Resilience of Micropollutant and Biological Effect Removal in an Aerated Horizontal Flow Treatment Wetland
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and System Description
2.2. Experimental Design
2.3. Water Quality Analysis
2.4. Chemical Analysis
2.5. Bioanalysis
2.6. Data Evaluation
3. Results and Discussion
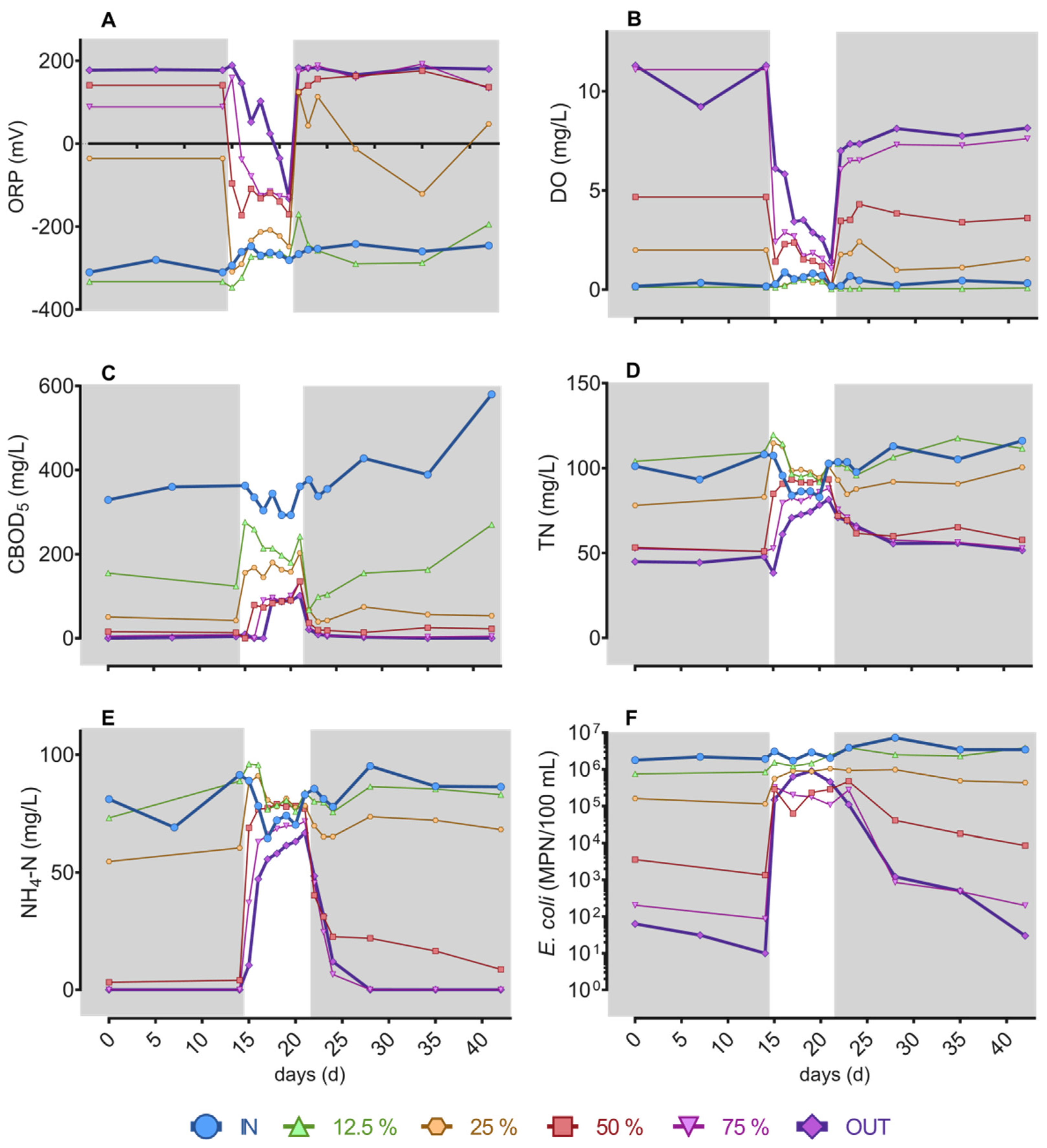
3.1. Weather Condition, Inflow Pollutant Concentrations, and Flow
3.2. Conventional Parameters and Pollutants
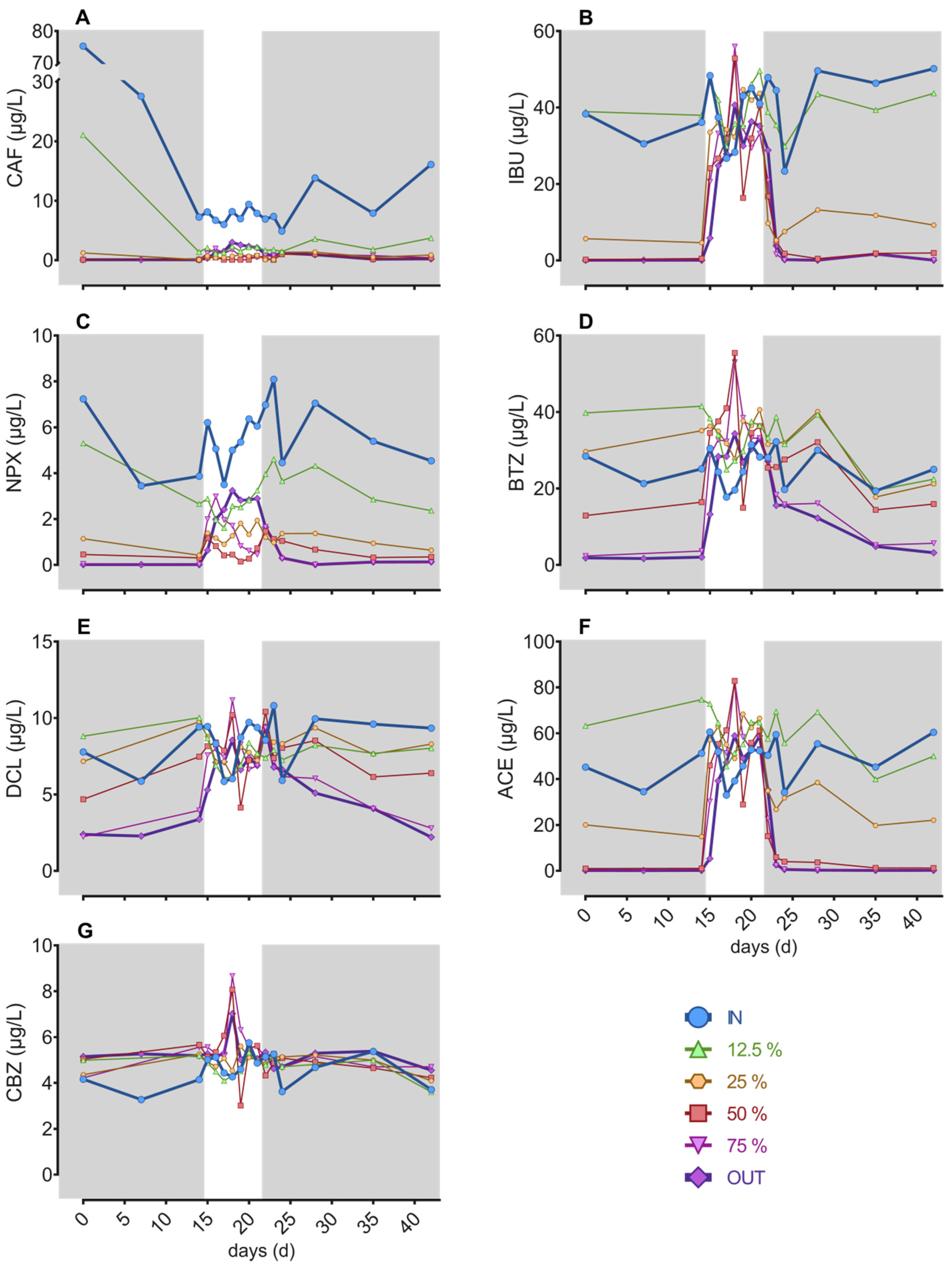
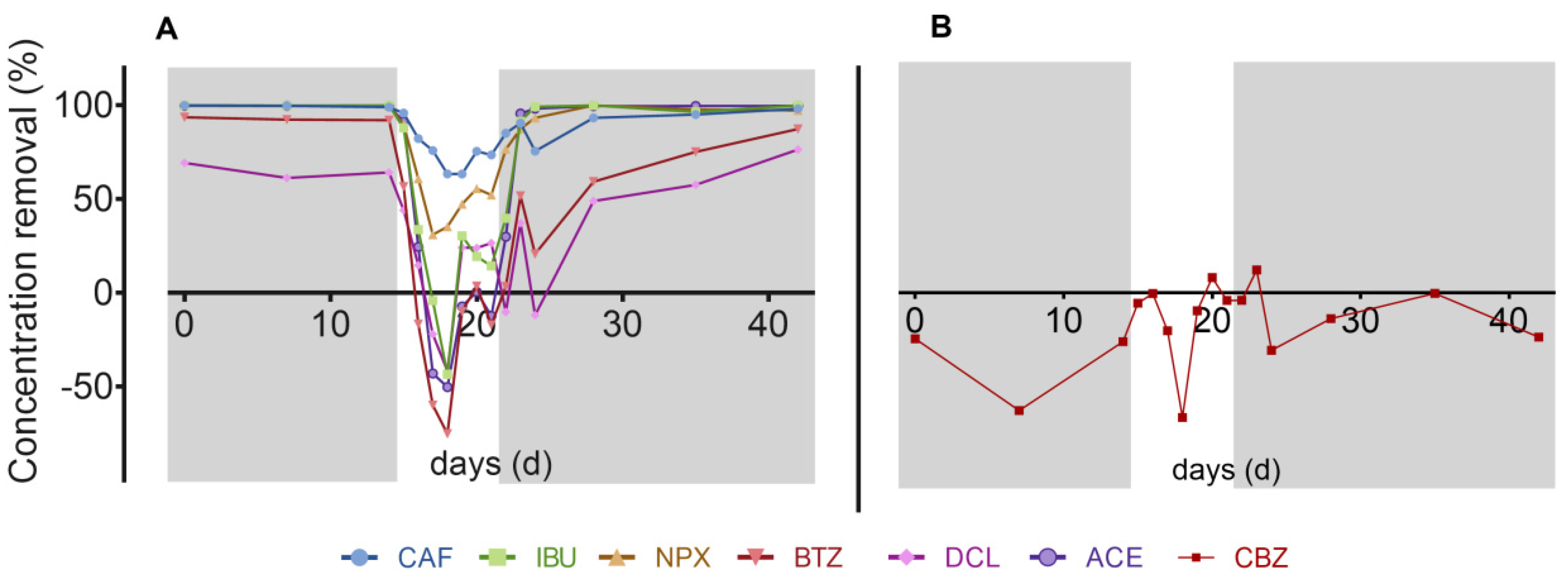
3.3. Contaminants of Emerging Concern
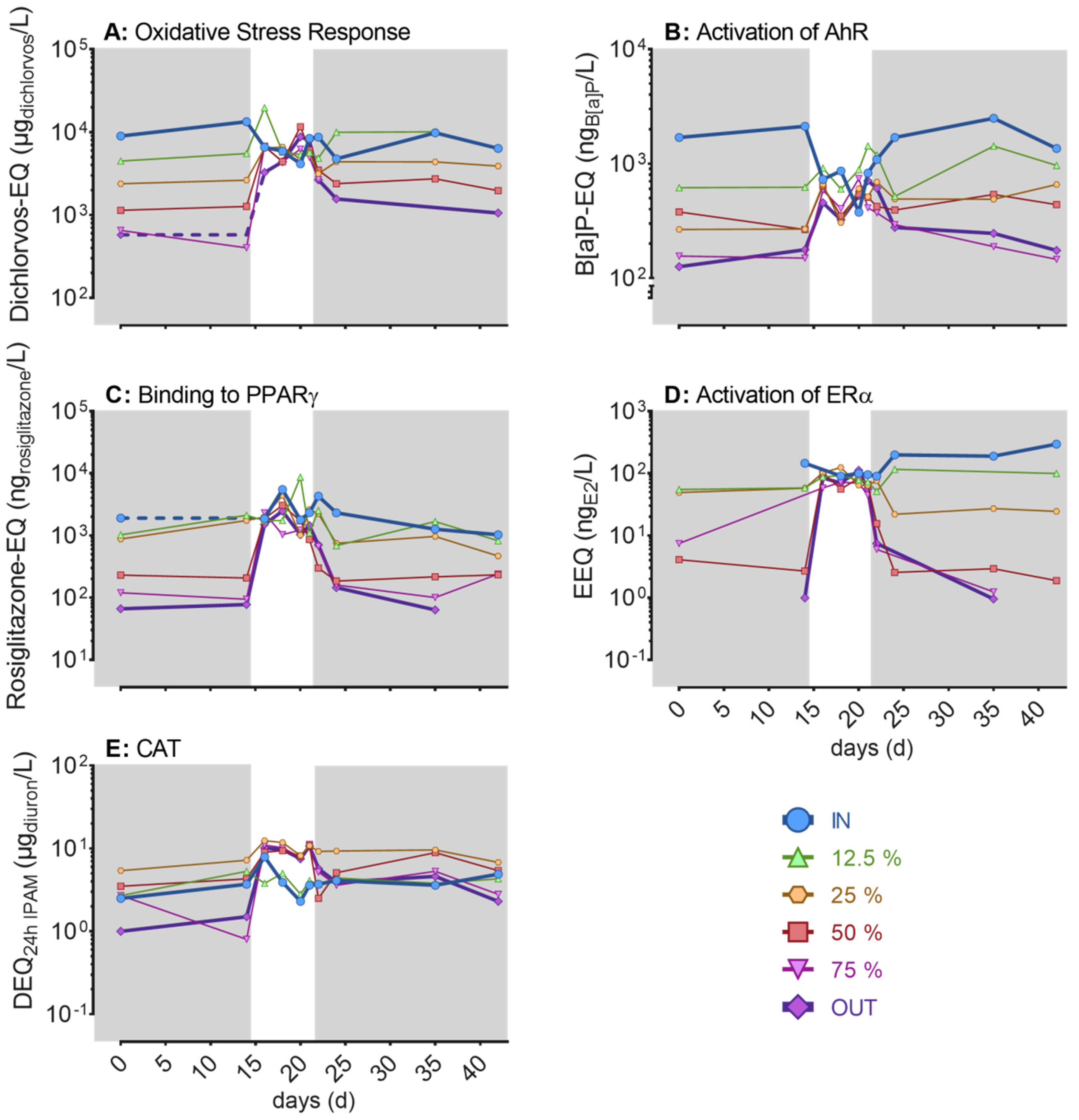
3.4. Bioanalysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2009; ISBN 978-1-56670-526-4. [Google Scholar]
- Nivala, J.; Headley, T.; Wallace, S.; Bernhard, K.; Brix, H.; van Afferden, M.; Müller, R.A. Comparative analysis of constructed wetlands: The design and construction of the ecotechnology research facility in Langenreichenbach, Germany. Ecol. Eng. 2013, 61, 527–543. [Google Scholar] [CrossRef]
- Dotro, G.; Langergraber, G.; Molle, P.; Nivala, J.; Puigagut, J.; Stein, O.; von Sperling, M. Treatment Wetlands; IWA Publishing: London, UK, 2017; Volume 7, ISBN 9781780408774. [Google Scholar]
- Fonder, N.; Headley, T. The taxonomy of treatment wetlands: A proposed classification and nomenclature system. Ecol. Eng. 2013, 51, 203–211. [Google Scholar] [CrossRef]
- Ávila, C.; Pelissari, C.; Sezerino, P.H.; Sgroi, M.; Roccaro, P.; García, J. Enhancement of total nitrogen removal through effluent recirculation and fate of PPCPs in a hybrid constructed wetland system treating urban wastewater. Sci. Total Environ. 2017, 584–585, 414–425. [Google Scholar]
- Nivala, J.; Boog, J.; Headley, T.; Aubron, T.; Wallace, S.; Brix, H.; Mothes, S.; van Afferden, M.; Müller, R.A. Side-by-side comparison of 15 pilot-scale conventional and intensified subsurface flow wetlands for treatment of domestic wastewater. Sci. Total Environ. 2019, 658, 1500–1513. [Google Scholar] [CrossRef]
