Spatial and Temporal Variability of Posidonia oceanica Monitoring Indicators, Valencian Community, Spain
Abstract
1. Introduction
2. Materials and Methods
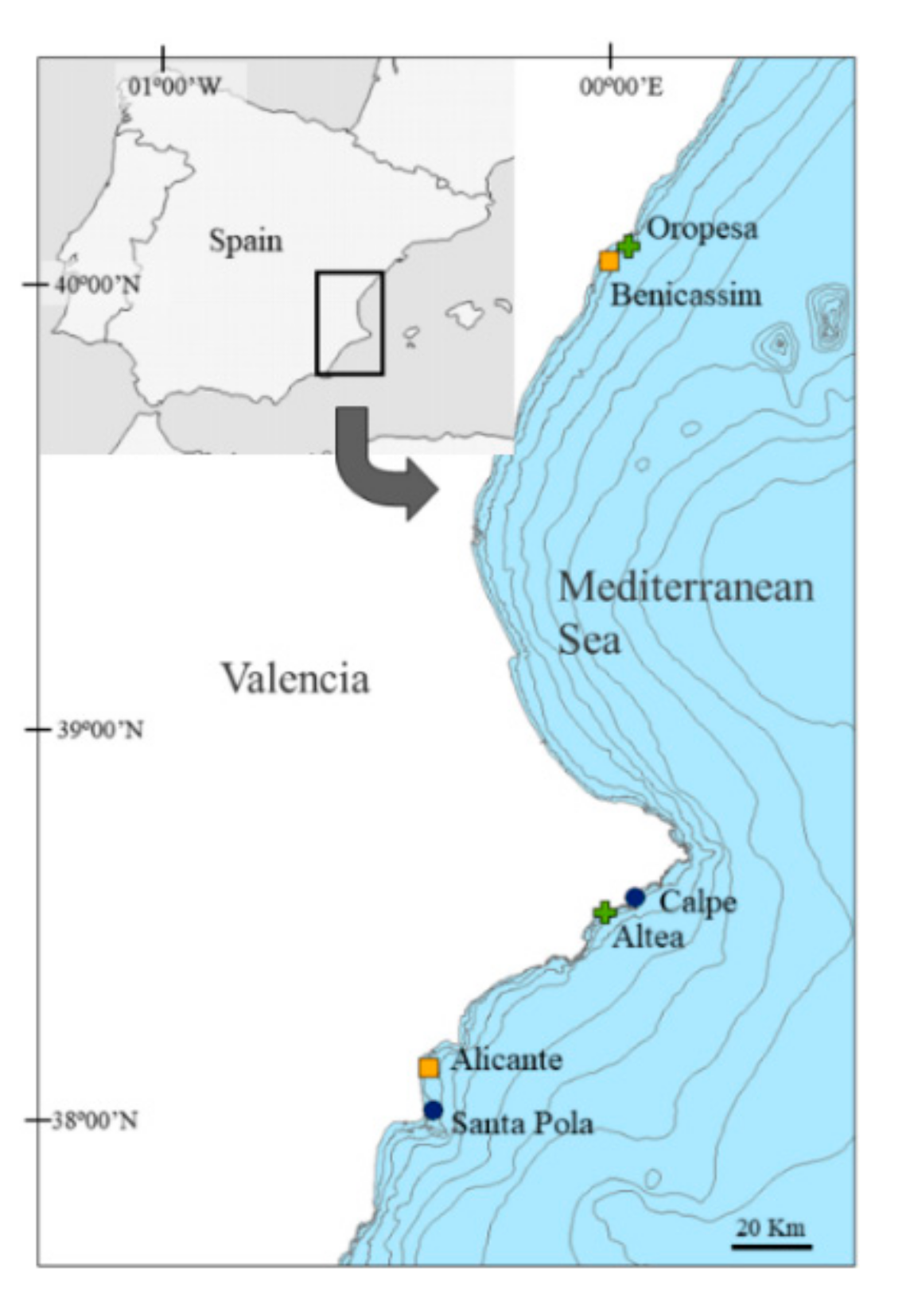
2.1. Study Area and Sampling Design
2.2. Posidonia Indicators
2.2.1. Structural Indicators
2.2.2. Shoot Morphology and Epiphytic Biomass
2.3. Data Analysis
3. Results
3.1. Spatial Variation of Posidonia Indicators
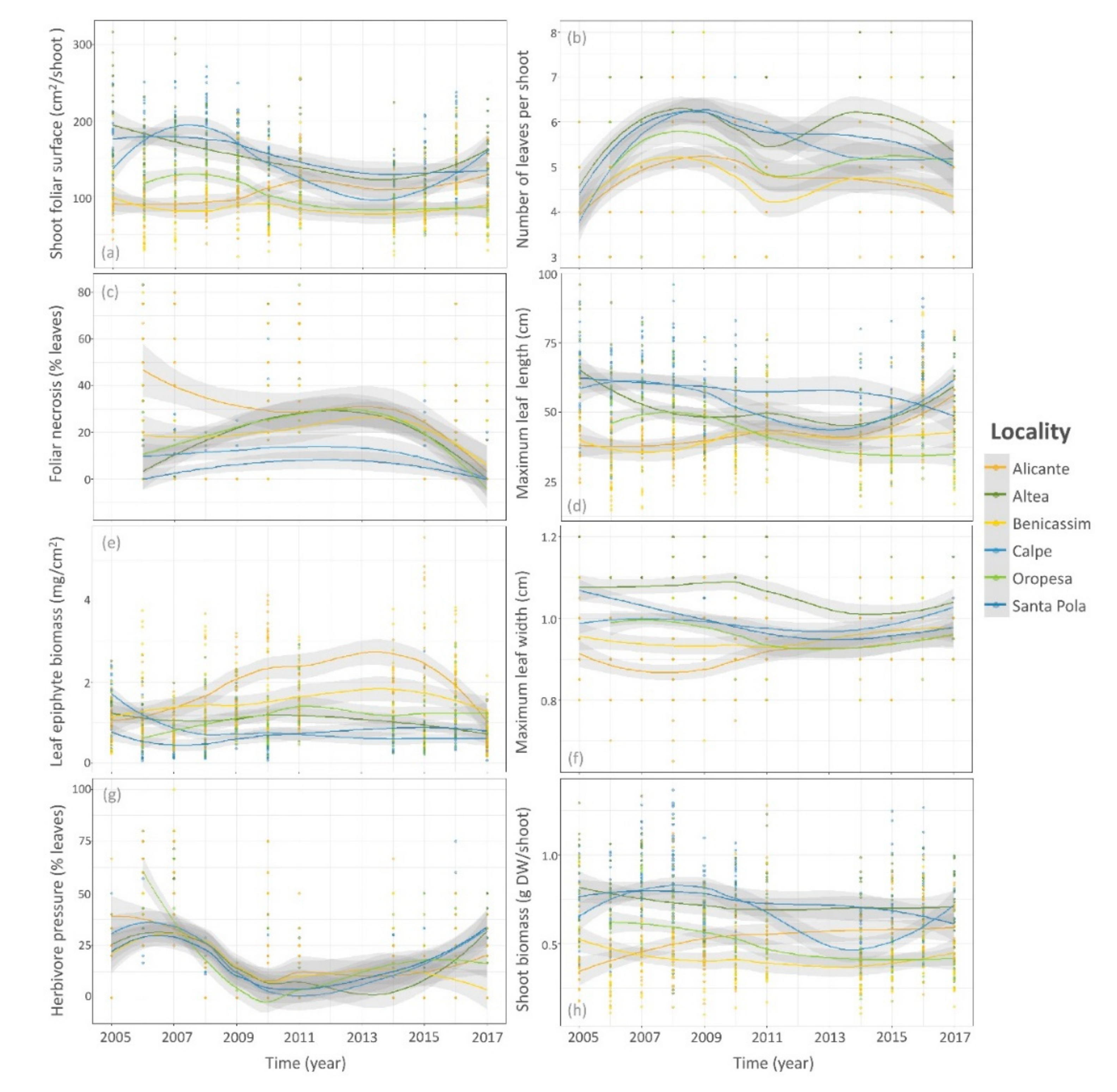
3.2. Temporal Trends of Posidonia Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems. BioScience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Romero, J.; Martínez-Crego, B.; Alcoverro, T.; Pérez, M. A multivariate index based on the seagrass Posidonia oceanica (POMI) to assess ecological status of coastal waters under the Water Framework Directive (WFD). Mar. Poll. Bull. 2007, 55, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Boudouresque, C.F.; Bernard, G.; Pergent, G.; Shili, A.; Verlaque, M. Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: A critical review. Bot. Mar. 2009, 52, 395–418. [Google Scholar] [CrossRef]
- Marbà, N.; Duarte, C.M.; Cebrian, J.; Gallegos, M.E.; Olesen, B.; Sand-Jensen, K. Growth and population dynamics of Posidonia oceanica on the Spanish Mediterranean coast: Elucidating seagrass decline. Mar. Ecol. Progr. Ser. 1996, 137, 203–213. [Google Scholar] [CrossRef]
- Jorda, G.; Marbà, N.; Duarte, C. Mediterranean seagrass vulnerable to regional climate warming. Nat. Clim. Chang. 2012, 2, 821–824. [Google Scholar] [CrossRef]
