Bacterial Diversity in the Asphalt Concrete Lining of the Upper Water Reservoir of a Pumped-Storage Scheme
Abstract
1. Introduction
2. Materials and Methods
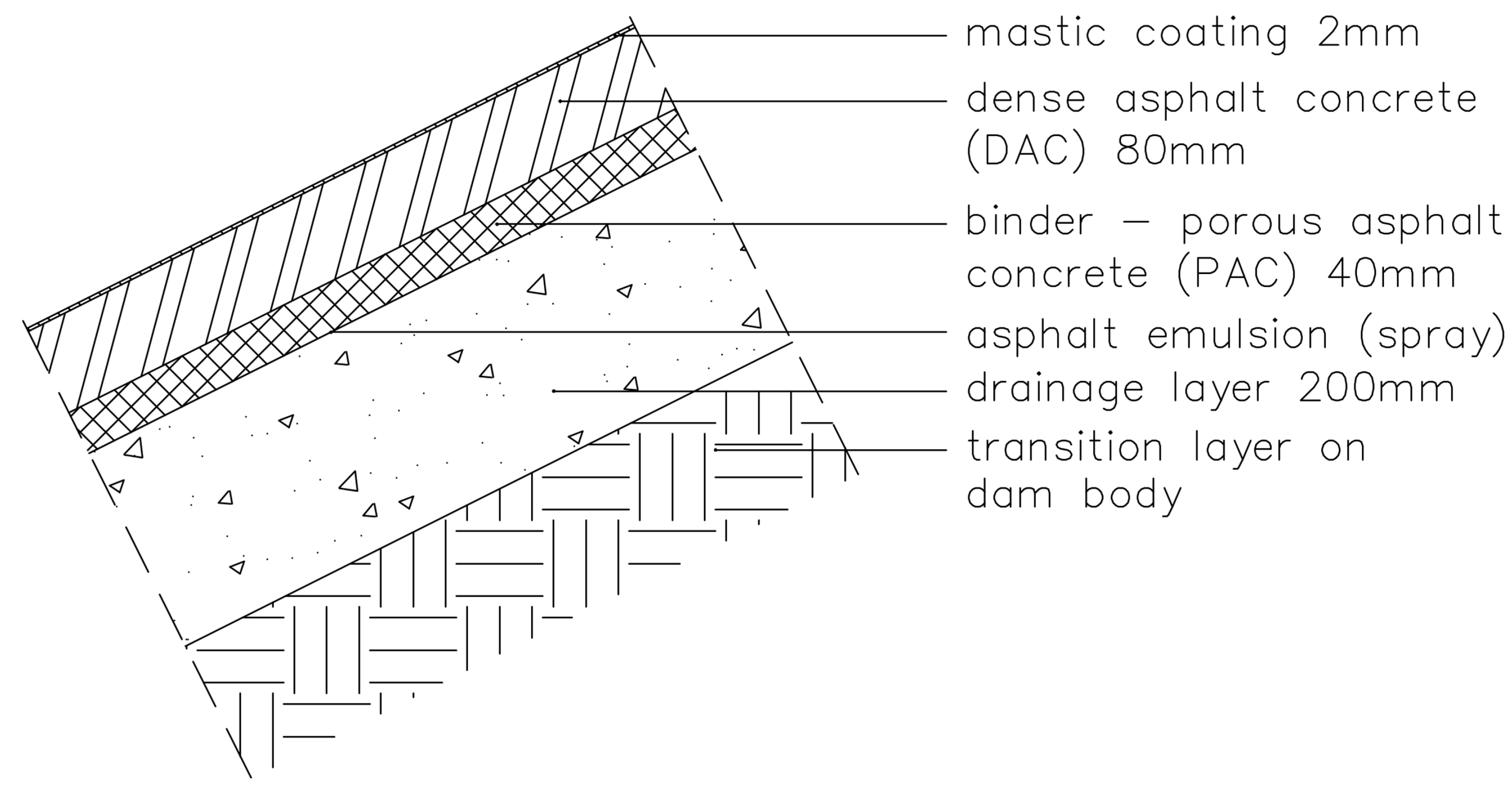
2.1. Description of the Study Area and Blisters
2.2. Sampling Method and Sampling Points
- Water from the upper reservoir. The water samples from the reservoir were taken into sterile bottles;
- Water, asphalt, asphalt with mastic remnants, and crumbled aggregate from inside the openings of two fully developed blisters in the damaged ACL. The samples were taken in two replicates. The water from the open blisters was taken using a sterile syringe. The solid samples were taken using sterile equipment (tweezers, hammers, chisels, and spatulas). The samples (pieces of asphalt concrete) were stored in sterile bottles or tubes. The equipment was wrapped in aluminium foil, sterilised via autoclaving, and transported in sterile plastic bags;
- Available components used for the preparation of ACL were taken from quarries: aggregates, sand, and pulverized limestone filler;
- A block of undamaged ACL (approximately 0.1 m wide × 0.1 m long × 0.12 m deep) was cut from the blister-free part of the lining using a sterilized titanium wheel. In the laboratory, the block was cut into slices with a sterilized disk after 3 h of pre-warming at 40 °C. Peeled samples of the ACL were taken from the upper face, including the mastic layer, the DAC at depths of 20 and 50 mm, the PAC (one sample), and the interface between the PAC and the drainage layer (back face, one sample). Mastic samples were taken from three different undamaged ACL elevations: one from the upper part of the slope, one from the middle zone subjected to water level fluctuation, and one from the lowest, permanently submerged part of the slope. The last sample was taken when the reservoir was emptied.
2.3. Isolation of DNA and Polymerase Chain Reaction (PCR)
2.4. Cultivation of Bacteria
2.5. MALDI-TOF MS Bacteria Identification
2.6. Data Analysis
3. Results
3.1. Demonstration of Bacterial Presence
3.2. Cultivation and Identification of Bacteria from the Undamaged ACL
3.3. Cultivation and Identification of Bacteria from the Damaged ACL
4. Discussion and Conclusions
4.1. Bacterial Strain Identification
4.2. Identification of Bacteria from the Undamaged ACL
