Influence of Warmer and Drier Environmental Conditions on Species-Specific Stem Circumference Dynamics and Water Status of Conifers in Submontane Zone of Central Slovakia
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Environmental Data
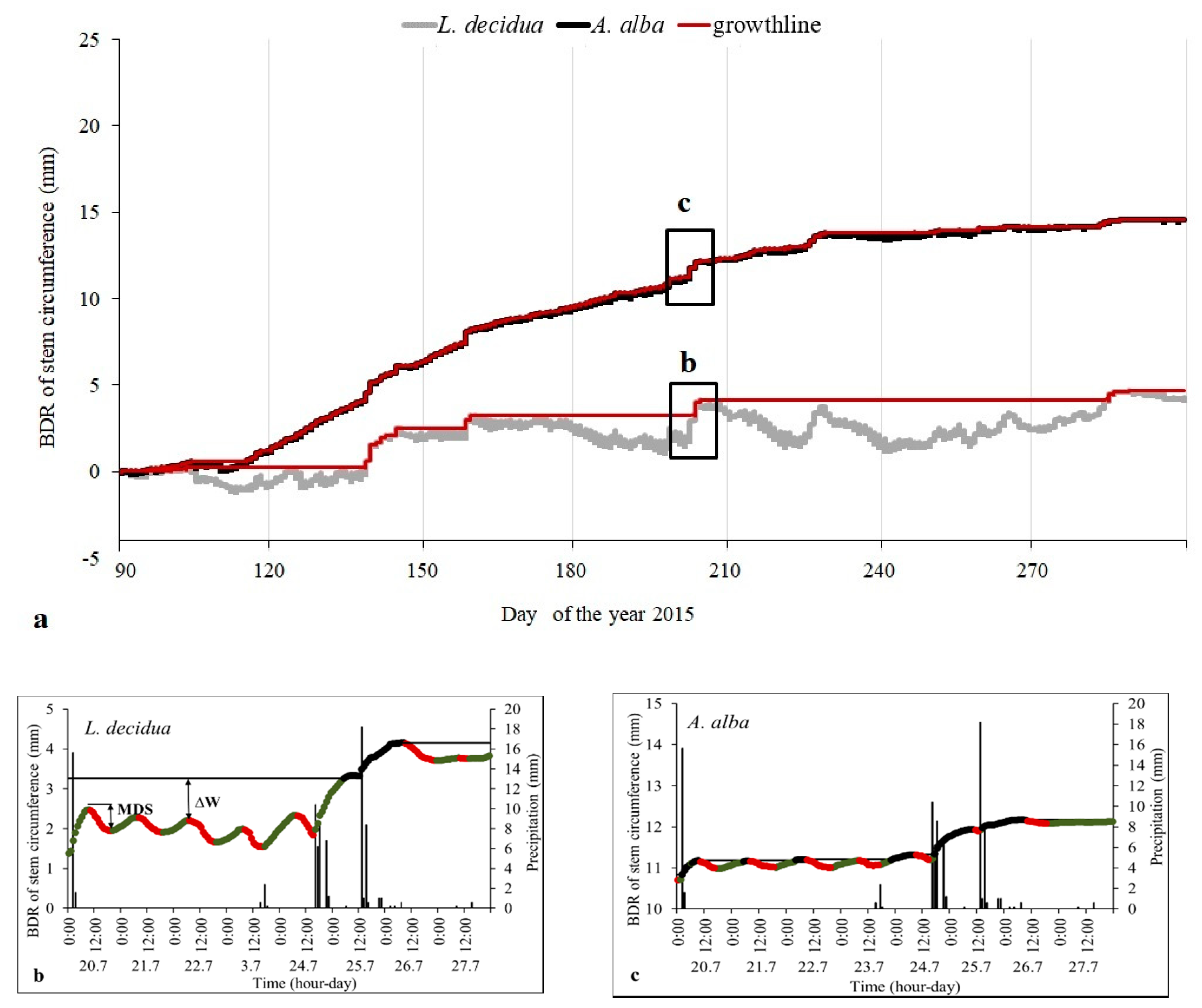
2.3. Band Dendrometer Records (BDR)
2.4. Tree Water Status
2.5. BDR and Environmental Variables
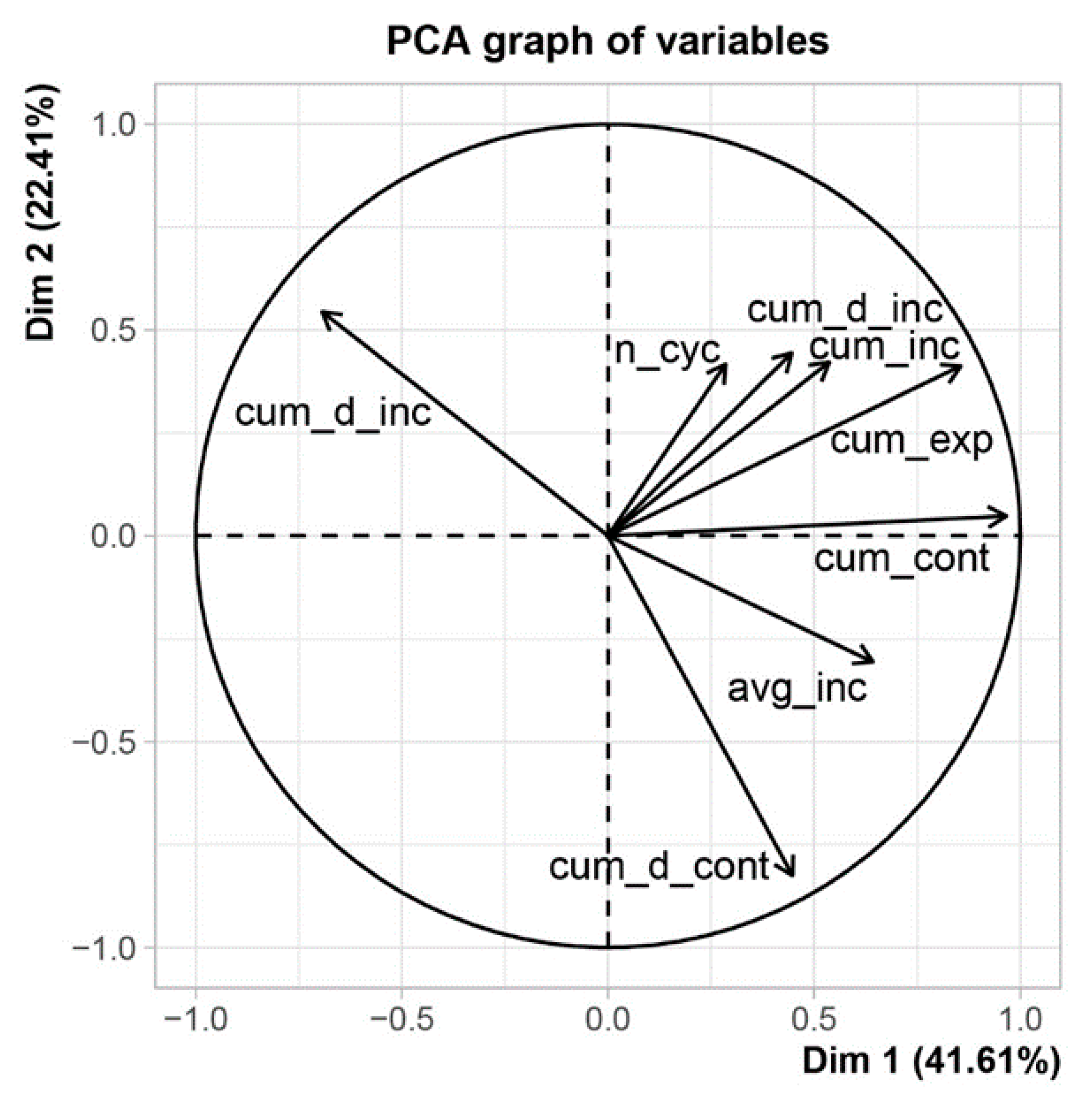
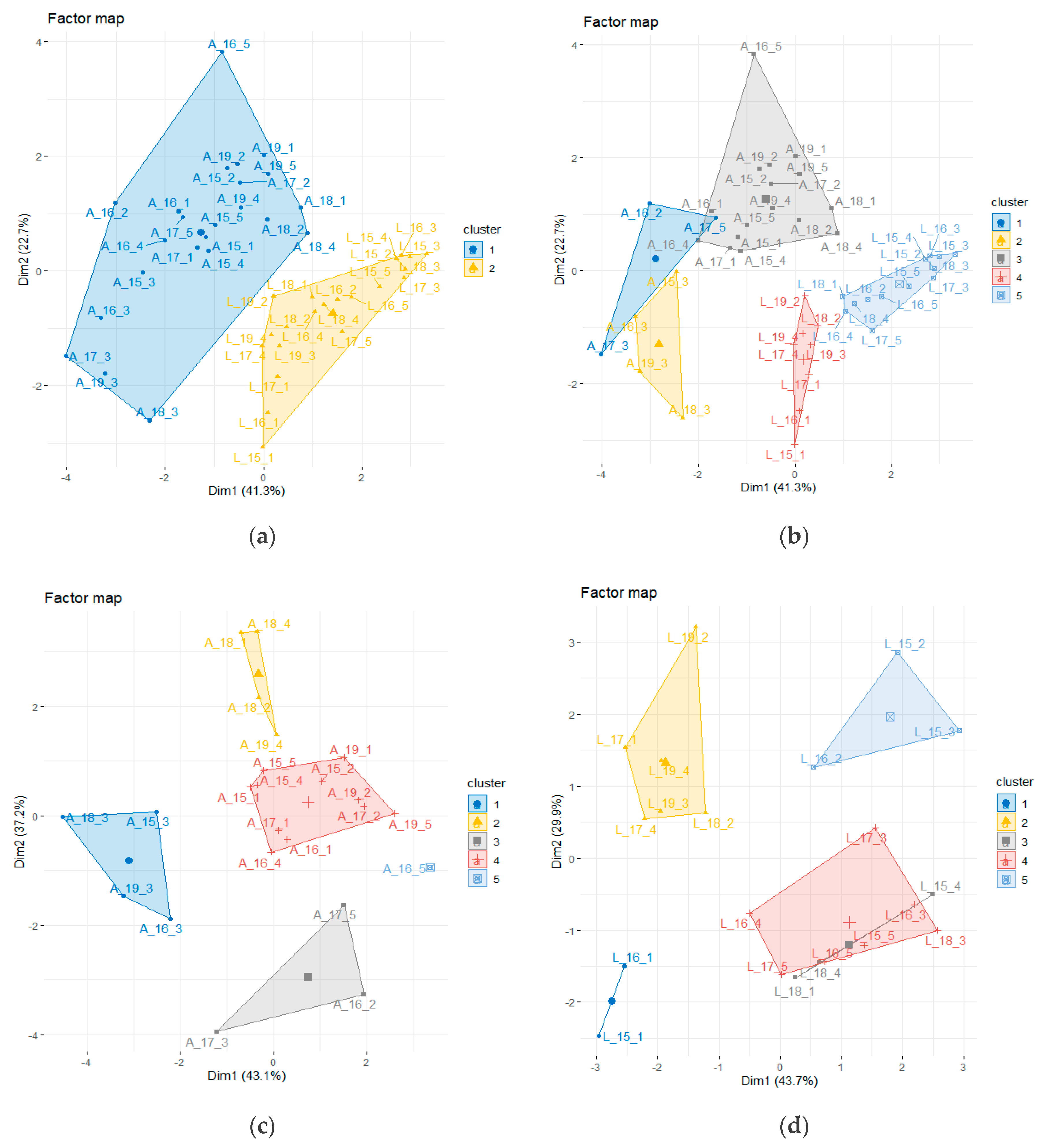
2.6. Species-Specific BDR Variability
- Number of cycles (n_cyc) (number) in the season—based on the stem cycle approach one cycle always comprises contraction and expansion phases, while the increment phase is optional;
- Cumulative duration of contraction (cum_d_cont) (hour)—seasonal sum of all contraction time lengths;
- Average duration of contraction (avg_d_cont) (hour)—cumulative duration of contraction divided by number of stem cycles;
- Cumulative amplitude of contraction (cum_cont) (mm)—seasonal sum of all contractions;
- Average amplitude of contraction (avg_cont) (mm)—seasonal sum of contractions divided by number of stem cycles;
- Cumulative duration of expansion (cum_d_exp) (hour)—seasonal sum of all expansion time lengths;
- Average duration of expansion (avg_d_exp) (hour)—cumulative duration of expansion divided by number of stem cycles;
- Cumulative amplitude of expansion (cum_exp) (mm)—seasonal sum of all expansions;
- Average amplitude of expansion (avg_exp) (mm)—cumulative amplitude of expansion divided by number of stem cycles;
- Cumulative duration of increment (cum_d_inc) (hour)—seasonal sum of all increment time lengths;
- Average duration of increment (avg_d_inc) (hour)—cumulative duration of increment divided by number of stem cycles;
- Cumulative increment (cum_inc) (mm)—seasonal sum of all daily increments;
- Average daily increment (avg_inc) (mm)—cumulative increment divided by number of stem cycles.
