Screening and Distribution of Contaminants of Emerging Concern and Regulated Organic Pollutants in the Heavily Modified Guadalhorce River Basin, Southern Spain
Abstract
1. Introduction
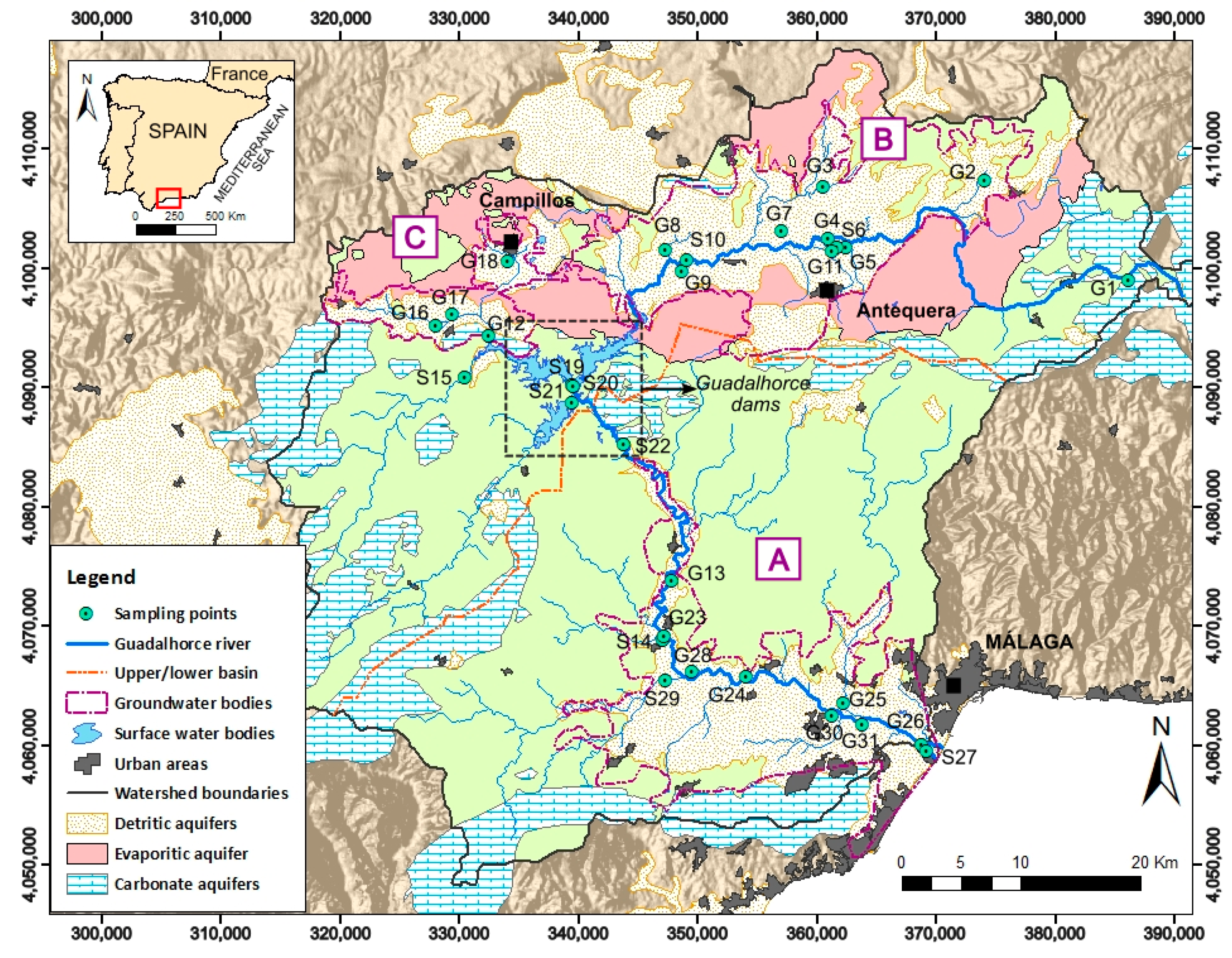
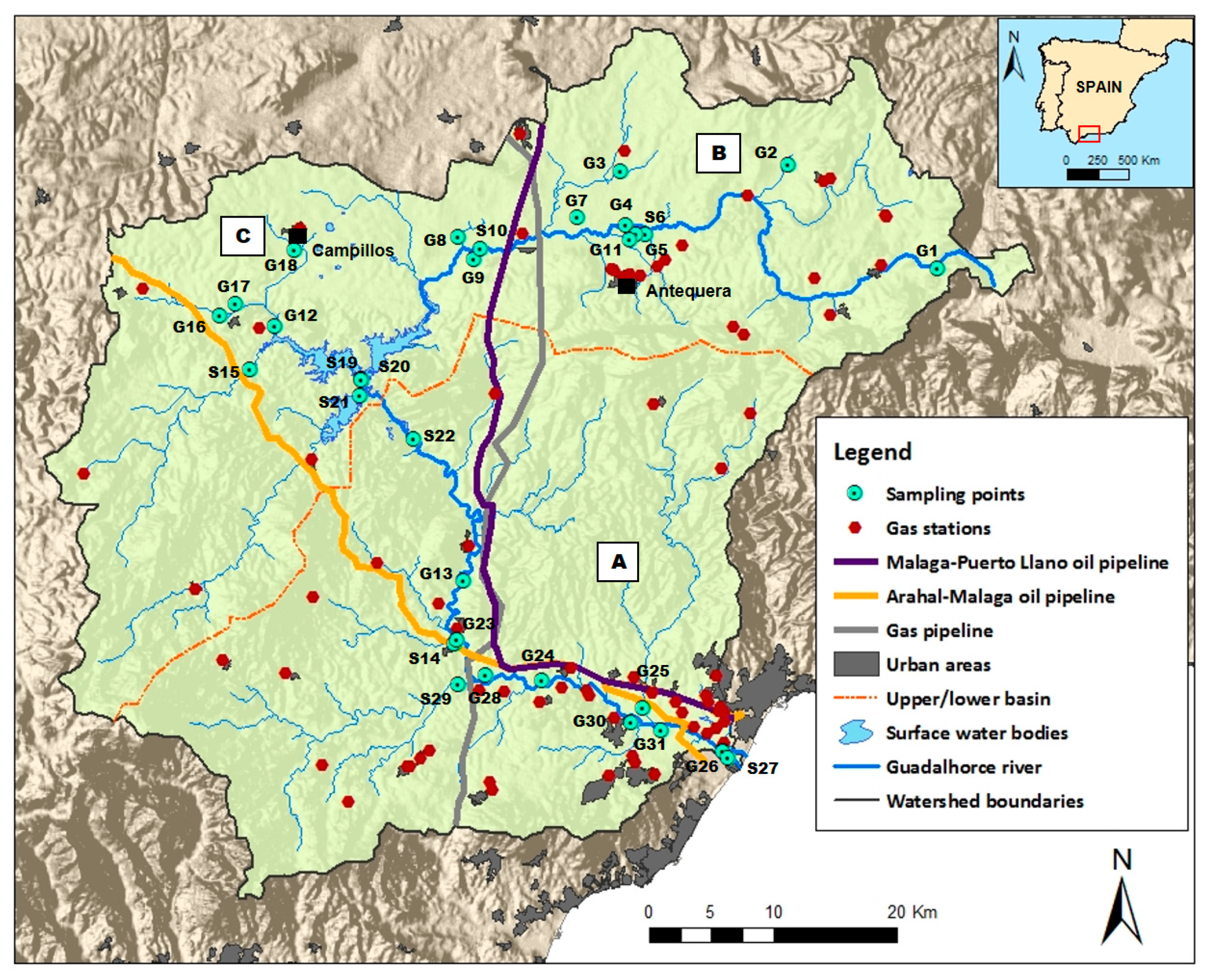
2. Study Area
3. Materials and Methods
3.1. Water Sample Collection and Preparation
3.2. Hydrochemical and Isotopic Analysis
3.3. Analysis of Emerging and Regulated Compounds
4. Results and Discussion
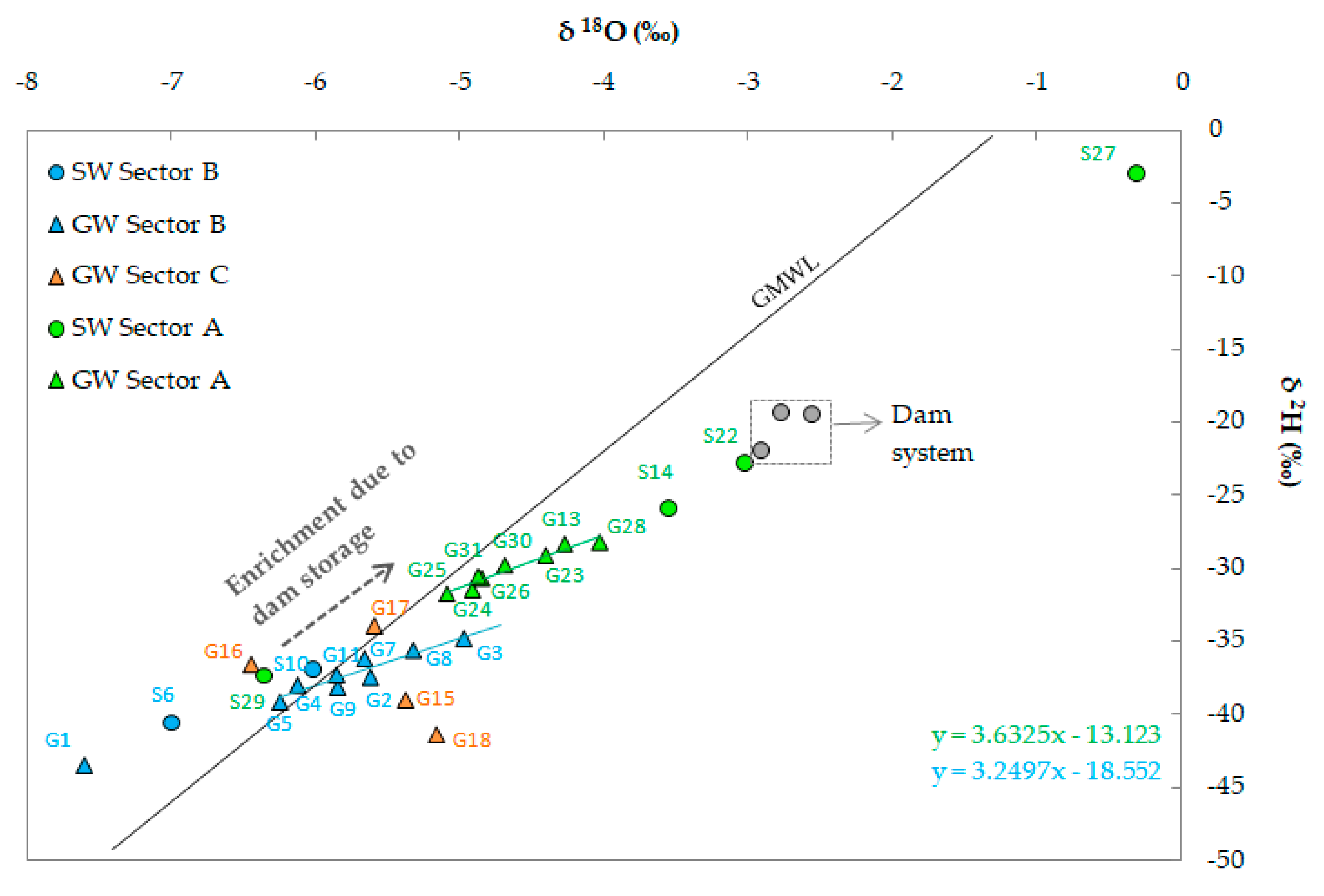
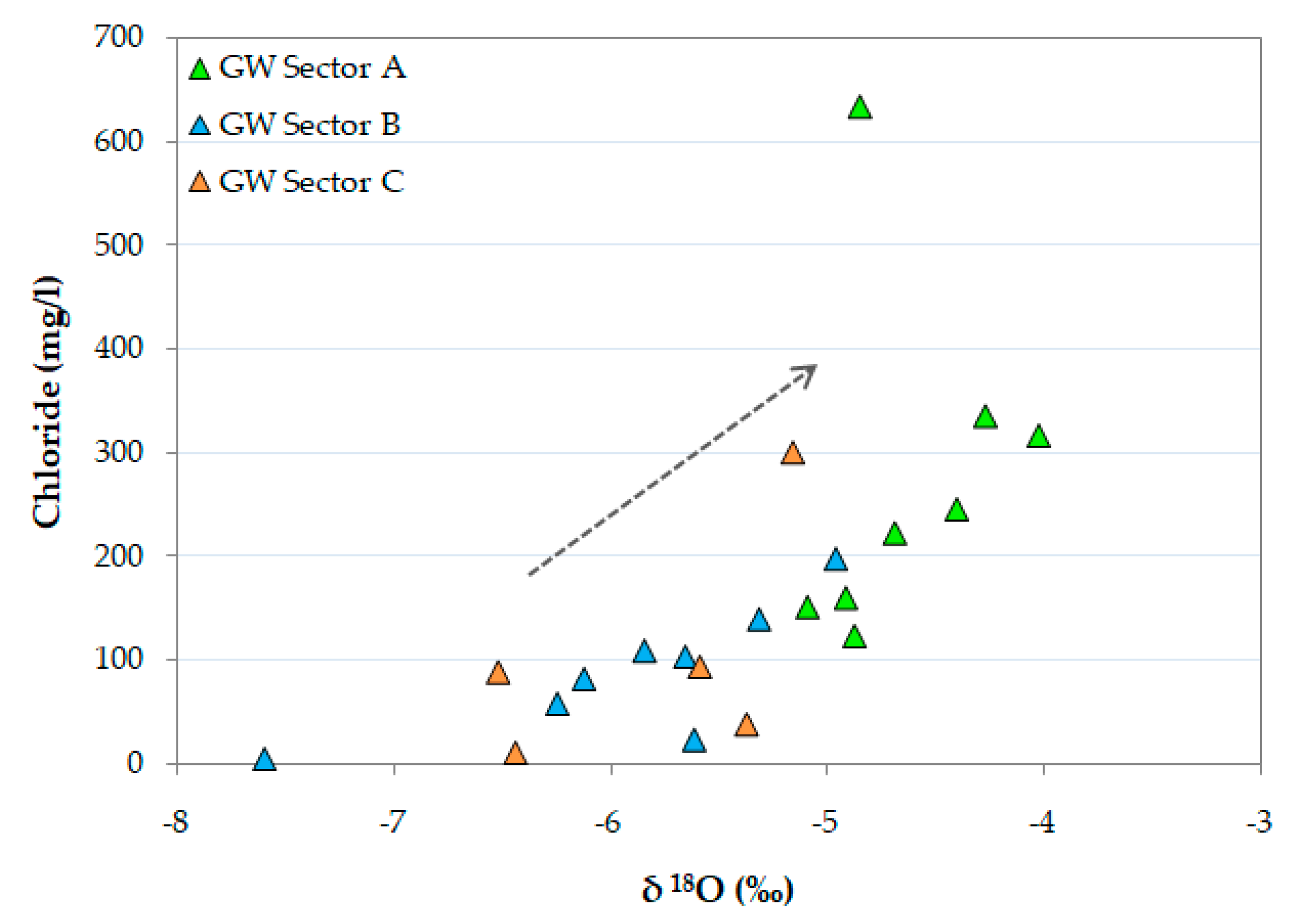
4.1. Water Chemistry and Hydrochemical Water Types
4.2. Organic Active Compounds
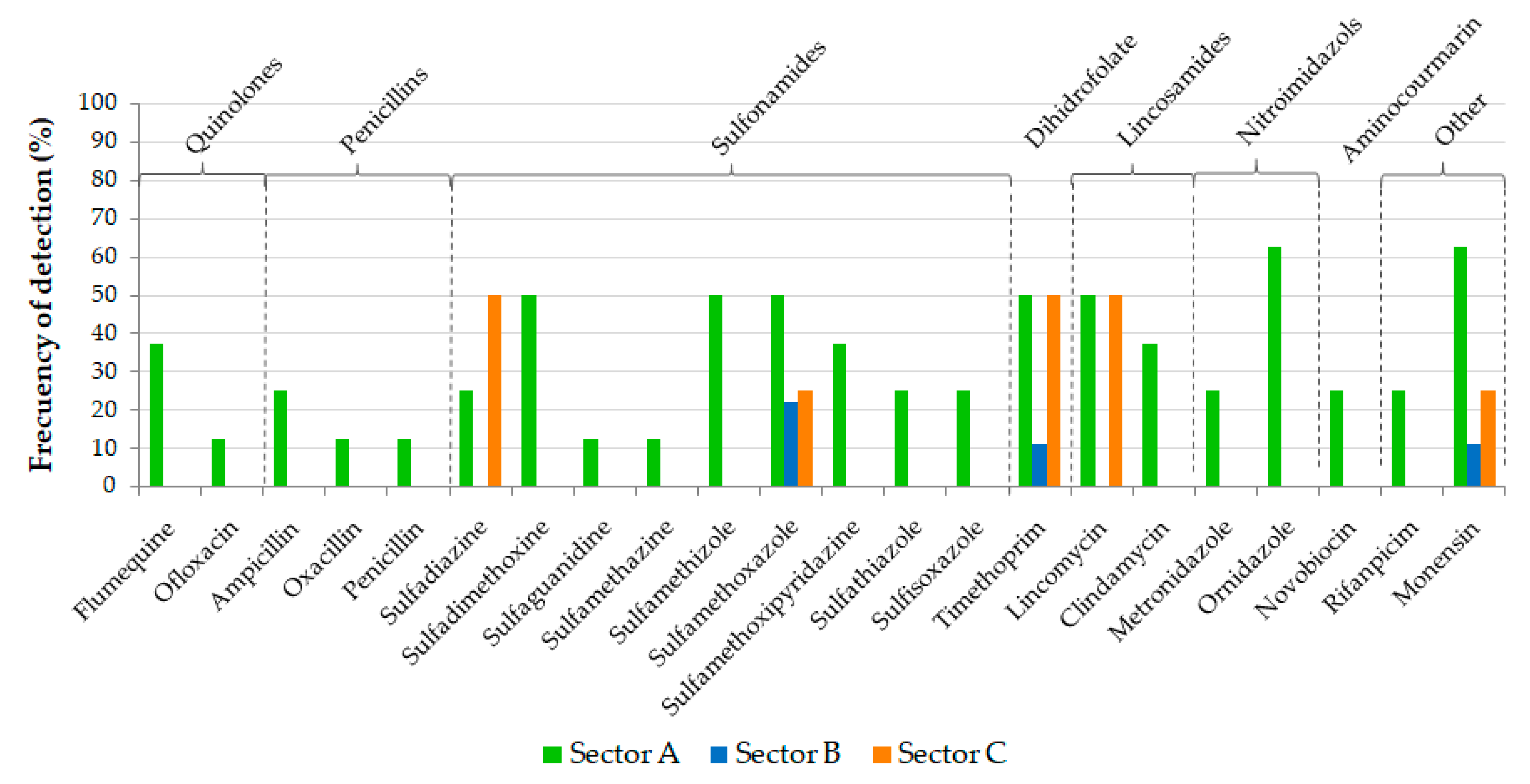
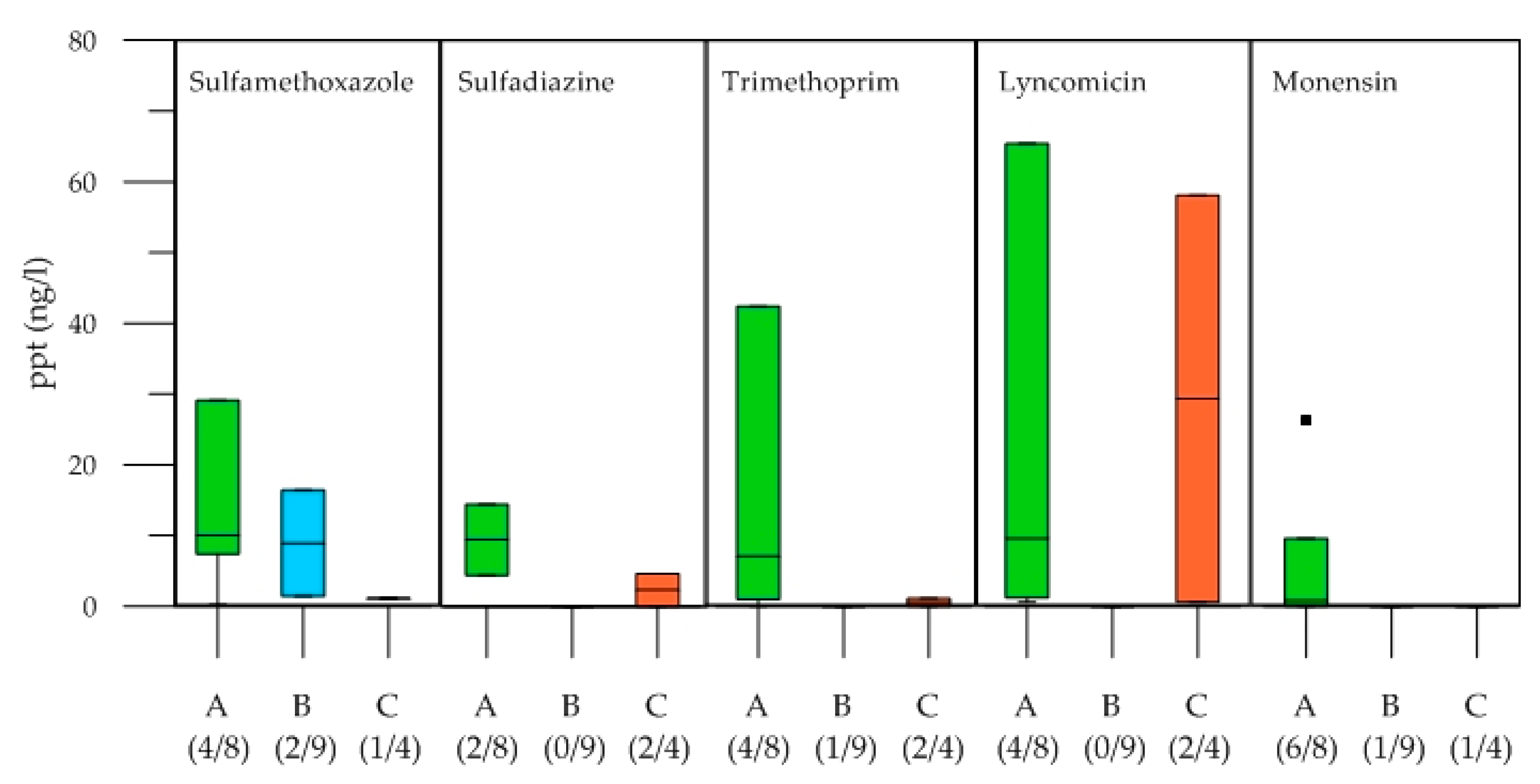
4.3. Antibiotics
4.4. Pesticides. Terbuthylazine
4.5. Personal Care Products. Triclosan
4.6. Polycyclic Aromatic Hydrocarbons. Acenaphthene
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef]
- European Parliament and the Council of the European Union. Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the protection of groundwater against pollution and deterioration. Off. J. Eur. Union 2006, L372/19. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32006L0118 (accessed on 20 June 2020).
- European Commission. Commission Implementing Decision (EU) 2015/495 of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of wáter policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2015, L78/40. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015D0495&from=PT (accessed on 20 June 2020).
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. An overview of UV-absorbing compounds (organic UV filters) in aquatic biota. Anal. Bioanal. Chem. 2012, 404, 2597–2610. [Google Scholar] [CrossRef]
- Lei, M.; Zhang, L.; Lei, J.; Zong, L.; Li, J.; Wu, Z.; Wang, Z. Overview of emerging contaminants and associated human health effects. Biomed. Res. Int. 2015, 2015, 404796. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Silva-Núñez, A.; Salinas-Salazar, C.; Arévalo-Gallegos, A.; Lizarazo-Holguin, L.A.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldívar, R. Anthropogenic contaminats of high concern: Existence in water resources and their adverse effects. Sci. Total Environ. 2019, 690, 1068–1088. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef]