4.3. Identification of Bacteria from the Damaged ACL
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swiss National Committee on Large Dams (SNCOLD). Longterm Behaviour of Bituminous and Cement Concrete Facings of Compensation Reservoirs in Switzerland; Question No. 61, R. 18; VII International Commission on Large Dams (ICOLD): San Francisco, CA, USA, 1988; pp. 311–325. [Google Scholar]
- Schonian, E. The Shell Bitumen Hydraulics Engineering Handbook; CD ROM Version; Thomas Telford: London, UK, 1999. [Google Scholar]
- Říha, J.; Buchtová, J. The asphalt and geomembrane dam lining database and its use at the Dlouhe Strane dam rehabilitation. In Proceedings of the Dams at the Beginning of the 21st Century, Annual Dresden Conference on Hydraulic Engineering, Dresden, Germany, 10–11 March 2005; Dresdner Wasserbauliche Mitteilungen: Dresden, Germany, 2005; pp. 277–286, ISBN 3-86005-461-9. [Google Scholar]
- Grätz, B.; Klimke, H.D.; Mock, P.; Seibert, C. Blasenbildung beim gussasphalt-einbau. einflussgrősse hohlraumgehalt der asphaltbinderschicht. Asphalt Jungle 2011, 46, 15–25. [Google Scholar]
- Wang, Z.; Hao, J.; Sun, Z.; Ma, B.; Xia, S.; Li, X. Blistering mechanism analysis of hydraulic asphalt concrete facing. Appl. Sci. 2019, 9, 2903. [Google Scholar]
- Adam, K.; Říha, J.; Špano, M. Investigation on the temperature of the asphalt-concrete facing of embankment dams. Int. J. Pav. Res. Technol. 2016, 9, 73–81. [Google Scholar]
- Sobolewski, T. PGE Energia Odnawialna SA; Monografia Elektrowni Szczytowo-Pompowej Porabka Žar Wydanie II: Biala, Polska, 2010. [Google Scholar]
- Akhtarpour, A.; Khodaii, A. Experimental study of asphaltic concrete dynamic properties as an impervious core in embankment dams. Constr. Build. Mater. 2013, 41, 319–334. [Google Scholar]
- Liu, H.; Yuan, G.; Zhang, Q.; Hao, P.; Dong, S.; Zhang, H. Study on influence factors of asphalt mixtures pavement blistering on Portland cement concrete bridge deck. Int. J. Pavement Eng. 2019. [Google Scholar] [CrossRef]
- Garcia-Gil, L.; Miró, R.; Pérez-Jiménez, F.E. Evaluating the role of aggregate gradation on cracking performance of asphalt concrete for thin overlays. Appl. Sci. 2019, 9, 628. [Google Scholar] [CrossRef]
