Adsorption of As(V) by the Novel and Efficient Adsorbent Cerium-Manganese Modified Biochar
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
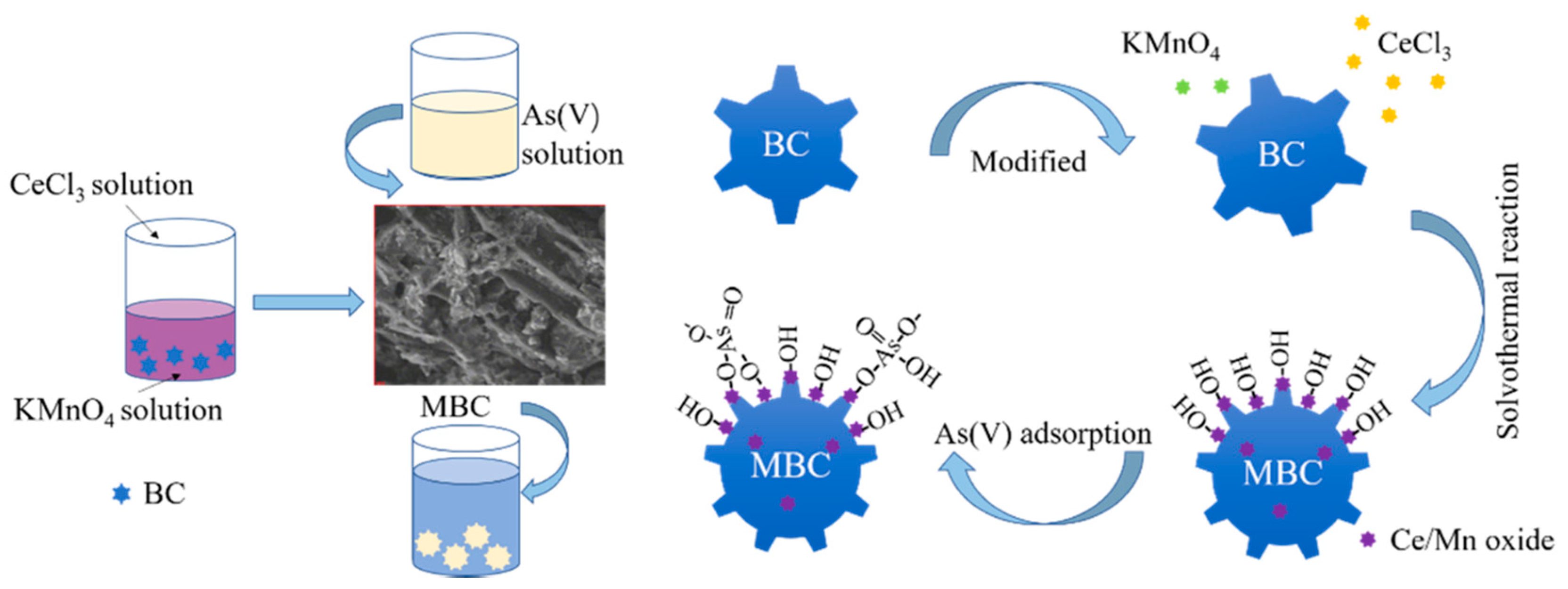
2.2. Biochar Modification
2.3. Characterizations
2.4. Adsorption Experiments
3. Results and Discussion
3.1. Adsorbent Characterization
3.1.1. SEM-EDS Analysis
3.1.2. XRD
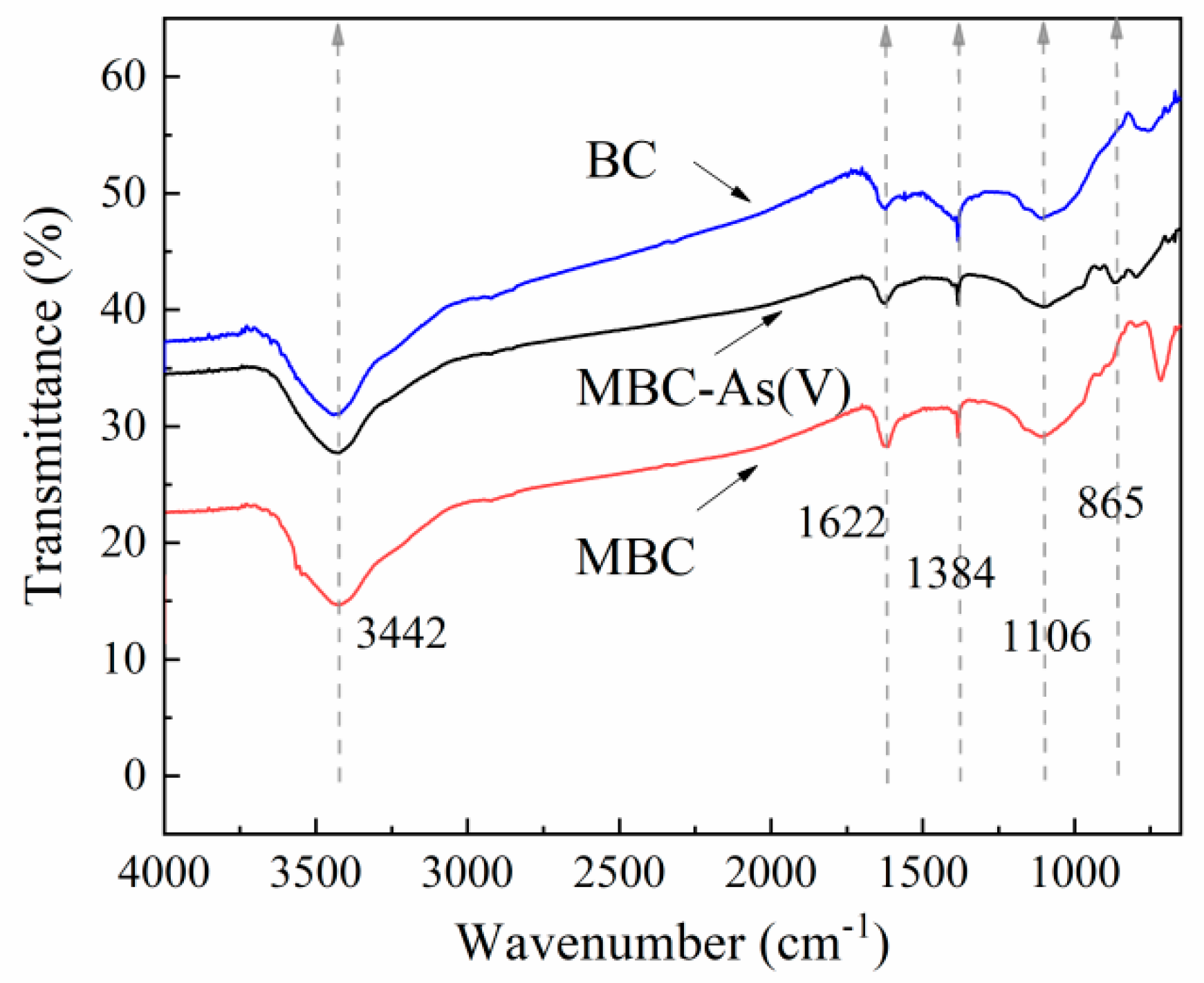
3.1.3. FT-IR
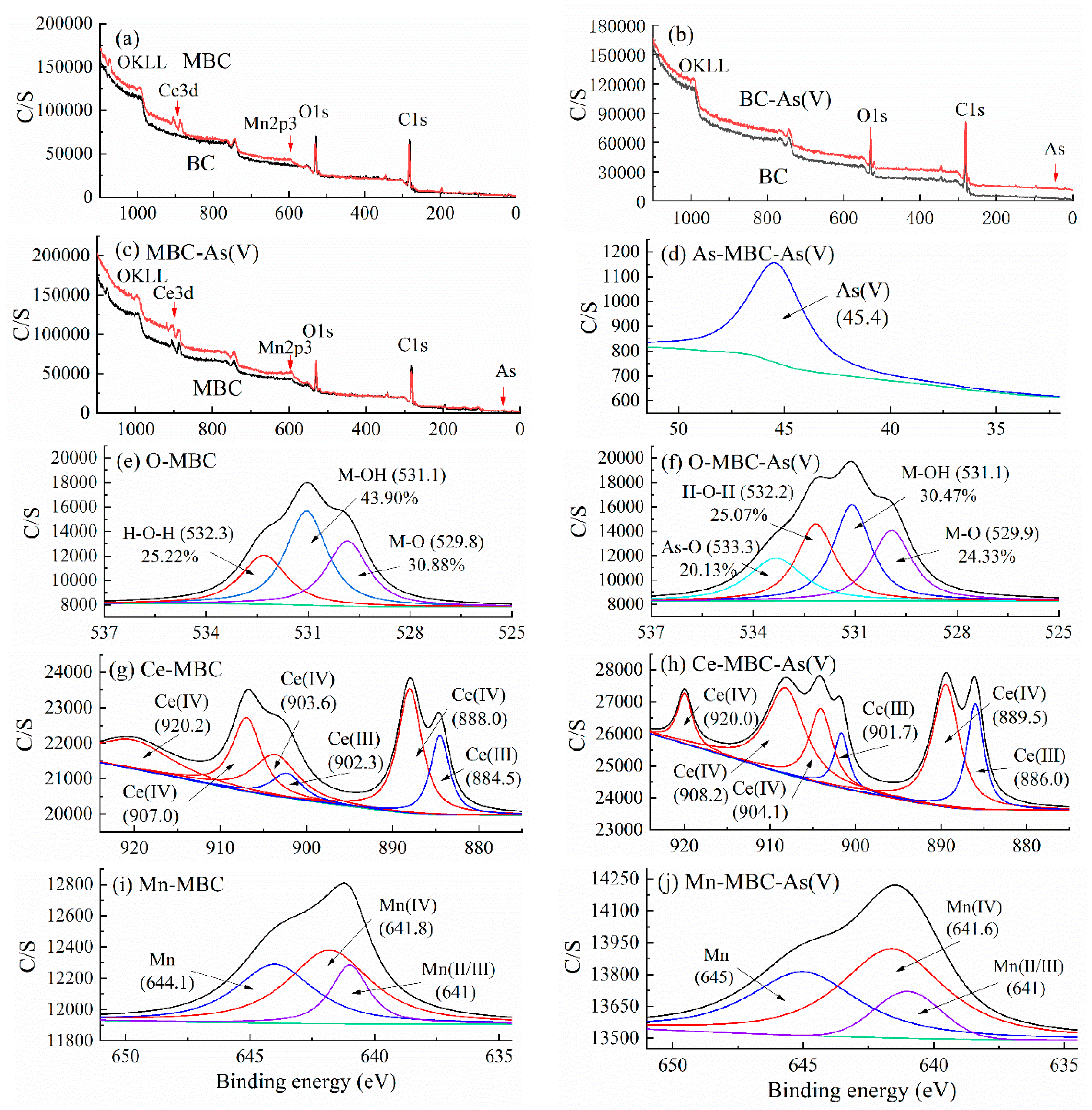
3.1.4. XPS
3.2. Effect of Experimental Conditions on As(V) Adsorption
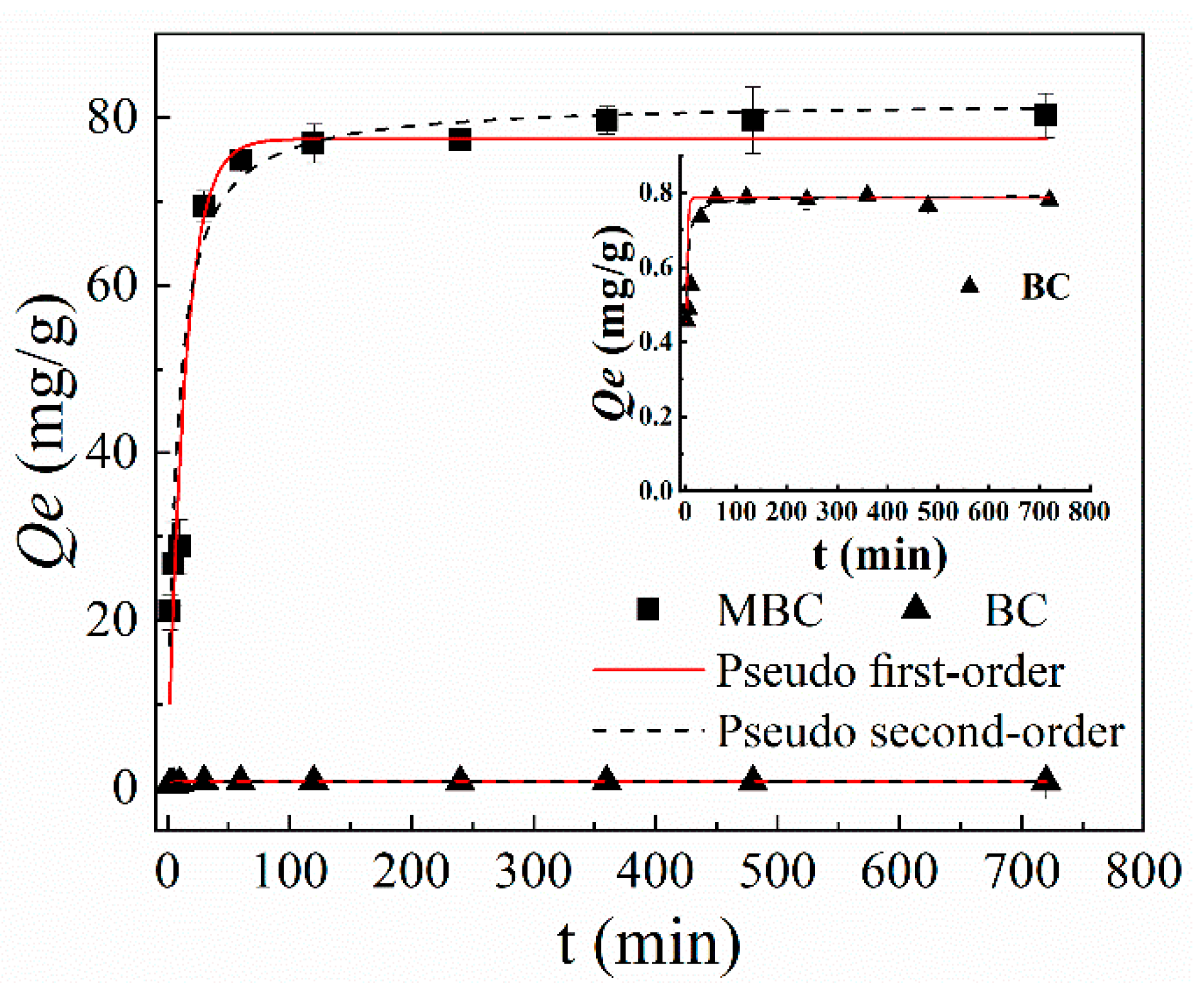
3.2.1. Contact Time
3.2.2. Initial As(V) Concentration and Temperature
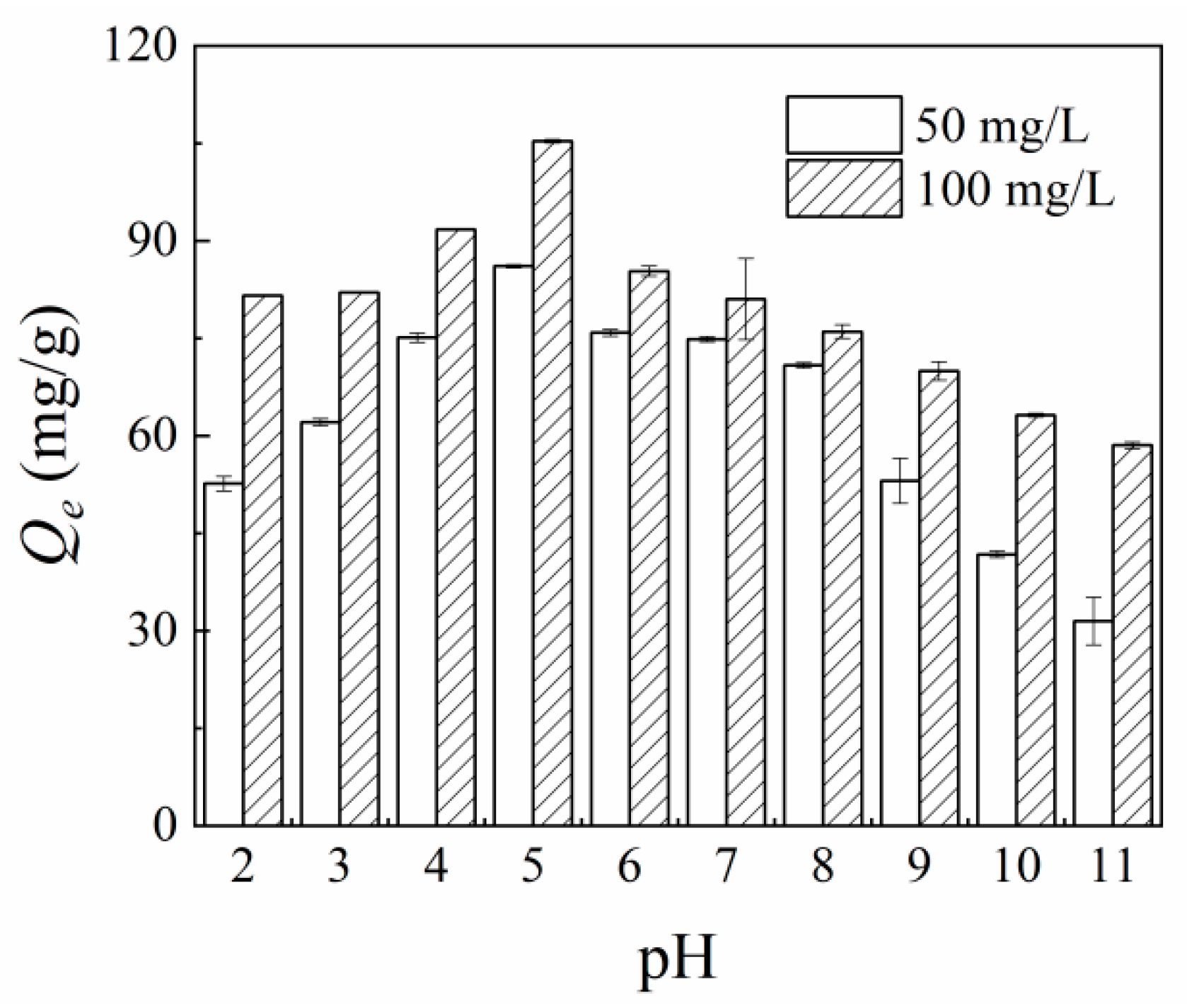
3.2.3. Effect of pH on As(V) Adsorption
3.2.4. Effect of Ionic Strength
3.2.5. Adsorbent Dosage
3.3. Possible Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.Y.; Su, Y.H.; McGrath, S.P.; Zhao, F.J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Bioresour. Technol. 2008, 105, 9931–9935. [Google Scholar] [CrossRef]
- Cui, J.; Jing, C. A review of arsenic interfacial geochemistry in groundwater and the role of organic matter. Ecotoxicol. Environ. Saf. 2019, 183, 1095502019. [Google Scholar] [CrossRef]
- Li, G.; Khan, S.; Ibrahim, M.; Sun, T.R.; Tang, J.F.; Cotner, J.B.; Xu, Y.Y. Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium. J. Hazard. Mater. 2018, 348, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; Pramanik, S.; Singh, P.; Mondal, P.; Chatterjee, D.; Nriagu, J. Arsenic in groundwater of West Bengal, India: A review of human health risks and assessment of possible intervention options. Sci. Total Environ. 2018, 612, 148–169. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Zhou, Q.W.; Liao, B.H.; Lin, L.N.; Qiu, W.W.; Song, Z.G. Adsorption of Cu(II) and Cd(II) from aqueous solutions by ferromanganese binary oxide-biochar composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Burton, E.D.; Wang, H.; Shaheen, S.M.; Rinklebe, J.; Lüttge, A. Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: An integrated spectroscopic and microscopic examination. Environ. Pollut. 2018, 232, 31–41. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, J.; Zhao, N.; Yang, X.; Chen, C.; Shang, J. Goethite modified biochar as a multifunctional amendment for cationic Cd(II), anionic As(III), roxarsone, and phosphorus in soil and water. J. Clean. Prod. 2020, 247, 1195792020. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Zhang, P.; O’Connor, D.; Wang, Y.N.; Jiang, L.; Xia, T.X.; Wang, L.W.; Tsang, D.C.W.; Ok, Y.S.; Hou, D.Y. A green biochar/iron oxide composite for methylene blue removal. J. Hazard. Mater. 2020, 384, 1212862020. [Google Scholar] [CrossRef] [PubMed]
- Vithanage, M.; Herath, I.; Joseph, S.; Bundschuh, J.; Bolan, N.; Ok, Y.S.; Kirkham, M.B.; Rinklebe, J. Interaction of arsenic with biochar in soil and water: A critical review. Carbon 2017, 113, 219–230. [Google Scholar] [CrossRef]
- Lu, H.L.; Zhang, W.H.; Yang, Y.X.; Huang, X.F.; Wang, S.Z.; Qiu, R.L. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Sohi, S.P. Agriculture. Carbon storage with benefits. Science 2012, 338, 1034–1035. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Titirici, M.M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; Rathke, S.J.; Laird, D.A. Arsenic sorption on zero-valent iron-biochar complexes. Water Res. 2018, 137, 153–163. [Google Scholar] [CrossRef]
- Wu, J.; Huang, D.; Liu, X.; Meng, J.; Tang, C.; Xu, J. Remediation of As(III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J. Hazard. Mater. 2018, 348, 10–19. [Google Scholar] [CrossRef]
- Du, Y.; Fan, H.; Wang, L.; Wang, J.; Wu, J.; Dai, H. α-Fe2O3 nanowires deposited diatomite: Highly efficient absorbents for the removal of arsenic. J. Mater. Chem. A 2013, 1, 77292013. [Google Scholar] [CrossRef]
- Song, Z.; Lian, F.; Yu, Z.; Zhu, L.; Xing, B.; Qiu, W. Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution. Chem. Eng. J. 2014, 242, 36–42. [Google Scholar] [CrossRef]
