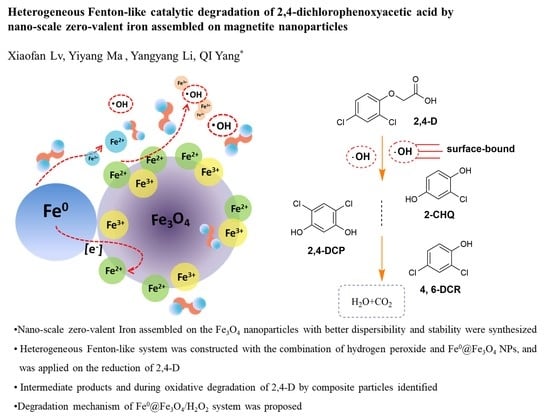
Heterogeneous Fenton-Like Catalytic Degradation of 2,4-Dichlorophenoxyacetic Acid by Nano-Scale Zero-Valent Iron Assembled on Magnetite Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
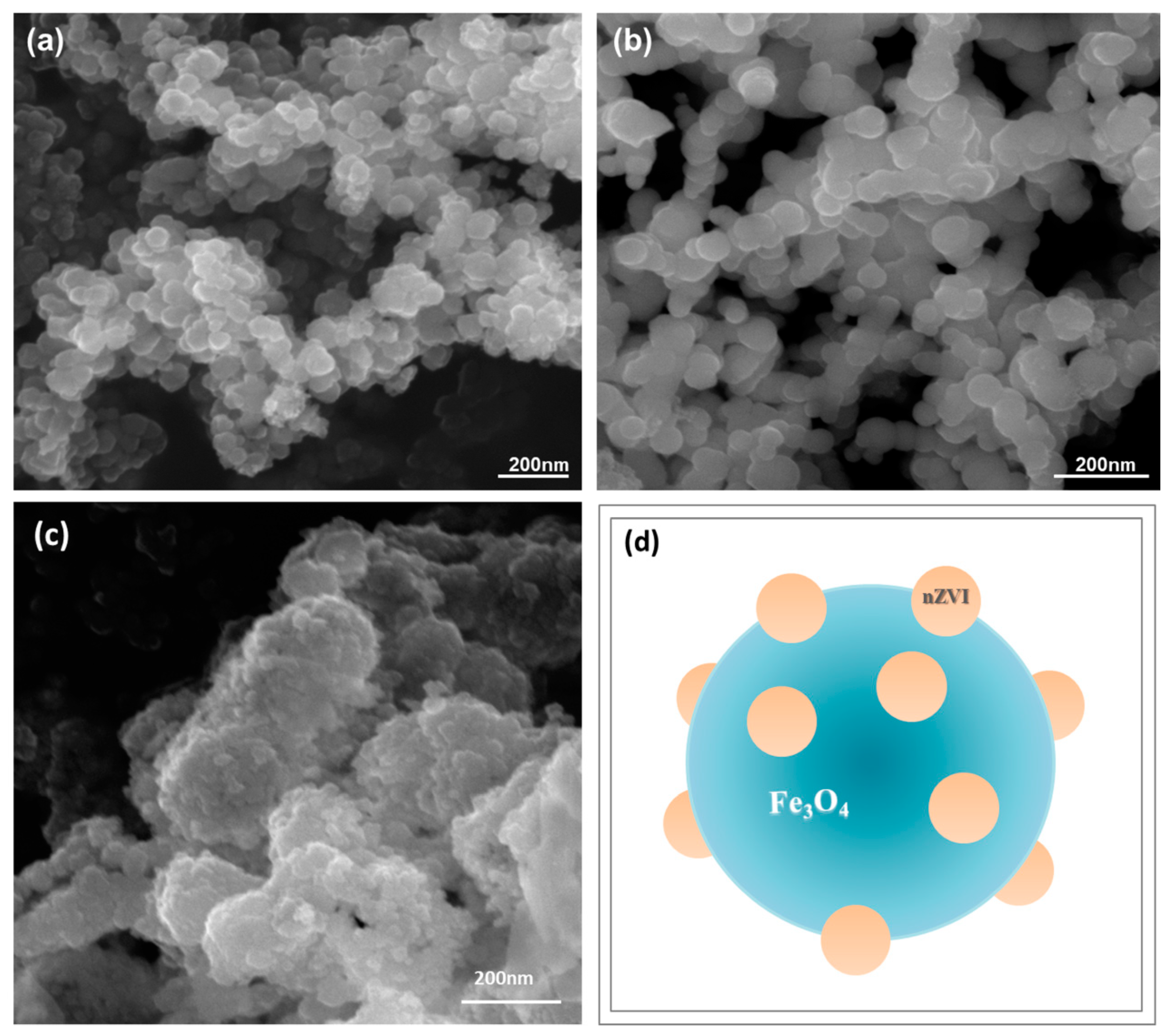
2.2. Preparation of Fe0@Fe3O4 Nanoparticles
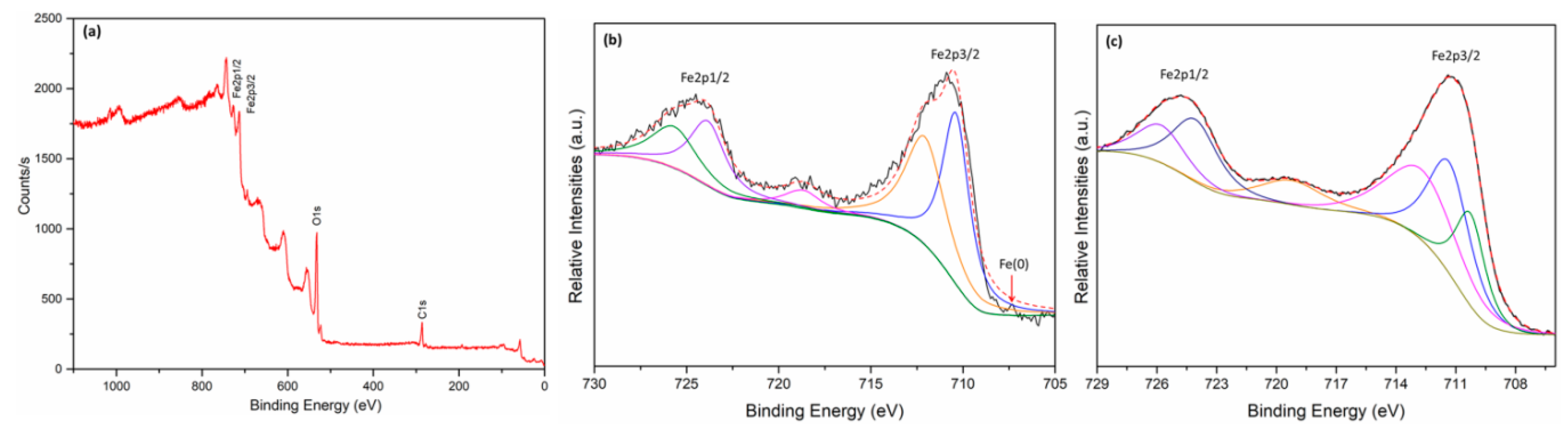
2.3. Characterization and Analysis Methods
2.4. Batch Experiments
3. Results and Discussion
3.1. Characterization of Fe0@Fe3O4 NPs
3.2. Batch Experiments
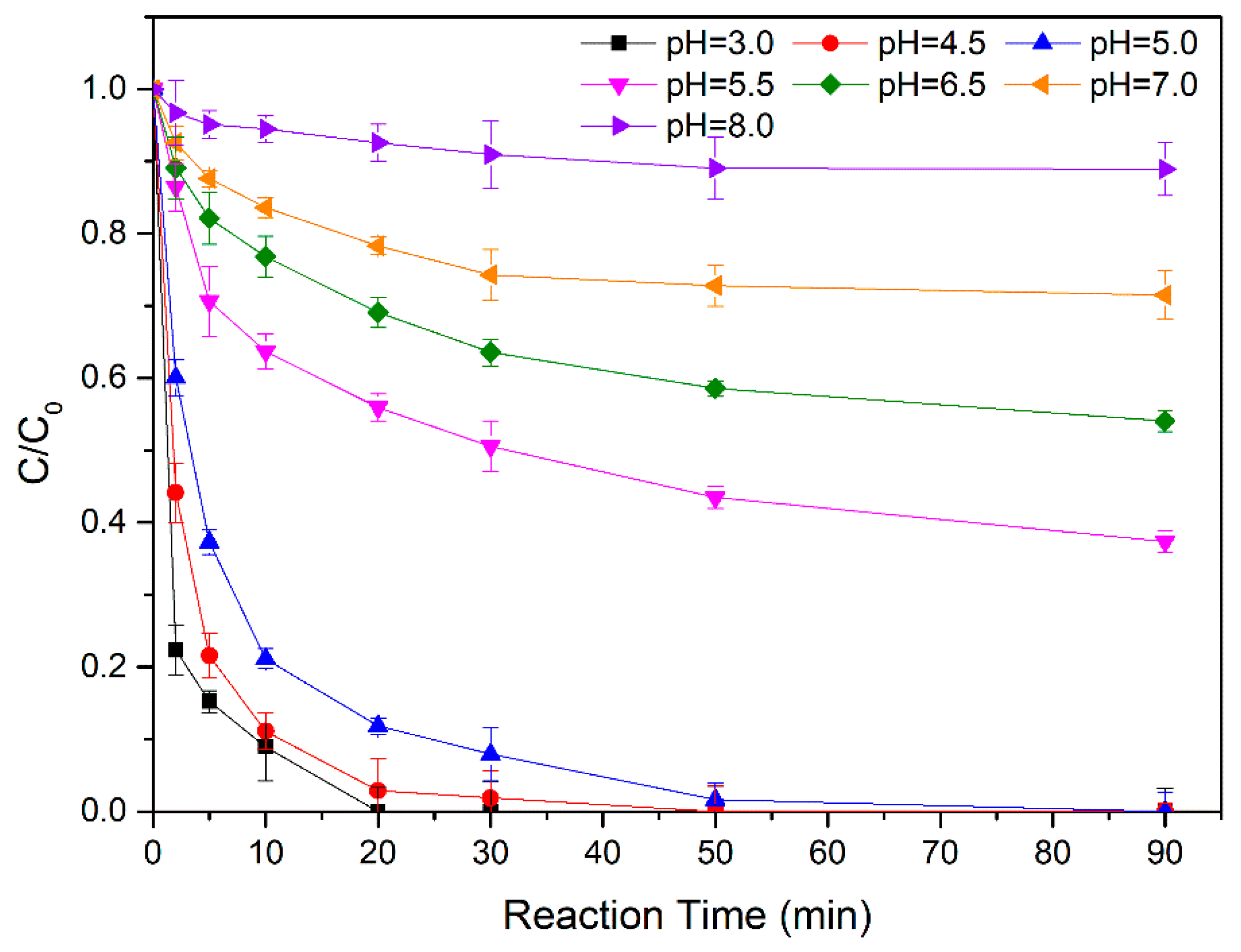
3.2.1. Effect of Initial pH
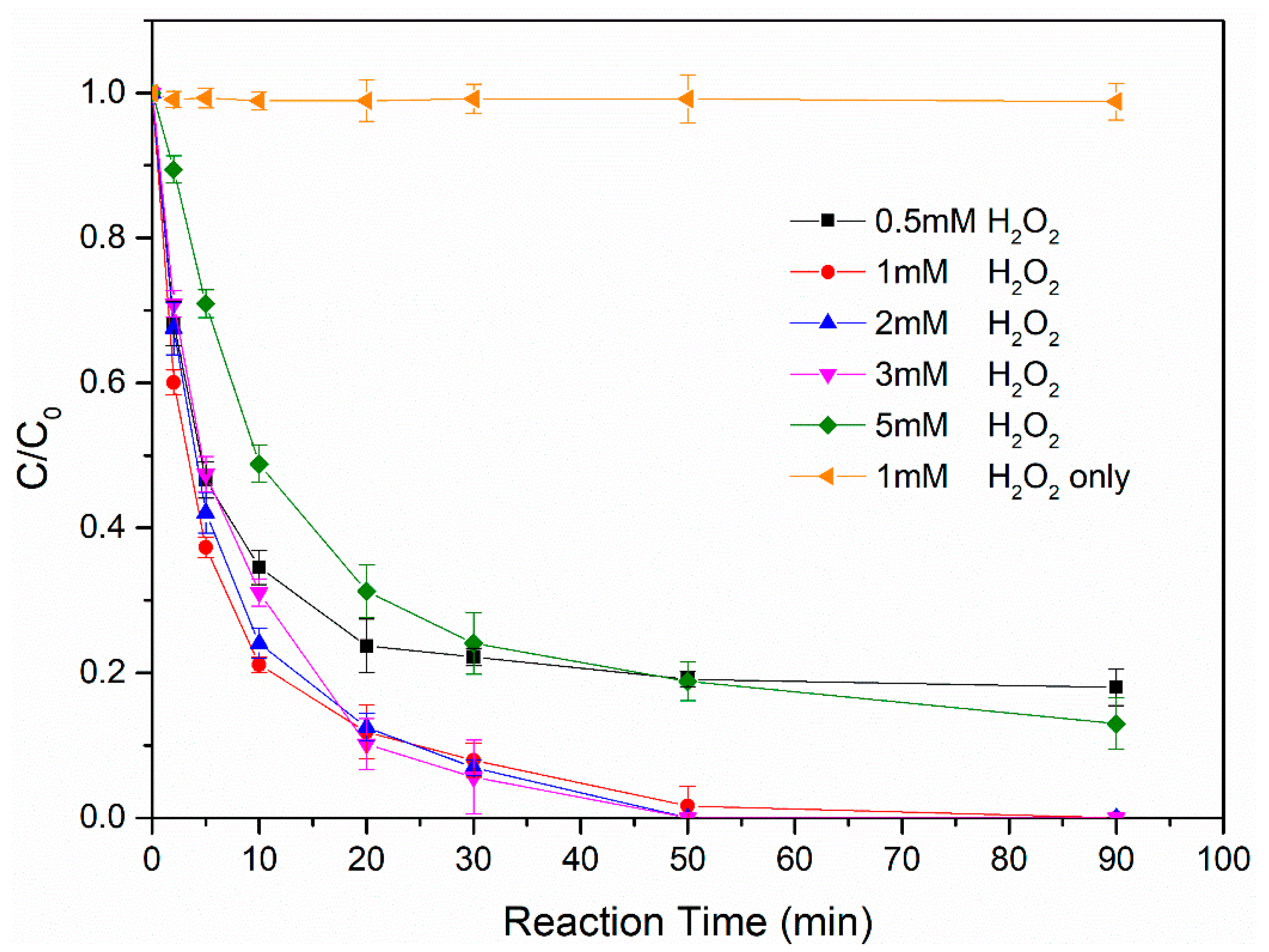
3.2.2. Effect of Hydrogen Peroxide Concentration
3.2.3. Effects of Temperature
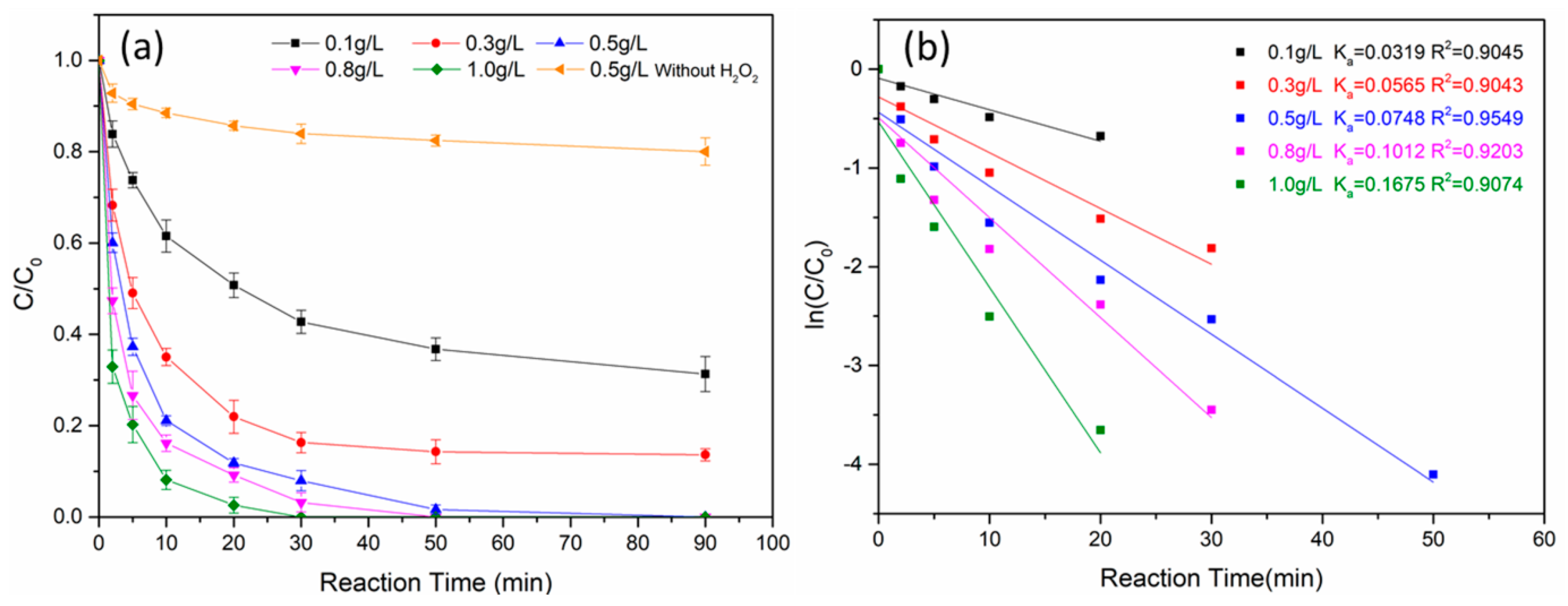
3.2.4. Effect of Fe0@Fe3O4 Dosage
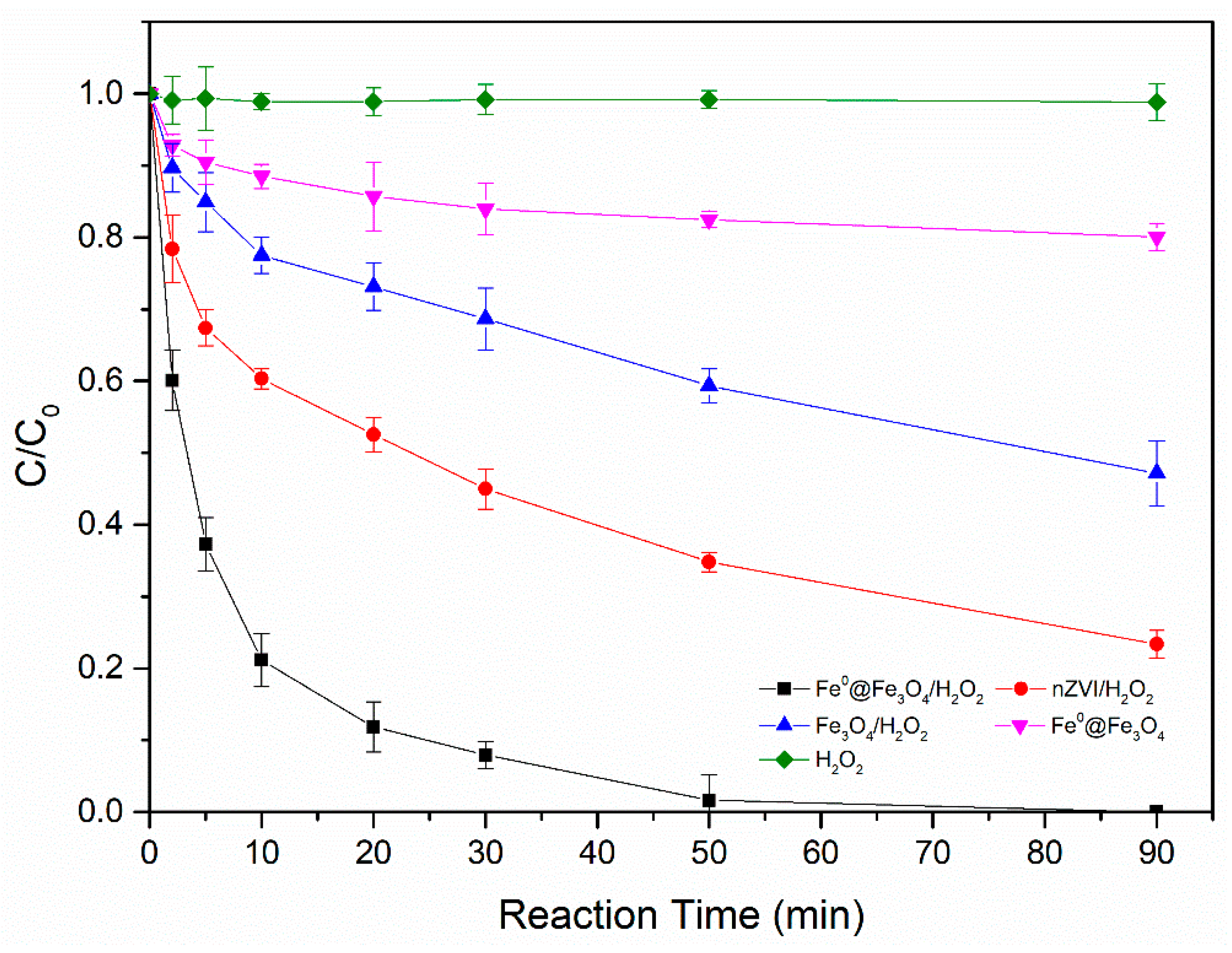
3.3. Comparison between Different Catalysts on the Degradation of 2,4-D
3.4. Degradation Products and Mechanism of 2,4-D in the Fe0@Fe3O4/H2O2 System
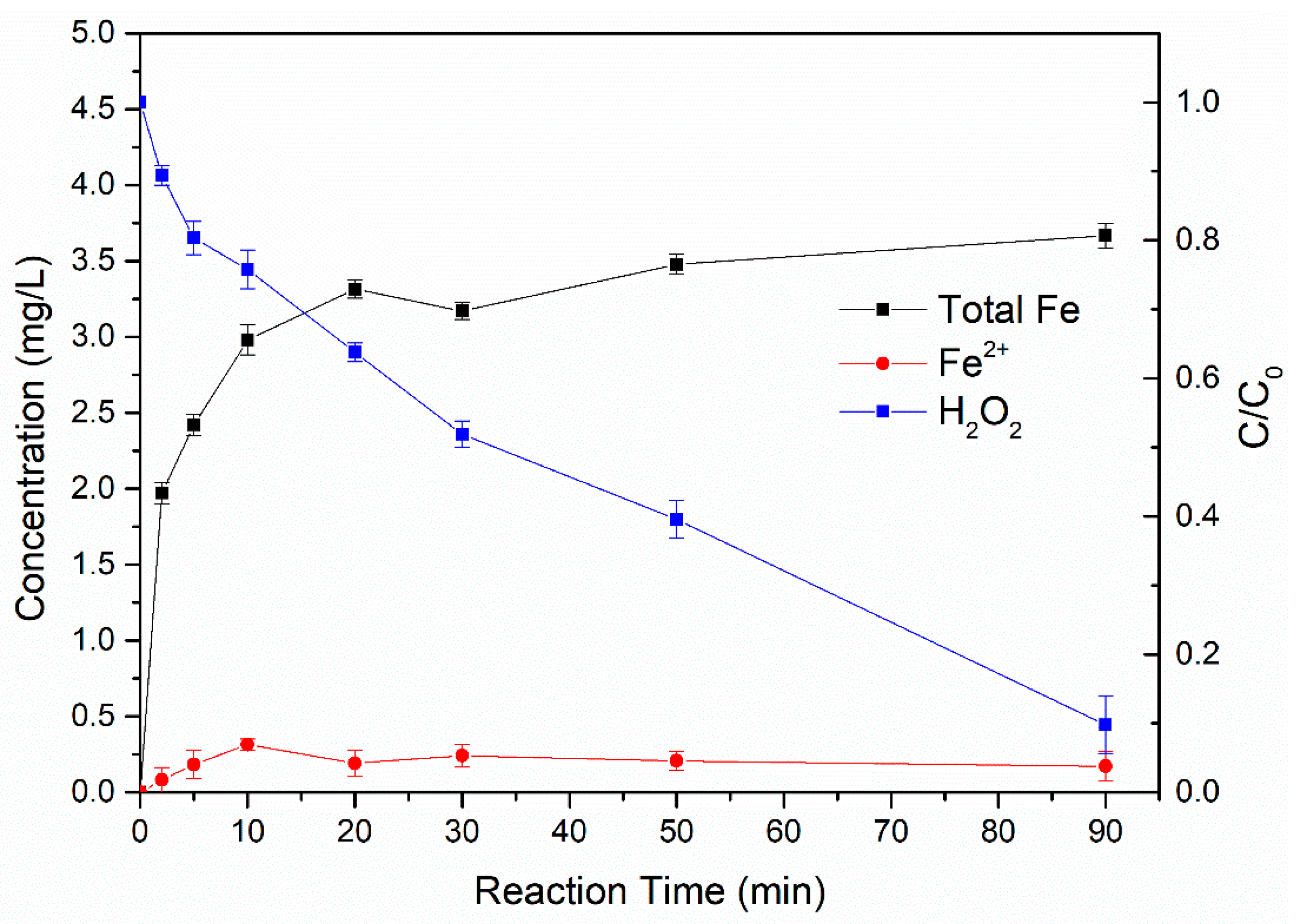
3.4.1. Changes of Ferrous/Ferric Ions Concentration
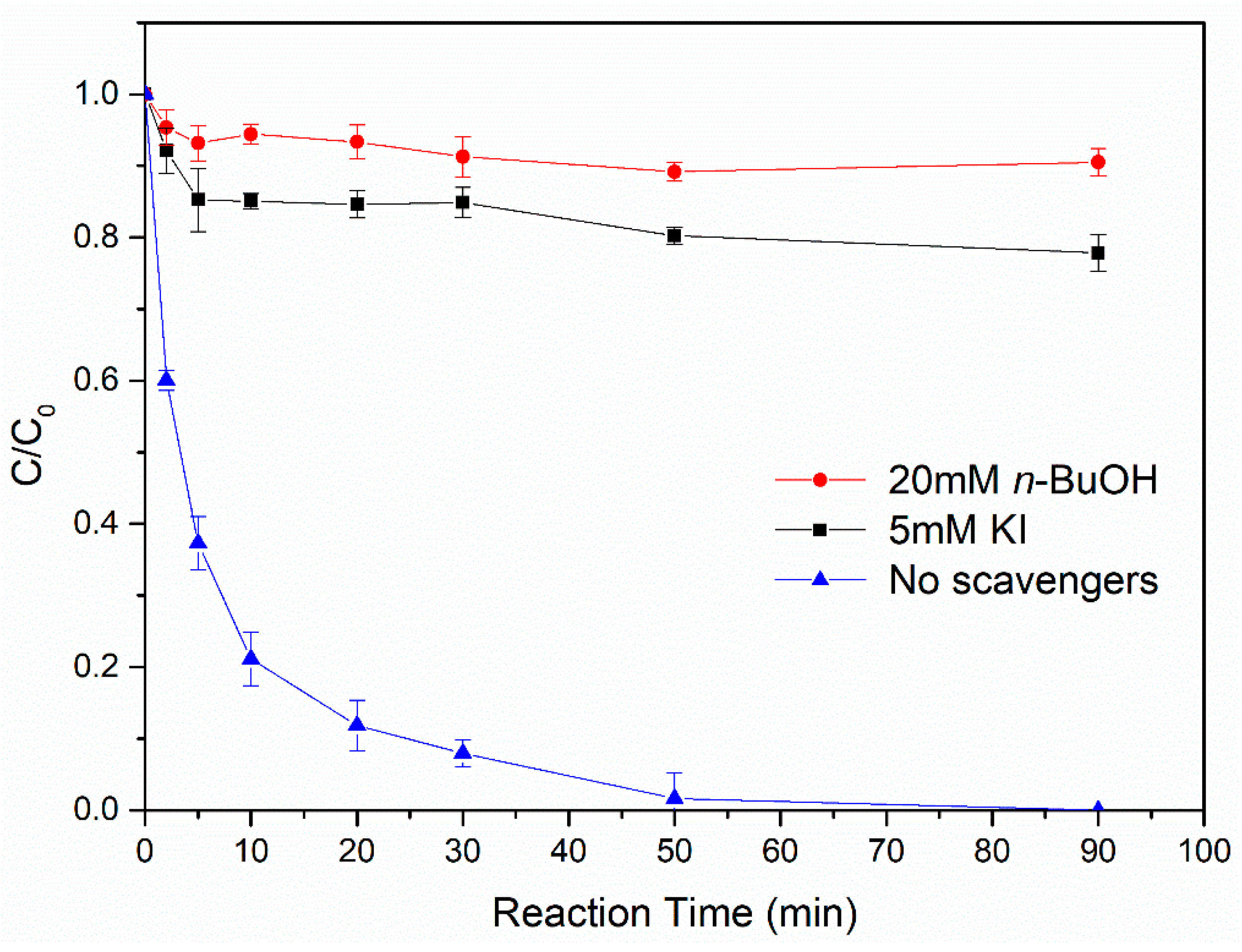
3.4.2. Identification of the Predominant Radical Species Generated in the Fe0@Fe3O4/H2O2 System
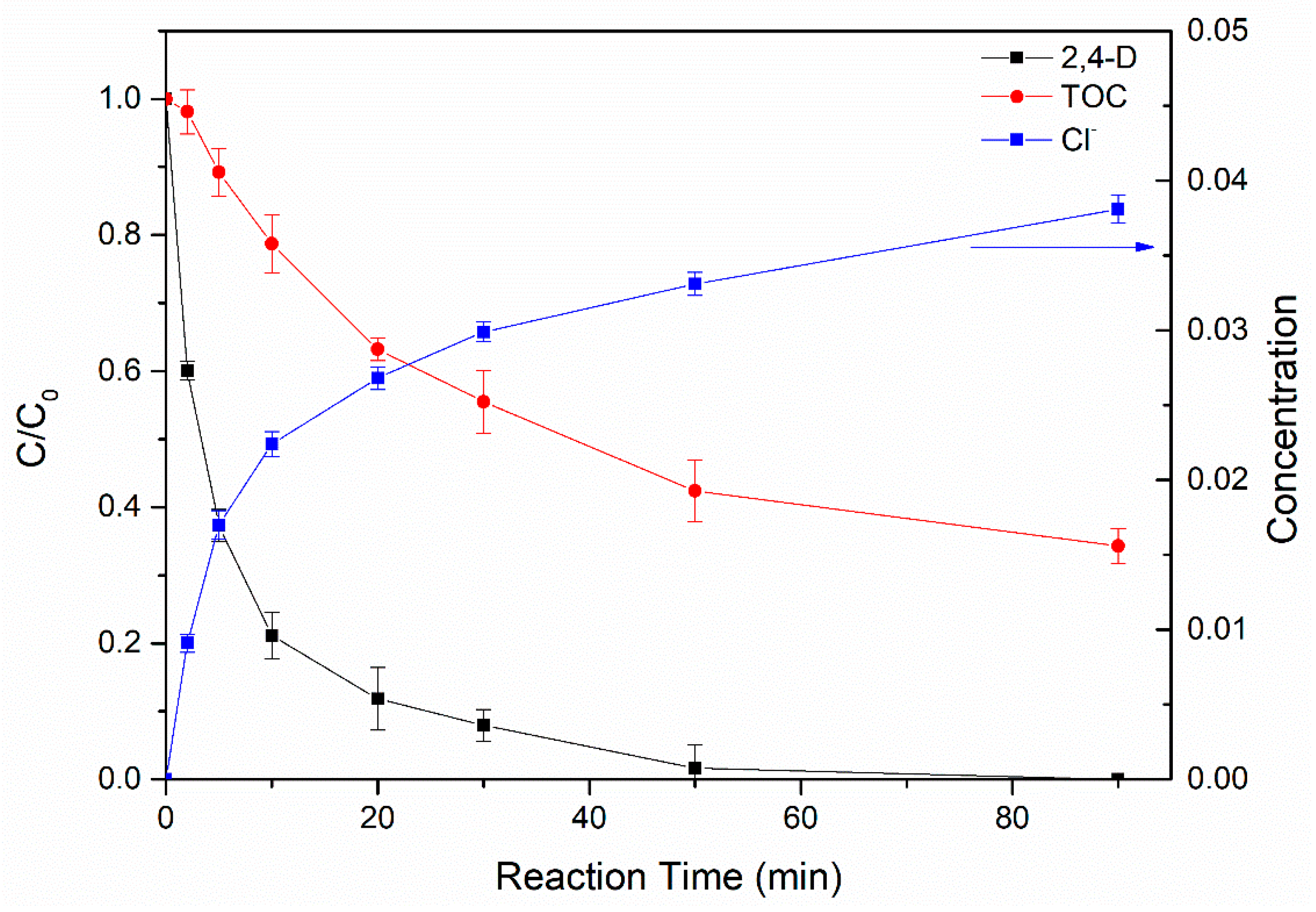
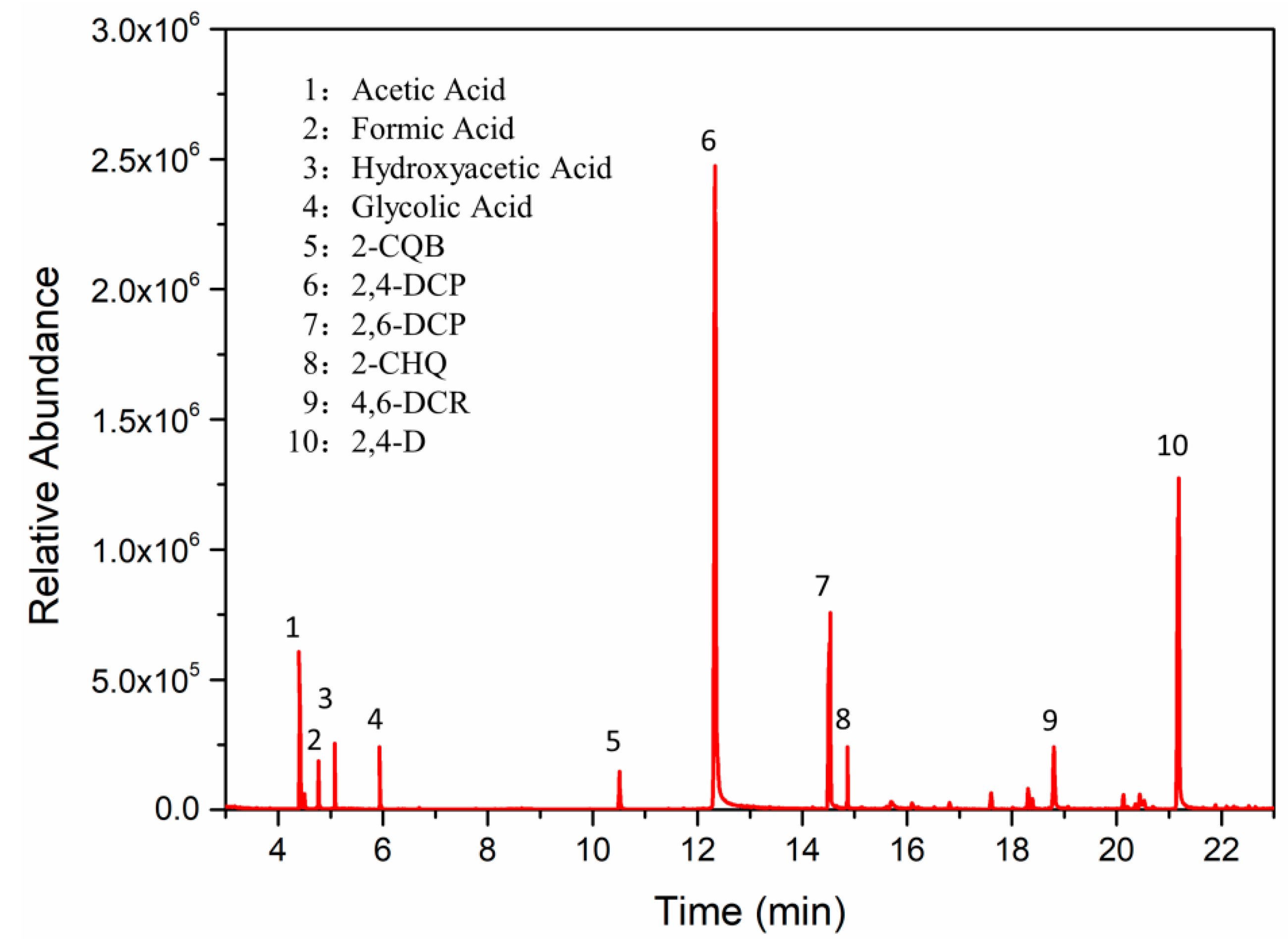
3.4.3. Degradation Products and Mineralization of 2,4-D