- Teltayev, B.B.; Rossi, C.O.; Izmailova, G.G.; Amirbayev, E.D. Effect of freeze-thaw cycles on mechanical characteristics of bitumens and stone mastic asphalts. Appl. Sci. 2019, 9, 458. [Google Scholar] [CrossRef]
- Croll, J.A. A new hypothesis for the development of blisters in asphalt pavements. Int. J. Pavement Eng. 2008, 9, 59–67. [Google Scholar]
- Hironaka, M.C.; Holland, T.J. Blistering of Asphalt Pavement Overlay on Runway 14–32 at MCAS; NCEL Technical Note N-1744; NCEL: Beaufort, SC, USA, 1986. [Google Scholar]
- Brown, L.R.; Darnell, T.R. Factors Affecting the Microbial Deterioration of Asphalt Overlays; Final Report to Mississippi State Highway Department; Report NO. MSHD-RD-86-84; Federal Highway Administration: Washington, DC, USA, 1986; p. 43.
- Brown, L.R.; Darnell, T.R. Blistering of asphalt overlays caused by microorganisms. J. Assoc. Asphalt Paving Technol. (AAPT) 1987, 56, 361–380. [Google Scholar]
- Phillips, U.H.; Traxler, R.W. Microbial degradation of asphalt. Appl. Microbiol. 1963, 11, 235–238. [Google Scholar]
- Pendrys, J.P. Biodegradation of asphalt cement-20 by aerobic bacteria. Appl. Environ. Microbiol. 1989, 55, 1357–1362. [Google Scholar]
- Koul, S.; Fulekar, M.H. Petrochemical industrial waste: Bioremediation techniques. Int. J. Adv. Res. Technol. 2013, 2, 211–257. [Google Scholar]
- Kim, J.S.; Crowley, D.E. Microbial diversity in natural asphalts of the Rancho La Brea Tar Pits. Appl. Environ. Microbiol. 2007, 73, 4579–4591. [Google Scholar] [CrossRef][Green Version]
- Balcon, I.N.; Crowley, D.E. Microbial diversity of asphalt-soil mixtures in the Rancho LA Brea asphalt seeps. Int. J. Phytoremediat. 2015, 12, 599–615. [Google Scholar]
- Hernández-López, E.L.; Ayala, M.; Vazques-Duhalt, R. Microbial and enzymatic biotransformation of asphaltenes. Pet. Sci. Technol. 2015, 33, 1017–1029. [Google Scholar] [CrossRef]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [PubMed]
- Hesham, A.E.-L.; Alamri, S.A.; Khan, S.; Mahmoud, M.E.; Mahmoud, H.M. Isolation and molecular genetic characterization of a yeast strain able to degrade petroleum polycyclic aromatic hydrocarbons. Afr. J. Biotechnol. 2009, 8, 2218–2223. [Google Scholar]
- Lawal, A.T. Polycyclic aromatic hydrocarbons. A review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar]
