Soil Salinity and Moisture Control the Processes of Soil Nitrification and Denitrification in a Riparian Wetlands in an Extremely Arid Regions in Northwestern China
Abstract
:1. Introduction
2. Materials and Methods
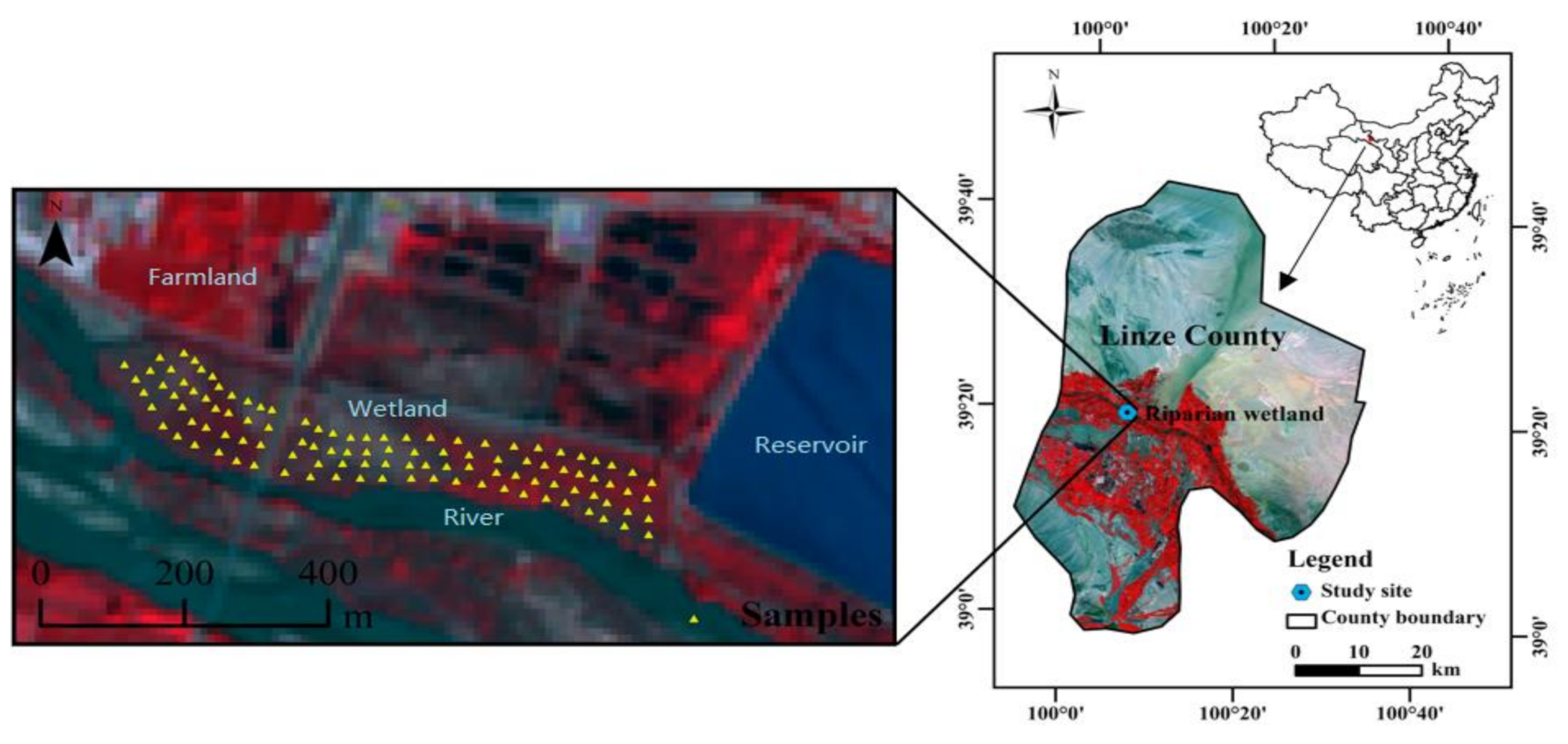
2.1. Study Site
2.2. Experimental Design and Soil Sampling
2.3. Measurements of Soil Nitrification and Denitrification Rates
2.4. Soil Analysis
2.5. Data Analysis
3. Results
3.1. Soil Nitrification, Denitrification and Environmental Factors
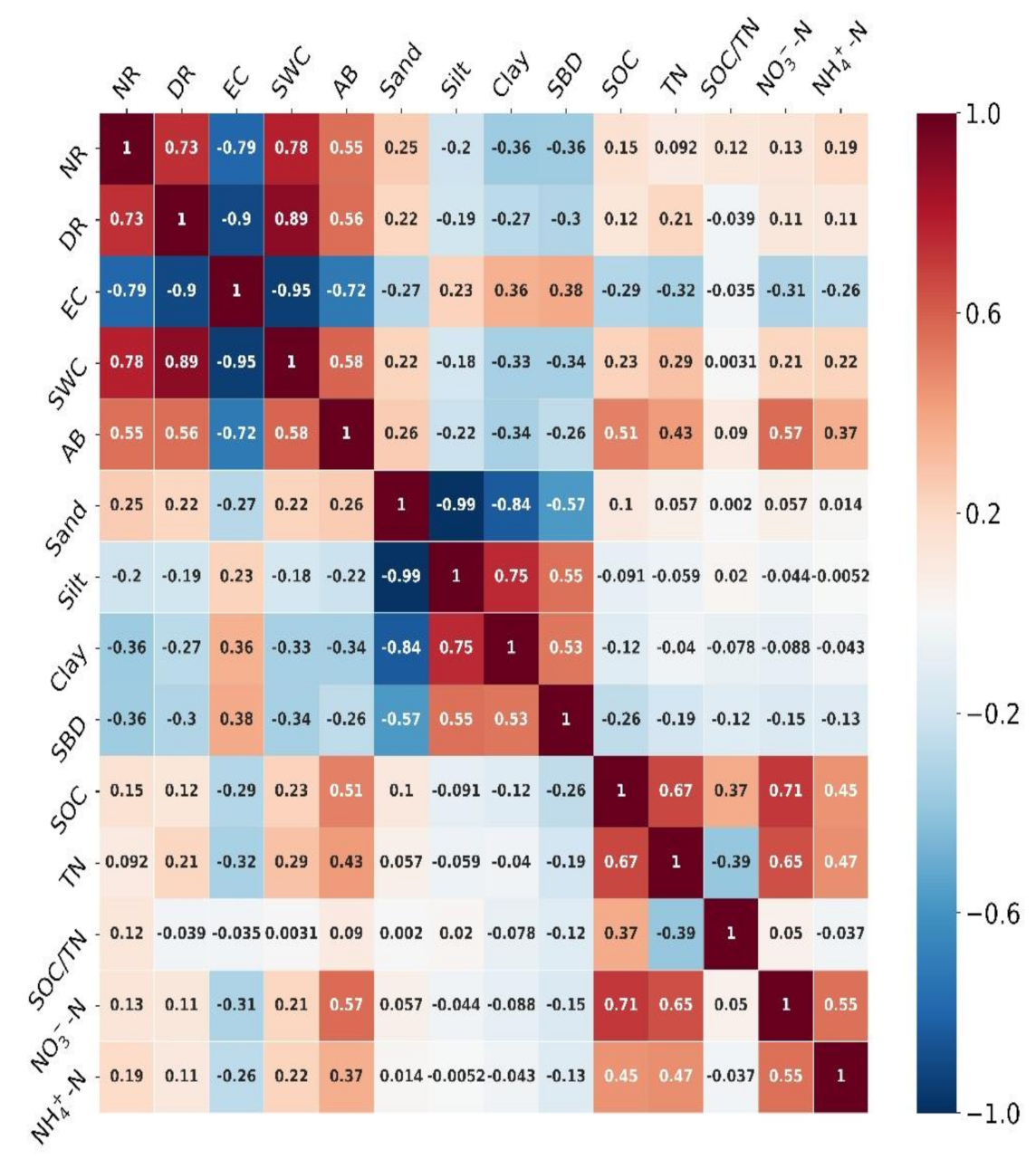
3.2. Correlations Between Soil Nitrification and Denitrification Rates and Environmental Variables
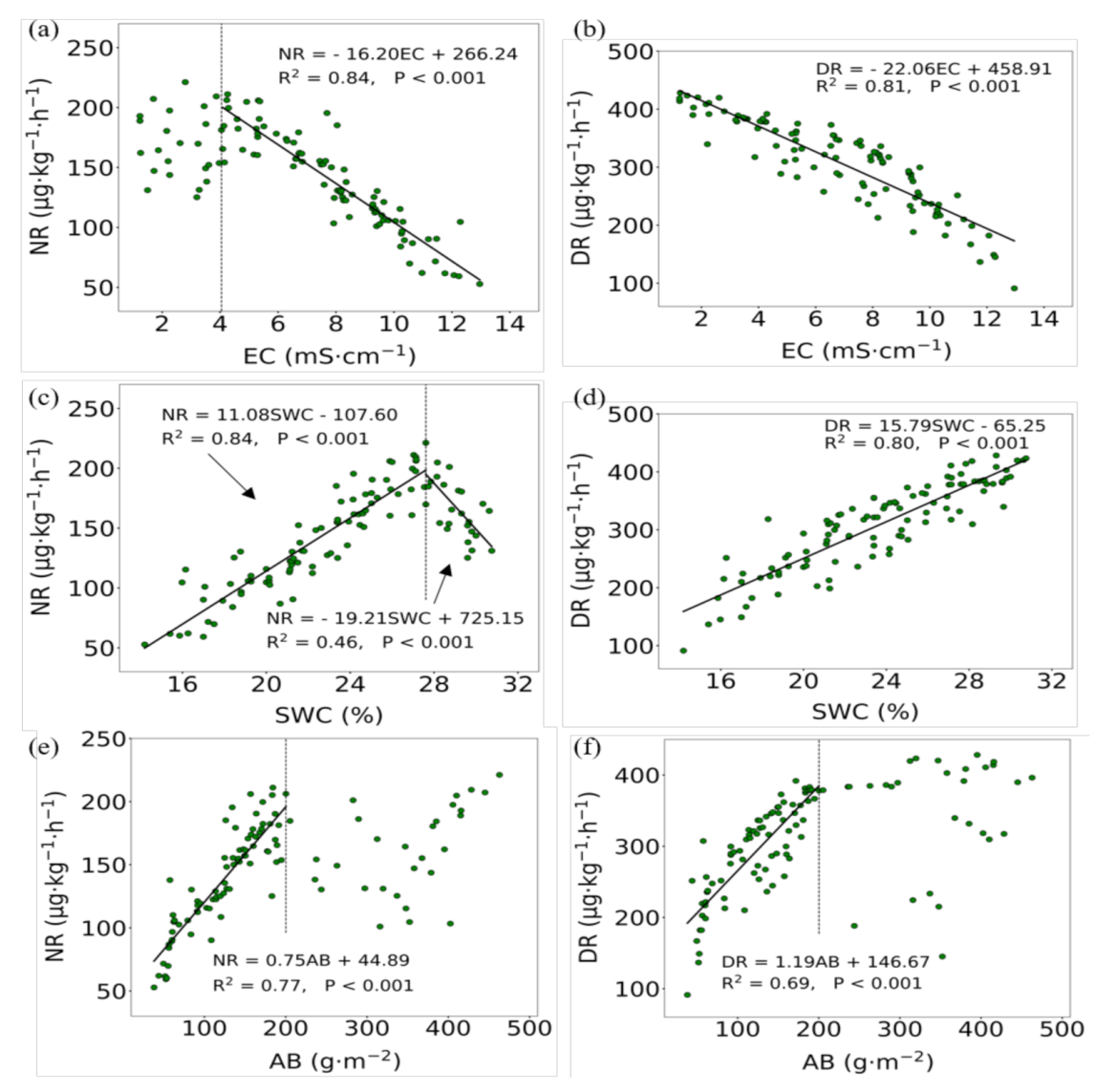
3.3. Relationships Between Soil Nitrification and Denitrification Rates, and Main Influencing Factors
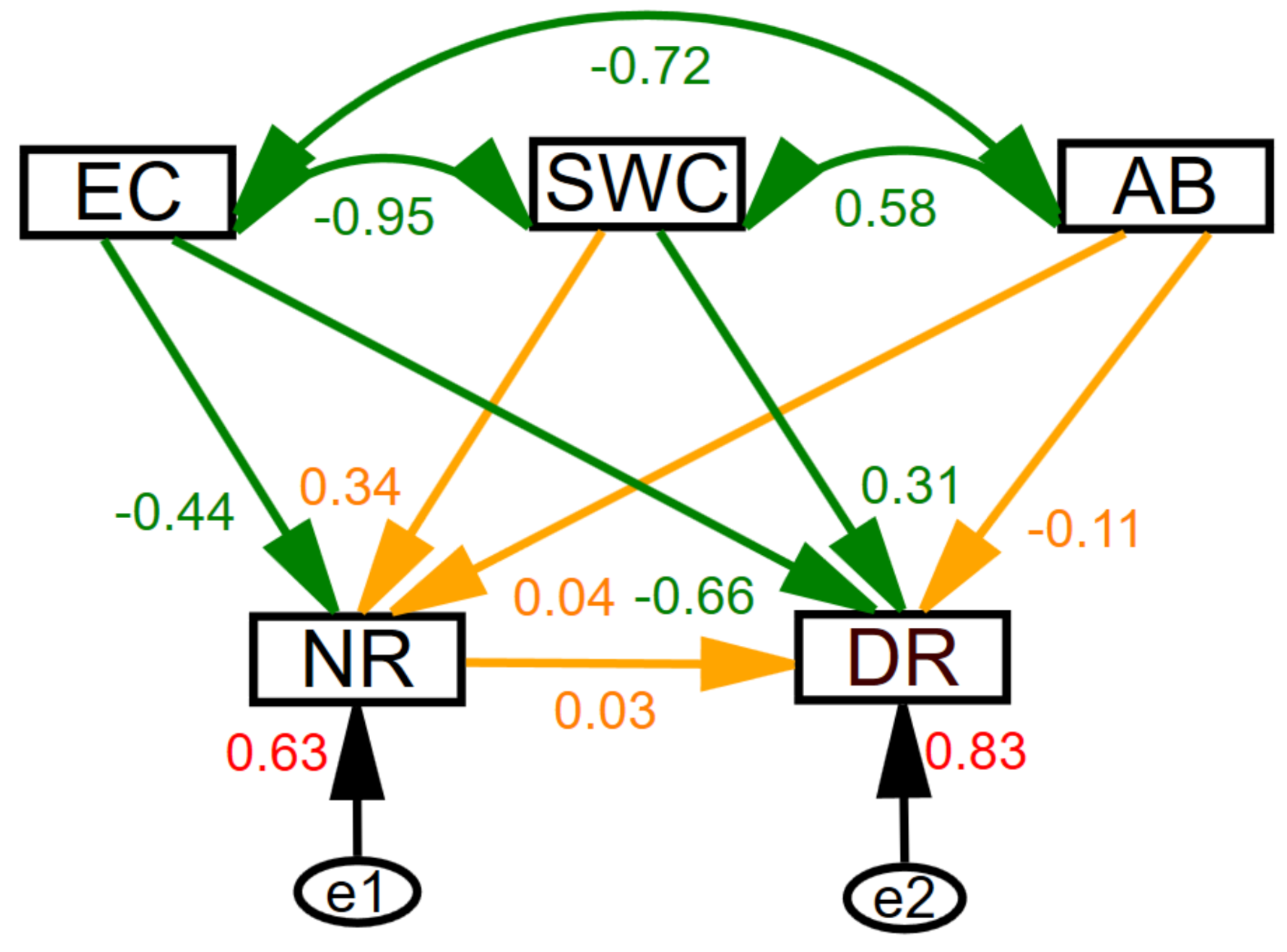
3.4. Interaction Among Main Influencing Factors of Soil Nitrification and Denitrification
4. Discussion
4.1. Soil Nitrification and Denitrification in a Riparian Wetland in an Extremely Arid Region
4.2. Driving factors in Soil Nitrification and Denitrification in a Riparian Wetland in an Extremely Arid Region
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, J.; He, Z.; Du, J.; Zhao, L.; Chen, L.; Zhu, X.; Lin, P.; Fang, S.; Zhao, M.; Tian, Q. Regional variability of agriculturally-derived nitrate-nitrogen in shallow groundwater in China, 2004–2014. Sustainability 2018, 10, 1393. [Google Scholar] [CrossRef] [Green Version]
- Burgin, A.J.; Groffman, P.M.; Lewis, D.N. Factors regulating denitrification in a riparian wetland. Soil Sci. Soc. Am. J. 2010. [Google Scholar] [CrossRef]