3.4.4. Mechanism of 2,4-D Degradation in the Fe0@Fe3O4/H2O2 System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boivin, A.; Amellal, S.; Schiavon, M.; Van Genuchten, M.T. 2,4-Dichlorophenoxyacetic acid (2,4-D) sorption and degradation dynamics in three agricultural soils. Environ. Pollut. 2005, 138, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Song, Y. Insight into the mode of action of 2, 4-dechlorophenoxyacetic acid (2,4-D) as an herbicide. J. Integr. Plant Biol. 2014, 56, 106–113. [Google Scholar] [CrossRef]
- Garabrant, D.H.; Philbert, M.A. Review of 2,4-Dichlorophenoxyacetic Acid (2,4-D) Epidemiology and Toxicology. Crit. Rev. Toxicol. 2002, 32, 233–257. [Google Scholar] [CrossRef]
- Chu, W.; Kwan, C.; Chan, K.; Chong, C. An unconventional approach to studying the reaction kinetics of the Fenton’s oxidation of 2,4-dichlorophenoxyacetic acid. Chemosphere 2004, 57, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Atamaniuk, T.M.; Kubrak, O.I.; Storey, K.B.; Lushchak, V.I. Oxidative stress as a mechanism for toxicity of 2,4-dichlorophenoxyacetic acid (2,4-D): Studies with goldfish gills. Ecotoxicology 2013, 22, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Brillas, E. Mineralization of 2,4-D by advanced electrochemical oxidation processes. Water Res. 2000, 34, 2253–2262. [Google Scholar] [CrossRef]