- Sánchez Lizaso, J.L.; Guillén Nieto, J.E.; Ramos Esplá, A.A. The regression of Posidonia oceanica meadow in El Campello (Spain). Rapp. Comm. Int. Mer Medit. 1990, 32, 7. [Google Scholar]
- Delgado, O.; Ruíz, J.M.; Pérez, M.; Romero, J.; Ballesteros, E. Effects of fish farming on seagrass (Posidonia oceanica) in a Mediterranean bay: Seagrass decline after organic loading cessation. Oceanol. Acta 1999, 22, 109–117. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Romero, J. Effects of disturbance caused by coastal constructions on spatial structure, growth, dynamics and photosynthesis of the seagrass Posidonia oceanica. Mar. Poll. Bull. 2003, 46, 1523–1533. [Google Scholar] [CrossRef]
- Fernández Torquemada, Y.; González Correa, J.M.; Martínez, J.E.; Sánchez Lizaso, J.L. Evaluation of the effects produced by the construction and expansion of marinas on Posidonia oceanica (L.) Delile meadows. J. Coast. Res. 2005, 49, 94–99. [Google Scholar]
- González Correa, J.M.; Fernández Torquemada, Y.; Sánchez Lizaso, J.L. Long-term effect of beach replenishment on natural recovery of shallow Posidonia oceanica meadows. Est. Coast. Shelf Sci. 2008, 76, 834–844. [Google Scholar] [CrossRef]
- González Correa, J.M.; Fernández Torquemada, Y.; Sánchez Lizaso, J.L. Short-term effect of beach replenishment on a shallow Posidonia oceanica meadow. Mar. Environm. Res. 2009, 68, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Guillen, J.E.; Sánchez Lizaso, J.L.; Jiménez, S.; Martínez, J.; Codina, A.; Montero, M.; Triviño, A.; Soler, G.; Zubcoff, J.J. Evolution of Posidonia oceanica seagrass meadows and its implications for management. J. Sea Res. 2013, 83, 65–71. [Google Scholar] [CrossRef]