- Rivas, A.; Singh, R.; Horne, D.; Roygard, J.; Matthews, A.; Hedley, M.J. Denitrification potential in the subsurface environment in the Manawatu River catchment, New Zealand: Indications from oxidation-reduction conditions, hydrogeological factors, and implications for nutrient management. J. Environ. Manag. 2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xiong, Z.; Liu, H.; Zhang, Q.; Liu, G. Catchment agriculture and local environment affecting the soil denitrification potential and nitrous oxide production of riparian zones in the Han River Basin, China. Agric. Ecosyst. Environ. 2016, 216, 147–154. [Google Scholar] [CrossRef]
- Mayer, P.M.; Reynolds, S.K.; McCutchen, M.D.; Canfield, T.J. Meta-analysis of nitrogen removal in riparian buffers. J. Environ. Qual. 2007. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xiong, Z.; Yao, L.; Liu, G.; Zhang, Q.; Liu, W. Soil properties alter plant and microbial communities to modulate denitrification rates in subtropical riparian wetlands. Land Degrad. Dev. 2020. [Google Scholar] [CrossRef]
- Yu, G.; Cheng, S.; Fang, H.; Tian, J.; Xu, M.; Yu, G.; He, S. European journal of soil biology responses of soil nitrous oxide fl ux to soil environmental factors in a subtropical coniferous plantation: A boundary line analysis. Eur. J. Soil Biol. 2018, 86, 16–25. [Google Scholar] [CrossRef]
- Philippot, L. Denitrifying genes in bacterial and Archaeal genomes. Biochim. Biophys. Acta Gene Struct. Expr. 2002. [Google Scholar] [CrossRef]
- Ranalli, A.J.; Macalady, D.L. The importance of the riparian zone and in-stream processes in nitrate attenuation in undisturbed and agricultural watersheds—a review of the scientific literature. J. Hydrol. 2010. [Google Scholar] [CrossRef]