2.7. Periodicity of BDR
3. Results
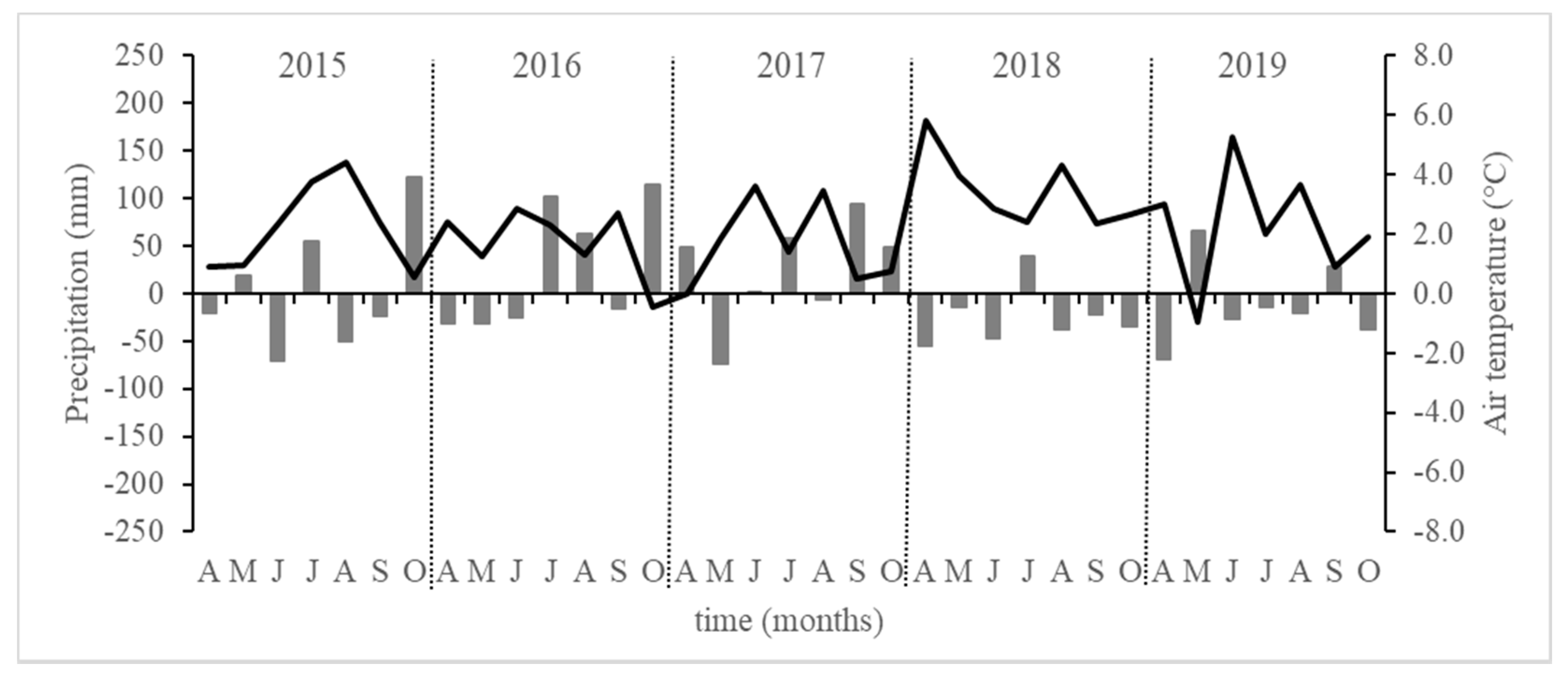
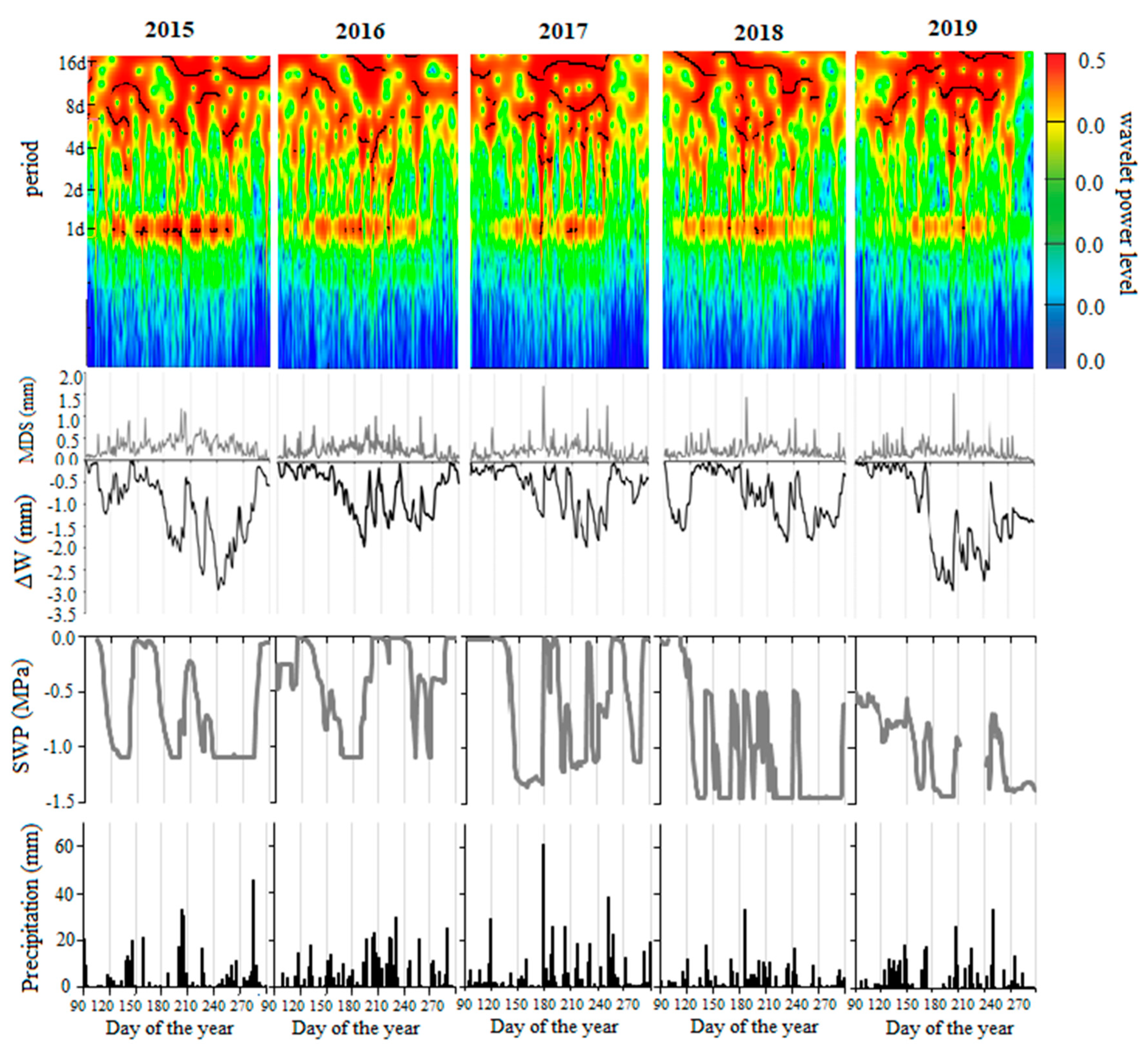
3.1. Environmental Conditions During the Studied Periods 2015–2019
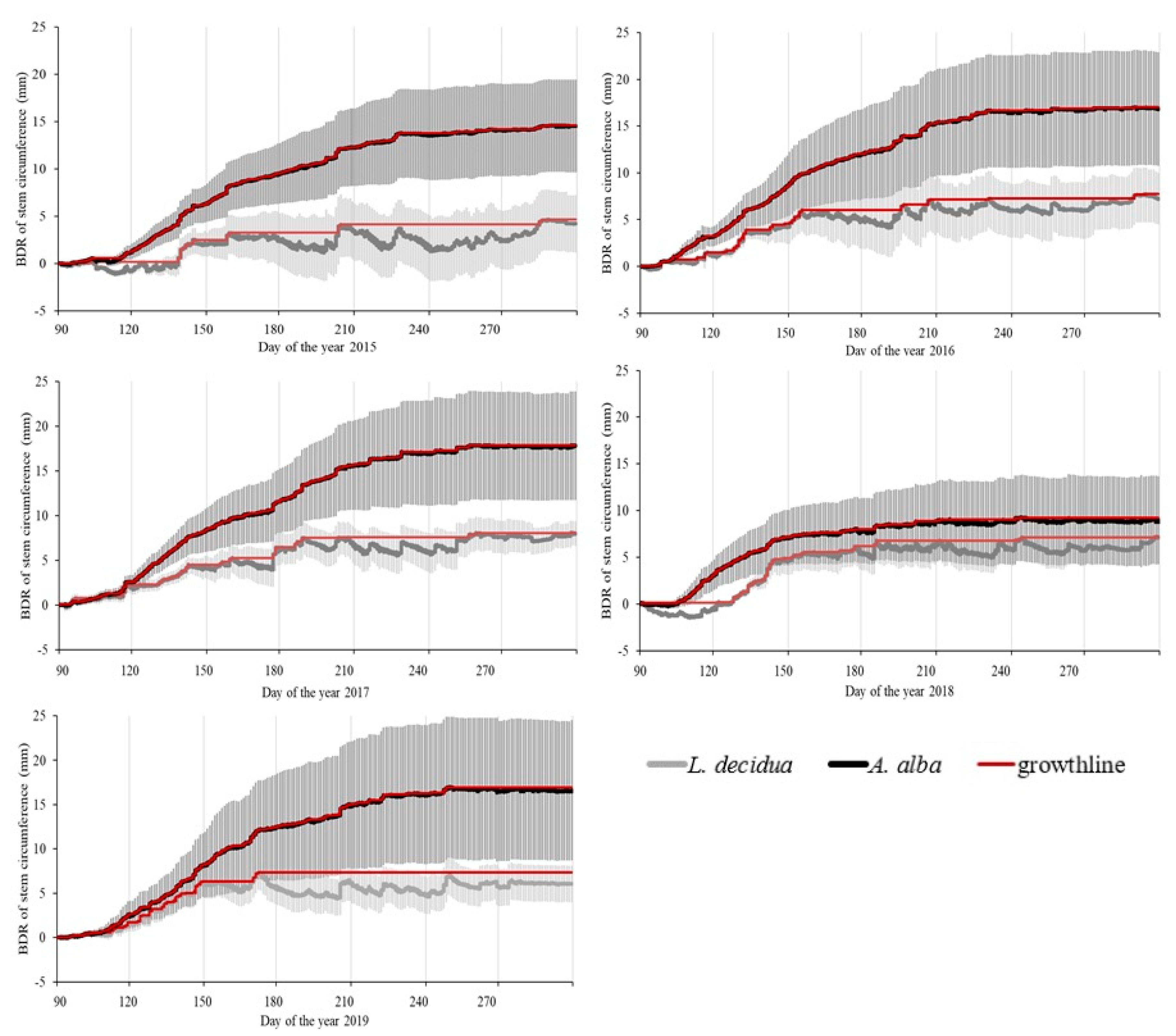
3.2. Stem Growth and Tree Water Status Derived from BDR
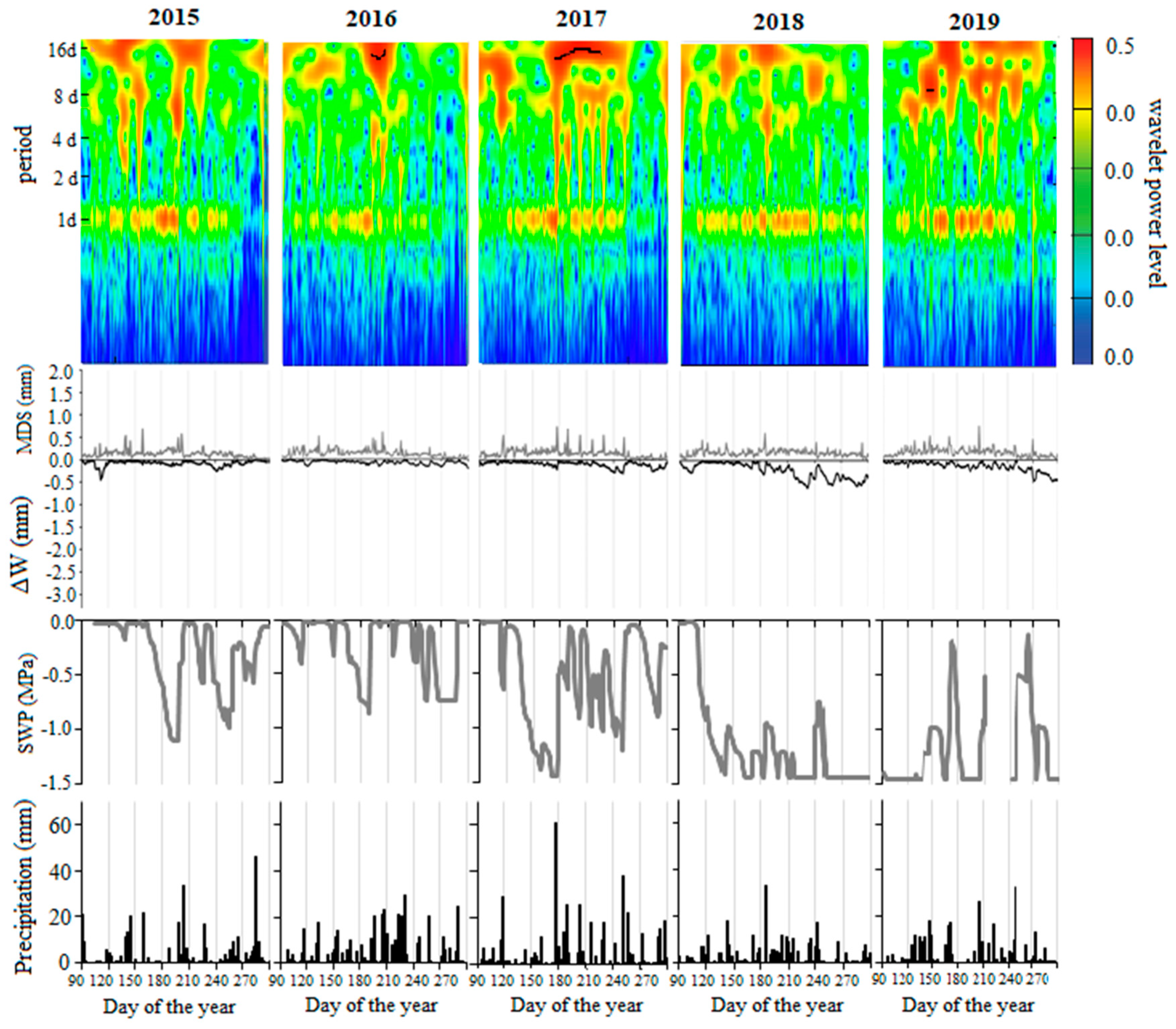
3.3. Tree Water Status and Environmental Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Will, R.E.; Wilson, S.M.; Zou, C.B.; Hennessey, T.C. Increased vapour pressure deficit due to higher temperature leads to greater transpiration and faster mortality during drought for tree seedlings common to the forest-grassland ecotone. New Phytol. 2013, 200, 366–374. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2013. The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Usoltsev, V.; Merganičová, K.; Konôpka, B.; Osmirko, A.A.; Tsepordey, I.S.; Chasovskikh, V.P. Fir (Abies spp.) stand biomass additive model for Eurasia sensitive to winter temperature and annual precipitation. Cent. Eur. For. J. 2019, 65, 166–172. [Google Scholar] [CrossRef]
- Albert, M.; Hansen, J.; Nagel, J.; Schmidt, M.; Spellmann, H. Assessing risks and uncertainties in forest dynamics under different management scenarios and climate change. For. Ecosyst. 2015, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Vitali, V.; Büntgen, U.; Bauhus, J. Silver fir and Douglas fir are more tolerant to extreme droughts than Norway spruce in south-western Germany. Glob. Chang. Biol. 2017, 23, 5108–5119. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, F.; Denvil, S. Changes in severe indices as simulated by two French coupled global climate models. Global Planet. Chang. 2007, 57, 96–117. [Google Scholar] [CrossRef] [Green Version]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Lebourgeois, F. Climatic signal in annual growth variation of silver fir (Abies alba Mill.) and spruce (Picea abies Karst.) from the French Permanent Plot Network (RENECOFOR). Ann. For. Sci. 2007, 64, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Lévesque, M.; Saurer, M.; Siegwolf, R.; Eilmann, B.; Brang, P.; Bugmann, H.; Rigling, A. Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Glob. Chang. Biol. 2013, 19, 3184–3199. [Google Scholar] [CrossRef]
- Danek, M.; Chuchro, M.; Walanus, A. Variability in Larch (Larix Decidua Mill.) Tree-ring growth response to climate in the polish Carpathian Mountains. Forests 2017, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Schulze, E.D.; Čermák, J.; Matyssek, R.; Penka, M.; Zimmermann, R.; Vasícek, F.; Gries, W.; Kučera, J. Canopy transpiration and water fluxes in the xylem of the trunk of Larix and Picea trees—A comparison of xylem flow, porometer and cuvette measurements. Oecologia 1985, 66, 475–483. [Google Scholar] [CrossRef]