- Gorito, A.M.; Ribeiro, A.R.; Almeida, C.M.R.; Silva, A.M.T. A review on the application of constructed wetlands for the removal of priority substances and contaminants of emerging concern listed in recently launched EU legislation. Environ. Pollut. 2017, 227, 428–443. [Google Scholar] [CrossRef]
- Kahl, S.; Nivala, J.; van Afferden, M.; Müller, R.A.; Reemtsma, T. Effect of design and operational conditions on the performance of subsurface flow treatment wetlands: Emerging organic contaminants as indicators. Water Res. 2017, 125, 490–500. [Google Scholar]
- Nivala, J.; Neale, P.A.; Haasis, T.; Kahl, S.; König, M.; Müller, R.A.; Reemtsma, T.; Schlichting, R.; Escher, B.I. Application of cell-based bioassays to evaluate treatment efficacy of conventional and intensified treatment wetlands. Environ. Sci. Water Res. Technol. 2018, 4, 206–217. [Google Scholar] [CrossRef]
- Weber, K.P.; Nivala, J. Treatment of micropollutants. In Wetland Technology: Practical Information on the Design and Application of Treatment Wetlands; Langergraber, G., Dotro, G., Nivala, J., Rizzo, A., Stein, O.R., Eds.; Scientific; IWA Publishing: London, UK, 2019; ISBN 9781789060171. [Google Scholar]
- König, M.; Escher, B.I.; Neale, P.A.; Krauss, M.; Hilscherová, K.; Novák, J.; Teodorović, I.; Schulze, T.; Seidensticker, S.; Hashmi, M.A.K.; et al. Impact of untreated wastewater on a major European river evaluated with a combination of In Vitro bioassays and chemical analysis. Environ. Pollut. 2017, 220, 1220–1230. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, G.; Ng, W.J.; Tan, S.K. A review on removing pharmaceutical contaminants from wastewater by constructed wetlands: Design, performance and mechanism. Sci. Total Environ. 2014, 468–469, 908–932. [Google Scholar]
- Carvalho, P.N. Interaction and Fate of Pharmaceuticals in Soil-Crop Systems: The Impact of Reclaimed Wastewater. In Handbook of Environmental Chemistry; Solsona, S.P., Montemurro, N., Chiron, S., Barceló, D., Eds.; Springer: Heidelberg, Germany, 2020; Volume 103, ISBN 978-3-030-61289-4. [Google Scholar]