- Ye, C.; Cheng, X.; Zhang, Y.; Wang, Z.; Zhang, Q. Soil nitrogen dynamics following short-term revegetation in the water level fluctuation zone of the Three Gorges Reservoir, China. Ecol. Eng. 2012. [Google Scholar] [CrossRef]
- Barnard, R.; Leadley, P.W.; Hungate, B.A. Global change, nitrification, and denitrification: A review. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Kjellin, J.; Hallin, S.; Wo, A. Spatial variations in denitrification activity in wetland sediments explained by hydrology and denitrifying community structure. Water Res. 2007, 41, 4710–4720. [Google Scholar] [CrossRef] [PubMed]
- Seitzinger, S.P. Linkages between organic matter mineralization denitrification in eight riparian wetlands. Biogeochemistry 1994, 25, 19–39. [Google Scholar] [CrossRef]
- Wang, X.; Hu, M.; Ren, H.; Li, J.; Tong, C.; Musenze, R.S. Seasonal variations of nitrous oxide fl uxes and soil denitri fi cation rates in subtropical freshwater and brackish tidal marshes of the Min River estuary. Sci. Total Environ. 2018, 616, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Balser, T.C. Linking soil process and microbial ecology in freshwater wetland ecosystems. Plant Soil 2006, 289, 17–34. [Google Scholar] [CrossRef]
- Yan, L.; Xie, C.; Xu, X.; Che, S. Effects of revetment type on the spatial distribution of soil nitrification and denitrification in adjacent tidal urban riparian zones. Ecol. Eng. 2019. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Benner, R. Denitrification, nutrient regeneration and carbon mineralization in sediments of Galveston Bay, Texas, USA. Mar. Ecol. Prog. Ser. 1994, 114, 275. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, W.; Wen, Z.; Yang, Y.; Chang, X.; Yang, Q.; Meng, Y.Y.; Liu, C. Mechanisms and feedbacks for evapotranspiration-induced salt accumulation and precipitation in an arid wetland of China. J. Hydrol. 2019, 568, 403–415. [Google Scholar] [CrossRef]
- Li, S.; Zhao, W. Landscape pattern changes of desert oasis wetlands in the middle reach of the heihe river, China. Arid Land Res. Manag. 2010. [Google Scholar] [CrossRef]
- Edmonds, J.W.; Weston, N.B.; Joye, S.B.; Mou, X.; Moran, M.A. Microbial community response to seawater amendment in low-salinity tidal sediments. Microb. Ecol. 2009, 58, 558–568. [Google Scholar] [CrossRef]
- Giblin, A.E.; Weston, N.B.; Banta, G.T.; Tucker, J.; Hopkinson, C.S. The effects of salinity on nitrogen losses from an oligohaline estuarine sediment. Estuaries Coasts 2010, 33, 1054–1068. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, L.; Tang, Y.; Li, Y.; Chen, J.; Xi, X.; Zhang, Y.; Fu, X.; Wu, J.; Sun, Y. Variability in soil microbial community and activity between coastal and riparian wetlands in the Yangtze River estuary e Potential impacts on carbon sequestration. Soil Biol. Biochem. 2014, 70, 221–228. [Google Scholar] [CrossRef]
- Benckiser, G.; Schartel, T.; Weiske, A. Control of NO3—and N2O emissions in agroecosystems: A review. Agron. Sustain. Dev. 2015, 35, 1059–1074. [Google Scholar] [CrossRef]
- Curtin, D.; Beare, M.H.; Hernandez-Ramirez, G. Temperature and moisture effects on microbial biomass and soil organic matter mineralization. Soil Sci. Soc. Am. J. 2012. [Google Scholar] [CrossRef]