- Ellenberg, H. Vegetation Ecology of Central Europe, 4th ed.; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- van der Maaten-Theunissen, M.; Kahle, H.-P.; van der Maaten, E. Drought sensitivity of Norway spruce is higher than that of silver fir along an altitudinal gradient in southwestern Germany. Ann. For. Sci. 2013, 70, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Ruosch, M.; Spahni, R.; Joos, F.; Henne, P.D.; van der Knaap, W.O.; Tinner, W. Past and future evolution of Abies alba forests in Europe-comparison of a dynamic vegetation model with palaeo data and observations. Glob. Chang. Biol. 2016, 22, 727–740. [Google Scholar] [CrossRef]
- Bošela, M.; Petráš, R.; Sitková, Z.; Priwitzer, T.; Pajtík, J.; Hlavatá, H.; Sedmák, R.; Tobin, B. Possible causes of the recent rapid increase in the radial increment of silver fir in the Western Carpathians. Environ. Pollut. 2014, 184, 211–221. [Google Scholar] [CrossRef]
- Büntgen, U.; Tegel, W.; Kaplan, J.O.; Schaub, M.; Hagedorn, F.; Bürgi, M.; Brázdil, R.; Helle, G.; Carrer, M.; Heussner, K.U.; et al. Placing unprecedented recent fir growth in a European-wide and Holocene-long context. Front. Ecol. Environ. 2014, 12, 100–106. [Google Scholar] [CrossRef]
- Hartmann, H.; Moura, C.F.; Anderegg, W.R.L.; Ruehr, N.K.; Salmon, Y.; Allen, C.D.; Arndt, S.K.; Breshears, D.D.; Davi, H.; Galbraith, D.; et al. Research frontier for improving our understanding of drought-induced tree and forest mortality. New Phytol. 2018, 218, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Y.; Wang, A.Y.; An, Y.N.; Lian, P.Y.; Wu, D.D.; Zhu, J.J.; Meinzer, F.C.; Hao, G.Y. Hydraulics play an important role in causing low growth rate and dieback of aging Pinus sylvestris var. mongolica trees in plantations of Northeast China. Plant Cell Environ. 2018, 41, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, W.M. Effects of water deficit on photosynthetic capacity. Physiol. Plant. 1987, 71, 142–149. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Kramer, P.J. Water Relation of Plants; Academic Press: New York, NY, USA, 1983. [Google Scholar]
- Peramaki, M.; Nikinmaa, E.; Sevanto, S.; Ilvesniemi, H.; Siivola, E.; Hari, P.; Vesala, T. Tree stem diameter variations and transpiration in Scots pine: An analysis using a dynamic sap flow model. Tree Physiol. 2001, 21, 889–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daudet, F.A.; Améglio, T.; Cochard, H.; Archilla, O.; Lacointe, A. Experimental analysis of the role of water and carbon in tree stem diameter variations. J. Exp. Bot. 2005, 56, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Čermák, J.; Kučera, J.; Bauerle, W.L.; Phillips, N.; Hinckley, T.M. Tree water storage and its diurnal dynamics related to sap flow and changes in stem volume in old-growth Douglas-fir trees. Tree Physiol. 2007, 27, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Peramaki, M.; Vesala, T.; Nikinmaa, E. Modeling the dynamics of pressure propagation and diameter variation in tree sapwood. Tree Physiol. 2005, 25, 1091–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinckley, T.; Lassoie, J. Radial growth in conifers and deciduous trees: A comparison. Mitt.-Vienna Forstl. Bundesversuchsanstalt 1981, 142, 17–56. [Google Scholar]