- Hijosa-Valsero, M.; Matamoros, V.; Sidrach-Cardona, R.; Martín-Villacorta, J.; Bécares, E.; Bayona, J.M. Comprehensive assessment of the design configuration of constructed wetlands for the removal of pharmaceuticals and personal care products from urban wastewaters. Water Res. 2010, 44, 3669–3678. [Google Scholar]
- Verlicchi, P.; Zambello, E. How efficient are constructed wetlands in removing pharmaceuticals from untreated and treated urban wastewaters? A review. Sci. Total Environ. 2014, 470–471, 1281–1306. [Google Scholar] [CrossRef]
- Ilyas, H.; Masih, I.; van Hullebusch, E.D. Pharmaceuticals’ removal by constructed wetlands: A critical evaluation and meta-analysis on performance, risk reduction, and role of physicochemical properties on removal mechanisms. J. Water Health 2020. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Jekel, M.; Dott, W.; Bergmann, A.; Dünnbier, U.; Gnirß, R.; Haist-Gulde, B.; Hamscher, G.; Letzel, M.; Licha, T.; Lyko, S.; et al. Selection of organic process and source indicator substances for the anthropogenically influenced water cycle. Chemosphere 2015, 125, 155–167. [Google Scholar] [CrossRef]
- Matamoros, V.; Bayona, J.M. Elimination of Pharmaceuticals and Personal Care Products in Subsurface Flow Constructed Wetlands. Environ. Sci. Technol. 2006, 40, 5811–5816. [Google Scholar] [CrossRef]
- Nivala, J.; Kahl, S.; Boog, J.; van Afferden, M.; Reemtsma, T.; Müller, R.A. Dynamics of emerging organic contaminant removal in conventional and intensified subsurface flow treatment wetlands. Sci. Total Environ. 2019, 649, 1144–1156. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.V.; Parsons, J.R.; Rijnaarts, H.H.M.; de Voogt, P.; Langenhoff, A.A.M. Benzotriazole removal mechanisms in pilot-scale constructed wetlands treating cooling tower water. J. Hazard. Mater. 2020, 384, 121314. [Google Scholar] [CrossRef]