- Junk, W.J.; An, S.; Finlayson, C.M.; Gopal, B.; Květ, J.; Mitchell, S.A.; Mitsch, W.J.; Robarts, R.D. Current state of knowledge regarding the world’s wetlands and their future under global climate change: A synthesis. Aquat. Sci. 2013, 75, 151–167. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.Z.; Zhao, W.Z.; Su, P.X.; Zhang, Z.H.; Wang, T.; Ram, R. Ecological effects of desertification control and desertified land reclamation in an oasis-desert ecotone in an arid region: A case study in Hexi Corridor, northwest China. Ecol. Eng. 2007. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1983; Volume 9, pp. 539–579. ISBN 978-0891188117. [Google Scholar]
- Arbuckle, J. Amos 17.0 User’s Guide; SPSS Inc.: Crawfordville, FL, USA, 2008. [Google Scholar]
- Paeri, H.W. Seasonal patterns of nitrification and denitrification in a natural and a restored salt marsh. Estuaries 1995, 18, 399–408. [Google Scholar]
- Rysgaard, S.; Thastum, P.; Dalsgaard, T.; Christensen, P.B.; Sloth, N.P.; Rysgaard, S. Effects of salinity on nh 4 + adsorption capacity, nitrification, and denitrification in danish estuarine sediments. Estuaries 1999, 22, 21–30. [Google Scholar] [CrossRef]
- He, Z.B.; Chen, L.F.; Du, J.; Zhu, X.; Lin, P.F.; Li, J.; Xiang, Y.Z. Responses of soil organic carbon, soil respiration, and associated soil properties to long-term thinning in a semiarid spruce plantation in northwestern China. Land Degrad. Dev. 2018, 29, 4387–4396. [Google Scholar] [CrossRef]
- Bastviken, S.K.; Eriksson, P.G.; Premrov, A.; Tonderski, K. Potential denitrification in wetland sediments with different plant species detritus. Ecol. Eng. 2005. [Google Scholar] [CrossRef]
