Adsorption of Organic Pollutants from Cold Meat Industry Wastewater by Electrochemical Coagulation: Application of Artificial Neural Networks
Abstract
1. Introduction
2. Materials and Methods
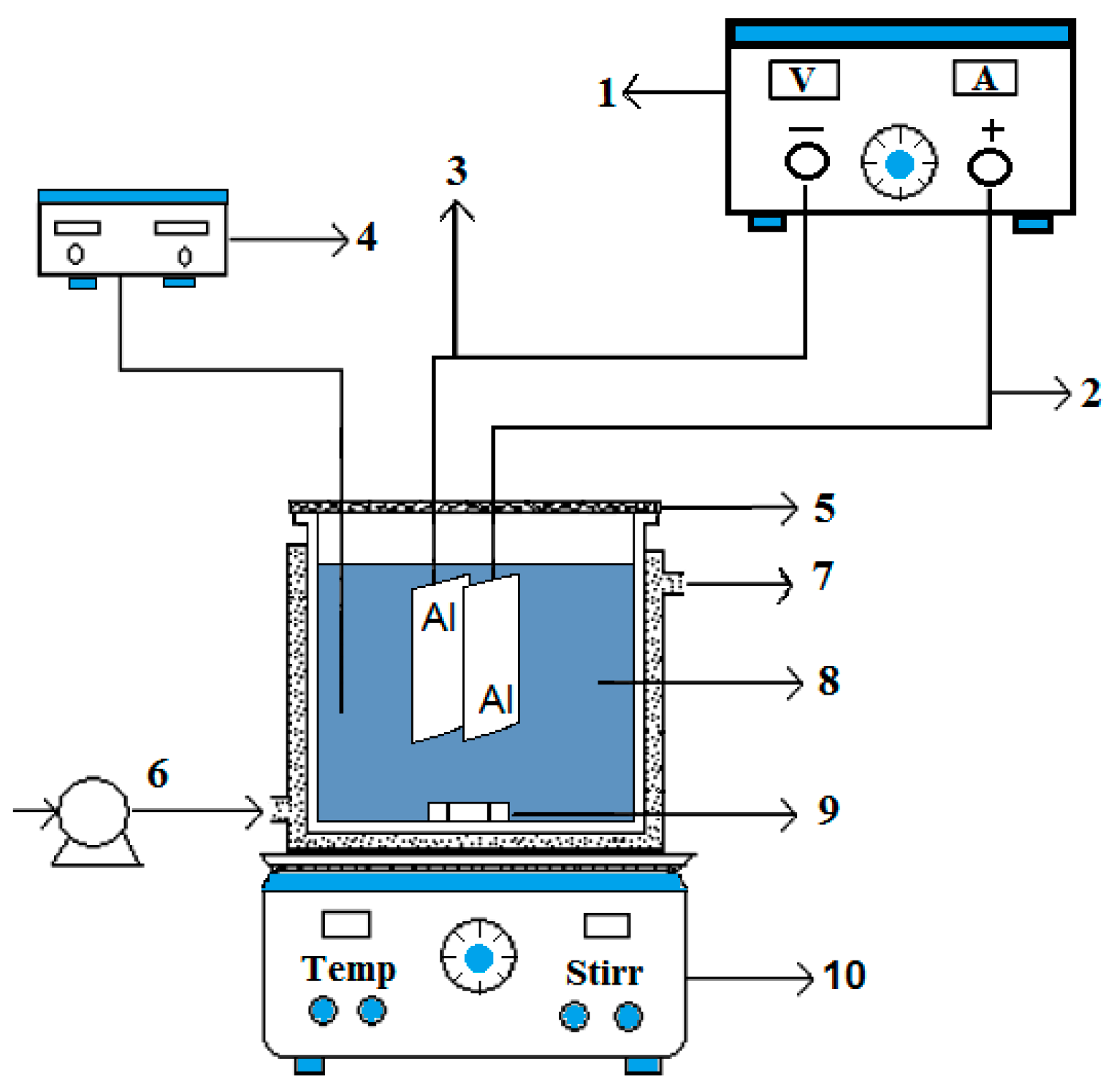
2.1. Electrochemical Monopolar Cell
2.2. Wastewater Sampling and Analyses
2.3. Experimental Setup
2.4. Development of Theoretical Models
3. Results and Discussion
3.1. Characterization of Wastewater
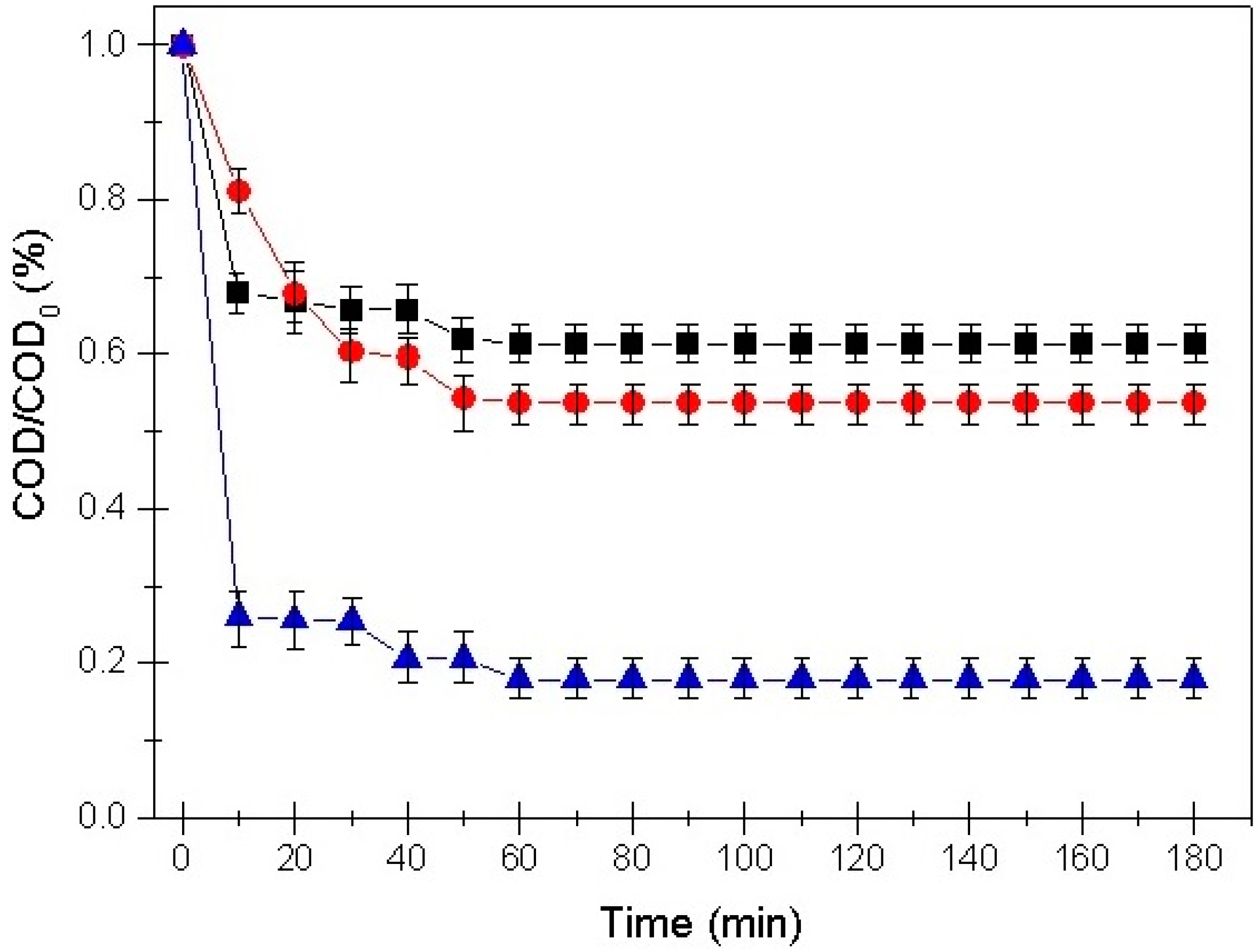
3.2. Adsorption Kinetic Models