- Neale, P.A.; Ait-Aissa, S.; Brack, W.; Creusot, N.; Denison, M.S.; Deutschmann, B.; Hilscherová, K.; Hollert, H.; Krauss, M.; Novák, J.; et al. Linking In Vitro Effects and Detected Organic Micropollutants in Surface Water Using Mixture-Toxicity Modeling. Environ. Sci. Technol. 2015, 49, 14614–14624. [Google Scholar] [CrossRef]
- Escher, B.I.; Van Daele, C.; Dutt, M.; Tang, J.Y.M.; Altenburger, R. Most Oxidative Stress Response In Water Samples Comes From Unknown Chemicals: The Need For Effect-Based Water Quality Trigger Values. Environ. Sci. Technol. 2013, 47, 7002–7011. [Google Scholar] [CrossRef]
- Wernersson, A.-S.; Carere, M.; Maggi, C.; Tušil, P.; Soldàn, P.; James, A.; Sanchez, W.; Dulio, V.; Broeg, K.; Reifferscheid, G.; et al. The European technical report on aquatic effect-based monitoring tools under the water framework directive. Environ. Sci. Eur. 2015, 27, 7. [Google Scholar] [CrossRef]
- Neale, P.A.; O’Brien, J.W.; Glauch, L.; König, M.; Krauss, M.; Mueller, J.F.; Tscharke, B.; Escher, B.I. Wastewater treatment efficacy evaluated with In Vitro bioassays. Water Res. X 2020, 9, 100072. [Google Scholar] [CrossRef]
- Boog, J.; Nivala, J.; Aubron, T.; Mothes, S.; van Afferden, M.; Müller, R.A. Resilience of carbon and nitrogen removal due to aeration interruption in aerated treatment wetlands. Sci. Total Environ. 2018, 621, 960–969. [Google Scholar] [CrossRef]
- Butterworth, E.; Richards, A.; Jones, M.; Mansi, G.; Ranieri, E.; Dotro, G.; Jefferson, B. Performance of four full-scale artificially aerated horizontal flow constructedwetlands for domestic wastewater treatment. Water 2016, 8, 365. [Google Scholar] [CrossRef]
- Murphy, C.; Rajabzadeh, A.R.; Weber, K.P.; Nivala, J.; Wallace, S.; Cooper, D.J. Nitrification cessation and recovery in an aerated saturated vertical subsurface flow treatment wetland: Field studies and microscale biofilm modeling. Bioresour. Technol. 2016, 209, 125–132. [Google Scholar] [CrossRef]