- Španová, A.; Rittich, B.; Štyriak, I.; Štyriaková, I.; Horák, D. Isolation of polymerase chain reaction-ready bacterial DNA from Lake Baikal sediments by carboxyl-functionalised magnetic polymer microspheres. J. Chromatogr. A 2006, 1130, 115–121. [Google Scholar] [CrossRef]
- Haarman, M.; Knol, J. Quantitative real-time PCR analysis of fecal Lactobacillus species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 2006, 72, 2359–2365. [Google Scholar]
- Freiwald, A.; Sauer, S. Phylogenetic classification and identification of bacteria by mass spectrometry. Nat. Protoc. 2009, 4, 732–742. [Google Scholar] [CrossRef]
- Peng, F.; Liu, Z.; Wang, L.; Shao, Z. An oil-degrading bacterium: Rhodococcus erythropolis strain 3C-9 and its biosurfactants. J. Appl. Microbiol. 2007, 102, 1603–1611. [Google Scholar] [CrossRef]
- Schippers, A.; Bosecker, K.; Sproer, C.; Schumann, P. Microbacterium oleivorans sp. nov. and Microbacterium hydrocarbonoxydans sp. nov., novel crude-oil-degrading Gram-positive bacteria. Int. J. Syst. Evol. Microbiol. 2005, 55, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Golby, S.; Ceri, H.; Gieg, L.M.; Chatterjee, I.; Marques, L.L.R.; Turner, R.J. Evaluation of microbial biofilm communities from an Alberta oil sands tailing pond. FEMS Microbiol. Ecol. 2012, 79, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef] [PubMed]
- Sauka, D.H.; Basurto-Ríos, R.E.; Ibarra, J.E.; Benintende, G.B. Characterization of an Argentine isolate of Bacillus thuringiensis similar to the HD-1 strain. Neotrop. Entomol. 2010, 39, 767–773. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gallegos-Monterrosa, R.; Kankel, S.; Götze, S.; Barnett, R.; Stallforth, P.; Kovács, Á.T. Lysinibacillus fusiformis M5 induces increased complexity in Bacillus subtilis 168 colony biofilms via hypoxanthine. J. Bacteriol. 2017, 199, e00204-17. [Google Scholar] [CrossRef]
- van Dijl, J.M.; Hecker, M. Bacillus subtilis: From soil bacterium to super-secreting cell factory. Microb. Cell Fact. 2013, 12, 3. [Google Scholar] [CrossRef]
- Kwon, S.W.; Kim, J.S.; Park, I.C.; Yoon, S.H.; Park, D.H.; Lim, C.K.; Go, S.J. Pseudomonas koreensis sp. nov., Pseudomonas umsongensis sp. nov. And Pseudomonas jinjuensis sp. nov., novel species from farm soils in Korea. Int. J. Syst. Evol. Microbiol. 2003, 53, 21–27. [Google Scholar] [CrossRef]