- Sirivedhin, T.; Gray, K.A. Factors affecting denitrification rates in experimental wetlands: Field and laboratory studies. Ecol. Eng. 2006, 26, 167–181. [Google Scholar] [CrossRef]
- Siddikee, M.A.; Tipayno, S.C.; Kim, K.; Chung, J.; Sa, T. Influence of varying degree of salinity-sodicity stress on enzyme activities and bacterial populations of coastal soils of yellow sea, South Korea. J. Microbiol. Biotechnol. 2011, 21, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Barrena, I.; Menéndez, S.; Correa-galeote, D.; Vega-mas, I.; Bedmar, E.J.; González-murua, C.; Estavillo, J.M. Geoderma soil water content modulates the e ff ect of the nitri fi cation inhibitor 3, 4- dimethylpyrazole phosphate (DMPP) on nitrifying and denitrifying bacteria. Geoderma 2017, 303, 1–8. [Google Scholar] [CrossRef]
- Flowers, T.H.; Arnold, P.W. Immobilization and mineralization of nitrogen in soils incubated with pig slurry or ammonium sulphate. Soil Biol. Biochem. 1983. [Google Scholar] [CrossRef]
- Linn, D.M.; Doran, J.W. Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils1. Soil Sci. Soc. Am. J. 1984. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, S.; Kumari, S.; Chakraborty, A.; Gupta, A.; Chakrabarti, K.; Bandyapadhyay, B.K. Microbial biomass and its activities in salt-affected coastal soils. Biol. Fertil. Soils 2006, 42, 273–277. [Google Scholar] [CrossRef]