- Daughton, C.G. Non-regulated water contaminants: Emerging research. Environ. Impact Assess. Rev. 2004, 24, 711–732. [Google Scholar] [CrossRef]
- Teijon, G.; Candela, L.; Tamoh, K.; Molina-Díaz, A.; Fernández-Alba, A.R. Occurrence of emerging contaminants, priority substances (2008/105/CE) and heavy metals in treated wastewater and groundwater at Depurbaix facility (Barcelona, Spain). Sci. Total Environ. 2010, 408, 3584–3595. [Google Scholar] [CrossRef]
- Estevez, E.; Cabrera, M.C.; Fernández-Vera, J.R.; Molina-Díaz, A.; Robles-Molina, J.; Palacios-Díaz, M.P. Monitoring priority substances, other organic contaminants and heavy metals in a volcanic aquifer from different sources and hydrological processes. Sci. Total Environ. 2016, 551–552, 186–196. [Google Scholar] [CrossRef]
- Watanabe, N.; Bergamaschi, B.A.; Loftin, K.A.; Meyer, M.T.; Harter, T. Use and environmental occurrence of antibiotics in freestall dairy farms with manured forage fields. Environ. Sci. Technol. 2010, 44, 6591–6600. [Google Scholar] [CrossRef]
- Corada-Fernández, C.; Candela, L.; Torres-Fuentes, N.; Pintado-Herrera, M.G.; Paniw, M.; González-Mazo, E. Effects of extreme rainfall events on the distribution of selected emerging contaminants in surface and groundwater: The Guadalete River basin (SW, Spain). Sci. Total Environ. 2017, 605–606, 770–783. [Google Scholar]
- Gurr, C.J.; Reinhard, M. Harnessing natural attenuation of pharmaceuticals and hormones in rivers. Environ. Sci. Technol. 2006, 40, 2872–2876. [Google Scholar] [CrossRef]
- Schaffer, M.; Licha, T. A framework for assessing the retardation of organic molecules in groundwater: Implications of the species distribution for the sorption-influenced transport. Sci. Total Environ. 2015, 524–525, 187–194. [Google Scholar] [CrossRef]
- Massman, G.; Greskowiak, J.; Dünnbier, U.; Zuehlke, S.; Knappe, A.; Pekdeger, A. The impact of variable temperaturas on the redox conditions and the behaviour of pharmaceutical residues during artificial recharge. J. Hydrol. 2006, 328, 141–156. [Google Scholar] [CrossRef]