- Boog, J.; Nivala, J.; Aubron, T.; Wallace, S.; Sullivan, C.; van Afferden, M.; Müller, R.A. Treatment wetland aeration without electricity? lessons learned from the first experiment using a wind-driven air pump. Water 2016, 8, 502. [Google Scholar] [CrossRef]
- Neale, P.A.; Altenburger, R.; Aït-Aïssa, S.; Brion, F.; Busch, W.; de Umbuzeiro, G.A.; Denison, M.S.; Du Pasquier, D.; Hilscherová, K.; Hollert, H.; et al. Development of a bioanalytical test battery for water quality monitoring: Fingerprinting identified micropollutants and their contribution to effects in surface water. Water Res. 2017, 123, 734–750. [Google Scholar] [CrossRef] [PubMed]
- Glauch, L.; Escher, B.I. The combined algae test for the evalution of mixture toxicity in environmental samples. Environ. Toxicol. Chem. 2020, 1–70. [Google Scholar] [CrossRef]
- Brennan, J.C.; He, G.; Tsutsumi, T.; Zhao, J.; Wirth, E.; Fulton, M.H.; Denison, M.S. Development of Species-Specific Ah Receptor-Responsive Third Generation CALUX Cell Lines with Enhanced Responsiveness and Improved Detection Limits. Environ. Sci. Technol. 2015, 49, 11903–11912. [Google Scholar] [CrossRef]
- Escher, B.I.; Dutt, M.; Maylin, E.; Tang, J.Y.M.; Toze, S.; Wolf, C.R.; Lang, M. Water quality assessment using the AREc32 reporter gene assay indicative of the oxidative stress response pathway. J. Environ. Monit. 2012, 14, 2877–2885. [Google Scholar] [CrossRef]
- Escher, B.I.; Aït-Aïssa, S.; Behnisch, P.A.; Brack, W.; Brion, F.; Brouwer, A.; Buchinger, S.; Crawford, S.E.; Du Pasquier, D.; Hamers, T.; et al. Effect-based trigger values for In Vitro and In Vivo bioassays performed on surface water extracts supporting the environmental quality standards (EQS) of the European Water Framework Directive. Sci. Total Environ. 2018, 628–629, 748–765. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, H.; Masih, I. Intensification of constructed wetlands for land area reduction: A review. Environ. Sci. Pollut. Res. 2017, 24, 12081–12091. [Google Scholar] [CrossRef]
- Kurzbaum, E.; Kirzhner, F.; Armon, R. Improvement of water quality using constructed wetland systems. Rev. Environ. Health 2012, 27, 59–64. [Google Scholar] [CrossRef]
