Compatibility of the Invasive Alien Lemna minuta and Its Potential Biocontrol Agent Cataclysta lemnata
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Sampling
2.2. Chemical and Physical Analyses
2.3. Statistical Analyses
3. Results
3.1. Characterization of L. minuta and C. lemnata Populations in the Sampling Sites
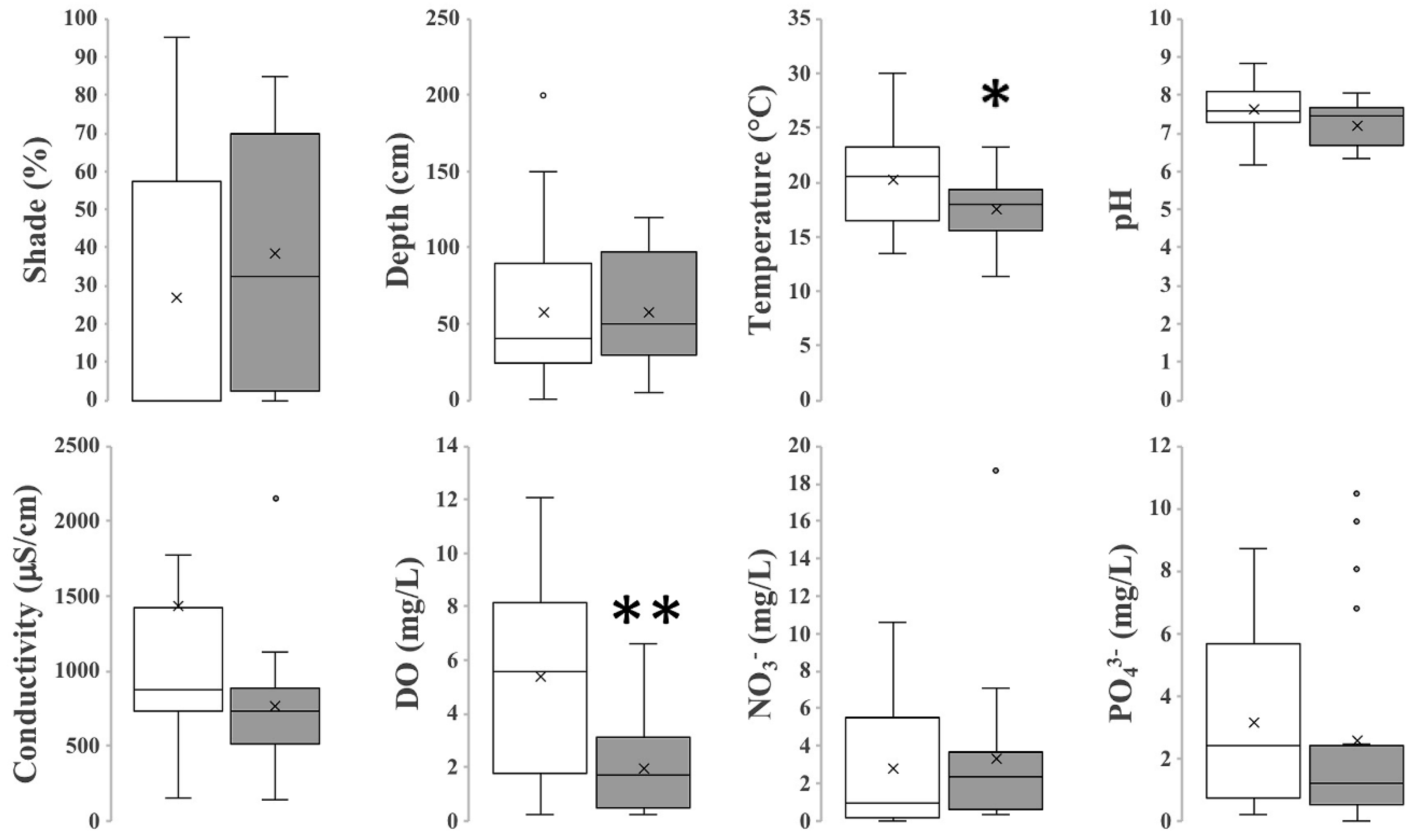