- Menció, A.; Mas-Pla, J. Assessing the influence of environmental factor son groundwater antibiotic occurrence by means of variation partitioning. Water 2019, 11, 1495. [Google Scholar] [CrossRef]
- Sánchez-García, D. Water Framework Directive 2000/60/CE Application in the Guadalhorce River Basin (Málaga). Ph.D. Thesis, University of Malaga, Malaga, Spain, 2010. Initial characterization. (In Spanish). [Google Scholar]
- Galvez, R.; Orozco, M. Strain determinations using deformed Radiolaria. Malaguide Complex, Southern Spain. Acta Geol. Hisp. 1979, 14, 129–134. [Google Scholar]
- Vadillo-Pérez, I.; Carrasco-Cantos, F.; Sánchez-García, D. Bajo Guadalhorce. In Atlas Hidrogeológico de la Provincia de Málaga, 1st ed.; Durán-Valsero, J.J., Andreo-Navarro, B., Eds.; IGME: Madrid, Spain; Diputación Provincial de Málaga: Málaga, Spain, 2007; Volume 2, pp. 180–184. [Google Scholar]
- IGME. Hydrogeological Investigation of the Basins from Southern Spain (Western Sector); Technical Report nº5, Aquifer System nº37 (Malaga Porous Aquifer); Spanish Geological Survey: Madrid, Spain, 1983; 130p. (In Spanish) [Google Scholar]
- Linares, L.; López-Arechavala, G.; López-Geta, J.A.; Campos-Rubio, J.C. Geometrical definition of the Pliocene-Quaternary aquifers of the Guadalhorce valley (Málaga). In Proceedings of the VI Hydrogeology Symposium, Sevilla, Spain, 23–27 October 1995; pp. 435–447. (In Spanish). [Google Scholar]
- Carrasco-Cantos, F. Contribution to the Knowledge of the Upper Basin of the Guadalhorce River: Physical Media. Ph.D. Thesis, University of Granada, Granada, Spain, 1986. (In Spainsh). [Google Scholar]
- Carrasco-Cantos, F.; Sánchez-García, D.; Vadillo-Pérez, I. Sierra de Teba-Almargen-Campillos. In Atlas Hidrogeológico de la Provincia de Málaga, 1st ed.; Durán-Valsero, J.J., Andreo-Navarro, B., Eds.; IGME: Madrid, Spain; Diputación Provincial de Málaga: Málaga, Spain, 2007; Volume 2, pp. 95–100. [Google Scholar]