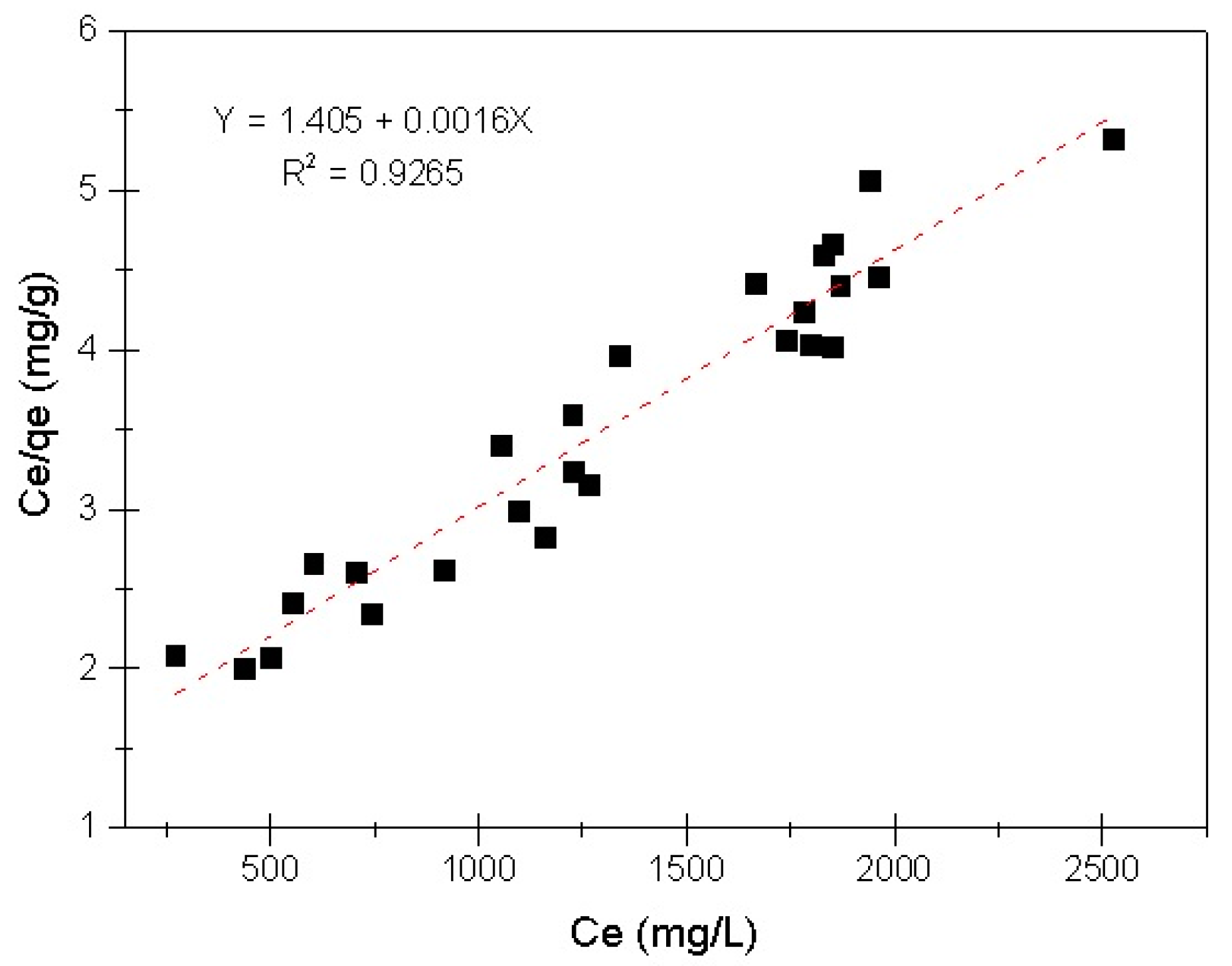
3.3. Evaluation of Adsorption Capacity
3.4. Influence of Initial pH on Removal Efficiency
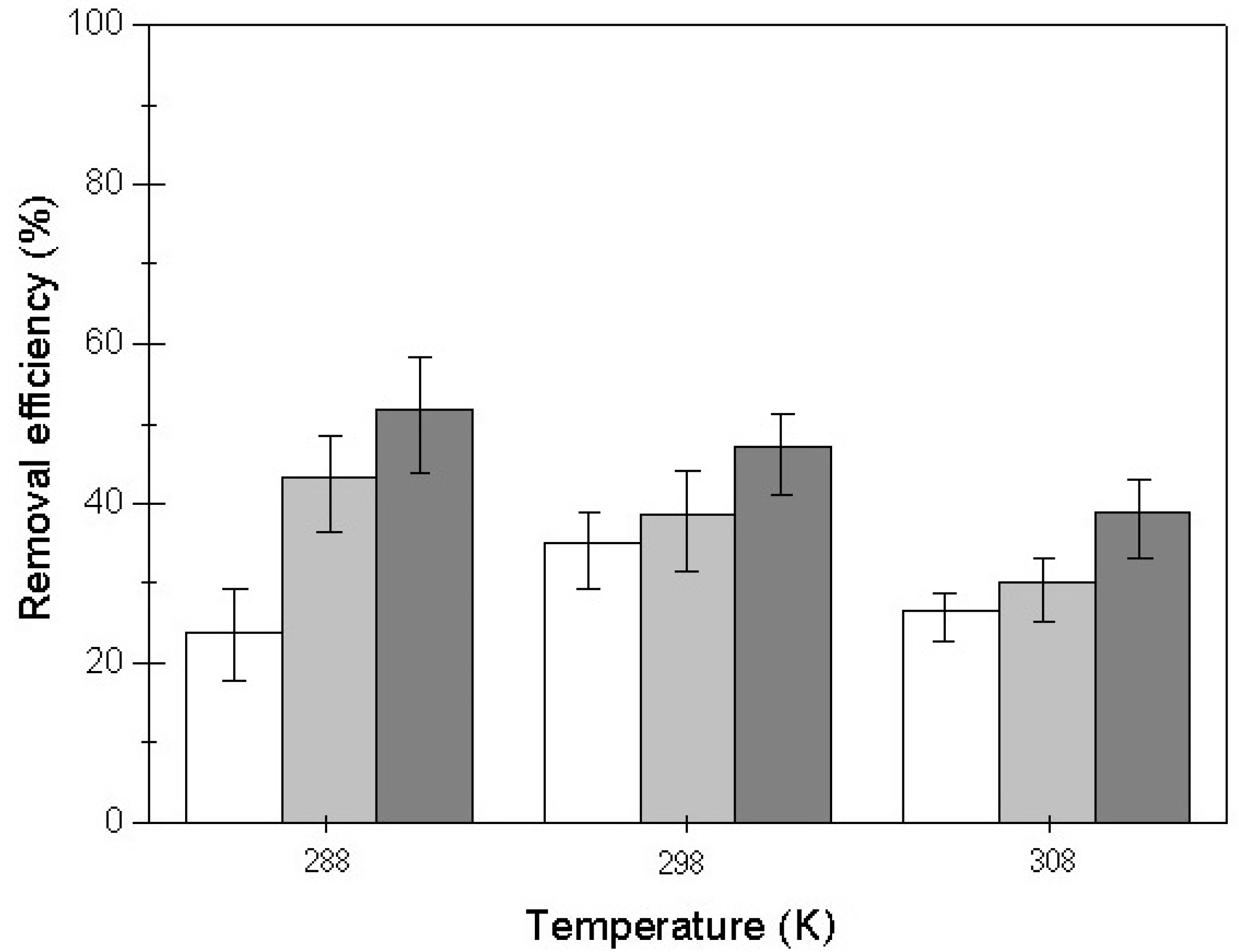
3.5. Effect of Temperature on the Adsorption Process
3.6. Proposed Mechanism for Adsorption
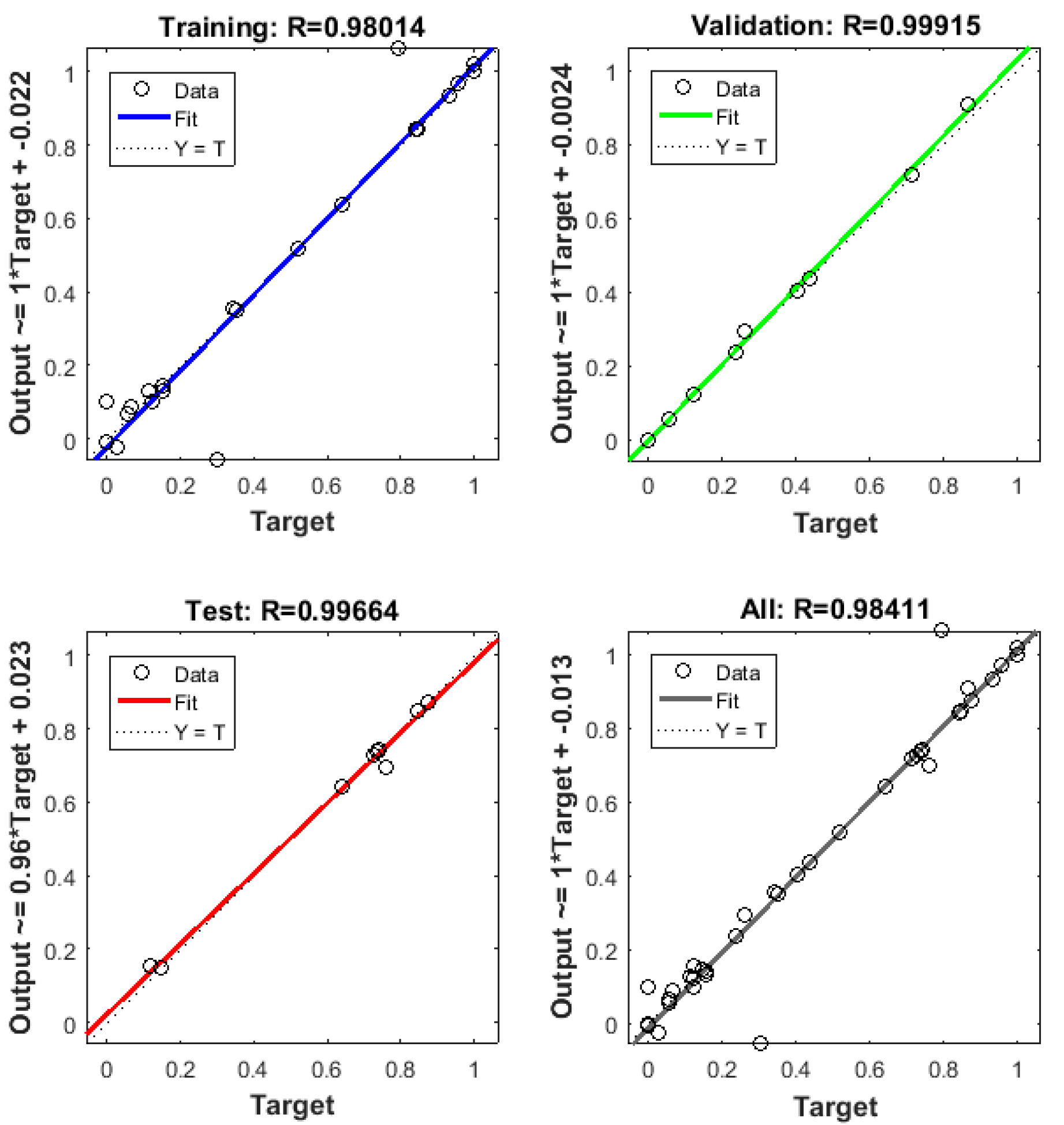
3.7. Neural Network Modeling
4. Conclusions
- Subsequent to employing Freundlich and Langmuir equations to fit the results, the linearized Langmuir isotherm equation was identified to be the principal fitting for the COD/EC system.
- The maximum adsorption capacity of COD by EC (qmax) was found to be 621.11 mg g−1. This value is similar to that obtained when treating diverse types of organic pollutants employing the EC process, which indicates the importance of doing further research into this application.
- The order of relevance for analyzed parameters was current density, initial pH, and contact time. In this case, the current density is the control parameter for COD adsorption by EC, which explained the mechanism through the adsorption rate.
- The pseudo-second-order kinetic equation permitted the accurate prediction of process trends and supported an indispensable comprehension of the treatment for possible upscaling the adsorption process.
- The best performance for ANN was found using 10 neurons in the hidden layer. This achieved a notable representation of all experimental data. With this ANN model, the better COD removal efficiency was 3262.7 mg L−1 (93.22%) with R2 = 0.984 under conditions of current density = 6 mA cm−2, pH = 7.8, EC = 30.22 min, and temperature = 298 K.
- The predominant mechanism proposed in this system is the adsorption mechanism, since when is increase the current density, improve the adsorption of COD. This no happened when are modified pH values, and therefore charge neutralization mechanism doesn’t seem to be relevant.
- These data indicated good removal efficiency of COD when the EC treatment is utilized, and ANN models are adequate for describing the adsorption behavior.