- Tang, X.; Xie, G.; Shao, K.; Bayartu, S.; Chen, Y.; Gao, G. Influence of salinity on the bacterial community composition in Lake Bosten, a large oligosaline lake in arid Northwestern China. Appl. Environ. Microbiol. 2012, 78, 4748–4751. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cheng, S.; Fang, H.; Yu, G.; Xu, M.; Dang, X.; Li, L.; Wang, L. Simulated nitrogen deposition reduces CH4 uptake and increases N2O emission from a subtropical plantation forest soil in Southern China. PLoS ONE 2014, 9, e93571. [Google Scholar] [CrossRef]
- Nielsen, L.P.; Christensen, P.B.; Revsbech, N.P.; Sørensen, J. Denitrification and oxygen respiration in biofilms studied with a microsensor for nitrous oxide and oxygen. Microb. Ecol. 1990. [Google Scholar] [CrossRef]

| N Processes and Environmental Factors | No. | Min | Max | Mean | SD | CV | Skew | Kurtosis | K-S | Distribution |
|---|---|---|---|---|---|---|---|---|---|---|
| NR (μg kg−1 h−1) | 108 | 52.77 | 221.18 | 144.11 | 40.11 | 0.49 | −0.258 | −0.64 | 0.20 | Normal |
| DR (μg kg−1 h−1) | 108 | 91.25 | 428.26 | 308.90 | 74.39 | 0.84 | −0.549 | −0.31 | 0.12 | Normal |
| EC (mScm−1) | 108 | 1.24 | 12.96 | 6.80 | 3.03 | 0.01 | −0.078 | −0.98 | 0.04 | Lognormal |
| SWC (%) | 108 | 14.20 | 30.75 | 23.69 | 4.21 | 0.13 | −0.214 | −0.92 | 0.03 | Lognormal |
| AB (g m−2) | 108 | 38.15 | 462.93 | 182.90 | 112.65 | 0.35 | 0.923 | −0.23 | 0.00 | Lognormal |
| Sand (%) | 108 | 1.72 | 67.26 | 31.21 | 11.79 | 0.02 | −0.137 | 0.22 | 0.20 | Normal |
| Silt (%) | 108 | 29.44 | 81.35 | 61.73 | 9.58 | 0.27 | −0.280 | 0.28 | 0.20 | Normal |
| Clay (%) | 108 | 3.30 | 18.29 | 7.06 | 2.77 | 0.03 | 1.815 | 3.84 | 0.00 | Lognormal |
| SBD (g cm−3) | 108 | 1.03 | 1.62 | 1.42 | 0.11 | 0.08 | −0.518 | 0.29 | 0.07 | Normal |