- Trakal, L.; Michalkova, Z.; Beesley, L.; Vitkova, M.; Ourednicek, P.; Barcelo, A.P.; Ettler, V.; Cihalova, S.; Komarek, M. AMOchar: Amorphous manganese oxide coating of biochar improves its efficiency at removing metal(loid)s from aqueous solutions. Sci. Total Environ. 2018, 625, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Alkurdi, S.S.A.; Herath, I.; Bundschuh, J.; Al-Juboori, R.A.; Vithanage, M.; Mohan, D. Biochar versus bone char for a sustainable inorganic arsenic mitigation in water: What needs to be done in future research? Environ. Int. 2019, 127, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, C.; Liu, X.; Li, F.; Li, H.; Ye, J. Biochar amendment immobilizes arsenic in farmland and reduces its bioavailability. Environ. Sci. Pollut. Res. Int. 2018, 25, 34091–34102. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Cao, Z.; Xu, J.; Zhou, X.; Zhu, J.; Liu, X.; Ali Baig, S.; Zhou, J.L.; Xu, X.H. Enhanced removal of As(III)/(V) from water by simultaneously supported and stabilized Fe-Mn binary oxide nanohybrids. Chem. Eng. J. 2017, 322, 710–721. [Google Scholar] [CrossRef]
- Banerjee, S.; Sharma, Y.C. Synthesis and application of Zn/Ce bimetallic oxides for the decontamination of arsenite (As-III) ions from aqueous solutions. J. Environ. Manag. 2019, 233, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Shabnam, N.; Kim, M.; Kim, H. Iron (III) oxide nanoparticles alleviate arsenic induced stunting in Vigna radiata. Ecotoxicol. Environ. Saf. 2019, 183, 1094962019. [Google Scholar] [CrossRef]
- Mishra, P.K.; Gahlyan, P.; Kumar, R.; Rai, P.K. Aero-Gel Based Cerium Doped Iron Oxide Solid Solution for Ultrafast Removal of Arsenic. ACS Sustain. Chem. Eng. 2018, 6, 10668–10678. [Google Scholar] [CrossRef]
- Olivera, S.; Chaitra, K.; Venkatesh, K.; Muralidhara, H.B.; Asiri, A.M.; Ahamed, M.I. Cerium dioxide and composites for the removal of toxic metal ions. Environ. Chem. Lett. 2018, 16, 1233–1246. [Google Scholar] [CrossRef]
