Crayfish as Bioindicators for Monitoring ClO2: A Case Study from a Brewery Water Treatment Facility
Abstract
1. Introduction
2. Materials and Methods
2.1. Monitoring Process
2.2. Monitoring System
2.3. Experimental Animals
2.4. Statistical Analysis
3. Results
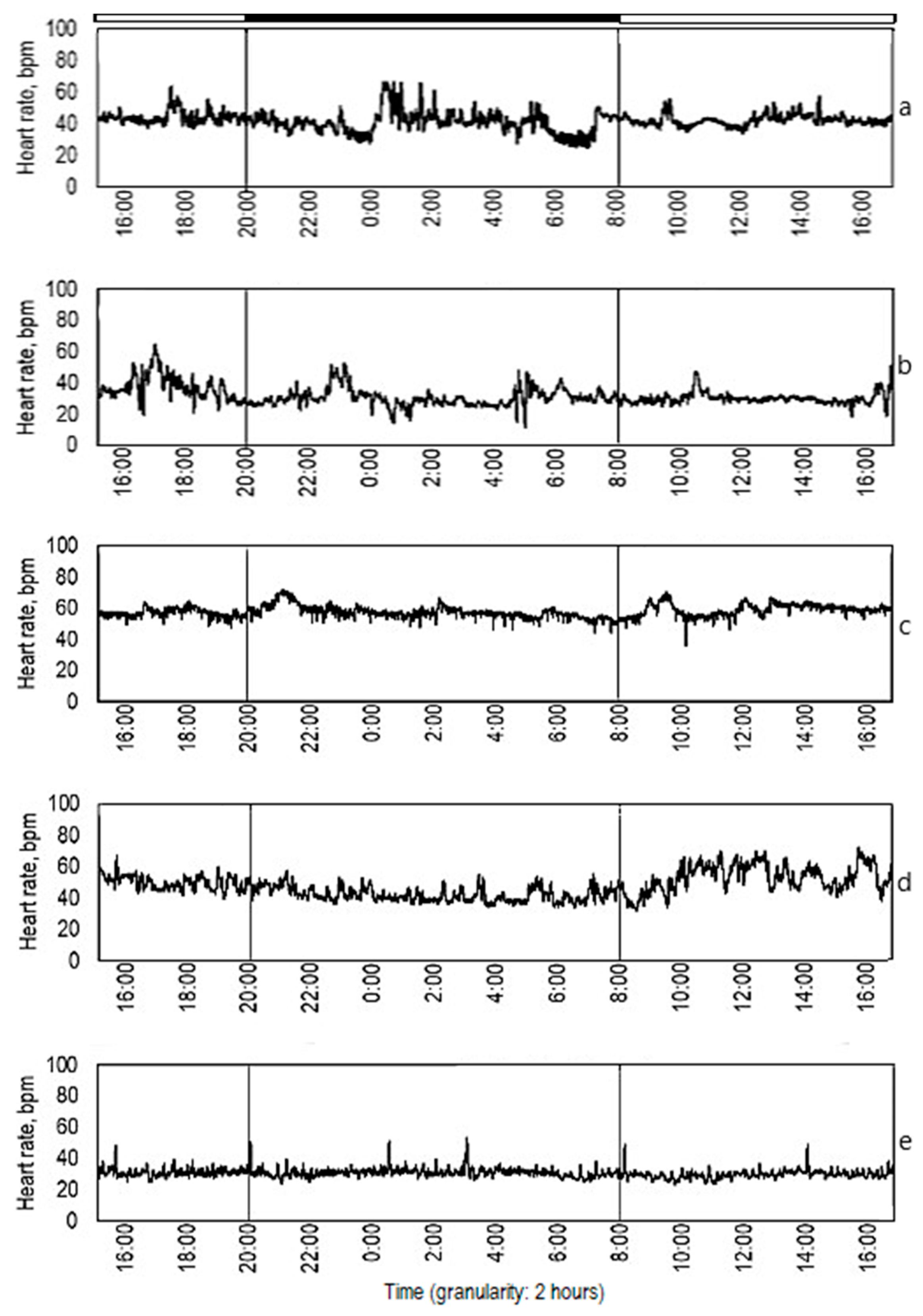
3.1. Ecdysis Period
3.2. Diurnal Rhythm
3.3. Mortality
Life Duration after Exposure to Cmax
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schilderman, P.A.E.L.; Moonen, E.J.C.; Maas, L.M.; Welle, I.; Kleinjans, J.C.S. Use of crayfish in biomonitoring studies of environmental pollution of the river Meuse. Ecotox. Environ. Safe 1999, 44, 241–252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soedarini, B.; Klaver, L.; Roessink, I.; Widianarko, B.; van Straalen, N.M.; van Gestel, C.A.M. Copper kinetics and internal distribution in the marbled crayfish (Procambarus sp.). Chemosphere 2012, 87, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Kuklina, I.; Sladkova, S.; Kouba, A.; Kholodkevich, S.; Kozak, P. Investigation of chloramine-T impact on crayfish Astacus leptodactylus (Esch., 1823) cardiac activity. Environ. Sci. Pollut. Res. 2014, 21, 10262–10269. [Google Scholar] [CrossRef] [PubMed]
- Pennuto, C.M.; Lane, O.P.; Evers, D.C.; Taylor, R.J.; Loukmas, J. Mercury in the northern crayfish, Orconectes virilis (Hagen), in New England, USA. Ecotoxicology 2005, 14, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Alcorlo, P.; Otero, M.; Crehuet, M.; Baltanas, A.; Montes, C. The use of the red swamp crayfish (Procambarus clarkii, Girard) as indicator of the bioavailability of heavy metals in environmental monitoring in the River Guadiamar (SW, Spain). Sci. Total Environ. 2006, 366, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Nathaniel, T.I.; Huber, R.; Panksepp, J. Repeated cocaine treatments induce distinct locomotor effects in Crayfish. Brain Res. Bull. 2012, 87, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Styrishave, B.; Rasmussen, A.D.; Depledge, M.H. The Influence of Bulk and Trace Metals on the Circadian Rhythm of Heart Rates in Fresh Water Crayfish. Astacus astacus. Mar. Pollut Bull. 1995, 31, 87–92. [Google Scholar] [CrossRef]
- Udalova, G.P.; Khodasevich, S.V.; Sladkova, S.V.; Ivanov, A.V.; Rymsha, V.A. Study of circadian activity in the crayfish Pontastacus leptodactylus during their multimonth maintenance in the river water flow. J. Evol. Biochem. Physiol. 2009, 45, 372–381. [Google Scholar] [CrossRef]
- Bojsen, B.H.; Witthofft, H.; Styrishave, B.; Andersen, O. In situ studies on heart rate and locomotor activity in the freshwater crayfish, Astacus astacus (L.) in relation to natural fluctuations in temperature and light intensity. Freshw. Biol. 1998, 39, 455–465. [Google Scholar] [CrossRef]