Author Contributions
Funding
Conflicts of Interest
References
- Hernández-Ramírez, D.A.; Herrera-López, E.J.; Rivera, A.L.; del Real-Olvera, J. Artificial Neural Network Modeling of Slaughterhouse Wastewater Removal of COD and TSS by Electrocoagulation; Springer International Publishing: Cham, Switzerland, 2014. [Google Scholar]
- León-Becerril, E.; García-Camacho, J.E.; del Real-Olvera, J.; López-López, A. Performance of an upflow anaerobic filter in the treatment of cold meat industry wastewater. Process Saf. Environ. Prot. 2016, 102, 385–391. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V.; Maran, J.P. Response surface modelling and optimization of treatment of meat industry wastewater using electrochemical treatment method. J. Taiwan Inst. Chem. Eng. 2015, 46, 160–167. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.; Bilyeu, B.; Roa, G.; Bernal-Martinez, L. Physicochemical Aspects of Electrocoagulation. Sep. Purif. Rev. 2011, 40, 1–24. [Google Scholar] [CrossRef]
- Sillanpää, M.; Shestakova, M. Chapter 2—Electrochemical Water Treatment Methods. In Electrochemical Water Treatment Methods; Sillanpää, M., Shestakova, M., Eds.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 47–130. [Google Scholar]
- Yasri, N.G.; Gunasekaran, S. Electrochemical Technologies for Environmental Remediation. In Enhancing Cleanup of Environmental Pollutants: Volume 2: Non-Biological Approaches; Anjum, N., Gill, S.S., Tuteja, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 5–73. [Google Scholar]
- Wang, J.; Shih, Y.; Wang, P.Y.; Yu, Y.H.; Su, J.F.; Huang, C.-P. Hazardous waste treatment technologies. Water Environ. Res. 2019, 91, 1177–1198. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Srivastava, V.C.; Mall, I.D. Electrochemical Treatment of Dye Bearing Effluent with Different Anode–Cathode Combinations: Mechanistic Study and Sludge Analysis. Ind. Eng. Chem. Res. 2014, 53, 10743–10752. [Google Scholar] [CrossRef]
- Ghazouani, M.; Akrout, H.; Jellali, S.; Bousselmi, L. Comparative study of electrochemical hybrid systems for the treatment of real wastewaters from agri-food activities. Sci. Total Environ. 2019, 647, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Tirado, L.; Gökkuş, Ö.; Brillas, E.; Sirés, I. Treatment of cheese whey wastewater by combined electrochemical processes. J. Appl. Electrochem. 2018, 48, 1307–1319. [Google Scholar] [CrossRef]
- Tolgayılmaz, M.; Bayar, S.; Özcan, S. Treatment of sugar industry wastewater by electrocoagulation using Fe and Al electrodes: A comparative study. Desalin. Water Treat. 2018, 131, 206–211. [Google Scholar] [CrossRef]
- Cañizares, P.; Carmona, M.; Lobato, J.; Martínez, F.; Rodrigo, M.A. Electrodissolution of Aluminum Electrodes in Electrocoagulation Processes. Ind. Eng. Chem. Res. 2005, 44, 4178–4185. [Google Scholar] [CrossRef]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of wastewater by electrocoagulation: A review. Environ. Sci. Pollut. Res. Int. 2014, 21, 2397–2413. [Google Scholar] [CrossRef]
- Reed, R.; MarksII, R.J. Neural Smithing: Supervised Learning in Feedforward Artificial Neural Networks; MIT Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Sebti, A.; Boutra, B.; Trari, M.; Aoudjit, L.; Igoud, S. Application of Artificial Neural Network for Modeling Wastewater Treatment Process. In Smart Energy Empowerment in Smart and Resilient Cities; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Valente, G.F.S.; Mendonca, R.C.S.; Pereira, J.A.M.; Felix, L.B. Artificial neural network prediction of chemical oxygen demand in dairy industry effluent treated by electrocoagulation. Sep. Purif. Technol. 2014, 132, 627–633. [Google Scholar] [CrossRef]