3.2. Chemical and Physical Characterization of L. minuta Sites and C. lemnata Sites
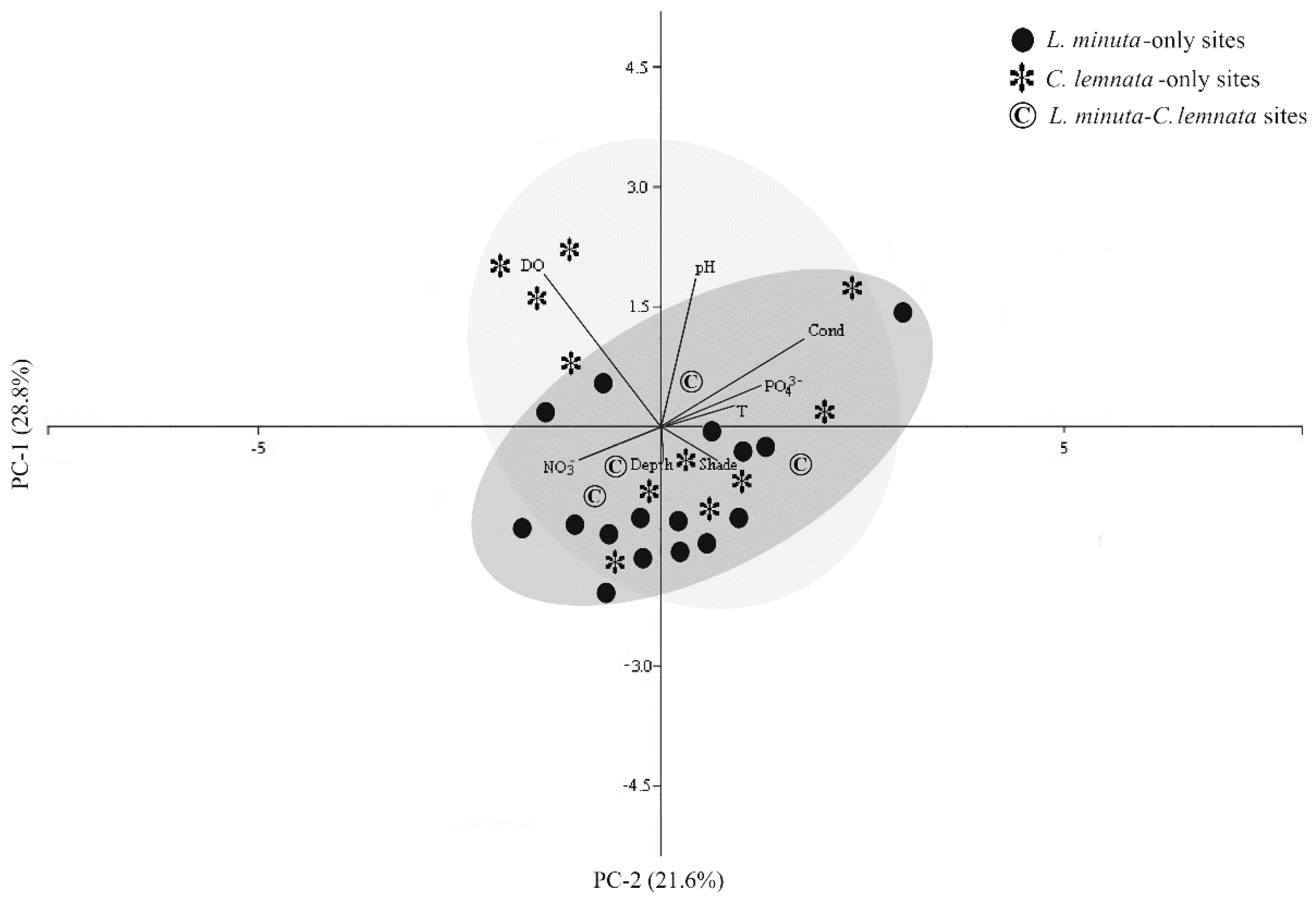
3.3. Main Ecological Driving Forces Affecting L. minuta and C. lemnata Distribution
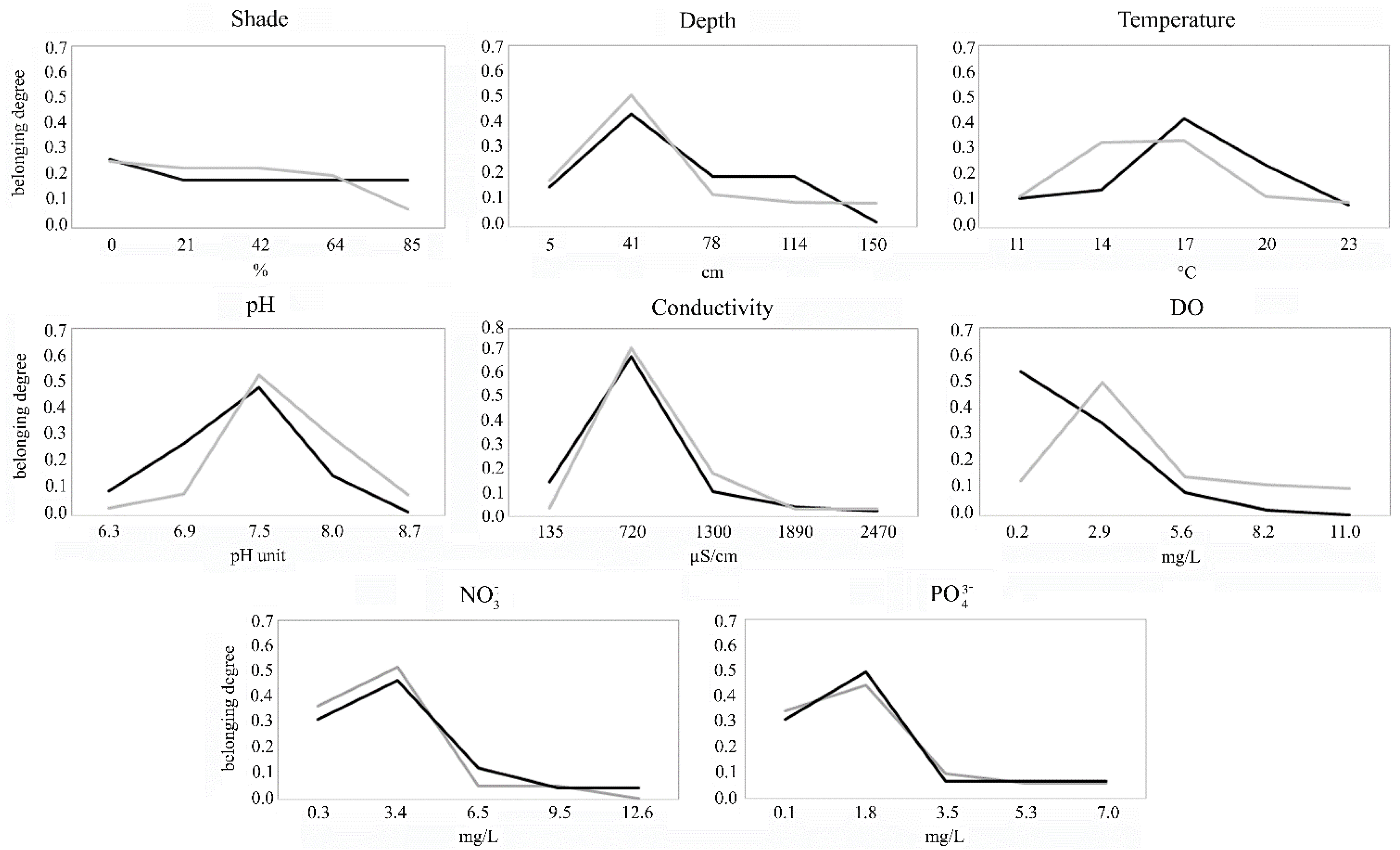
3.4. Ecological Response Curves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef]
- IUCN. IUCN guidelines for the prevention of biodiversity loss caused by alien invasive species. In Proceedings of the 5th Meeting of the Conference of the Parties to the Convention on Biological Diversity, Nairobi, Kenya, 15–26 May 2000; Available online: https://portals.iucn.org/library/node/12673 (accessed on 15 May 2020).