- Baena-Nogueras, R.M.; Pintado-Herrera, M.G.; González-Mazo, E.; Lara-Martín, P.A. Determination of pharmaceuticals in coastal systems using solid phase extraction (SPE) followed by ultraperformance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS). Curr. Anal. Chem. 2016, 12, 183–201. [Google Scholar] [CrossRef]
- Pintado-Herrera, M.G.; González-Mazo, E.; Lara-Martín, P.A. Atmospheric pressure gas chromatography-time-of-flight-mass spectrometry (APGC-Tof-MS) for the determination of regulated and emerging contaminants in aqueous samples after stir bar sorptive extraction (SBSE). Anal. Chim. Acta 2014, 851, 1–13. [Google Scholar] [CrossRef]
- Pintado-Herrera, M.G.; González-Mazo, E.; Lara-Martín, P.A. In-cell clean-up pressurized liquid extraction and gas chromatography ‒ tandem mass spectrometry determination of hydrophobic persistent and emerging organic pollutants in coastal sediments. J. Chromatogr. A 2016, 1429, 107–118. [Google Scholar] [CrossRef]
- Urresti-Estala, B.; Vadillo-Pérez, I.; Jiménez-Gavilán, P.; Soler, A.; Sánchez-García, D.; Carrasco-Cantos, F. Application of stable isotopes (δ34S-SO4, δ18O-SO4, δ15N-NO3, δ18O-NO3) to determine natural background and contamination sources in the Guadalhorce River Basin (southern Spain). Sci. Total Environ. 2015, 506–507, 46–57. [Google Scholar] [CrossRef]
- European Parliament and the Council of the European Union. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Union 2013, L226/1. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:226:0001:0017:EN:PDF (accessed on 20 September 2020).
- Demeneix, B.; Slama, R. Endocrine disruptors: From scientific evidence to human health protection. Policy Department for Citizens’ Rights and Constitutional Affairs. In Directorate General for Internal Policies of the Union; European Union: Brussel, Belgium, 2019; Available online: https://www.europarl.europa.eu/thinktank/es/document.html?reference=IPOL_STU%282019%29608866 (accessed on 20 September 2020).
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Photochemical fate of sulfa drugs in the aquatic environment: Sulfa drugs containing five-membered heterocyclic groups. Environ. Sci. Technol. 2004, 38, 3933–3940. [Google Scholar] [CrossRef]