- Lee, C.; Keenan, C.R.; Sedlak, D.L. Polyoxometalate-Enhanced Oxidation of Organic Compounds by Nanoparticulate Zero-Valent Iron and Ferrous Ion in the Presence of Oxygen. Environ. Sci. Technol. 2008, 42, 4921–4926. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; Mackay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Benatti, C.T.; da Costa, A.C.S.; Tavares, C.R.G. Characterization of solids originating from the Fenton’s process. J. Hazard. Mater. 2009, 163, 1246–1253. [Google Scholar]
- Chu, L.; Wang, J.; Dong, J.; Liu, H.; Sun, X. Treatment of coking wastewater by an advanced Fenton oxidation process using iron powder and hydrogen peroxide. Chemosphere 2012, 86, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.H.; Feitz, A.J.; Sedlak, D.L.; Waite, T.D. Quantification of the Oxidizing Capacity of Nanoparticulate Zero-Valent Iron. Environ. Sci. Technol. 2005, 39, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J.L. A heterogeneous Fenton-like system with nanoparticulate zero-valent iron for removal of 4-chloro-3-methyl phenol. J. Hazard. Mater. 2011, 186, 256–264. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Carraway, E.R. Dechlorination of Pentachlorophenol by Zero Valent Iron and Modified Zero Valent Irons. Environ. Sci. Technol. 2000, 34, 2014–2017. [Google Scholar] [CrossRef]