- Whitehead, D.; Jarvis, P.G. Coniferous Forests and Plantations. In Water Deficits and Plant Growth; Kozlowski, T.T., Ed.; Academic Press: New York, NY, USA, 1981; Volume VI, pp. 49–152. [Google Scholar]
- Herzog, K.M.; Thum, R.; Häsler, R. Diurnal changes in the radius of a subalpine Norway spruce stem: Their relation to the sap flow and their use to estimate transpiration. Trees 1995, 10, 94–101. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Newbery, D.M. Modeling tree water deficit from microclimate: An approach to quantifying drought stress. Tree Physiol. 2005, 25, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrenberger, W.; Rüger, S.; Fitzke, R.; Vollenweider, P.; Günthardt-Goerg, M.S.; Kuster, T.; Zimmermann, U.; Arend, M. Concomitant dendrometer and leaf patch pressure probe measurements reveal the effect of microclimate and soil moisture on diurnal stem water and leaf turgor variations in young oak trees. Funct. Plant. Biol. 2012, 39, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Costa, E.; Poyatos, R.; Sabaté, S. Contrasting growth and water use strategies in four co-occurring Mediterranean tree species revealed by concurrent measurements of sap flow and stem diameter variations. Agric. For. Meteorol. 2015, 207, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Sevanto, S.; Hölttä, T.; Markkanen, T.; Perämäki, M.; Nikinmaa, E.; Vesala, T. Relationships between diurnal xylem diameter variation and environmental factors in Scots pine. Boreal Environ. Res. 2005, 10, 447–458. [Google Scholar]
- Steppe, K.; Sterck, F.; Deslauriers, A. Diel growth dynamics in tree stems: Linking anatomy and ecophysiology. Trends Plant Sci. 2015, 20, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Zweifel, R.; Drew, D.M.; Schweingruber, F.; Downes, G.M. Xylem as the main origin of stem radius changes in eucalyptus. Funct. Plant Biol. 2014, 41, 520–534. [Google Scholar] [CrossRef] [Green Version]
- Zweifel, R.; Item, H.; Häsler, R. Stem radius changes and their relation to stored water in stems of young Norway spruce trees. Trees Struct. Funct. 2000, 15, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Deslauriers, A.; Morin, H.; Urbinati, C.; Carrer, M. Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Quebec (Canada). Trees 2003, 17, 477–484. [Google Scholar] [CrossRef]
- Turcotte, A.; Morin, H.; Krause, C.; Deslauriers, A.; Thibeault-Martel, M. The timing of spring rehydration and its relation with the onset of wood formation in black spruce. Agric. For. Meteorol. 2009, 149, 1403–1409. [Google Scholar] [CrossRef]