- Avisar, D.; Lester, Y.; Ronen, D. Sulfamethoxazole contamination of a deep phreatic aquifer. Sci. Total. Environ. 2009, 407, 4278–4282. [Google Scholar] [CrossRef] [PubMed]
- Baena-Nogueras, R.M.; González-Mazo, E.; Lara-Martín, P.A. Degradation kinetics of pharmaceuticals and personal care products in surface waters: Photolisis vs biodegradation. Sci. Total Environ. 2017, 590–591, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Candela, L.; Tamoh, K.; Vadillo, I.; Valdes-Abellan, J. Monitoring of selected pharmaceuticals over 3 years in a detrital aquifer during artificial groundwater recharge. Environ. Earth Sci. 2016, 75, 274. [Google Scholar] [CrossRef]
- Tasca, A.L.; Puccini, M.; Fletcher, A. Terbuthylazine and desethylterbuthylazine: Recent occurrence, mobility and removal techniques. Chemosphere 2018, 202, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Dousset, S.; Mouvet, C.; Schiavon, M. Sorption of terbuthylazine and atrazine in relation to the physico-chemical properties of three soils. Chemosphere 1994, 28, 467–476. [Google Scholar] [CrossRef]
- Navarro, S.; Vela, N.; Giménez, M.J.; Navarro, G. Persistence of four s-triazine herbicides in river, sea and groundwater samples exposed to sunlight and darkness under laboratory conditions. Sci. Total Environ. 2004, 329, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Kronvang, B.; Iversen, H.L.; Vejrup, K.; Mogensen, B.B.; Hansen, A.M.; Hansen, L.B. Pesticides in streams and subsurface drainage water within two arable catchments in Denmark. In Pesticide Application, Concentration, Transport and Fate; Danish Environmental Protection Agency: København, Denmark, 2003. [Google Scholar]
- Junta de Andalucía (Andalusian Government). Cartographic Database SIOSE. Available online: http://www.juntadeandalucia.es/medioambiente/site/rediam/menuitem.04dc44281e5d53cf8ca78ca731525ea0/?vgnextoid=83539c8e47b3c310VgnVCM1000001325e50aRCRD&vgnextchannel=c2770219f560f210VgnVCM1000001325e50aRCRD&vgnextfmt=rediam&lr=lang_es (accessed on 20 September 2020).