- Levine, J.M.; D’Antonio, C.M. Forecasting biological invasions with increasing international trade. Conserv. Biol. 2003, 17, 322–326. [Google Scholar] [CrossRef]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends. Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Howard, G.W.; Harley, K.L.S. How do floating aquatic weeds affect wetland and conservation and development? How can these effects be minimised? Wetl. Ecol. Manag. 1998, 5, 215–225. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin III, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Stiers, I.; Triest, L. Impact of non-native invasive plant species cover on phytoplankton and zooplankton communities in temperate ponds. Aquat. Invasions 2017, 12, 385–395. [Google Scholar] [CrossRef]
- Capers, R.S.; Selsky, R.; Bugbee, G.J.; White, J.C. Aquatic plant community invasibility and scale-dependent patterns in native and invasive species richness. Ecology 2007, 88, 3135–3143. [Google Scholar] [CrossRef]
- DAISIE. Handbook of alien species in Europe; Springer: Berlin, Germany, 2009. [Google Scholar]
- Ceschin, S.; Abati, S.; Ellwood, N.T.W.; Zuccarello, V. Riding invasion waves: Spatial and temporal patterns of the invasive Lemna minuta from its arrival to its spread across Europe. Aquat. Bot. 2018, 150, 1–8. [Google Scholar] [CrossRef]
- Desfayes, M. Segnalazioni floristiche italiane: 677. Inform. Bot. Ital. 1992, 24, 52. [Google Scholar]
- Celesti-Grapow, L.; Alessandrini, A.; Arrigoni, P.V.; Banfi, E.; Bernardo, L.; Bovio, M.; Brundu, G.; Cagiotti, M.R.; Camarda, I.; Carli, E.; et al. Inventory of the non-native flora of Italy. Plant Biosyst. 2009, 143, 386–430. [Google Scholar] [CrossRef]
- Marrone, G.; Naselli-Flores, L. Primo reperto di una lenticchia d’acqua alloctona in Sicilia: Lemna minuta Kunth (Araceae Lemnoideae). Natur. Sicil. 2011, 35, 179–235. [Google Scholar]
- Ceschin, S.; Mariani, F. Notulae to the Italian alien vascular flora. Floristic records: Lemna minuta Kunth (Araceae). Ital. Bot. 2020, 9, 58. [Google Scholar]
- Landolt, E. The Family of Lemnaceae—A Monographic Study; 1. Veröffentlichungen des Geobotanischen Institutes der ETH, Stiftung Rübel: Zürich, Switzerland, 1986. [Google Scholar]
- Njambuya, J.; Stiers, I.; Triest, L. Competition between Lemna minuta and Lemna minor at different nutrient concentrations. Aquat. Bot. 2011, 94, 158–164. [Google Scholar] [CrossRef]
- Ceschin, S.; Della Bella, V.; Piccari, F.; Abati, S. Colonization dynamics of the alien macrophyte Lemna minuta Kunth: A case study from a semi-natural pond in Appia Antica Regional Park (Rome, Italy). Fundam. Appl. Limnol. 2016, 188, 93–101. [Google Scholar] [CrossRef]
- Dussart, G.; Robertson, J.; Bramley, J. Death of a lake. Biol. Sci. Rev. 1993, 5, 8–10. [Google Scholar]
- Janes, A.R.; Eaton, W.J.; Hardwick, K. The effects of floating mats of Azolla filiculoides Lam and Lemna minuta Kunth on the growth of submerged macrophytes. Hydrobiologia 1996, 340, 23–26. [Google Scholar] [CrossRef]
- Ceschin, S.; Abati, S.; Traversetti, L.; Spani, F.; Del Grosso, F.; Scalici, M. Effects of the invasive duckweed Lemna minuta on aquatic animals: Evidence from an indoor experiment. Plant Biosyst. 2019, 153, 749–755. [Google Scholar] [CrossRef]