- Wu, S.; Carvalho, P.N.; Müller, J.A.; Manoj, V.R.; Dong, R. Sanitation in constructed wetlands: A review on the removal of human pathogens and fecal indicators. Sci. Total Environ. 2016, 541, 8–22. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of drug in german sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Vieno, N.; Tuhkanen, T.; Kronberg, L. Elimination of pharmaceuticals in sewage treatment plants in Finland. Water Res. 2007, 41, 1001–1012. [Google Scholar] [CrossRef]
- Hashmi, M.A.K.; Krauss, M.; Escher, B.I.; Teodorović, I.; Brack, W. Effect-Directed Analysis of Progestogens and Glucocorticoids at Trace Concentrations in River Water. Environ. Toxicol. Chem. 2020, 39, 189–199. [Google Scholar] [CrossRef]
- Macova, M.; Toze, S.; Hodgers, L.; Mueller, J.F.; Bartkow, M.E.; Escher, B.I. Bioanalytical tools for the evaluation of organic micropollutants during sewage treatment, water recycling and drinking water generation. Water Res. 2011, 45, 4238–4247. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.M.; Escher, B.I. Realistic environmental mixtures of micropollutants in surface, drinking, and recycled water: Herbicides dominate the mixture toxicity toward algae. Environ. Toxicol. Chem. 2014, 33, 1427–1436. [Google Scholar] [CrossRef]
- Neale, P.A.; Braun, G.; Brack, W.; Carmona, E.; Gunold, R.; König, M.; Krauss, M.; Liebmann, L.; Liess, M.; Link, M.; et al. Assessing the mixture effects in In-Vitro bioassays of chemicals occurring in small agricultural streams during rain events. Environ. Sci. Technol. 2020, 54, 8280–8290. [Google Scholar] [CrossRef]
- Felis, E.; Sochacki, A.; Magiera, S. Degradation of benzotriazole and benzothiazole in treatment wetlands and by artificial sunlight. Water Res. 2016, 104, 441–448. [Google Scholar] [CrossRef]
- Button, M.; Nivala, J.; Weber, K.P.; Aubron, T.; Müller, R.A. Microbial community metabolic function in subsurface flow constructed wetlands of different designs. Ecol. Eng. 2015, 80, 162–171. [Google Scholar] [CrossRef]