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledón, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current status, occurrence, environmental risks and bioaccumulation potential. Int. J. Environ. Res. Public Health 2015, 12, 5657–5684. [Google Scholar] [CrossRef]
- Chalew, T.E.A.; Halden, R.U. Environmental exposure of aquatic and terrestrial biota to triclosan and triclocarban. J. Am. Water Resourc. Assoc. 2009, 45, 4–13. [Google Scholar] [CrossRef]
- Fernandes, M.B.; Sicre, M.A.; Boireau, A.; Tronczynski, J. Polyaromatic hydrocarbon (PAH) distributions in the Seine River and its estuary. Mar. Poll. Bull. 1997, 34, 857–867. [Google Scholar] [CrossRef]
- Li, J.; Li, F.; Liu, Q. PAHs behavior in surface water and groundwater of the Yellow River estuary: Evidence from isotopes and hydrochemistry. Chemosphere 2017, 178, 143–153. [Google Scholar] [CrossRef]
- Rodríguez-Paradinas, E.; Orozco-Vallejo, E. Málaga-Puerto Llano oil pipeline. Project and main pipeline construction. Revista de Obras Públicas 1967, 115, 97–111. Available online: http://ropdigital.ciccp.es/revista_op/detalle_articulo.php?registro=16925&anio=1967&numero_revista=3022 (accessed on 20 September 2020). (In Spanish).
- Luque-Espinar, J.A.; Navas, N.; Chica-Olmo, M.; Cantarero-Malagón, S.; Chica-Rivas, L. Seasonal occurrence and distribution of a group of ECs in the water resources of Granada city metropolitana reas (South of Spain): Pollution of raw drinking water. J. Hydrol. 2015, 531, 612–625. [Google Scholar] [CrossRef]
- You, L.; Nguyen, V.T.; Pal, A.; Chen, H.; He, Y.; Reinhard, M.; Gin, K.Y. Investigation of pharmaceuticals, personal care products and endocrine disrupting chemicals in a tropical urban catchment and the influence of environmental factors. Sci. Total. Environ. 2015, 536, 955–963. [Google Scholar] [CrossRef]



| pH | EC (µS/cm) | Temperature (°C) | Eh (mV) | O2 (mg/L) | |
|---|---|---|---|---|---|
| Sector A | |||||
| Min. | 7.0 | 1877 | 16.2 | 105 | 3.4 |
| Max. | 9.1 | 4100 | 19.0 | 203 | 7.6 |
| Median | 7.4 | 2280 | 18.3 | 190 | 5.0 |
| Mean | 7.5 | 2640 | 18.0 | 181 | 5.5 |
| Std. dev. | 0.6 | 777 | 1.0 | 32 | 1.7 |
| Sector B | |||||
| Min. | 7.0 | 305 | 14.2 | 187 | 7.0 |
| Max. | 7.7 | 2300 | 18.0 | 277 | 8.9 |
| Median | 7.1 | 1626 | 15.0 | 241 | 8.8 |
| Mean | 7.2 | 1518 | 15.5 | 235 | 8.2 |
| Std. dev. | 0.2 | 631 | 1.2 | 27 | 0.8 |
| Sector C | |||||
| Min. | 7.1 | 495 | 11.6 | 160 | 3.2 |
| Max. | 8.3 | 3730 | 18.5 | 235 | 9.8 |
| Median | 7.4 | 1454 | 15.2 | 198 | 5.9 |
| Mean | 7.5 | 1775 | 15.2 | 201 | 6.0 |
| Std. dev. | 0.4 | 1192 | 2.4 | 28 | 2.7 |
| Ca2+ (mg/L) | Mg2+ (mg/L) | Na+ (mg/L) | K+ (mg/L) | HCO3− (mg/L) | SO42− (mg/L) | Cl− (mg/L) | NO3− (mg/) | δ2H (‰) | δ18O (‰) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sector A | ||||||||||
| Min. | 7.4 | 0.8 | 5.6 | 0.4 | 96.2 | 66.7 | 123.1 | 2.9 | −31.8 | −5.1 |
| Max. | 283.8 | 138.9 | 526.3 | 7.6 | 474.4 | 233.5 | 634.3 | 58.5 | −28.2 | −4.0 |
| Median | 115.5 | 80.7 | 207.5 | 3.9 | 371.7 | 186.2 | 234.1 | 18.9 | −30.2 | −4.8 |
| Mean | 112.2 | 81.8 | 243.3 | 3.7 | 353.7 | 169.3 | 273.7 | 25.0 | −30.0 | −4.6 |
| Std. dev. | 95.3 | 44.9 | 189.3 | 2.5 | 112.8 | 55.3 | 164.6 | 20.4 | 1.4 | 0.4 |
| Sector B | ||||||||||
| Min. | 54.3 | 6.3 | 1.8 | 0.3 | 191.1 | 8.5 | 3.6 | 3.8 | −43.5 | −7.6 |
| Max. | 316.0 | 82.2 | 165.9 | 3.7 | 301.0 | 2007.1 | 197.5 | 155.9 | −34.8 | −5.0 |
| Median | 244.2 | 50.3 | 58.6 | 2.3 | 270.1 | 291.2 | 81.2 | 75.6 | −37.5 | −5.8 |
| Mean | 208.3 | 48.6 | 67.2 | 2.3 | 260.7 | 786.9 | 82.4 | 70.0 | −37.8 | −5.9 |
| Std. dev. | 99.2 | 26.7 | 50.7 | 1.0 | 40.9 | 823.2 | 62.0 | 44.8 | 2.5 | 0.7 |
| Sector C | ||||||||||
| Min. | 57.6 | 10.6 | 13.6 | 1.6 | 243.7 | 20.3 | 10.4 | 5.5 | −41.4 | −6.5 |
| Max. | 381.9 | 136.7 | 306.0 | 4.8 | 340.8 | 664.2 | 300.1 | 433.8 | −33.3 | −5.2 |
| Median | 135.7 | 45.4 | 120.1 | 2.6 | 284.5 | 143.5 | 88.1 | 44.8 | −36.5 | −5.6 |
| Mean | 160.6 | 57.8 | 130.2 | 2.8 | 285.5 | 242.3 | 105.9 | 108.4 | −36.8 | −5.8 |
| Std. dev. | 129.0 | 52.2 | 113.8 | 1.3 | 35.7 | 265.3 | 113.9 | 183.2 | 3.4 | 0.6 |
| Compounds | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pharmaceuticals | CAS | Use | PCPs | CAS | Use | Pesticides | CAS | Use | |
| 17α-ethynylestradiol ab | 57-63-6 | Estrogen | OD-PABA | 21245-02-3 | UV filter | Aldrin a | 309-00-2 | Organochlorine pesticide | |
| 17-β estradiol ab | 50-28-2 | Estrogen | 2-OHBP | 117-99-7 | UV filter | Ametryn | 834-12-8 | Triazine | |
| Acetaminophen | 103-90-2 | Analgesic/anti-inflammatory | 3-OHPB | 13020-57-0 | UV filter | Atraton | 1610-17-9 | Triazine | |
| Albuterol | 18559-94-9 | Other PhACs | 4-OHPB | 1137-42-4 | UV filter | Atrazineab | 1912-24-9 | Triazine | |