- Köcher, P.; Horna, V.; Leuschner, C. Environmental control of daily stem growth patterns in five temperate broad-leaved tree species. Tree Physiol. 2012, 32, 1021–1032. [Google Scholar] [CrossRef] [Green Version]
- Zweifel, R.; Item, H.; Häsler, R. Link between diurnal stem radius changes and tree water relations. Tree Physiol. 2001, 21, 869–877. [Google Scholar] [CrossRef] [Green Version]
- Drew, D.M.; Downes, G.M. The use precision dendrometers in research on daily stem size and wood property variation: A review. Dendrochronologia 2009, 27, 159–172. [Google Scholar] [CrossRef]
- Downes, G.; Beadle, C.; Worledge, D. Daily stem growth patterns in irrigated Eucalyptus globulus and E. nitens in relation to climate. Trees 1999, 14, 102–111. [Google Scholar] [CrossRef]
- Deslauriers, A.; Rossi, S.; Anfondillo, T. Dendrometer and intra-annual tree growth: What kind of information can be inferred? Dendrochronologia 2007, 25, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Leštianska, A.; Merganičová, K.; Merganič, J.; Střelcová, K. Intra-annual patterns of weather and daily radial growth changes of Norway spruce and their relationship in the Western Carpathian mountain region over a period of 2008–2012. J. For. Sci. 2015, 61, 315–324. [Google Scholar] [CrossRef]
- Oberhuber, W.; Gruber, A.; Kofler, W.; Swidrak, I. Radial stem growth in response to microclimate and soil moisture in a drought-prone mixed coniferous forest at an inner Alpine site. Eur. J. For. Res. 2014, 133, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Lukáčik, I. Arborétum Borová hora—História, súčasnosť a perspektívy. In Dendroflora of Central Europe—Utilization of Knowledge in Research, Education and Practice; Lukáčik, I., Sarvašová, I., Eds.; Vydavateľstvo TU vo Zvolene: Zvolen, Slovakia, 2015; pp. 9–19. [Google Scholar]
- Kölling, C. Klimahüllen für 27 Baumarten. AFZ-DerWald 2007, 62, 1242–1245. [Google Scholar]
- Oberhuber, W.; Hammerle, A.; Kofler, W. Tree water status and growth of saplings and mature Norway spruce (Picea abies) at a dry distribution limit. Front. Plant Sci. 2015, 6, 703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Maaten, E.; van der Maaten-Theunissen, M.; Smiljanić, M.; Rossi, S.; Simard, S.; Wilmking, M.; Deslauriers, A.; Fonti, P.; von Arx, G.; Bouriaud, O. dendrometeR: Analyzing the pulse of tree in R. Dendrochronologia 2016, 40, 12–16. [Google Scholar] [CrossRef]
- Torrence, C.; Compo, G.P. A practical guide to wavelet analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef] [Green Version]
- Rösch, A.; Schmidbauer, H. WaveletComp1.1: A Guided Tour through the Rpackage. 2018. Available online: http://www.Hsstat.Com/Projects/WaveletComp/WaveletComp_guided_tour.Pdf (accessed on 12 September 2020).