- Yi, S.; Sun, Y.; Hu, X.; Xu, H.; Gao, B.; Wu, J. Porous nano-cerium oxide wood chip biochar composites for aqueous levofloxacin removal and sorption mechanism insights. Environ. Sci. Pollut. Res. Int. 2018, 25, 25629–25637. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, C.; Yang, L.; Paul Chen, J. Cerium oxide modified activated carbon as an efficient and effective adsorbent for rapid uptake of arsenate and arsenite: Material development and study of performance and mechanisms. Chem. Eng. J. 2017, 315, 630–638. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, G.; Liu, X.; Khan, Z.H.; Qiu, W.; Song, Z. Synthesis and adsorption of FeMnLa-impregnated biochar composite as an adsorbent for As(III) removal from aqueous solutions. Environ. Pollut. 2019, 247, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhang, Y.; Cheng, G.; Wang, Y.; Chen, R. Simultaneous removal of As(V)/Cr(VI) and acid orange 7 (AO7) by nanosized ordered magnetic mesoporous Fe-Ce bimetal oxides: Behavior and mechanism. Chemosphere 2019, 218, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Peng, Z.; Lyu, H.; Huang, H.; Nan, Q.; Tang, J. Synthesis and characterization of an iron-impregnated biochar for aqueous arsenic removal. Sci. Total Environ. 2018, 612, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Lin, W.; Ying, W.C. Preparation of iron-impregnated granular activated carbon for arsenic removal from drinking water. J. Hazard. Mater. 2010, 184, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Qiu, W.; Wang, F.; Lei, M.; Wang, D.; Song, Z. Effects of manganese oxide-modified biochar composites on arsenic speciation and accumulation in an indica rice (Oryza sativa L.) cultivar. Chemosphere 2017, 168, 341–349. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, W.; Wang, D.; Huang, Q.; Song, Z.; Chau, H.W. Arsenic removal in aqueous solution by a novel Fe-Mn modified biochar composite: Characterization and mechanism. Ecotoxicol. Environ. Saf. 2017, 144, 514–521. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, Z.; Guo, Y.; Qiu, Y.; Zhao, J. Facile synthesis of mesoporous Ce-Fe bimetal oxide and its enhanced adsorption of arsenate from aqueous solutions. J. Colloid Interface Sci. 2013, 398, 142–151. [Google Scholar] [CrossRef]