- Roca, G.; Alcoverro, T.; De Torres, M.; Manzanera, M.; Martínez-Crego, B.; Bennett, S.; Farina, S.; Pérez, M.; Romero, J. Detecting water quality improvement along the Catalan coast (Spain) using stress-specific biochemical seagrass indicators. Ecol. Indic. 2015, 54, 161–170. [Google Scholar] [CrossRef]
- de-los-Santos, C.B.; Krause-Jensen, D.; Alcoverro, T.; Marbà, N.; Duarte, C.M.; van-Katwijk, M.M.; Pérez, M.; Romero, J.; Sánchez-Lizaso, J.L.; Roca, G.; et al. Recent trend reversal for declining European seagrass meadows. Nat. Commun. 2019, 10, 3356. [Google Scholar] [CrossRef]
- Casazza, G.; Lopez Royo, C.; Silvestri, C. Seagrasses as key coastal ecosystems: An overview of the recent EU WFD requirements and current applications. Biol. Mar. Med. 2006, 13, 189–193. [Google Scholar]
- Fernández-Torquemada, Y.; Díaz-Valdés, M.; Colilla, F.; Luna, B.; Sánchez-Lizaso, J.L.; Ramos-Esplá, A.A. Descriptors from Posidonia oceanica (L.) Delile meadows in coastal waters of Valencia, Spain, in the context of the EU Water Framework Directive. ICES J. Mar. Scien. 2008, 65, 1492–1497. [Google Scholar] [CrossRef]