- Noubactep, C.; Carè, S. On nanoscale metallic iron for groundwater remediation. J. Hazard. Mater. 2010, 182, 923–927. [Google Scholar] [CrossRef]
- Garbou, A.M.; Liu, M.; Zou, S.; Yestrebsky, C. Degradation kinetics of hexachlorobenzene over zero-valent magnesium/graphite in protic solvent system and modeling of degradation pathways using density functional theory. Chemosphere 2019, 222, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Shea, P.J.; Yang, J.E.; Kim, J.-E. Halide salts accelerate degradation of high explosives by zerovalent iron. Environ. Pollut. 2007, 147, 634–641. [Google Scholar] [CrossRef]
- Bae, S.; Lee, W. Influence of Riboflavin on Nanoscale Zero-Valent Iron Reactivity during the Degradation of Carbon Tetrachloride. Environ. Sci. Technol. 2014, 48, 2368–2376. [Google Scholar] [CrossRef]
- Lv, X.; Li, H.; Ma, Y.; Yang, H.; Yang, Q. Degradation of Carbon Tetrachloride by nanoscale Zero-Valent Iron@ magnetic Fe3O4: Impact of reaction condition, Kinetics, Thermodynamics and Mechanism. Appl. Organomet. Chem. 2018, 32, 4139. [Google Scholar] [CrossRef]
- Huang, R.; Fang, Z.; Yan, X.; Cheng, W. Heterogeneous sono-Fenton catalytic degradation of bisphenol A by Fe3O4 magnetic nanoparticles under neutral condition. Chem. Eng. J. 2012, 197, 242–249. [Google Scholar] [CrossRef]