- Kholodkevich, S.V.; Ivanov, A.V.; Kurakin, A.S.; Kornienko, E.L.; Fedotov, V.P. Real time biomonitoring of surface water toxicity level at water supply stations. Environ. Bioindic. 2008, 3, 23–34. [Google Scholar] [CrossRef]
- Kozak, P.; Policar, T.; Fedotov, V.P.; Kuznetsova, T.V.; Buric, M.; Kholodkevich, S.V. Effect of chloride content in water on heart rate in narrow-clawed crayfish (Astacus leptodactylus). Knowl. Manag. Aquat. Ecosyst. 2009, 394–395, 10. [Google Scholar] [CrossRef][Green Version]
- Lalezary, S.; Pirbazari, M.; Mcguire, M.J. Oxidation of 5 Earthy Musty Taste and Odor Compounds. J. Am. Water Works Assoc. 1986, 78, 62–69. [Google Scholar] [CrossRef]
- Aieta, E.M.; Berg, J.D. A Review of Chlorine Dioxide in Drinking-Water Treatment. J. Am. Water Works Assoc. 1986, 78, 62–72. [Google Scholar] [CrossRef]
- World Health Organization. Chlorine Dioxide, Chlorite and Chlorate in Drinking-water. In Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2016; p. 24. [Google Scholar]
- Knocke, W.R.; Vanbenschoten, J.E.; Kearney, M.J.; Soborski, A.W.; Reckhow, D.A. Kinetics of Manganese and Iron Oxidation by Potassium-Permanganate and Chlorine Dioxide. J. Am. Water Works Assoc. 1991, 83, 80–87. [Google Scholar] [CrossRef]
- Fisher, D.J.; Burton, D.T.; Yonkos, L.T.; Turley, S.D.; Ziegler, G.P.; Turley, B.S. Derivation of acute ecological risk criteria for chlorite in freshwater ecosystems. Water Res. 2003, 37, 4359–4368. [Google Scholar] [CrossRef]
- Svecevicius, G.; Syvokiene, J.; Stasiunaite, P.; Mickeniene, L. Acute and chronic toxicity of chlorine dioxide (ClO2) and chlorite (ClO2-) to rainbow trout (Oncorhynchus mykiss). Environ. Sci. Pollut. Res. 2005, 12, 302–305. [Google Scholar] [CrossRef]
- Elia, A.C.; Anastasi, V.; Dorr, A.J.M. Hepatic antioxidant enzymes and total glutathione of Cyprinus carpio exposed to three disinfectants, chlorine dioxide, sodium hypochlorite and peracetic acid, for superficial water potabilization. Chemosphere 2006, 64, 1633–1641. [Google Scholar] [CrossRef]
- Tkachenko, H.; Kurhaluk, N.; Grudniewska, J. Biomarkers of oxidative stress and antioxidant defences as indicators of different disinfectants exposure in the heart of rainbow trout (Oncorhynchus mykiss Walbaum). Aquac. Res. 2015, 46, 679–689. [Google Scholar] [CrossRef]
- Matisoff, G.; Brooks, G.; Bourland, B.I. Toxicity of chlorine dioxide to adult zebra mussels. J. Am. Water Works Assoc. 1996, 88, 93–106. [Google Scholar] [CrossRef]
- Kuznetsova, T.V.; Sladkova, G.V.; Kholodkevich, S.V. Evaluation of functional state of crayfish Pontastacus leptodactylus in normal and toxic environment by characteristics of their cardiac activity and hemolymph biochemical parameters. J. Evol. Biochem. Physiol. 2010, 46, 241–250. [Google Scholar] [CrossRef]