- Wen, Z.; Ke, J.; Xu, J.; Guo, S.; Zhang, Y.; Chen, R. One-step facile hydrothermal synthesis of flowerlike Ce/Fe bimetallic oxides for efficient As(V) and Cr(VI) remediation: Performance and mechanism. Chem. Eng. J. 2018, 343, 416–426. [Google Scholar] [CrossRef]
- McCann, C.M.; Peacock, C.L.; Hudson-Edwards, K.A.; Shrimpton, T.; Gray, N.D.; Johnson, K.L. In situ arsenic oxidation and sorption by a Fe-Mn binary oxide waste in soil. J. Hazard. Mater. 2018, 342, 724–731. [Google Scholar] [CrossRef]
- Gupta, K.; Bhattacharya, S.; Nandi, D.; Dhar, A.; Maity, A.; Mukhopadhyay, A.; Chattopadhyay, D.J.; Ray, N.R.; Sen, P.; Ghosh, U.C. Arsenic(III) sorption on nanostructured cerium incorporated manganese oxide (NCMO): A physical insight into the mechanistic pathway. J. Colloid Interface Sci. 2012, 377, 269–276. [Google Scholar] [CrossRef]
- Hu, X.; Ding, Z.; Zimmerman, A.R.; Wang, S.; Gao, B. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2015, 68, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Q.; Gao, S.; Shang, J.K. Exceptional arsenic adsorption performance of hydrous cerium oxide nanoparticles: Part A. Adsorption capacity and mechanism. Chem. Eng. J. 2012, 185–186, 127–135. [Google Scholar] [CrossRef]
- Pawar, R.R.; Kim, M.; Kim, J.G.; Hong, S.M.; Sawant, S.Y.; Lee, S.M. Efficient removal of hazardous lead, cadmium, and arsenic from aqueous environment by iron oxide modified clay-activated carbon composite beads. Appl. Clay Sci. 2018, 162, 339–350. [Google Scholar] [CrossRef]
- Mishra, P.K.; Rai, P.K. Ultrafast removal of arsenic using solid solution of aero-gel based Ce1-XTixO2-Y oxide nanoparticles. Chemosphere 2019, 217, 483–495. [Google Scholar] [CrossRef]