- Tan, L.; Lu, S.; Fang, Z.; Cheng, W.; Tsang, E.P. Enhanced reductive debromination and subsequent oxidative ring-opening of decabromodiphenyl ether by integrated catalyst of nZVI supported on magnetic Fe3O4 nanoparticles. Appl. Catal. B Environ. 2017, 200, 200–210. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Feng, M.; Liu, W.; Wang, W.; Yang, Q.; Hu, Y. Degradation of 2,4-dichlorophenoxyacetic acid in water by persulfate activated with FeS (mackinawite). Chem. Eng. J. 2017, 313, 498–507. [Google Scholar] [CrossRef]
- Lv, X.; Prastistho, W.; Yang, Q.; Tokoro, C. Application of nano-scale zero-valent iron adsorbed on magnetite nanoparticles for removal of carbon tetrachloride: Products and degradation pathway. Appl. Organomet. Chem. 2020, 34, e5592. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Y.; Wu, X.; Wang, H. Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J. Hazard. Mater. 2009, 172, 1591–1596. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Sun, M.; Li, X.; Lu, F.; Qiu, H. Preparation of novel magnetic chitosan/graphene oxide composite as effective adsorbents toward methylene blue. Bioresour. Technol. 2012, 114, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Rivas, F.J.; Beltrán, F.J.; Frades, J.; Buxeda, P. Oxidation of p-hydroxybenzoic acid by Fenton’s reagent. Water Res. 2001, 35, 387–396. [Google Scholar] [CrossRef]