- Calderón, C.E.; Ramos, C.; de Vicente, A.; Cazorla, F.M. Comparative genomic analysis of Pseudomonas chlororaphis PCL 1606 reveals new insight into antifungal compounds involved in biocontrol. Mol. Plant Microbe Interact. 2015, 28, 249–260. [Google Scholar] [CrossRef]
- Keskin, N.O.S.; Han, D.; Ozkan, A.D.; Angun, P.; Umu, O.C.O.; Tekinay, T. Production and structural characterization of biosurfactant produced by newly isolated Staphylococcus xylosus STF1 from petroleum contaminated soil. J. Petrol. Sci. Eng. 2015, 133, 689–694. [Google Scholar] [CrossRef]
- Ahmed, I.; Yokota, A.; Yamazoe, A.; Fujiwara, T. Proposal of Lysinibacillus boronitolerans gen. nov. sp. nov., and transfer of Bacillus fusiformis to Lysinibacillus fusiformis comb. nov. and Bacillus sphaericus to Lysinibacillus sphaericus comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 1117–1125. [Google Scholar] [CrossRef]
- Josic, D.; Porobic, M.; Milicevic, M.; Vukovic, D.; Pivic, R.; Zdravkovic, M.; Coric, T. RAPD fingerprinting of indigenous Lysinibacillus fusiformis isolates from stabilized sludge and oil-polluted soil. In Proceedings of the 6th International Meeting on Soil Fertility Land Management and Agroclimatology, Kusadasi-Aydin, Turkey, 29–31 October 2008; pp. 927–933. [Google Scholar]
- Tuleva, B.; Christova, N.; Cohen, R.; Antonova, D.; Todorov, T.; Stoineva, I. Isolation and characterization of trehalose tetraester biosurfactants from a soil strain Micrococcus luteus BN56. Process Biochem. 2009, 44, 135–141. [Google Scholar] [CrossRef]
- Ting, A.S.Y.; Tan, H.C.; Aw, C.S. Hydrocarbon-degradation by Isolate Pseudomanas lundensis UTAR FPE2. Malays. J. Microbiol. 2009, 5, 104–108. [Google Scholar]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 211, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Basu, B.; Ramasamy, R.; Viswanathan, S.; Priya, I.D.; Jeyan, J.; Reddy, A.; Kumar, V.V. Analysis of aromatic hydrocarbon degrading capacity by thermophilic bacteria isolated from oil contaminated soil. Int. J. ChemTech Res. 2014, 6, 4556–4563. [Google Scholar]
- Rabus, R.; Boll, M.; Heider, J.; Meckenstock, R.U.; Buckel, W.; Einsle, O.; Ermler, U.; Golding, B.T.; Gunsalus, R.P.; Kroneck, P.M.; et al. Anaerobic microbial degradation of hydrocarbons. From enzymatic reactions to the environment. J. Mol. Microbiol. Biotechnol. 2016, 26, 5–28. [Google Scholar] [CrossRef]