- Xue, Q.; Ran, Y.; Tan, Y.; Peacock, C.L.; Du, H. Arsenite and arsenate binding to ferrihydrite organo-mineral coprecipitate: Implications for arsenic mobility and fate in natural environments. Chemosphere 2019, 224, 103–110. [Google Scholar] [CrossRef]
- Asere, T.G.; Verbeken, K.; Tessema, D.A.; Fufa, F.; Stevens, C.V.; Du Laing, G. Adsorption of As(III) versus As(V) from aqueous solutions by cerium-loaded volcanic rocks. Environ. Sci. Pollut. Res. Int. 2017, 24, 20446–20458. [Google Scholar] [CrossRef]
- Pamphile, N.; Xuejiao, L.; Guangwei, Y.; Yin, W. Synthesis of a novel core-shell-structure activated carbon material and its application in sulfamethoxazole adsorption. J. Hazard. Mater. 2019, 368, 602–612. [Google Scholar] [CrossRef]
- Aravindhan, R.; Rao, J.R.; Nair, B.U. Removal of basic yellow dye from aqueous solution by sorption on green alga Caulerpa scalpelliformis. J. Hazard. Mater. 2007, 142, 68–76. [Google Scholar] [CrossRef]
- Gupta, K.; Bhattacharya, S.; Chattopadhyay, D.; Mukhopadhyay, A.; Biswas, H.; Dutta, J.; Ray, N.R.; Ghosh, U.C. Ceria associated manganese oxide nanoparticles: Synthesis, characterization and arsenic(V) sorption behavior. Chem. Eng. J. 2011, 172, 219–229. [Google Scholar] [CrossRef]
- Liu, C.H.; Chuang, Y.H.; Chen, T.Y.; Tian, Y.; Li, H.; Wang, M.K.; Zhang, W. Mechanism of Arsenic Adsorption on Magnetite Nanoparticles from Water: Thermodynamic and Spectroscopic Studies. Environ. Sci. Technol. 2015, 49, 7726–7734. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Shaheen, S.M.; Rinklebe, J.; Wang, H.; Murtaza, B.; Islam, E.; Farrakh Nawaz, M.; et al. Arsenic removal by Japanese oak wood biochar in aqueous solutions and well water: Investigating arsenic fate using integrated spectroscopic and microscopic techniques. Sci. Total Environ. 2018, 621, 1642–1651. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M. Biosorption of As(III) and As(V) from aqueous solution by macrofungus (Inonotus hispidus) biomass: Equilibrium and kinetic studies. J. Hazard. Mater. 2009, 164, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Y.G.; Liu, S.B.; Liu, H.Y.; Zeng, G.M.; Tan, X.F.; Yang, C.P.; Ding, Y.; Yan, Z.L.; Cai, X.X. Sorption performance and mechanisms of arsenic(V) removal by magnetic gelatin-modified biochar. Chem. Eng. J. 2017, 314, 223–231. [Google Scholar] [CrossRef]