- Pautsina, A.; Kuklina, I.; Stys, D.; Cisar, P.; Kozak, P. Noninvasive crayfish cardiac activity monitoring system. Limnol. Oceanogr. Meth. 2014, 12, 670–679. [Google Scholar] [CrossRef]
- Kuklina, I.; Lozek, F.; Cisar, P.; Kouba, A.; Kozak, P. Crayfish can distinguish between natural and chemical stimuli as assessed by cardiac and locomotor reactions. Environ. Sci. Pollut. Res. 2018, 25, 8396–8403. [Google Scholar] [CrossRef]
- Kuramoto, T. Cardiac Activation and Inhibition Involved in Molting Behavior of a Spiny Lobster. Experientia 1993, 49, 682–685. [Google Scholar] [CrossRef]
- Yonkos, L.T.; Fisher, D.J.; Wright, D.A.; Kane, A.S. Pathology of fathead minnows (Pimephales promelas) exposed to chlorine dioxide and chlorite. Mar. Environ. Res. 2000, 50, 267–271. [Google Scholar] [CrossRef]
- Chupani, L.; Zuskova, E.; Stara, A.; Velisek, J.; Kouba, A. Histological changes and antioxidant enzyme activity in signal crayfish (Pacifastacus leniusculus) associated with sub-acute peracetic acid exposure. Fish. Shellfish Immun. 2016, 48, 190–195. [Google Scholar] [CrossRef]
- Chupani, L.; Stara, A.; Velisek, J.; Zuskova, E. Evaluation of the toxic effect of peracetic acid on grass carp (Ctenopharyngodon idella) juveniles. Neuroendocrinol. Lett. 2014, 35, 86–92. [Google Scholar]
- Straus, D.L.; Meinelt, T.; Farmer, B.D.; Beck, B.H. Acute toxicity and histopathology of channel catfish fry exposed to peracetic acid. Aquaculture 2012, 342, 134–138. [Google Scholar] [CrossRef]

 ) indicate crayfish mortalities; the fluctuating line indicates levels of ClO2 concentration; the solid horizontal line indicates level of ClO2 concentration 0.2 mg L−1; the punctuated vertical line divides exposure period in two parts: First period (89 days), when high ClO2 concentrations (up to 0.2 mg L−1) were found five times and four crayfish died; and the second period (113 days), when high ClO2 concentrations occurred 23 times and 21 crayfish died.
) indicate crayfish mortalities; the fluctuating line indicates levels of ClO2 concentration; the solid horizontal line indicates level of ClO2 concentration 0.2 mg L−1; the punctuated vertical line divides exposure period in two parts: First period (89 days), when high ClO2 concentrations (up to 0.2 mg L−1) were found five times and four crayfish died; and the second period (113 days), when high ClO2 concentrations occurred 23 times and 21 crayfish died.
 ) indicate crayfish mortalities; the fluctuating line indicates levels of ClO2 concentration; the solid horizontal line indicates level of ClO2 concentration 0.2 mg L−1; the punctuated vertical line divides exposure period in two parts: First period (89 days), when high ClO2 concentrations (up to 0.2 mg L−1) were found five times and four crayfish died; and the second period (113 days), when high ClO2 concentrations occurred 23 times and 21 crayfish died.