- Eilmann, B.; Rigling, A. Tree-growth analyses to estimate tree species’ drought tolerance. Tree Physiol. 2012, 32, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Vieira, J.; Rossi, S.; Campelo, F.; Freitas, H.; Nabais, C. Seasonal and daily cycles of stem radial variation of pinus pinaster in a drought-prone environment. Agric. For. Meteorol. 2013, 180, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Ibáñez, I.; Clark, J.S.; Dietze, M.C.; Feeley, K.; Hersh, M.; LaDeau, S.; McBride, A.; Welch, N.E.; Wolosin, M.S. Predicting biodiversity change: Outside the climate envelope, beyond the species-area curve. Ecology 2006, 87, 1896–1906. [Google Scholar] [CrossRef]
- Walentowski, H.; Falk, W.; Mette, T.; Kunz, J.; Bräuning, A.; Melnardus, C.; Zang, C.; Sucliffe, L.; Leuschner, C. Assessing future suitability of tree species under climate change by multiple methods: A case study in southern Germany. Ann. For. Res 2017, 60, 101–126. [Google Scholar] [CrossRef] [Green Version]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiol. Plant. 2013, 147, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Körner, C.; Basler, D. Phenology under global warming. Science 2010, 327, 1461–1462. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Water Deficits and Plant Growth. Vol III. Plant Responce and Control of Water Balance; Academic Press: New York, NY, USA, 1976; 368p. [Google Scholar]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newberry, D.M. Intra-annual radial growth and water relations of trees: Implications towards a growth mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devine, W.D.; Harrington, C.A. Factors affecting diurnal stem contraction in young Douglas-fir. Agric. For. Meteorol. 2011, 151, 414–419. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osorio, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherubini, P.; Gartner, B.L.; Tognetti, R.; Bräker, O.U.; Schoch, W.; Innes, J.L. Identification, measurement and interpretation of tree rings in woody species from Mediterranean climates. Biol. Rev. 2003, 78, 119–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Gričar, J.; Čufar, K. Seasonal dynamics of phloem and xylem formation in silver fir and Norway spruce as affected by drought. Russ. J. Plant Physiol. 2008, 55, 538–543. [Google Scholar] [CrossRef]
- Swidrak, I.; Gruber, A.; Oberhuber, W. Xylem and phloem phenology in co-occurring conifers exposed to drought. Trees 2014, 28, 1161–1171. [Google Scholar] [CrossRef] [Green Version]
- Köcher, P.; Horna, V.; Leuschner, C. Stem water storage in five coexisting temperate broad-leaved tree species: Significance, temporal dynamics and dependence on tree functional traits. Tree Physiol. 2013, 33, 817–832. [Google Scholar] [CrossRef]
- Anfodillo, T.; Rento, S.; Carraro, V.; Furlanetto, L.; Urbinati, C.; Carrer, M. Tree water relations and climatic variations at the alpine timberline: Seasonal changes of sap flux and xylem water potential in Larix decidua Miller, Picea abies (L.) Karst and Pinus cembra L. Ann. For. Sci. 1998, 55, 159–172. [Google Scholar] [CrossRef]
- Saulnier, M.; Corona, C.; Stoffel, M.; Guibal, F.; Edouard, J.-L. Climate-growth relationships in a Larix decidua Mill. Network in the French Alps. Sci. Total Environ. 2019, 664, 554–566. [Google Scholar] [CrossRef]
- Migliavacca, M.; Cremonese, E.; Colombo, R.; Busetto, L.; Galvagno, M.; Ganis, L.; Meroni, M.; Pari, E.; Rossini, M.; Siniscalco, C.; et al. European larch phenology in the Alps: Can we grasp the role of ecological factors by combining field observations and inverse modelling? Int. J. Biometeorol. 2008, 52, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Battipaglia, G.; Saurer, M.; Cherubini, P.; Siegwolf, R.T.W.; Cotrufo, M.F. Tree rings indicate different drought resistance of a native (Abies alba Mill.) and a non-native (Picea abies (L.) Karst.) species co-occurring at a dry site in Southern Italy. For. Ecol. Manag. 2009, 257, 820–828. [Google Scholar] [CrossRef]
- Breshears, D.D.; Adams, H.D.; Eamus, D.; McDowell, N.; Law, D.J.; Will, R.E.; Williams, A.P.; Zou, C.B. The critical amplifying role of increasing atmospheric moisture demand on tree mortality and associated regional die-off. Front. Plant Sci. 2013, 4, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberhuber, W.; Gruber, A. Climatic influences on intra-annual stem radial increment of Pinus sylvestris (L.) exposed to drought. Trees 2010, 24, 887–898. [Google Scholar] [CrossRef] [Green Version]
- Steppe, K.; De Pauw, D.J.W.; Lemeur, R.; Vanrolleghem, P.A. A mathematical model linking tree sap flow dynamics to daily stem diameter fluctuations and radial stem growth. Tree Physiol. 2006, 26, 257–273. [Google Scholar] [CrossRef] [Green Version]