- Lopez y Royo, C.; Pergent, G.; Pergent-Martini, C.; Casazza, G. Seagrass (Posidonia oceanica) monitoring in western Mediterranean: Implications for management and conservation. Environ. Monit. Assess. 2010, 171, 365–380. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Leoni, V.; Pasqualini, V.; Ardizzone, G.D.; Balestri, E.; Bedini, R.; Belluscio, A.; Belsher, T.; Borg, J.; Boudouresque, C.; et al. Descriptors of Posidonia oceanica meadows: Use and application. Ecol. Ind. 2005, 5, 213–230. [Google Scholar] [CrossRef]
- González-Correa, J.M.; Sánchez Lizaso, J.L.; Fernández Torquemada, Y.; Forcada, A. Long-term population dynamics in a healthy Posidonia oceanica meadow. Thalassas 2015, 31, 63–72. [Google Scholar]
- Hurlbert, S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- Moreno, D.; Aguilera, P.A.; Castro, H. Assessment of the conservation status of seagrass (Posidonia oceanica) meadows: Implications for monitoring strategy and the decision-making process. Biol. Conserv. 2001, 102, 325–332. [Google Scholar] [CrossRef]
- Underwood, A.J. Experiments in Ecology. Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997; p. 522. [Google Scholar]
- Fisher, R.; Wilson, S.K.; Sin, T.M.; Lee, A.C.; Langlois, T.J. A simple function for full-subsets multiple regression in ecology with R. Ecol. Evol. 2018, 8, 6104–6113. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.L. Modelling palaeoecological time series using generalised additive models. Front. Ecol. Evol. 2018, 6, 149. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; CRC Press: Boca Raton, FL, USA, 2017; p. 496. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 10 July 2020).
