A.; Tranvik, L.J. Inner filter correction of dissolved organic matter fluorescence. Limnol. Oceanogr. Methods 2013, 11, 616–630. [Google Scholar] [CrossRef]
- Duhamel, S.; Jacquet, S. Flow cytometric analysis of bacteria- and virus-like particles in lake sediments. J. Microbiol. Methods 2006, 64, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Browne, J.L. Comparison of Chemotaxonomic Methods for the Determination of Periphyton Community Composition. Master’s Thesis, Florida Atlantic University, Florida, FL, USA, 2010. [Google Scholar]
- Greisberger, S.; Teubner, K. Does pigment composition reflect phytoplankton community structure in differing temperature and light conditions in a deep alpine lake? An approach using hplc and delayed fluorescence techniques. J. Phycol. 2007, 43, 1108–1119. [Google Scholar] [CrossRef]
- Weigelhofer, G.; Pedro, J.; Pitzl, B.; Bondar-kunze, E.; Keeffe, J.O. Decoupled water-sediment interactions restrict the phosphorus buffer mechanism in agricultural streams. Sci. Total Environ. 2018, 628–629, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Pucher, M.; Wünsch, U.; Weigelhofer, G.; Murphy, K.; Hein, T.; Graeber, D. StaRdom: Versatile software for analyzing spectroscopic data of dissolved organic matter in R. Water 2019, 11, 2366. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef]
- Riu, J.; Bro, R. Jack-knife technique for outlier detection and estimation of standard errors in PARAFAC models. Chemom. Intell. Lab. Syst. 2003, 65, 35–49. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Wenig, P.; Bro, R. OpenFluor—An online spectral library of auto-fluorescence by organic compounds in the environment. Anal. Methods 2014, 6, 658–661. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S. Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnol. Ocean. 2005, 50, 686–697. [Google Scholar] [CrossRef]
- Yamashita, Y.; Kloeppel, B.D.; Knoepp, J.; Zausen, G.L.; Jaffé, R. Effects of Watershed History on Dissolved Organic Matter Characteristics in Headwater Streams. Ecosystems 2011, 14, 1110–1122. [Google Scholar] [CrossRef]
- Ejarque, E.; Khan, S.; Steniczka, G.; Schelker, J.; Kainz, M.J.; Battin, T.J. Climate-induced hydrological variation controls the transformation of dissolved organic matter in a subalpine lake. Limnol. Oceanogr. 2018, 63, 1355–1371. [Google Scholar] [CrossRef]
- Gabor, R.S.; Baker, A.; McKnight, D.M.; Miller, M.P. Fluorescence Indices and Their Interpretation. In Aquatic Organic Matter Fluorescence; Coble, P.G., Lead, J., Baker, A., Reynolds, D.M., Spencer, R.G.M., Eds.; Cambridge Universiy Press: New York, NY, USA, 2014; pp. 303–338. ISBN 978-0-521-76461-2. [Google Scholar]
- Helms, J.R.J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.E.C.; Kieber, D.J.D.J.; Mopper, K.; Stubbings, A.; Ritchie, J.D.; Minor, E.C.E.C.; Kieber, D.J.; et al. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Lennon, J.T.; Pfaff, L.E. Source and supply of terrestrial carbon affects aquatic microbial metabolism. Aquat. Microb. Ecol. 2005, 39, 107–119. [Google Scholar] [CrossRef]
- O’Brien, J.M.; Dodds, W.K. Saturation of NO3− uptake in prairie streams as a function of acute and chronic N exposure. J. N. Am. Benthol. Soc. 2010, 29, 627–635. [Google Scholar] [CrossRef]
- Trentman, M.T.; Dodds, W.K.; Fencl, J.S.; Gerber, K.; Guarneri, J.; Hitchman, S.M.; Peterson, Z.; Rüegg, J. Quantifying ambient nitrogen uptake and functional relationships of uptake versus concentration in streams: A comparison of stable isotope, pulse, and plateau approaches. Biogeochemistry 2015, 125, 65–79. [Google Scholar] [CrossRef]