| SOC (g Kg−1) | 108 | 3.03 | 13.51 | 7.78 | 2.88 | 0.03 | 0.197 | −0.85 | 0.20 | Normal |
| TN (g Kg−1) | 108 | 0.33 | 1.92 | 0.95 | 0.37 | 0.00 | 0.546 | −0.18 | 0.20 | Normal |
| SOC/TN | 108 | 3.01 | 19.04 | 8.55 | 2.50 | 0.03 | 1.243 | 3.38 | 0.00 | Lognormal |
| NO3−-N (mg Kg−1) | 108 | 5.24 | 27.56 | 16.47 | 5.92 | 0.05 | 0.158 | −0.84 | 0.07 | Normal |
| NH4+-N (mg Kg−1) | 108 | 2.05 | 12.73 | 7.18 | 2.79 | 0.02 | 0.079 | −0.84 | 0.20 | Normal |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; He, Z.; Liu, B.; Chen, L.; Lin, P.; Luo, W. Soil Salinity and Moisture Control the Processes of Soil Nitrification and Denitrification in a Riparian Wetlands in an Extremely Arid Regions in Northwestern China. Water 2020, 12, 2815. https://doi.org/10.3390/w12102815
Meng Y, He Z, Liu B, Chen L, Lin P, Luo W. Soil Salinity and Moisture Control the Processes of Soil Nitrification and Denitrification in a Riparian Wetlands in an Extremely Arid Regions in Northwestern China. Water. 2020; 12(10):2815. https://doi.org/10.3390/w12102815
Chicago/Turabian StyleMeng, Yangyang, Zhibin He, Bing Liu, Longfei Chen, Pengfei Lin, and Weicheng Luo. 2020. "Soil Salinity and Moisture Control the Processes of Soil Nitrification and Denitrification in a Riparian Wetlands in an Extremely Arid Regions in Northwestern China" Water 12, no. 10: 2815. https://doi.org/10.3390/w12102815
APA StyleMeng, Y., He, Z., Liu, B., Chen, L., Lin, P., & Luo, W. (2020). Soil Salinity and Moisture Control the Processes of Soil Nitrification and Denitrification in a Riparian Wetlands in an Extremely Arid Regions in Northwestern China. Water, 12(10), 2815. https://doi.org/10.3390/w12102815