- Ceschin, S.; Ferrante, G.; Mariani, F.; Traversetti, L.; Ellwood, N.T.W. Habitat change and alteration of plant and invertebrate communities in waterbodies dominated by the invasive alien macrophyte Lemna minuta Kunth. Biol. Invasions 2020, 22, 1325–1337. [Google Scholar] [CrossRef]
- Ceschin, S.; Abati, S.; Leacche, I.; Iamonico, D.; Iberite, M.; Zuccarello, V. Does the alien L. minuta show an invasive behaviour outside its original range? Evidence of antagonism with the native L. minor L. in Central Italy. Int. Rev. Hydrobiol. 2016, 101, 173–181. [Google Scholar] [CrossRef]
- Ceschin, S.; Abati, S.; Leacche, I.; Zuccarello, V. Ecological comparison between duckweeds in Central Italy: The invasive Lemna minuta vs the native L. minor. Plant Biosyst. 2018, 152, 674–683. [Google Scholar] [CrossRef]
- Mariani, F.; Ceschin, S. Potenzialità del biocontrollo per contenere in Italia l’espansione della macrofita esotica invasiva Lemna minuta. Not. Soc. Bot. Ital. 2019, 3, 15–16. [Google Scholar]
- Mariani, F.; Di Giulio, A.; Fattorini, S.; Ceschin, S. Development and reproduction of C. lemnata (Lepidoptera: Crambidae), a potential natural enemy of the invasive alien duckweed Lemna minuta in Italy. Eur. Zool. J. under review.
- Carpenter, S.R. Microcosm experiments have limited relevance for community and ecosystem ecology. Ecology 1996, 77, 677–680. [Google Scholar] [CrossRef]
- Schindler, D.W. Replication versus realism: The need for ecosystem-scale experiments. Ecosystems 1998, 1, 323–334. [Google Scholar] [CrossRef]
- Cullen, J.M. Predicting effectiveness: Fact and fantasy. In Biological Control of Weeds, Proceedings of the 8th International Symposium on Biological Control of Weeds, Lincoln University, Canterbury, New Zealand, 2–7 February 1992; Delfosse, E.S., Scott, R.R., Eds.; CSIRO: Melbourne, Australia, 1995; pp. 103–110. [Google Scholar]
- Sun, Y.; Bronnimann, O.; Roderick, G.K.; Poltavsky, A.; Lommen, S.T.E.; Müller-Schärer, H. Climatic suitability ranking of biological control candidates: A biogeographic approach for ragweed management in Europe. Ecosphere 2017, 8, e01731. [Google Scholar] [CrossRef]
- Björkman, C.; Niemelä, P. Climate Change and Insect Pests; CAB International: Wallingford, UK, 2015. [Google Scholar]
- Hoy, M.A. Augmentative biological control. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Hokkanen, H.; Pimentel, D. New associations in biological control: Theory and practice. Can. Entomol. 1989, 121, 829–840. [Google Scholar] [CrossRef]
- Mariani, F.; Di Giulio, A.; Fattorini, S.; Ceschin, S. Experimental evidence of the consumption of the invasive alien duckweed Lemna minuta by herbivorous larvae of the moth C. lemnata in Italy. Aquat. Bot. 2020, 161, 103172. [Google Scholar] [CrossRef]
- Vallenduuk, H.J.; Cuppen, H.M.J. The aquatic living caterpillars (Lepidoptera: Pyraloidea: Crambidae) of Central Europe. A key to the larvae and autecology. Lauterbornia 2004, 49, 1–17. [Google Scholar]
- Ceschin, S.; Leacche, I.; Pascucci, S.; Abati, S. Morphological study of Lemna minuta Kunth, an alien species often mistaken for the native L. minor L. (Araceae). Aquat. Bot. 2016, 131, 51–56. [Google Scholar] [CrossRef]