- Knapik, H.G.; Fernandes, C.V.S.; de Azevedo, J.C.R.; dos Santos, M.M.; Dall’Agnol, P.; Fontane, D.G. Biodegradability of anthropogenic organic matter in polluted rivers using fluorescence, UV, and BDOC measurements. Environ. Monit. Assess. 2015, 187, 1–15. [Google Scholar] [CrossRef]
- Kamjunke, N.; Lechtenfeld, O.J.; Herzsprung, P. Quality of dissolved organic matter driven by autotrophic and heterotrophic microbial processes in a large river. Water 2020, 12, 1577. [Google Scholar] [CrossRef]
- Kuehn, K.A.; Francoeur, S.N.; Findlay, R.H.; Neely, R.K. Priming in the microbial landscape: Periphytic algal stimulation of litter-associated microbial decomposers. Ecology 2014, 95, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Howard-Parker, B.; White, B.; Halvorson, H.M.; Evans-White, M.A. Light and dissolved nutrients mediate recalcitrant organic matter decomposition via microbial priming in experimental streams. Freshw. Biol. 2020, 1–11. [Google Scholar] [CrossRef]
- Danger, M.; Cornut, J.; Chauvet, E.; Chavez, P.; Elger, A.; Danger, M.; Cornut, J.; Chauvet, E.; Chavez, P.; Elger, A. Benthic algae stimulate leaf litter decomposition in detritus-based headwater streams: A case of aquatic priming effect ? Ecology 2013, 94, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Guenet, B.; Danger, M.; Abbadie, L.; Lacroix, G. Priming effect: Bridging the gap between terrestrial and aquatic ecology. Ecology 2010, 91, 2850–2861. [Google Scholar] [CrossRef]
- Wyatt, K.H.; Rober, A.R. Warming enhances the stimulatory effect of algal exudates on dissolved organic carbon decomposition. Freshw. Biol. 2019, 1–10. [Google Scholar] [CrossRef]
- Catalán, N.; Kellerman, A.M.; Peter, H.; Carmona, F.; Tranvik, L.J. Absence of a priming effect on dissolved organic carbon degradation in lake water. Limnol. Oceanogr. 2015, 60, 159–168. [Google Scholar] [CrossRef]
- Halvorson, H.M.; Barry, J.R.; Lodato, M.B.; Findlay, R.H.; Francoeur, S.N.; Kuehn, K.A. Periphytic algae decouple fungal activity from leaf litter decomposition via negative priming. Funct. Ecol. 2019, 33, 188–201. [Google Scholar] [CrossRef]
- Romaní, A.M.; Amalfitano, S.; Artigas, J.; Fazi, S.; Sabater, S.; Timoner, X.; Ylla, I.; Zoppini, A. Microbial biofilm structure and organic matter use in mediterranean streams. Hydrobiologia 2013, 719, 43–58. [Google Scholar] [CrossRef]
- Mineau, M.M.; Wollheim, W.M.; Buffam, I.; Findlay, S.E.G.; Hall, R.O., Jr.; Hotchkiss, E.R.; Koenig, L.E.; McDowell, W.H.; Parr, T.B. Dissolved organic carbon uptake in streams: A review and assessment of reach-scale measurements. J. Geophys. Res. Biogeosci. 2016, 121, 2019–2029. [Google Scholar] [CrossRef]
| Component | Ex/Em Wavelengths | Description | References |
|---|---|---|---|
| C1 | ex <230 (320), em 420 | humic-like, terrestrial, common in freshwaters, exported from agricultural catchments (C peak) | [4,53,54] |
| C2 | ex 285 (<230), em 350 | tryptophan-like, microbial delivered autochthonous; correlated to terrestrial fluorophores | [4,53] |
| C3 | ex 240/370/295, em 472 | humic-like, terrestrial delivered, mixture of A and C peaks; high molecular weight | [54,55] |
| C4 | ex 275 (<230), em 308 | tyrosine-like; autochthonous sources; produced and removed by the same processes as tryptophan; correlated to terrestrial fluorophores | [4,53] |