- Lindner, M.; Garcia-Gonzalo, J.; Kolstrom, M.; Green, T.; Reguera, R.; Maroschek, M.; Seidl, R.; Lexer, M.J.; Netherer, S.; Schopf, A.; et al. Impacts of Climate Change on European Forests and Options for Adaptation; Report to the European Commission Directorate-General for Agriculture and Rural Development; European Forestry Institute: Joensuu, Finland, 2008; 173p. [Google Scholar]
- Bošeľa, M.; Lukáč, M.; Castagneri, D.; Sedmák, R.; Biber, P.; Carrer, P.; Konôpka, B.; Nola, P.; Thomas, A.; Nagel, T.A.; et al. Contrasting effects of environmental change on the radial growth of co-occurring beech and fir trees across Europe. Sci. Total Environ. 2018, 615, 1460–1469. [Google Scholar] [CrossRef]
- Moreno, F.; Conejero, W.; Martín-Palomo, M.J.; Girón, I.F.; Torrecillas, A. Maximum daily trunk shrinkage reference values for irrigation scheduling in olive trees. Agric. Water Manag. 2006, 84, 290–294. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Brock, W.A. Rising variance: A leading indicator of ecological transition. Ecol. Lett. 2006, 9, 311–318. [Google Scholar] [CrossRef]
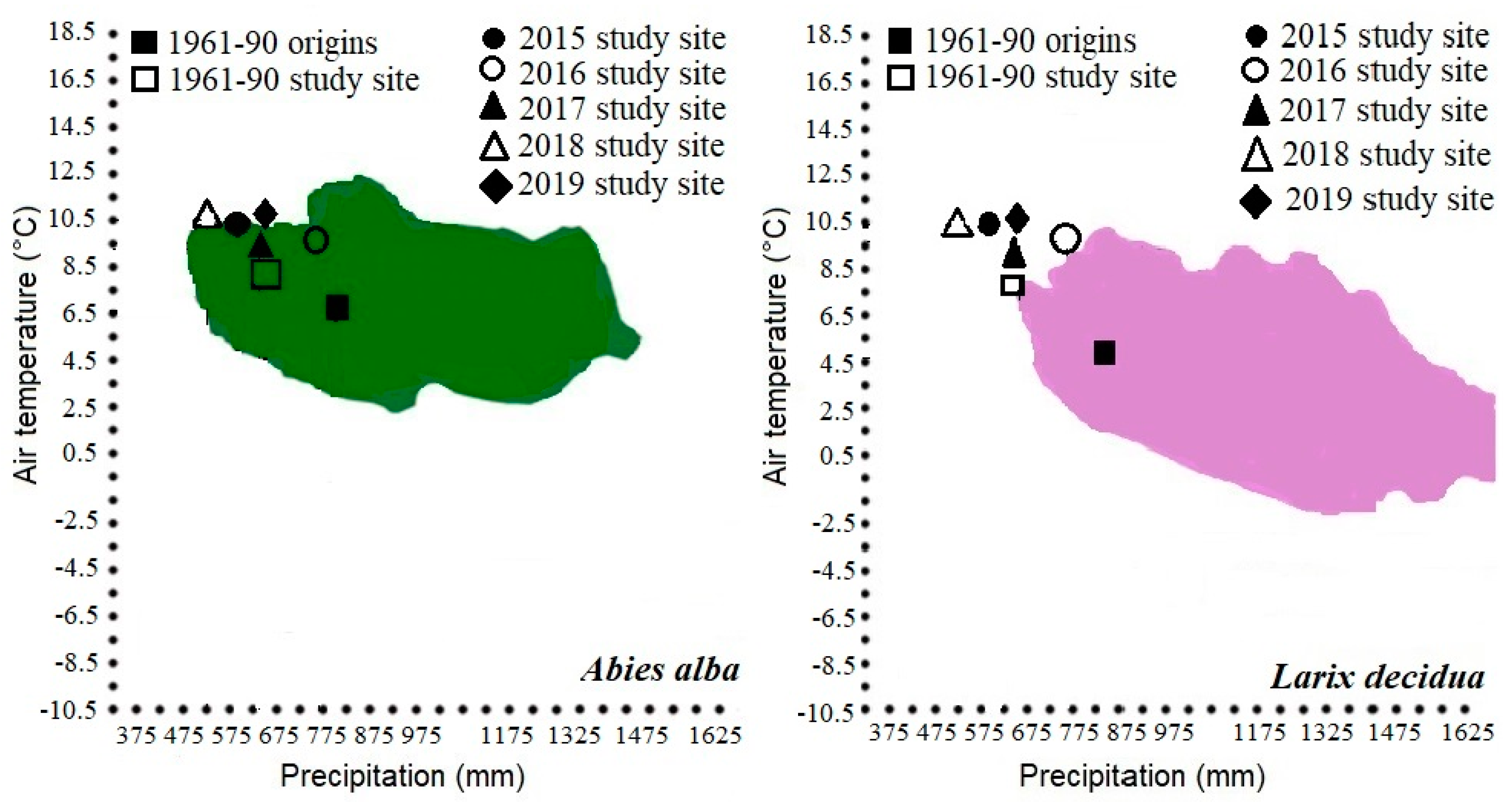
| Species | Original Location of Selected Species | ||||
|---|---|---|---|---|---|
| Orographic Unit | Locality | Elevation | Air Temperature | Precipitation | |
| (m a.s.l.) | (°C) | (mm) | |||
| L. decidua | Spišsko-gemerský kras | Voniaca dolina | 900 | 4.7 | 835 |
| A. alba | Kremnica mountains | Flochovský back | 950 | 6.5 | 786 |
| Month | Precipitation (mm) | Air Temperature (°C) | VPD | ||
|---|---|---|---|---|---|
| Precipitation Total | N | Mean | Difference from Normal | ||
| (mm) | (%) | (°C) | (°C) | (kPa) | |
| (A-O) 2015 | 412.2 | 105 | 15.7 | 2.2 | 0.552 |
| (A-O) 2016 | 512.3 | 125 | 15.3 | 1.8 | 0.455 |
| (A-O) 2017 | 501.0 | 124 | 15.2 | 1.6 | 0.500 |
| (A-O) 2018 | 320.8 | 75 | 17.0 | 3.5 | 0.557 |
| (A-O) 2019 | 387.2 | 92 | 15.8 | 2.3 | 0.480 |
| A. Alba | L. Decidua | |||||
|---|---|---|---|---|---|---|
| Cum_Inc ± SE | ∆Wcum ± SE | MDScum ± SE | Cum_Inc ± SE | ∆Wcum ± SE | MDScum ± SE | |
| 2015 | 14.59 ± 2.18 | −17.34 ± 0.54 | 29.35 ± 3.67 | 4.79 ± 1.33 | −234.78 ± 28.33 | 68.11 ± 7.34 |
| 2016 | 17.04 ± 2.74 | −14.69 ± 1.79 | 29.26 ± 4.49 | 7.74 ± 1.28 | −146.51 ± 11.97 | 58.53 ± 4.63 |
| 2017 | 17.88 ± 2.71 | −19.17 ± 1.61 | 32.52 ± 5.39 | 8.36 ± 0.72 | −122.32 ± 11.35 | 53.29 ± 4.21 |
| 2018 | 9.34 ± 2.01 | −49.60 ± 6.75 | 35.07 ± 6.32 | 7.41 ± 0.75 | −169.86 ± 13.25 | 56.00 ± 3.60 |
| 2019 | 16.96 ± 3.55 | −29.76 ± 3.71 | 37.31 ± 6.59 | 7.38 ± 0.82 | −241.92 ± 22.03 | 48.12 ± 1.74 |
| p-values | |||
|---|---|---|---|
| Cum_Inc | ∆Wcum | MDScum | |
| 2015 | 0.005 ** | 0.000 *** | 0.002 ** |
| 2016 | 0.015 * | 0.000 *** | 0.002 ** |
| 2017 | 0.009 ** | 0.000 *** | 0.016 * |
| 2018 | 0.400 | 0.000 *** | 0.021 * |
| 2019 | 0.030 * | 0.000 *** | 0.024 * |
| GR | ATmean | ATmin | ATmax | P | P-1 | RAH | VPD | SWP | ||
|---|---|---|---|---|---|---|---|---|---|---|
| A. alba | ∆W | −0.04 | −0.17 *** | 0.08 * | −0.37 *** | 0.20 *** | 0.27 *** | 0.17 *** | −0.24 *** | 0.41 *** |
| MDS | 0.40 *** | 0.48 *** | 0.42 *** | 0.44 *** | 0.12 *** | −0.04 | −0.25 *** | 0.39 *** | −0.24 *** | |
| L. decidua | ∆W | −0.08 ** | −0.37 *** | −0.30 *** | −0.38 *** | 0.15 *** | 0.28 *** | 0.14 *** | −0.30 *** | 0.48 *** |
| MDS | 0.34 *** | 0.57 *** | 0.61 *** | 0.49 *** | 0.22 *** | 0.09 ** | −0.11 *** | 0.33 *** | −0.09 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leštianska, A.; Fleischer, P., Jr.; Merganičová, K.; Fleischer, P.; Střelcová, K. Influence of Warmer and Drier Environmental Conditions on Species-Specific Stem Circumference Dynamics and Water Status of Conifers in Submontane Zone of Central Slovakia. Water 2020, 12, 2945. https://doi.org/10.3390/w12102945
Leštianska A, Fleischer P Jr., Merganičová K, Fleischer P, Střelcová K. Influence of Warmer and Drier Environmental Conditions on Species-Specific Stem Circumference Dynamics and Water Status of Conifers in Submontane Zone of Central Slovakia. Water. 2020; 12(10):2945. https://doi.org/10.3390/w12102945
Chicago/Turabian StyleLeštianska, Adriana, Peter Fleischer, Jr., Katarína Merganičová, Peter Fleischer, and Katarína Střelcová. 2020. "Influence of Warmer and Drier Environmental Conditions on Species-Specific Stem Circumference Dynamics and Water Status of Conifers in Submontane Zone of Central Slovakia" Water 12, no. 10: 2945. https://doi.org/10.3390/w12102945
APA StyleLeštianska, A., Fleischer, P., Jr., Merganičová, K., Fleischer, P., & Střelcová, K. (2020). Influence of Warmer and Drier Environmental Conditions on Species-Specific Stem Circumference Dynamics and Water Status of Conifers in Submontane Zone of Central Slovakia. Water, 12(10), 2945. https://doi.org/10.3390/w12102945