| Amitriptyline | 50-48-6 | Psychiatric drug and stimulant | 4MBC | 36861-47-9 | UV filter | Bifenthrin | 82657-04-3 | Pyrethroid | |
| Amoxicillin | 26787-78-0 | Penicillin (antibiotic) | Benzyl salicylate | 118-58-1 | UV filter | Carbophenothion | 786-19-6 | Organophosphate pesticide | |
| Ampicillin | 69-53-4 | Penicillin (antibiotic) | Bisphenol Ab | 80-05-7 | Plasticizer | Chlorpyrifos ab | 2921-88-2 | Organophosphate pesticide | |
| Atenolol | 29122-68-7 | β-blocker (antihypertensive) | Cashmeran | 33704-61-9 | Other fragrances | Cyfluthrin (I-IV) | 68359-37-5 | Pyrethroid | |
| Atorvastatin | 134523-00-5 | Lipid regulator | Celestolide | 13171-00-1 | Polycyclic musk | Cypermethrin (I-IV) | 52315-07-8 | Pyrethroid | |
| Azithromycin | 83905-01-5 | Macrolide | DEET | 134-62-3 | Insect repellent | Deltamethrin I,II | 52918-63-5 | Pyrethroid | |
| Bezafibrate | 41859-67-0 | Lipid regulator | EHMC | 83834-59-7 | UV filter | Dieldrin a | 60-57-1 | Organochlorine pesticide | |
| Caffeine | 58-08-2 | Psychiatric drug and stimulant | Ethylhexyl salicylate | 118-60-5 | UV filter | Endosulfan Sulfate a | 1031-07-8 | Organochlorine pesticide | |
| Carbamazepine | 298-46-4 | Psychiatric drugs and stimulants | Exaltenone | 14595-54-1 | Macrocyclic musk | Endrina | 72-20-8 | Organochlorine pesticide | |
| Cefaclor | 53994-73-3 | Cephalosporin | Galaxolide | 1222-05-5 | Polycyclic musks | Endrin Ketone | 53494-70-5 | Organochlorine pesticide | |
| Cefadroxil | 50370-12-2 | Cephalosporin | Habanolide | 34902-57-3 | Macrocyclic musk | Ethion | 563-12-2 | Organophosphate pesticide | |
| Cefdinir | 91832-40-5 | Cephalosporin | Helvetolide | 141773-73-1 | Other fragrances | Fenvalerate I,II | 51630-58-1 | Pyrethroid | |
| Cefquinome | 84957-30-2 | Cephalosporin | Homosalate | 118-56-9 | UV filter | Heptachlor | 76-44-8 | Organochlorine pesticide | |
| Ceftiofur | 80370-57-6 | Cephalosporin | IRGAROL | 28159-98-0 | Insect repellent | Heptachlor Epoxide Isomer B | 1024-57-3 | Organochlorine pesticide | |
| Chloramphenicol | 56-75-7 | Amphenicol | Mexenone | 1641-17-4 | UV filter | Lindane b | 58-89-9 | Organochlorine pesticide | |
| Chlortetracycline | 57-62-5 | Tetracyclines | MTCS b | 4640-01-1 | Antibacterial | Metoxychlor | 72-43-5 | Organochlorine pesticide | |
| Ciprofloxacin | 85721-33-1 | Quinolone (antibiotic) | Muscenone | 63314-79-4 | Macrocyclic musk | o,p’-DDT ab | 789-02-6 | Organochlorine pesticide | |
| Clarithromycin | 81103-11-9 | Macrolide | Muscone | 541-91-3 | Macrocyclic musk | p,p’-DDT ab | 72-54-8 | Organochlorine pesticide | |
| Clindamycin | 18323-44-9 | Lincosamides (antibiotics) | Musk ambrette | 83-66-9 | Nitro musk | p,p’-DDE a | 72-55-9 | Organochlorine pesticide | |
| Clofibric acid | 882-09-7 | Lipid regulator | Musk ketone | 81-14-1 | Nitro musks | Parathion | 56-38-2 | Organophosphate pesticide | |
| Danofloxacin | 112398-08-0 | Quinolone (antibiotic) | Musk moskene | 116-66-5 | Nitro musk | Permethrin I,II | 52645-53-1 | Pyrethroid | |
| Diclofenac b | 15307-86-5 | Analgesic/anti-inflammatory | Musk R1 | 3391-83-1 | Macrocyclic musk | Phenothrin I,II | 26002-80-2 | Pyrethroid | |
| Doxycicline | 564-25-0 | Tetracyclines | Musk tibetene | 145-39-1 | Nitro musk | Prometon | 1610-18-0 | Triazine | |
| Enrofloxacin | 93106-60-6 | Quinolone (antibiotic) | Musk xylene | 81-15-2 | Nitro musk | Prometryn | 7287-19-6 | Triazine | |
| Erythromycin | 114-07-8 | Macrolide | Nonylphenol ab | 84852-15-3 | Plasticizer | Propazine | 139-40-2 | Triazine | |
| Estrone b | 53-16-7 | Estrogen | Octylphenol ab | 1806-26-4 | Plastizicer | Secbumeton | 26259-45-0 | Triazine | |
| Famotidine | 76824-35-6 | Histamine H2/receptor antagonists | OTNE | 54464-57-2 | Other fragrances | Simazine a | 122-34-9 | Triazine | |
| Fenofibrate | 49562-28-9 | Lipid regulator | Oxybenzone | 131-57-7 | UV filter | Simetryn | 1014-70-6 | Triazine | |
| Fenoprofen | 29679-58-1 | Analgesic/anti-inflammatory | Phantolide | 15323-35-0 | Polycyclic musk | Terbuthylazine | 5915-41-3 | Triazine | |
| Flumequine | 42835-25-6 | Quinolone (antibiotic) | Tonalide b | 1506-02-1 | Polycyclic musks | Terbutryn a | 886-50-0 | Triazine | |
| Fluoxetine | 54910-89-3 | Psychiatric drug and stimulant | Traseolide | 6814-48-7 | Polycyclic musk | α-chlordane | 5103-71-9 | Organochlorine pesticide | |
| Furosemide | 54-31-9 | Diuretic | Triclocarban | 101-20-2 | Antibacterial | α-endosulfan | 959-98-8 | Organochlorine pesticide | |
| Gemfibrozil | 25812-30-0 | Lipid regulator | Triclosan b | 3380-34-5 | Antibacterial | β-endosulfan | 33213-65-9 | Organochlorine pesticide | |
| Glibenclamide | 10238-21-8 | Anti-diabetic medication | γ-chlordane | 5103-74-2 | Organochlorine pesticide | ||||
| Hydrochlorotiazide | 58-93-5 | Diuretic | |||||||
| Ibuprofen b | 15687-27-1 | Analgesic/anti-inflammatory | |||||||