- Filatov, D.; Kopytov, M.; Ovsyannikova, V.; Elchaninova, E. Microbial oxidation of high viscosity bitumen in soil. Eurasian Chem. Technol. J. 2018, 20, 159–169. [Google Scholar] [CrossRef]
- Vinithini, O.; Sudhakar, S.; Ravikumar, R. Biodegradation of petroleum and crude oil by Pseudomonas putida and Bacillus cereus. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 318–329. [Google Scholar]
- Kim, D.; Choi, K.Y.; Yoo, M.; Zylstra, G.J.; Kim, E. Biotechnological potential of Rhodococcus biodegradative pathways. J. Microbiol. Biotechnol. 2018, 28, 1037–1051. [Google Scholar] [CrossRef]
- Olowomofe, T.O.; Oluyege, J.O.; Aderiye, B.I.; Oluwole, O.A. Degradation of poly aromatic fractions of crude oil and detection of catabolic genes in hydrocarbon-degrading bacteria isolated from Agbabu bitumen sediments in Ondo State. AIMS Microbiol. 2019, 5, 308–323. [Google Scholar] [CrossRef]
- Bento, F.M.; de Oliveira Camargo, F.A.; Okeke, B.C.; Frankenberger, W.T. Diversity of biosurfactant producing microorganisms isolated from soils contaminated with diesel oil. Microbiol. Res. 2005, 160, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Patowary, K.; Patowary, R.; Kalita, M.C.; Deka, S. Characterisation of biosurfactant produced during degradation of hydrocarbons using crude oil as sole source of carbon. Front. Microbiol. 2017, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Soare, M.G.; Lakatos, E.S.; Ene, N.; Malo, N.; Popa, O.; Băbeanu, N. The potential applications of Bacillus sp. and Pseudomonas sp. strains with antimicrobial activity against phytopathogens, in waste oils and the bioremediation of hydrocarbons. Catalysts 2019, 9, 959. [Google Scholar] [CrossRef]
- Truskewycz, A.; Gundry, T.D.; Khudur, L.S.; Kolobaric, A.; Taha, M.; Aburto-Medina, A.; Ball, A.S.; Shahsavari, E. Petroleum hydrocarbon contamination in terrestrial ecosystems fate and microbial responses. Molecules 2019, 24, 3400. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.; Jain, K.; Madamwar, D. Metabolism of pyrene through phthalic acid pathway by enriched bacterial consortium composed of Pseudomonas, Burkholderia, and Rhodococcus (PBR). Biotechnology 2017, 7, 29. [Google Scholar] [CrossRef]