- Kahl, S.; Kleinsteuber, S.; Nivala, J.; van Afferden, M.; Reemtsma, T. Emerging Biodegradation of the Previously Persistent Artificial Sweetener Acesulfame in Biological Wastewater Treatment. Environ. Sci. Technol. 2018, 52, 2717–2725. [Google Scholar] [CrossRef]
- Burke, V.; Greskowiak, J.; Grünenbaum, N.; Massmann, G. Redox and Temperature Dependent Attenuation of Twenty Organic Micropollutants—A Systematic Column Study. Water Environ. Res. 2016, 89, 155–167. [Google Scholar] [CrossRef]
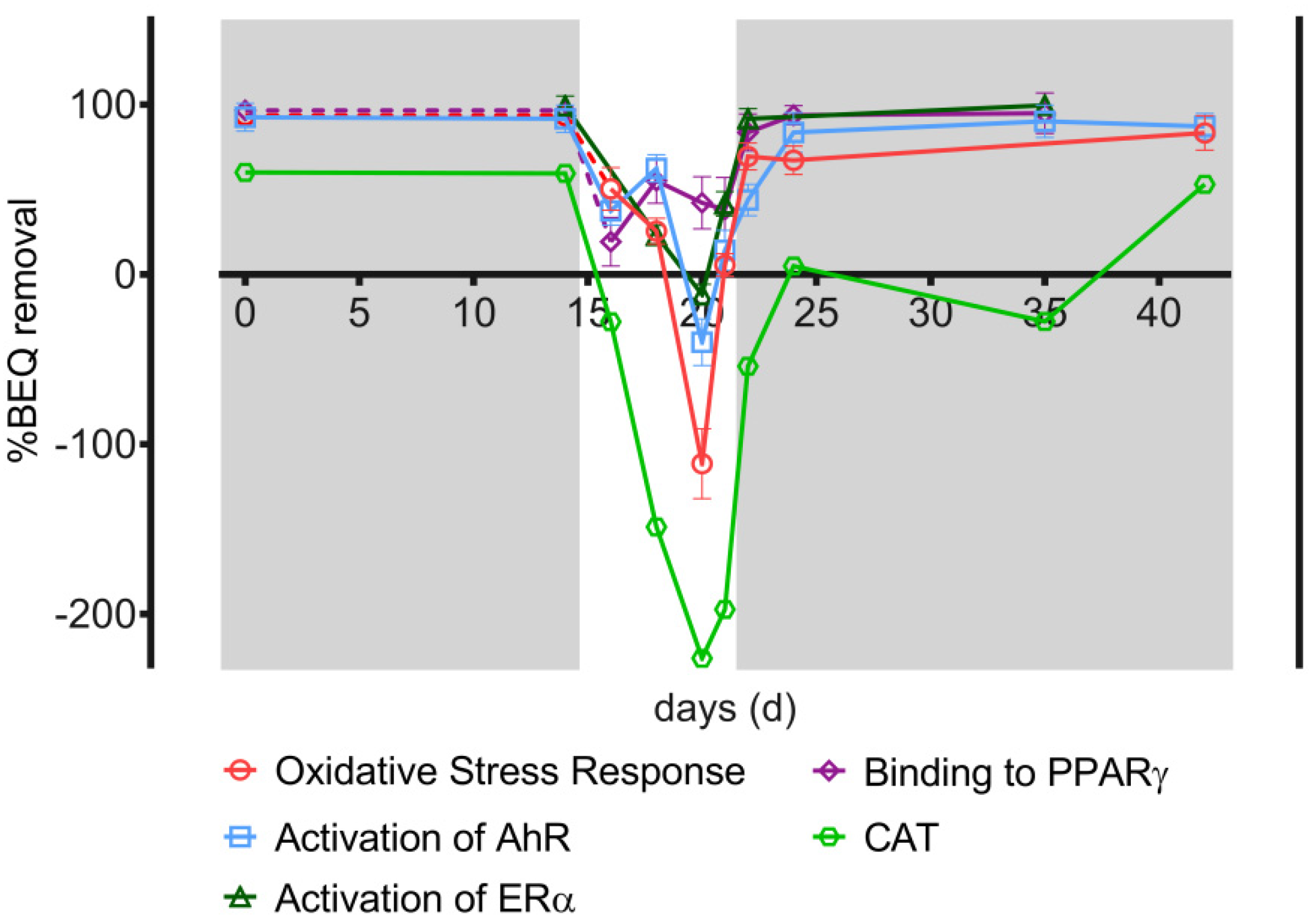
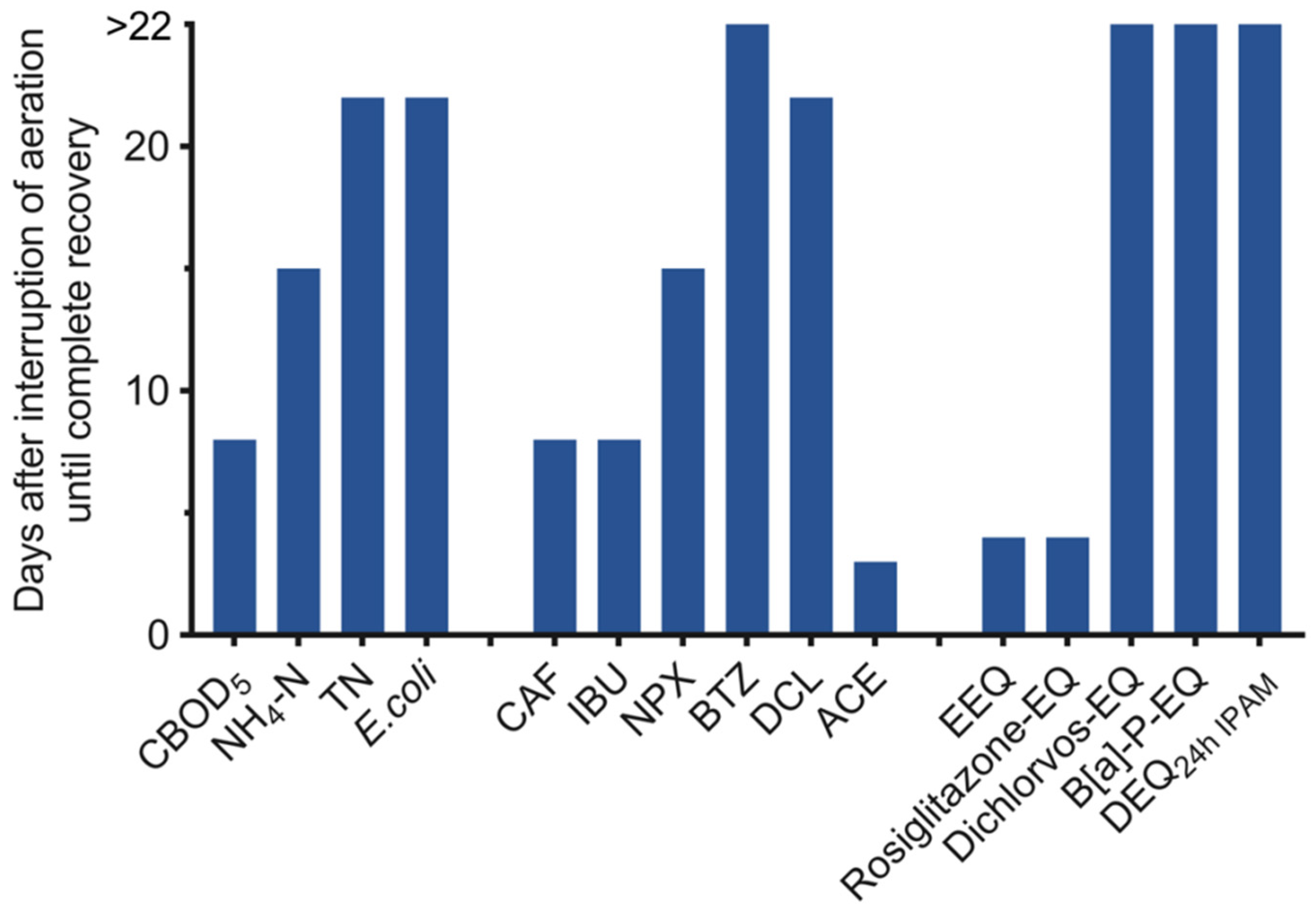
| Time | Phase | Sampling Routine | |
|---|---|---|---|
| Conventional Wastewater Parameters and CECs | Bioassays | ||
| Day 0–14 | Baseline | weekly | every other week |
| Day 15–21 | Interruption of aeration | daily | every other day |
| Day 22–24 | Recovery phase | daily | every other day |
| Day 25–42 | Recovered-baseline | weekly | every other week |
| Assay | Endpoint | Reference Compound | EC Value | Reference Compound EC Value (M) | Bioanalytical Equivalent (BEQ) |
|---|---|---|---|---|---|
| AhR CALUX [32] | Activation of the aryl hydrocarbon receptor | Benzo[a]pyrene | EC10 | (8.38 ± 0.55) × 10−10 | Benzo[a]pyrene-EQ (B[a]P-EQ) |
| AREc32 [33] | Oxidative Stress Response | Dichlorvos | ECIR1.5 | (7.70 ± 0.30) × 10−6 | Dichlorvos-EQ |
| ERα-GeneBLAzer [11] | Activation of the estrogen receptor | 17β-Estradiol | EC10 | (1.57 ± 0.03) × 10−11 | 17β-Estradiol-EQ (EEQ) |
| GR-GeneBLAzer [11] | Activation of glucocorticoid receptor | Dexamethasone | EC10 | (4.69 ± 0.06) × 10−10 | Dexamethasone-EQ |
| PPARγ-GeneBLAzer [30] | Binding to the peroxisome proliferator-activated receptor gamma | Rosiglitazone | EC10 | (4.97 ± 0.08) × 10−10 | Rosiglitazone-EQ |
| Combined algae test [31] | Inhibition of IPAM 2 h | Diuron | EC50 | (2.91) × 10−8 (2.76–3.06) × 10−8 a | Diuron-EQ2 h IPAM (DEQ2 h IPAM) |