| Light | Dark | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Runs | Cont | Maize | Leaf | Dung | Cont | Maize | Leaf | Dung | |
| DOC 1 | 1 | 1.9 (0.1) | 8.9 (1.9) | 7.3 (0.4) | 7.1 (0.9) | 2.4 (0.1) | 5.0 (0.3) | 4.8 (0.9) | 5.7 (0.5) |
| mg L−1 | 2 | 1.4 (0) | 8.7 (0.0) | 6.4 (0.1) | 6.5 (0.2) | 1.9 (0) | 8.5 (0.1) | 6.5 (0.4) | 7.0 (0.5) |
| NH4-N | 1 | - | 7.5 (0.4) | 7.7 (3.3) | 30.9 (11) | - | 8.5 (0.1) | 15.2 (2.1) | 46.0 (2.1) |
| µg L−1 | 2 | - | 8.2 (2.1) | 9.1 (4.3) | 13.7 (11) | - | 7.9 (0.9) | 32.2 (1.9) | 150.8 (6) |
| NO3-N | 1 | 1.8 (0) | 1.8 (0.3) | 1.6 (0.6) | 1.7 (0.3) | 2.5 (0.3) | 2.6 (0.2) | 2.7 (0.1) | 2.6 (0.1) |
| mg L−1 | 2 | 1.2 (0) | 1.3 (0.3) | 1.2 (0.2) | 1.2 (0.3) | 1.7 (0) | 1.4 (0.1) | 1.6 (0.2) | 1.7 (0.1) |
| C1 (%) 2 | 1 | 29.1 | 33.3 | 28.1 | 30.6 | 24.9 | 27.6 | 27.2 | 28.0 |
| 2 | 31.8 | 26.6 | 30.5 | 29.4 | 28.2 | 23.0 | 22.6 | 23.2 | |
| C2 (%) | 1 | 45.1 | 38.8 | 44.1 | 42.9 | 41.7 | 35.6 | 36.0 | 35.1 |
| 2 | 37.1 | 44.4 | 41.9 | 44.8 | 34.2 | 38.1 | 39.8 | 40.1 | |
| C3 (%) | 1 | 5.1 | 7.1 | 5.7 | 7.1 | 8.0 | 12.6 | 10.5 | 13.5 |
| 2 | 8.9 | 7.8 | 8.7 | 8.0 | 14.6 | 10.5 | 10.1 | 11.2 | |
| C4 (%) | 1 | 20.6 | 20.8 | 22.1 | 19.4 | 25.3 | 24.2 | 26.3 | 23.5 |
| 2 | 22.2 | 21.3 | 18.9 | 17.8 | 22.9 | 28.4 | 27.5 | 25.6 | |
| SUVA254 3 | 1 | 9.2 | 1.4 | 2.4 | 2.0 | 5.6 | 2.2 | 3.2 | 2.4 |
| 2 | 4.7 | 1.3 | 2.4 | 2.1 | 4.3 | 1.6 | 2.0 | 1.8 | |
| E2:E3 4 | 1 | 5.54 | 12.6 | 10.6 | 9.6 | 5.8 | 8.3 | 8.0 | 9.0 |
| 2 | 7.73 | 5.6 | 5.6 | 5.3 | 7.1 | 5.6 | 5.7 | 6.6 | |
| HIX 5 | 1 | 0.58 | 0.66 | 0.61 | 0.67 | 0.58 | 0.64 | 0.61 | 0.65 |
| 2 | 0.66 | 0.60 | 0.64 | 0.61 | 0.67 | 0.59 | 0.58 | 0.62 | |
| FIX 6 | 1 | 1.63 | 1.71 | 1.71 | 1.71 | 1.65 | 1.60 | 1.65 | 1.59 |
| 2 | 1.72 | 1.60 | 1.63 | 1.61 | 1.57 | 1.59 | 1.59 | 1.60 | |
| BIX 7 | 1 | 1.15 | 0.95 | 1.00 | 1.03 | 1.10 | 1.10 | 1.12 | 1.06 |
| 2 | 0.92 | 1.13 | 1.11 | 1.05 | 1.03 | 1.10 | 1.12 | 1.06 | |
| Run 1 | Run 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rate | T50 | Rate | T50 | ||||||
| df | F | p | F | p | F | p | F | p | |
| Model | 6 | 17.3 | 0.000 | 23.3 | 0.000 | 81.6 | 0.000 | 39.0 | 0.000 |
| DOC | 1 | 0.9 | 0.347 | 0.2 | 0.632 | 13.6 | 0.004 | 0.4 | 0.539 |
| Source | 1 | 28.4 | 0.000 | 44.8 | 0.000 | 110.6 | 0.000 | 36.2 | 0.000 |
| Light | 2 | 4.9 | 0.048 | 9.4 | 0.011 | 1.9 | 0.199 | 2.4 | 0.148 |
| Light x Source | 2 | 0.8 | 0.482 | 0.2 | 0.862 | 52.2 | 0.000 | 18.1 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weigelhofer, G.; Jirón, T.S.; Yeh, T.-C.; Steniczka, G.; Pucher, M. Dissolved Organic Matter Quality and Biofilm Composition Affect Microbial Organic Matter Uptake in Stream Flumes. Water 2020, 12, 3246. https://doi.org/10.3390/w12113246
Weigelhofer G, Jirón TS, Yeh T-C, Steniczka G, Pucher M. Dissolved Organic Matter Quality and Biofilm Composition Affect Microbial Organic Matter Uptake in Stream Flumes. Water. 2020; 12(11):3246. https://doi.org/10.3390/w12113246
Chicago/Turabian StyleWeigelhofer, Gabriele, Tania Sosa Jirón, Tz-Ching Yeh, Gertraud Steniczka, and Matthias Pucher. 2020. "Dissolved Organic Matter Quality and Biofilm Composition Affect Microbial Organic Matter Uptake in Stream Flumes" Water 12, no. 11: 3246. https://doi.org/10.3390/w12113246
APA StyleWeigelhofer, G., Jirón, T. S., Yeh, T.-C., Steniczka, G., & Pucher, M. (2020). Dissolved Organic Matter Quality and Biofilm Composition Affect Microbial Organic Matter Uptake in Stream Flumes. Water, 12(11), 3246. https://doi.org/10.3390/w12113246