- Dasgupta, D.; Jublee, J.; Suparna, M. Characterization, phylogenetic distribution and evolutionary trajectories of diverse hydrocarbon degrading microorganisms isolated from refinery sludge. Biotechnology 2018, 8, 273. [Google Scholar] [CrossRef]
- Hagai, E.; Dvora, R.; Havkin-Blank, T.; Zelinger, E.; Porat, Z.; Schulz, S.; Helman, Z. Surface-mobility induction, attraction and hitchhiking between bacterial species promote dispersal on solid surfaces. ISME J. 2014, 8, 1147–1151. [Google Scholar] [CrossRef]

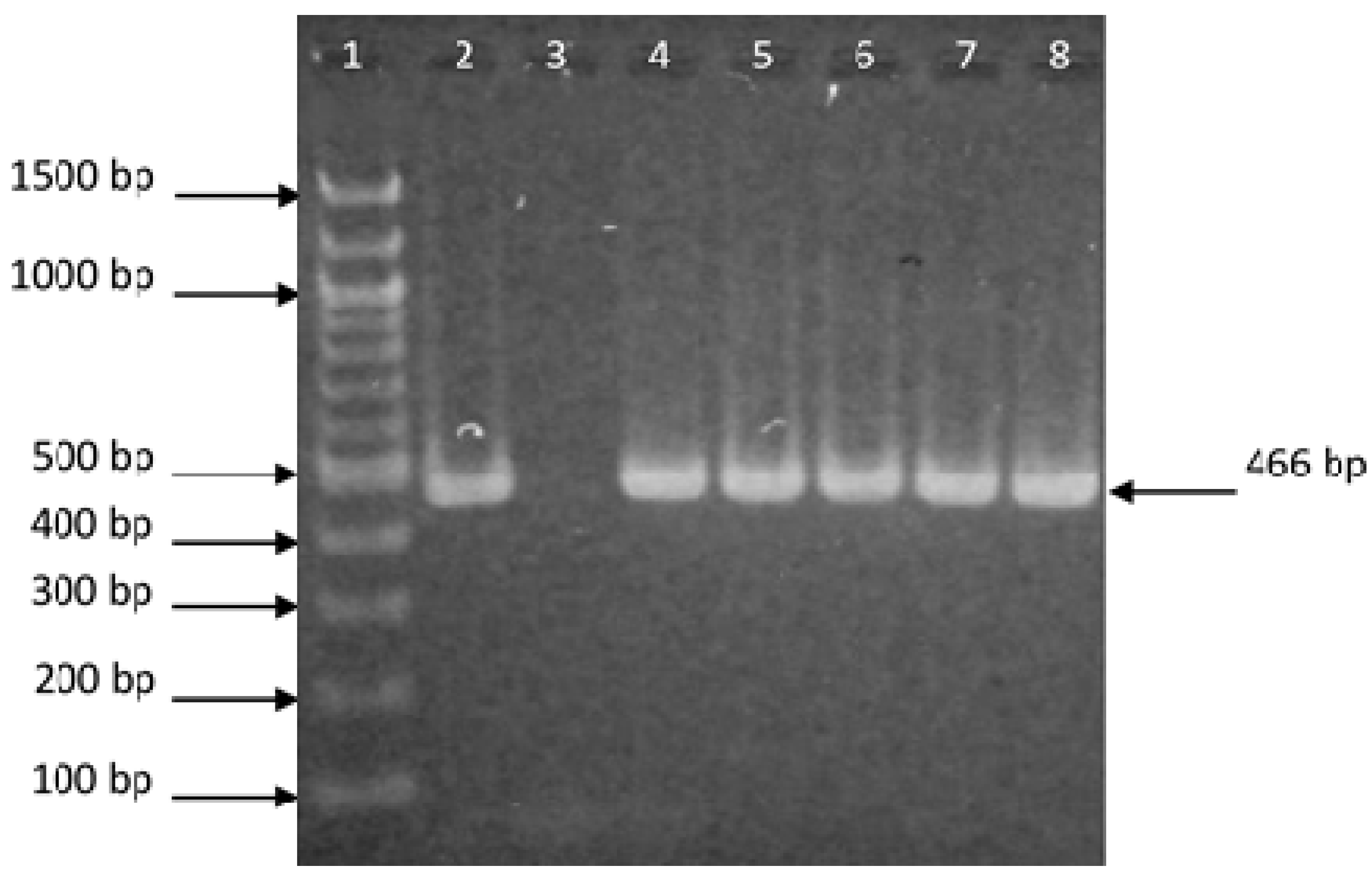
| Resource | Source of Bacteria/Sample | PCR Product Detection | Growth (°C) | |||
|---|---|---|---|---|---|---|
| 10 | 30 | 37 | 50 | |||
| Water | Reservoir | + | + | + | - | - |
| Damaged ACL, open blister, inside hole | Water | + | + | + | + | + |
| Asphalt | + | + g | + | + | + | |
| Asphalt with mastic remnants | + | + | + g | + g | + g | |
| Crumbled aggregate | + | + | + | + | + | |
| ACL components | Aggregates | + | + | + | + | - |
| Sand | + | + | + | + | + | |
| Pulverized limestone filler | + | + | + | + | - | |
| Block of ACL, blister-free (undamaged) | Upper face with mastic layer | + | + | + | + | + |
| 20 mm depth inner portion of DAC | + | - | - | - | - | |
| 50 mm depth inner portion of DAC | + | - | - | - | - | |
| 100 mm depth inside of PAC | + | - | - | - | - | |
| Asphalt from PAC layer—back face | + | + | + | - | - | |
| Undamaged ACL mastic, different elevations | Upper part without reservoir water | + | + | + | + | + |
| Middle zone, alternately under and above water | + | + | + | + g | + g | |
| Lowest part, permanently under water | + | + | + | + | + g | |
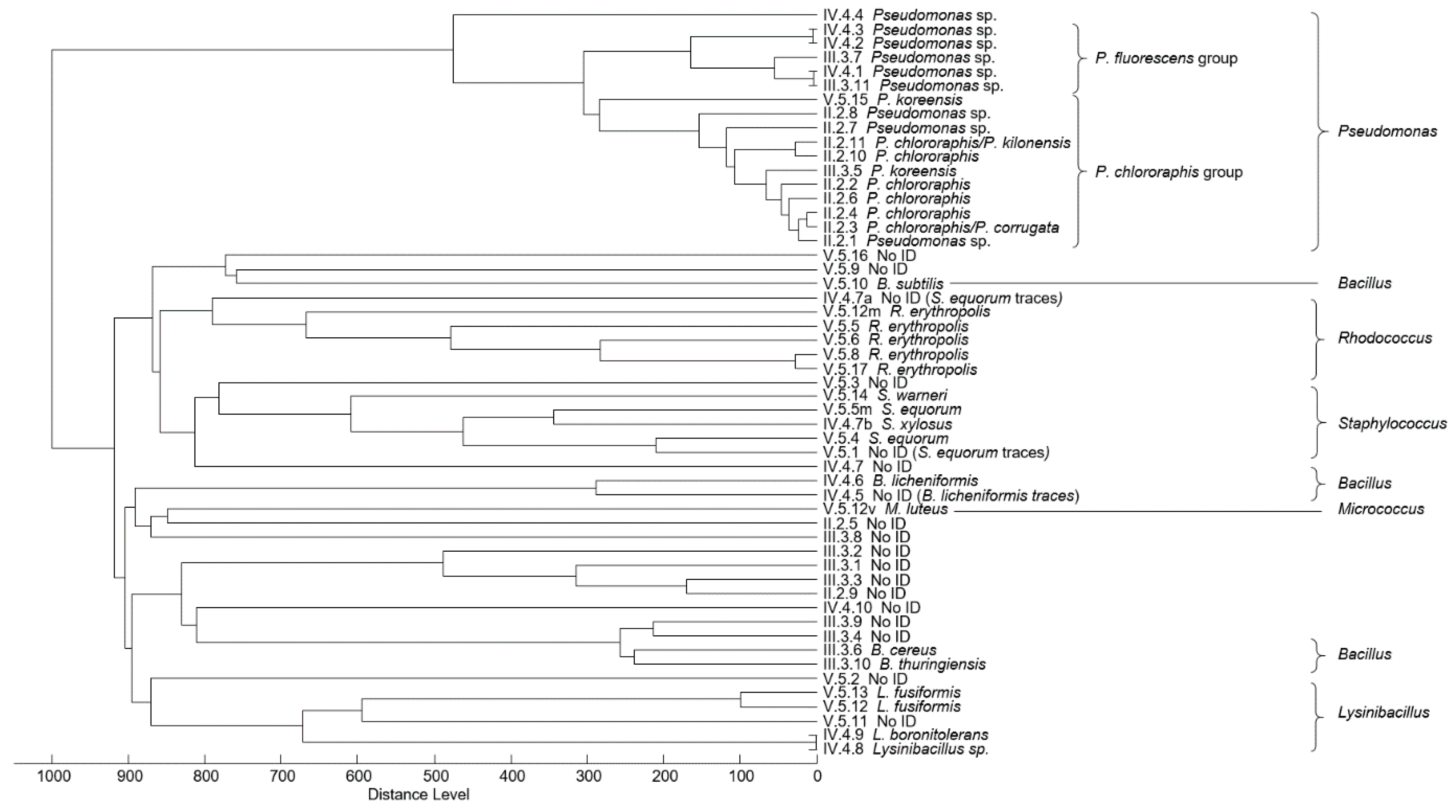
| Resource | Source of Bacteria/Group | Morpho Types | Genus Identification | Species Identification/ No. of Strains | Ambiguous Identification/ No. of Strains | Without Identification/ No. of Strains |
|---|---|---|---|---|---|---|
| Water | Reservoir/I | 7 | Pseudomonas | P. chlororaphis P. fragi | Pseudomonas (4) a | 1 |
| Damaged ACL, open blister, inside hole | Water/II | 11 | Pseudomonas | P.chlororaphis (4) | Pseudomonas (5) b | 2 |
| Asphalt/III | 11 | Pseudomonas Bacillus | P. koreensis B. cereus B. thuringiensis | Pseudomonas (2) c1 Bacillus (1) c2 | 6 | |
| Asphalt with mastic remnants/IV | 12 | Pseudomonas Bacillus Lysinibacillus Staphylococcus | B. licheniformis L. fusiformis L. boronitolerans (4.9 g) S. xylosus | Pseudomonas (4) d1 Lysinibacillus (1) d2 | 4 (4.10 g) | |