| Indomethacine | 53-86-1 | Analgesic/anti-inflammatory | |||||||
| Ivermectin | 71827-03-7 | Other antibiotics | |||||||
| Ketoprofen | 22071-15-4 | Analgesic/anti-inflammatory | |||||||
| Lincomycin | 154-21-2 | Lincosamide (antibiotic) | |||||||
| Mefenamic Acid | 61-68-7 | Analgesic/anti-inflammatory | |||||||
| Metoprolol | 51384-51-1 | β-blocker (antihypertensive) | |||||||
| Methotrexate | 59-05-2 | Other PhACs | |||||||
| Metronidazole | 443-48-1 | Nitroimidazol (antibiotic) | |||||||
| Monensin | 17090-79-8 | Other antibiotics | |||||||
| Nadolol | 42200-33-9 | β-blocker (antihypertensive) | |||||||
| Naproxen b | 22204-53-1 | Analgesic/anti-inflammatory | |||||||
| Nitrofurantoin | 67-20-9 | Nitroimidazols | |||||||
| Norfloxacin | 70458-96-7 | Quinolone (antibiotic) | |||||||
| Novobiocin | 303-81-1 | Aminocoumarin antibiotic | |||||||
| Ofloxacin | 82419-36-1 | Quinolone (antibiotic) | |||||||
| Ornidazole | 16773-42-5 | Nitroimidazol (antibiotic) | |||||||
| Oxacillin | 66-79-5 | Penicillin (antibiotic) | |||||||
| Oxytetracycline | 79-57-2 | Tetracyclines | |||||||
| Penicillin-G | 61-33-6 | Penicillin (antibiotic) | |||||||
| Pharmaceuticals | CAS | Use | OPFRs | CAS | Use | PAHs | CAS | PCBs | CAS |
| Phenazone | 60-80-0 | Phenazone type | TBP-N | 126-73-8 | Flame retardant | Acenaphthene | 83-32-9 | PCB28 | 7012-37-5 |
| Phenylbutazone | 50-33-9 | Phenazone type | TPP b | 115-86-6 | Flame retardant | Acenaphthylene | 208-96-8 | PCB52 | 35693-99-3 |
| Pindolol | 13523-86-9 | β-blocker (antihypertensive) | Anthracene a | 120-12-7 | PCB101 | 37680-73-2 | |||
| Pravastatin | 81093-37-0 | Lipid regulator | Benzo [b] fluoranthene a | 205-99-2 | PCB138 | 35065-28-2 | |||
| Propanolol | 525-66-6 | β-blocker (antihypertensive) | Benzo [k] fluoranthene a | 207-08-9 | PCB153 | 35065-27-1 | |||
| Ranitidine | 66357-35-5 | Histamine H2/receptor antagonists | Benzo[a] anthracene | 56-55-3 | PCB180 | 35065-29-3 | |||
| Rifanpicim | 13292-46-1 | Other antibiotics | Benzo[a] pyrene a | 50-32-8 | |||||
| Roxithromycin | 80214-83-1 | Macrolide | Benzo[g,h,i] perylene a | 191-24-2 | |||||
| Salicylic Acid b | 69-72-7 | Analgesic/anti-inflammatory | Chrysene | 218-01-9 | |||||
| Sparfloxacin | 110871-86-8 | Quinolone (antibiotic) | Dibenzo[a,h] anthracene | 53-70-3 | |||||
| Spyramycin | 8025-81-8 | Macrolide | Fluoranthene a | 206-44-0 | |||||
| Sulfadiazine | 68-35-9 | Sulfonamide (antibiotic) | Fluorene | 86-73-7 | |||||
| Sulfadimethoxine | 122-11-2 | Sulfonamide (antibiotic) | Indeno[1,2,3-Cd] pyrene a | 193-39-5 | |||||
| Sulfaguanidine | 57-67-0 | Sulfonamide (antibiotic) | Phenanthrene | 85-01-8 | |||||
| Sulfamethazine | 57-68-1 | Sulfonamide (antibiotic) | Pyrene | 129-00-0 | |||||
| Sulfamethizole | 144-82-1 | Sulfonamide (antibiotic) | |||||||
| Sulfamethoxazole | 723-46-6 | Sulfonamide (antibiotic) | |||||||
| Sulfamethoxipyridazine | 80-35-3 | Sulfonamide (antibiotic) | |||||||
| Sulfanilamide | 63-74-1 | Sulfonamide (antibiotic) | |||||||
| Sulfathiazole | 72-14-0 | Sulfonamide (antibiotic) | |||||||
| Sulfisoxazole | 127-69-5 | Sulfonamide (antibiotic) | |||||||
| Tetracycline | 60-54-8 | Tetracyclines | |||||||
| Tiamulin | 55297-95-5 | Amphenicol | |||||||
| Timolol | 26839-75-8 | β-blocker (antihypertensive) | |||||||
| Trimethoprim | 738-70-5 | Dihydrofolate (antibiotic) | |||||||
| Tylosin | 1401-69-0 | Macrolide | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llamas, M.; Vadillo-Pérez, I.; Candela, L.; Jiménez-Gavilán, P.; Corada-Fernández, C.; Castro-Gámez, A.F. Screening and Distribution of Contaminants of Emerging Concern and Regulated Organic Pollutants in the Heavily Modified Guadalhorce River Basin, Southern Spain. Water 2020, 12, 3012. https://doi.org/10.3390/w12113012
Llamas M, Vadillo-Pérez I, Candela L, Jiménez-Gavilán P, Corada-Fernández C, Castro-Gámez AF. Screening and Distribution of Contaminants of Emerging Concern and Regulated Organic Pollutants in the Heavily Modified Guadalhorce River Basin, Southern Spain. Water. 2020; 12(11):3012. https://doi.org/10.3390/w12113012
Chicago/Turabian StyleLlamas, Marta, Iñaki Vadillo-Pérez, Lucila Candela, Pablo Jiménez-Gavilán, Carmen Corada-Fernández, and Antonio F. Castro-Gámez. 2020. "Screening and Distribution of Contaminants of Emerging Concern and Regulated Organic Pollutants in the Heavily Modified Guadalhorce River Basin, Southern Spain" Water 12, no. 11: 3012. https://doi.org/10.3390/w12113012
APA StyleLlamas, M., Vadillo-Pérez, I., Candela, L., Jiménez-Gavilán, P., Corada-Fernández, C., & Castro-Gámez, A. F. (2020). Screening and Distribution of Contaminants of Emerging Concern and Regulated Organic Pollutants in the Heavily Modified Guadalhorce River Basin, Southern Spain. Water, 12(11), 3012. https://doi.org/10.3390/w12113012