- Xu, X.R.; Li, X.Y.; Li, X.Z.; Li, H.B. Degradation of melatonin by UV, UV/H2O2, Fe2+/H2O2 and UV/Fe2+/H2O2 processes. Sep. Purif. Technol. 2009, 68, 261–266. [Google Scholar] [CrossRef]
- Ma, Y.S.; Huang, S.T.; Lin, J.G. Degradation of 4-nitro phenol using the Fenton process. Water Sci. Technol. 2000, 42, 155–160. [Google Scholar]
- Babuponnusami, A.; Muthukumar, K. Degradation of Phenol in Aqueous Solution by Fenton, Sono-Fenton and Sono-photo-Fenton Methods. Clean-Soil Air Water 2011, 39, 142–147. [Google Scholar] [CrossRef]
- Kwan, W.P.; Voelker, B.M. Decomposition of Hydrogen Peroxide and Organic Compounds in the Presence of Dissolved Iron and Ferrihydrite. Environ. Sci. Technol. 2002, 36, 1467–1476. [Google Scholar] [CrossRef]
- De La Plata, G.B.O.; Alfano, O.M.; Cassano, A.E. Decomposition of 2-chlorophenol employing goethite as Fenton catalyst II: Reaction kinetics of the heterogeneous Fenton and photo-Fenton mechanisms. Appl. Catal. B Environ. 2010, 95, 14–25. [Google Scholar] [CrossRef]
- Lin, S.H.; Lo, C.C. Fenton process for treatment of desizing wastewater. Water Res. 1997, 31, 2050–2056. [Google Scholar] [CrossRef]