| Crumbled aggregate/V | 19 | Pseudomonas Bacillus Lysinibacillus Staphylococcus Rhodococcus Micrococcus | P. koreensis B. subtilis L. fusiformis (2) S. equorum (2) S. warneri R. erytropolis (5) M. luteus | Pseudomonas (1) e | 6 | |
| ACL components | Aggregates/VI | 4 | Pseudomonas Bacillus Lysinibacillus | P. chlororaphis B. licheniformis L. fusiformis | 1 | |
| Sand/VII | 9 | Pseudomonas Lysinibacillus Aeromonas | P. chlororaphis P. frederiksbergenesis L. sphaericus L. fusiformis (3) A. salmonicida | Pseudomonas (2) f1 | ||
| Pulverized limestone filler/VIII | 2 | Bacillus | Bacillus (2) f2 | |||
| Block of ACL, blister-free (undamaged) | Upper face with mastic layer/IX | 4 | Bacillus | B. licheniformis (2) B. pumilus | 1 | |
| Asphalt from PAC layer back face/X | 3 | Microbacterium Micrococcus | M. hydrocarbonooxydans (2) M. luteus | |||
| Undamaged ACL mastic, different elevations | Upper part without reservoir water/XI | 2 | 2 | |||
| Middle zone, alternately under and above water/XII | 16 | Bacillus Staphylococcus | B. licheniformis (2) B. cereus B. pumilus S. equorum (3) | 9 (8.11 g) | ||
| Lowest part, permanently under water/XIII | 4 | Bacillus | B. thuringiensis (2) (8.2 g, 8.3 g) | 2 (8.1 g) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Špano, M.; Říha, J.; Španová, A.; Šedo, O.; Rittich, B. Bacterial Diversity in the Asphalt Concrete Lining of the Upper Water Reservoir of a Pumped-Storage Scheme. Water 2020, 12, 3045. https://doi.org/10.3390/w12113045
Špano M, Říha J, Španová A, Šedo O, Rittich B. Bacterial Diversity in the Asphalt Concrete Lining of the Upper Water Reservoir of a Pumped-Storage Scheme. Water. 2020; 12(11):3045. https://doi.org/10.3390/w12113045
Chicago/Turabian StyleŠpano, Miroslav, Jaromír Říha, Alena Španová, Ondrej Šedo, and Bohuslav Rittich. 2020. "Bacterial Diversity in the Asphalt Concrete Lining of the Upper Water Reservoir of a Pumped-Storage Scheme" Water 12, no. 11: 3045. https://doi.org/10.3390/w12113045
APA StyleŠpano, M., Říha, J., Španová, A., Šedo, O., & Rittich, B. (2020). Bacterial Diversity in the Asphalt Concrete Lining of the Upper Water Reservoir of a Pumped-Storage Scheme. Water, 12(11), 3045. https://doi.org/10.3390/w12113045