- Zhang, G.; Qu, J.; Liu, H.; Liu, R.; Wu, R. Preparation and evaluation of a novel Fe-Mn binary oxide adsorbent for effective arsenite removal. Water Res. 2007, 41, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, B.; Li, Y.; Mosa, A.; Zimmerman, A.R.; Ma, L.Q.; Harris, W.G.; Migliaccio, K.W. Manganese oxide-modified biochars: Preparation, characterization, and sorption of arsenate and lead. Bioresour. Technol. 2015, 181, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Lonappan, L.; Rouissi, T.; Kaur Brar, S.; Verma, M.; Surampalli, R.Y. An insight into the adsorption of diclofenac on different biochars: Mechanisms, surface chemistry, and thermodynamics. Bioresour. Technol. 2018, 249, 386–394. [Google Scholar] [CrossRef]
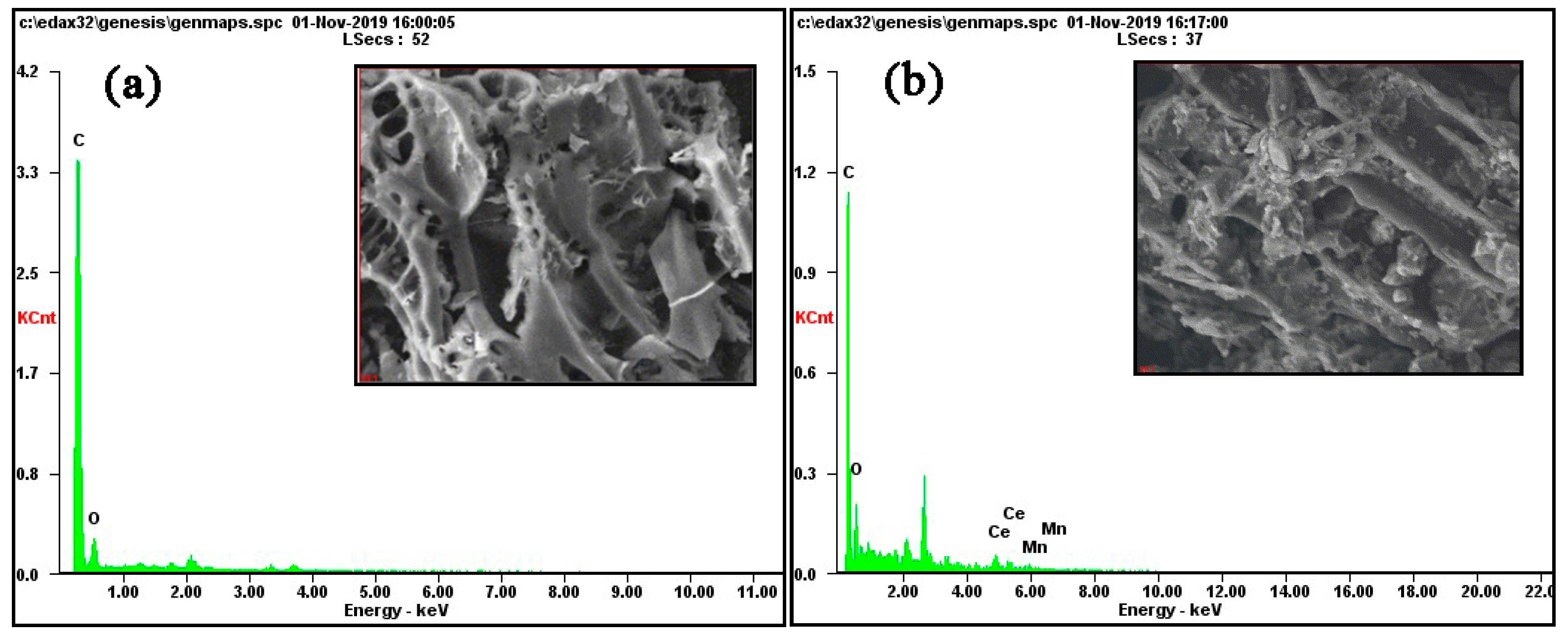
| Samples | Weight (%) | Atom (%) | SBET | Pore Width | Pore Volume | pH | pHzpc | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C(K) | O(K) | Ce(L) | Mn(K) | C(K) | O(K) | Ce(L) | Mn(K) | (m2/g) | (nm) | (cm3/g) | - | ||
| MBC | 55.99 | 8.05 | 31.04 | 4.92 | 85.13 | 9.19 | 4.05 | 1.63 | 6.881 | 10.681 | 0.024 | 6.06 | 5.28 ± 0.16 |
| BC | 93.83 | 6.17 | 0 | 0 | 96.29 | 3.71 | 0 | 0 | 5.525 | 36.000 | 0.005 | 9.26 | 6.58 ± 0.11 |
| Adsorbent | Temperature | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| (K) | Qm (mg/g) | kl (L/mg) | R2 | kf (mg1−n·Ln·g−1) | 1/n - | R2 - | |
| 278 | 67.62 ± 1.20 | 3.25 ± 0.34 | 0.9910 | 34.23 ± 5.32 | 0.15 ± 0.04 | 0.8337 | |
| 288 | 69.71 ± 1.93 | 5.21 ± 0.82 | 0.9778 | 36.35 ± 5.12 | 0.14 ± 0.03 | 0.8539 | |
| MBC | 298 | 79.77 ± 2.74 | 5.99 ± 1.17 | 0.9688 | 40.19 ± 5.89 | 0.15 ± 0.03 | 0.8685 |
| 308 | 84.30 ± 2.70 | 11.49 ± 2.57 | 0.9743 | 45.24 ± 4.90 | 0.14 ± 0.02 | 0.9222 | |