- Liu, C.-C.; Tseng, D.-H.; Wang, C.-Y. Effects of ferrous ions on the reductive dechlorination of trichloroethylene by zero-valent iron. J. Hazard. Mater. 2006, 136, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Lookman, R.; Bastiaens, L.; Borremans, B.; Maesen, M.; Gemoets, J.; Diels, L. Batch-test study on the dechlorination of 1, 1, 1-trichloroethane in contaminated aquifer material by zero-valent iron. J. Contam. Hydrol. 2004, 74, 133–144. [Google Scholar] [CrossRef]
- Zhou, T.; Li, Y.; Lim, T.-T. Catalytic hydrodechlorination of chlorophenols by Pd/Fe nanoparticles: Comparisons with other bimetallic systems, kinetics and mechanism. Sep. Purif. Technol. 2010, 76, 206–214. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Pham, A.L.-T.; Lee, C.; Doyle, F.M.; Sedlak, D.L. A Silica-Supported Iron Oxide Catalyst Capable of Activating Hydrogen Peroxide at Neutral pH Values. Environ. Sci. Technol. 2009, 43, 8930–8935. [Google Scholar] [CrossRef] [PubMed]
- Navalón, S.; Alvaro, M.; Garcia, H. Heterogeneous Fenton catalysts based on clays, silicas and zeolites. Appl. Catal. B Environ. 2010, 99, 1–26. [Google Scholar] [CrossRef]
- Sychev, A.Y.; Isak, V.G. Iron compounds and the mechanisms of the homogeneous catalysis of the activation of O2 and H2O2and of the oxidation of organic substrates. Russ. Chem. Rev. 1995, 64, 1105–1129. [Google Scholar] [CrossRef]