- Morales-Rivera, J.; Sulbarán-Rangel, B.; Gurubel-Tun, K.J.; del Real-Olvera, J.; Zúñiga-Grajeda, V. Modeling and Optimization of COD Removal from Cold Meat Industry Wastewater by Electrocoagulation Using Computational Techniques. Processes 2020, 8, 1139. [Google Scholar] [CrossRef]
- Nasr, M.; Ateia, M.; Hassan, K. Artificial intelligence for greywater treatment using electrocoagulation process. Sep. Sci. Technol. 2016, 51, 96–105. [Google Scholar] [CrossRef]
- Besharati Fard, M.; Mirbagheri, S.A.; Pendashteh, A.; Alavi, J. Biological treatment of slaughterhouse wastewater: Kinetic modeling and prediction of effluent. J. Environ. Health Sci. Eng. 2019, 17, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Gadekar, M.R.; Ahammed, M.M. Modelling dye removal by adsorption onto water treatment residuals using combined response surface methodology-artificial neural network approach. J. Environ. Manag. 2019, 231, 241–248. [Google Scholar] [CrossRef]
- Aber, S.; Amani-Ghadim, A.R.; Mirzajani, V. Removal of Cr(VI) from polluted solutions by electrocoagulation: Modeling of experimental results using artificial neural network. J. Hazard. Mater. 2009, 171, 484–490. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Shine, K. Investigation on the Removal of Chromium from Wastewater using Electrocoagulation. Int. J. Chem. React. Eng. 2018, 16. [Google Scholar] [CrossRef]
- Hasani, G.; Daraei, H.; Shahmoradi, B.; Gharibi, F.; Maleki, A.; Yetilmezsoy, K.; McKay, G. A novel ANN approach for modeling of alternating pulse current electrocoagulation-flotation (APC-ECF) process: Humic acid removal from aqueous media. Process Saf. Environ. Prot. 2018, 117, 111–124. [Google Scholar] [CrossRef]
- Peralta-Hernández, J.M.; Picos, A. Genetic algorithm and artificial neural network model for prediction of discoloration dye from an electro-oxidation process in a press-type reactor. Water Sci. Technol. 2018, 78, 925–935. [Google Scholar] [CrossRef]
- Da Silva Ribeiro, T.; Grossi, C.D.; Merma, A.G.; Santos, B.F.D.; Torem, M.L. Removal of boron from mining wastewaters by electrocoagulation method: Modelling experimental data using artificial neural networks. Miner. Eng. 2019, 131, 8–13. [Google Scholar] [CrossRef]
- David, C.; Thangavelu, A. Degradation of distillery effluent by twisted-type Iron electrodes: Experimental with ANN approach. Int. J. Environ. Anal. Chem. 2020, 1–13. [Google Scholar] [CrossRef]
- Kuokkanen, V.; Kuokkanen, T.; Rämö, J.; Lassi, U. Recent Applications of Electrocoagulation in Treatment of Water and Wastewater-A Review. Green Sustain. Chem. 2013, 3, 89–121. [Google Scholar] [CrossRef]
- Baird, R.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Kaur, P.; Sangal, V.K.; Kushwaha, J.P. Modeling and evaluation of electro-oxidation of dye wastewater using artificial neural networks. RSC Adv. 2015, 5, 34663–34671. [Google Scholar] [CrossRef]
- Mirsoleimani-azizi, S.M.; Amooey, A.A.; Ghasemi, S.; Salkhordeh-panbechouleh, S. Modeling the Removal of Endosulfan from Aqueous Solution by Electrocoagulation Process Using Artificial Neural Network (ANN). Ind. Eng. Chem. Res. 2015, 54, 9844–9849. [Google Scholar] [CrossRef]
- Betiku, E.; Odude, V.O.; Ishola, N.B.; Bamimore, A.; Osunleke, A.S.; Okeleye, A.A. Predictive capability evaluation of RSM, ANFIS and ANN: A case of reduction of high free fatty acid of palm kernel oil via esterification process. Energy Convers. Manag. 2016, 219–230. [Google Scholar] [CrossRef]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of electrocoagulation in wastewater treatment: A developmental review. J. Water Process Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Tawalbeh, M.; Al-Shannag, M.; Al-Anber, Z.; Bani-Melhem, K. Combined electrocoagulation processes as a novel approach for enhanced pollutants removal: A state-of-the-art review. Sci. Total Environ. 2020, 744, 140806. [Google Scholar] [CrossRef]