| 323 | 89.97 ± 2.54 | 46.31 ± 8.28 | 0.9806 | 51.20 ± 5.37 | 0.13 ± 0.02 | 0.9183 | |
| Adsorbents | Experimental Conditions | Adsorption Capacities (mg/g) | Reference | ||
|---|---|---|---|---|---|
| pH | Dosage (g/L) | As(V) Concentration (mg/L) | |||
| Cerium-loaded pumice (Ce-Pu) | 7.0 | 5 | 0.25–25 | 0.893 | [46] |
| Iron-impregnated biochar | 5.8 | 2 | 0–55 | 2.16 | [41] |
| Zero valent iron-red oak biochar complexes (ZVI-RO) | 7.0–7.5 | 1 | 0–25 | 15.58 | [17] |
| Cerium oxide modified activated carbon | 5.0 | 0.1 | 1–150 | 43.6 | [30] |
| Magnetic gelatin-modified biochar (MG-CSB) | 3.0/4.0 | 0.04 | 0.2–50 | 45.8 | [53] |
| Fe-Mn binary oxide nanohybrids(Starch-FeMnOx/RGO (reduced graphene oxide)) | 7.0 | 0.2 | 0.2–7 | 55.56 | [24] |
| Cerium-manganese modified biochar (MBC) | 5.0 | 0.5 | 5–200 | 104.58 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, T.; Li, L.; Zhu, C.; Liu, X.; Li, H.; Su, Q.; Ye, J.; Geng, B.; Tian, Y.; Sardar, M.F.; et al. Adsorption of As(V) by the Novel and Efficient Adsorbent Cerium-Manganese Modified Biochar. Water 2020, 12, 2720. https://doi.org/10.3390/w12102720
Liang T, Li L, Zhu C, Liu X, Li H, Su Q, Ye J, Geng B, Tian Y, Sardar MF, et al. Adsorption of As(V) by the Novel and Efficient Adsorbent Cerium-Manganese Modified Biochar. Water. 2020; 12(10):2720. https://doi.org/10.3390/w12102720
Chicago/Turabian StyleLiang, Ting, Lianfang Li, Changxiong Zhu, Xue Liu, Hongna Li, Qianqian Su, Jing Ye, Bing Geng, Yunlong Tian, Muhammad Fahad Sardar, and et al. 2020. "Adsorption of As(V) by the Novel and Efficient Adsorbent Cerium-Manganese Modified Biochar" Water 12, no. 10: 2720. https://doi.org/10.3390/w12102720
APA StyleLiang, T., Li, L., Zhu, C., Liu, X., Li, H., Su, Q., Ye, J., Geng, B., Tian, Y., Sardar, M. F., Huang, X., & Li, F. (2020). Adsorption of As(V) by the Novel and Efficient Adsorbent Cerium-Manganese Modified Biochar. Water, 12(10), 2720. https://doi.org/10.3390/w12102720