- Leriche, A.; Boudouresque, C.F.; Gravez, V.; Mayot, N. Does coverage matter at mesoscale within a Posidonia oceanica seagrass meadow? C. R. Biol. 2006, 329, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Panayotidis, P.; Boudouresque, C.F.; Marcot-Coqueugniot, J. Microstructure de l’herbier de Posidonia oceanica (Linnaeus) Delile. Bot. Mar. 1981, 24, 115–124. [Google Scholar] [CrossRef]
- Balestri, E.; Cinelli, F.; Lardicci, C. Spatial variation in Posidonia oceanica structural, morphological and dynamic features in a northwestern Mediterranean coastal area: A multi scale analysis. Mar. Ecol. Progr. Ser. 2003, 250, 51–60. [Google Scholar] [CrossRef]
- Darmaraki, S.; Somot, S.; Sevault, F.; Nabat, P.; Cabos Narvaez, W.D.; Cavicchia, L.; Djurdjevic, V.; Li, L.; Sannino, G.; Sein, D.V. Future evolution of Marine Heatwaves in the Mediterranean Sea. Clim. Dyn. 2019, 53, 1371–1392. [Google Scholar] [CrossRef]
- Roca, G.; Alcoverro, T.; Krause-Jensen, D.; Balsby, T.J.S.; van Katwijk, M.M.; Marba, N.; Santos, R.; Arthur, R.; Mascaró, O.; Fernández-Torquemada, Y.; et al. Response of seagrass indicators to shifts in environmental stressors: A global review and management synthesis. Ecol. Indic. 2016, 63, 310–323. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Quaresma, A.L.; Cunha, A.H.; Greve, T.M. How are seagrass distribution and abundance monitored? In European Seagrasses: An Introduction to Monitoring and Management; Borum, J., Duarte, C.M., Krause-Jensen, D., Greve, T.M., Eds.; EU Project Monitoring and Managing of European Seagrasses (M & MS): Copenhagen, Denmark, 2004; pp. 45–53. Available online: http://www.seagrasses.org (accessed on 2 March 2020).
- González-Correa, J.M.; Bayle, J.T.; Sánchez-Lizaso, J.L.; Valle, C.; Sánchez-Jerez, P.; Ruiz, J.M. Recovery of deep Posidonia oceanica meadows degraded by trawling. J. Exp. Mar. Biol. Ecol. 2005, 320, 65–76. [Google Scholar] [CrossRef]



| Indicator | p-Valor Locality | p-Valor Site | p-Valor Time |
|---|---|---|---|
| Shoot density | *** | *** | *** |
| Plagiotropic rhizomes | *** | *** | *** |
| Rhizome baring/burial | *** | *** | *** |
| Posidonia cover | *** | *** | *** |
| Dead-matte cover | *** | *** | *** |
| Conservation Index | *** | *** | *** |
| Shoot foliar surface | *** | 0.2616 | *** |
| Foliar necrosis | *** | 0.4591 | *** |
| Leaf epiphyte biomass | *** | *** | *** |
| Herbivore pressure | 0.0524 | 0.5117 | *** |
| Number of leaves | *** | 0.0815 | *** |
| Maximum leaf length | *** | ** | *** |
| Maximum leaf width | *** | * | *** |
| Shoot biomass | *** | * | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Torquemada, Y.; Díaz-Valdés, M.; Izquierdo-Muñoz, A.; Sánchez-Lizaso, J.L.; Ramos-Esplá, A.A. Spatial and Temporal Variability of Posidonia oceanica Monitoring Indicators, Valencian Community, Spain. Water 2020, 12, 3235. https://doi.org/10.3390/w12113235
Fernández-Torquemada Y, Díaz-Valdés M, Izquierdo-Muñoz A, Sánchez-Lizaso JL, Ramos-Esplá AA. Spatial and Temporal Variability of Posidonia oceanica Monitoring Indicators, Valencian Community, Spain. Water. 2020; 12(11):3235. https://doi.org/10.3390/w12113235
Chicago/Turabian StyleFernández-Torquemada, Yolanda, Marta Díaz-Valdés, Andrés Izquierdo-Muñoz, José Luis Sánchez-Lizaso, and Alfonso A. Ramos-Esplá. 2020. "Spatial and Temporal Variability of Posidonia oceanica Monitoring Indicators, Valencian Community, Spain" Water 12, no. 11: 3235. https://doi.org/10.3390/w12113235
APA StyleFernández-Torquemada, Y., Díaz-Valdés, M., Izquierdo-Muñoz, A., Sánchez-Lizaso, J. L., & Ramos-Esplá, A. A. (2020). Spatial and Temporal Variability of Posidonia oceanica Monitoring Indicators, Valencian Community, Spain. Water, 12(11), 3235. https://doi.org/10.3390/w12113235