- Speidel, W. Acentropinae. In Microlepidoptera of Europe 4, Pyraloidea I (Crambidae: Acentropinae, Evergestinae, Heliothelinae, Schoenobiinae, Scopariinae); Goater, B., Nuss, M., Speidel, W., Eds.; Apollo Books: Vester Skerninge, Denmark, 2005. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Zadeh, L.A. Fuzzy sets as a basis for a theory of possibility. Fuzzy Sets Syst. 1978, 1, 3–28. [Google Scholar] [CrossRef]
- Andreucci, F.; Biondi, E.; Feoli, E.; Zuccarello, V. Modelling environmental responses of plant associations by fuzzy set theory. Community Ecol. 2000, 1, 73–80. [Google Scholar] [CrossRef]
- Peruzzi, L.; Savio, L. Notulae alla flora esotica d’Italia. Inform. Bot. Ital. 2011, 43, 149. [Google Scholar]
- Abati, S.; Evangelista, M.; Minciardi, M.R.; Olivieri, L.; Selvaggi, A.; Spada, C.D. Nota floristica piemontese n. 447. Lemna minuta Kunth (Araceae). Riv. Piem. St. Nat. 2012, 33, 441–442. [Google Scholar]
- Pérez, G.L.; Vera, M.S.; Miranda, L. Effects of herbicide glyphosate and glyphosate-based formulations on aquatic ecosystems. In Herbicides and Environment; Kortekamp, A., Ed.; IntechOpen: London, UK, 2010; pp. 343–368. [Google Scholar]
- Rzymski, P.; Klimaszyk, P.; Kubacki, T.; Poniedziałek, B. The effect of glyphosate-based herbicide on aquatic organisms—A case study. Limnol. Rev. 2013, 13, 215–220. [Google Scholar] [CrossRef]
- Janse, J.H.; Van Puijenbroek, P.J.T.M. Effects of eutrophication in drainage ditches. Environ. Pollut. 1998, 102, 547–552. [Google Scholar] [CrossRef]
- Misfud, S. First occurrences of Lemna minuta Kunth (Fam. Lemnaceae) in the Maltese Islands. Central. Med. Nat. 2010, 5, 1–4. [Google Scholar]
| Lemna minuta Coverage (%), Thickness (mm) | ||||
|---|---|---|---|---|
| Site | Winter | Spring | Summer | Autumn |
| FOG | 80%, 10 mm | 100%, 19 mm | 100%, 15 mm | 80%, 13 mm |
| CAF3 | 100%, 16 mm | 100%, 25 mm | 100%, 21 mm | 100%, 18 mm |
| CAF7 | 100%, 12 mm | 100%, 12 mm | 100%, 14 mm | 100%, 15 mm |
| OST | 80%, 8 mm | 100%, 12 mm | 90%, 12 mm | 85%, 10 mm |
| Cataclysta lemnataAbundance (Class) | ||||
| Site | Winter | Spring | Summer | Autumn |
| MET2 | 2 | 2 | 3 | 2 |
| PAQ1 | 2 | 2 | 2 | 1 |
| TMA2 | 2 | 4 | 3 | 4 |
| WNU1 | 0 | 2 | 2 | 2 |
| L. minuta-Only Sites | C. lemnata-Only Sites | L. minuta-C. lemnata | |
|---|---|---|---|
| L. minuta-only sites | 1.000 | 0.325 | 0.820 |
| C. lemnata-only sites | 0.325 | 1.000 | 0.683 |
| L. minuta-C. lemnata | 0.820 | 0.683 | 1.000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariani, F.; Ellwood, N.T.W.; Zuccarello, V.; Ceschin, S. Compatibility of the Invasive Alien Lemna minuta and Its Potential Biocontrol Agent Cataclysta lemnata. Water 2020, 12, 2719. https://doi.org/10.3390/w12102719
Mariani F, Ellwood NTW, Zuccarello V, Ceschin S. Compatibility of the Invasive Alien Lemna minuta and Its Potential Biocontrol Agent Cataclysta lemnata. Water. 2020; 12(10):2719. https://doi.org/10.3390/w12102719
Chicago/Turabian StyleMariani, Flaminia, Neil Thomas William Ellwood, Vincenzo Zuccarello, and Simona Ceschin. 2020. "Compatibility of the Invasive Alien Lemna minuta and Its Potential Biocontrol Agent Cataclysta lemnata" Water 12, no. 10: 2719. https://doi.org/10.3390/w12102719
APA StyleMariani, F., Ellwood, N. T. W., Zuccarello, V., & Ceschin, S. (2020). Compatibility of the Invasive Alien Lemna minuta and Its Potential Biocontrol Agent Cataclysta lemnata. Water, 12(10), 2719. https://doi.org/10.3390/w12102719