- Bekkari, N.; Zeddouri, A. Using artificial neural network for predicting and controlling the effluent chemical oxygen demand in wastewater treatment plant. Manag. Environ. Qual. Int. J. 2019, 30, 593–608. [Google Scholar] [CrossRef]


| Factor | Variable | Coded Variables | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| A | Initial pH (P.U) 1 | 5 | 7 | 9 |
| B | Current density (mA cm−2) | 2 | 4 | 6 |
| C | EC time (min) | 20 | 40 | 60 |
| Parameter | Units | Mean Value ±SD 1 | Method |
|---|---|---|---|
| pH | P.U 2 | 5 ± 0.2 | pH-meter |
| Turbidity | NTU 4 | 423 ± 34.2 | 2130B |
| Color | u. Pt-Co 3 | 8043 ± 805 | 2120C |
| BOD | mg L−1 | 2035 ± 264 | 5210B |
| COD | mg L−1 | 3500 ± 125 | 5220D |
| FOG | mg L−1 | 1114 ± 220 | 5520D |
| TSS | mg L−1 | 776 ± 140 | 2540D |
| Total phosphorus | mg L−1 | 25 ± 1.5 | 4500-P E |
| Total nitrogen | mg L−1 | 135 ± 7 | 4500-N C |
| Alkalinity | mg CaCO3 L−1 | 300 ± 17 | 2320B |
| Cl− | mg L−1 | 365.5 ± 35 | Ion chromatography |
| K+ | mg L−1 | 54.4 ± 0.7 | Ion chromatography |
| Sulfate | mg L−1 | 108.9 ± 1.9 | Ion chromatography |
| Ca2+ | mg L−1 | 101.1 ± 8 | Ion chromatography |
| Na+ | mg L−1 | 295.5 ± 0.45 | Ion chromatography |
| Model | pH 5 | pH 7 | pH 9 | |
|---|---|---|---|---|
| Pseudo-first-order | qe (mg g−1) | 2098.9 | 2069.7 | 2423.8 |
| k1 (min−1) | 0.0171 | 0.0197 | 0.0218 | |
| R2 | 0.7903 | 0.8037 | 0.7730 | |
| Pseudo-second-order | qe (mg g−1) | 726.067 | 703.55 | 1028.92 |
| k2 (g mg−1 min−1) | 1.284 × 10−5 | 1.238 × 10−5 | 1.176 × 10−5 | |
| R2 | 0.8339 | 0.8749 | 0.8605 |
| Model | ||||
|---|---|---|---|---|
| Freundlich | Langmuir | |||
| n (dimensionless) | Kf (L g−1) | qmax (mg g−1) | KL (L g−1) | |
| 1.99 | 9.89 | 621.11 | 1.146 × 10−3 | |
| R2 | 0.890 | 0.926 | ||
| Parameter | Number of Hidden Layers (Neurons) | ||||
|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | |
| R2 | 0.9233 | 0.9142 | 0.9576 | 0.9523 | 0.9841 |
| Adjusted R2 | 0.9104 | 0.9134 | 0.9528 | 0.9562 | 0.9734 |
| MSE | 14.77 | 15.63 | 8.41 | 8.22 | 5.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Real-Olvera, J.; Morales-Rivera, J.; González-López, A.P.; Sulbarán-Rangel, B.; Zúñiga-Grajeda, V. Adsorption of Organic Pollutants from Cold Meat Industry Wastewater by Electrochemical Coagulation: Application of Artificial Neural Networks. Water 2020, 12, 3040. https://doi.org/10.3390/w12113040
del Real-Olvera J, Morales-Rivera J, González-López AP, Sulbarán-Rangel B, Zúñiga-Grajeda V. Adsorption of Organic Pollutants from Cold Meat Industry Wastewater by Electrochemical Coagulation: Application of Artificial Neural Networks. Water. 2020; 12(11):3040. https://doi.org/10.3390/w12113040
Chicago/Turabian Styledel Real-Olvera, Jorge, Juan Morales-Rivera, Ana Patricia González-López, Belkis Sulbarán-Rangel, and Virgilio Zúñiga-Grajeda. 2020. "Adsorption of Organic Pollutants from Cold Meat Industry Wastewater by Electrochemical Coagulation: Application of Artificial Neural Networks" Water 12, no. 11: 3040. https://doi.org/10.3390/w12113040
APA Styledel Real-Olvera, J., Morales-Rivera, J., González-López, A. P., Sulbarán-Rangel, B., & Zúñiga-Grajeda, V. (2020). Adsorption of Organic Pollutants from Cold Meat Industry Wastewater by Electrochemical Coagulation: Application of Artificial Neural Networks. Water, 12(11), 3040. https://doi.org/10.3390/w12113040