- Wang, H.; Liang, H.S.; Chang, M.B. Chlorobenzene oxidation using ozone over iron oxide and manganese oxide catalysts. J. Hazard. Mater. 2011, 186, 1781–1787. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Ma, Y.; Li, Y.; Yang, Q. Heterogeneous Fenton-Like Catalytic Degradation of 2,4-Dichlorophenoxyacetic Acid by Nano-Scale Zero-Valent Iron Assembled on Magnetite Nanoparticles. Water 2020, 12, 2909. https://doi.org/10.3390/w12102909
Lv X, Ma Y, Li Y, Yang Q. Heterogeneous Fenton-Like Catalytic Degradation of 2,4-Dichlorophenoxyacetic Acid by Nano-Scale Zero-Valent Iron Assembled on Magnetite Nanoparticles. Water. 2020; 12(10):2909. https://doi.org/10.3390/w12102909
Chicago/Turabian StyleLv, Xiaofan, Yiyang Ma, Yangyang Li, and Qi Yang. 2020. "Heterogeneous Fenton-Like Catalytic Degradation of 2,4-Dichlorophenoxyacetic Acid by Nano-Scale Zero-Valent Iron Assembled on Magnetite Nanoparticles" Water 12, no. 10: 2909. https://doi.org/10.3390/w12102909
APA StyleLv, X., Ma, Y., Li, Y., & Yang, Q. (2020). Heterogeneous Fenton-Like Catalytic Degradation of 2,4-Dichlorophenoxyacetic Acid by Nano-Scale Zero-Valent Iron Assembled on Magnetite Nanoparticles. Water, 12(10), 2909. https://doi.org/10.3390/w12102909