| Inhibition of IPAM 24 h | Diuron | EC50 | (4.66) × 10−8 (4.24–5.12) × 10−8 a | Diuron-EQ24 h IPAM (DEQ24 h IPAM) | |
| Inhibition of growth | Diuron | EC50 | (1.26) × 10−6 (0.75–1.95) × 10−6 a | Diuron-EQ24 h growth (DEQ24 h growth) |
| pH | EC µS/cm | DO mg/L | ORP mV | CBOD5 mg/L | TOC mg/L | TN mg/L | NH4-N mg/L | NO3-N mg/L | NO2-N mg/L | E. coli MPN/100 mL | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 6.9 | 2008 | 0.4 | −269 | 341 | 185 | 99 | 80 | 0.04 | 0.01 | 6.45 × 106 |
| SD | 0.1 | 262 | 0.2 | 21 | 113 | 41 | 10 | 9 | 0.00 | 0.00 | 0.19 × 106 |
| n | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 11 |
| CAF (µg/L) | IBU (µg/L) | NPX (µg/L) | BTZ (µg/L) | DCL (µg/L) | ACE (µg/L) | CBZ (µg/L) | |
|---|---|---|---|---|---|---|---|
| Mean | 13.8 | 39.8 | 5.5 | 25.3 | 8.4 | 48.2 | 4.6 |
| SD | 17.3 | 8.7 | 1.4 | 4.7 | 1.6 | 9.2 | 0.7 |
| n | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sossalla, N.A.; Nivala, J.; Escher, B.I.; Reemtsma, T.; Schlichting, R.; van Afferden, M.; Müller, R.A. Resilience of Micropollutant and Biological Effect Removal in an Aerated Horizontal Flow Treatment Wetland. Water 2020, 12, 3050. https://doi.org/10.3390/w12113050
Sossalla NA, Nivala J, Escher BI, Reemtsma T, Schlichting R, van Afferden M, Müller RA. Resilience of Micropollutant and Biological Effect Removal in an Aerated Horizontal Flow Treatment Wetland. Water. 2020; 12(11):3050. https://doi.org/10.3390/w12113050
Chicago/Turabian StyleSossalla, Nadine A., Jaime Nivala, Beate I. Escher, Thorsten Reemtsma, Rita Schlichting, Manfred van Afferden, and Roland A. Müller. 2020. "Resilience of Micropollutant and Biological Effect Removal in an Aerated Horizontal Flow Treatment Wetland" Water 12, no. 11: 3050. https://doi.org/10.3390/w12113050
APA StyleSossalla, N. A., Nivala, J., Escher, B. I., Reemtsma, T., Schlichting, R., van Afferden, M., & Müller, R. A. (2020). Resilience of Micropollutant and Biological Effect Removal in an Aerated Horizontal Flow Treatment Wetland. Water, 12(11), 3050. https://doi.org/10.3390/w12113050