) indicate crayfish mortalities; the fluctuating line indicates levels of ClO2 concentration; the solid horizontal line indicates level of ClO2 concentration 0.2 mg L−1; the punctuated vertical line divides exposure period in two parts: First period (89 days), when high ClO2 concentrations (up to 0.2 mg L−1) were found five times and four crayfish died; and the second period (113 days), when high ClO2 concentrations occurred 23 times and 21 crayfish died.
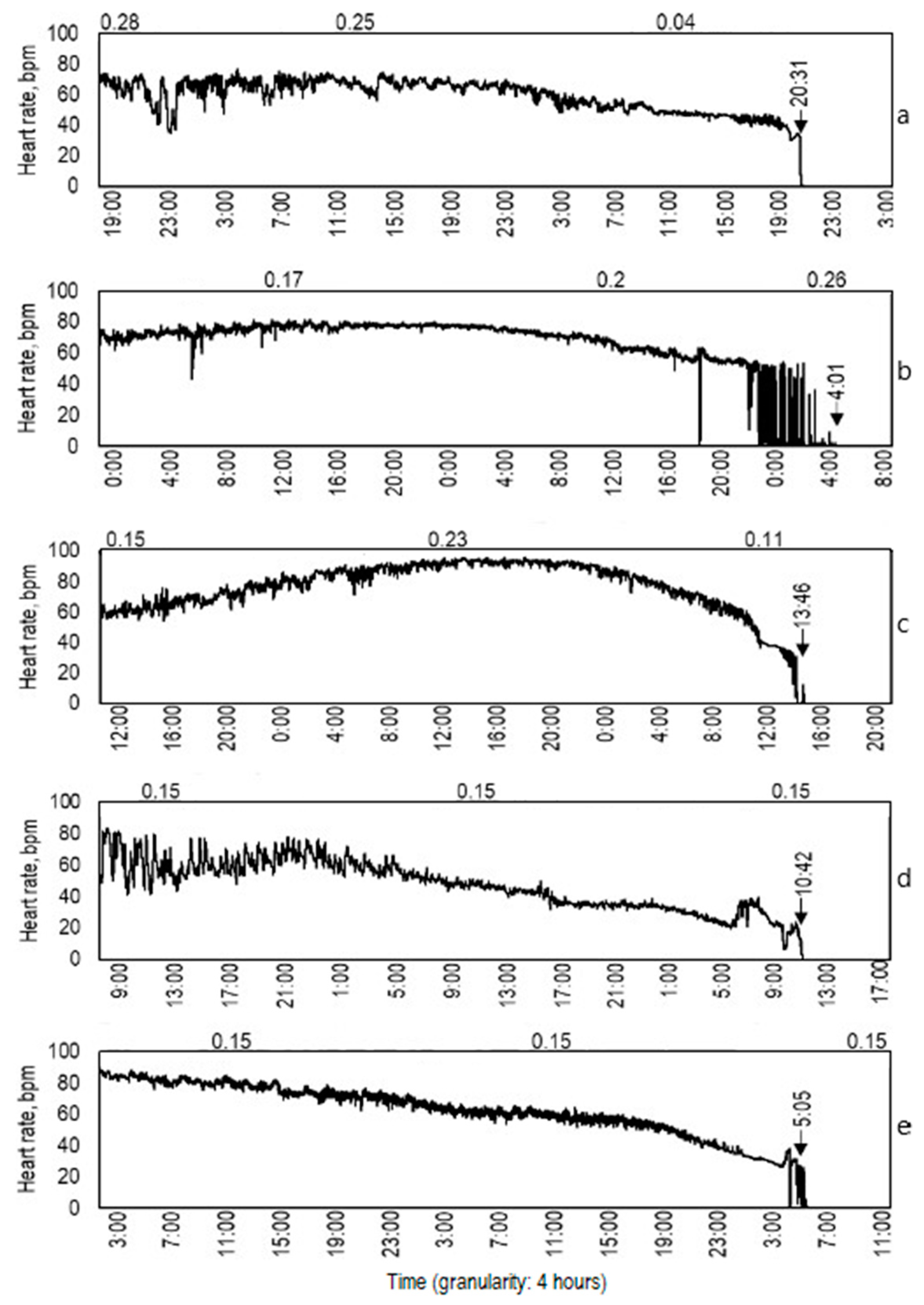

| Crayfish Group | N | Cmax of ClO2, mg L−1 | Ordinal Day, When Cmax Occurred | Exposure Period Before Mortality, Days | Life Duration After Cmax Treatment, Days |
|---|---|---|---|---|---|
| 1 | 13 | 0.21 ± 0.04 | 4 ± 2 | 13 ± 8 | 9 ± 7 |
| 2 | 6 | 0.26 ± 0.02 | 13 ± 1 | 29 ± 8 | 16 ± 8 |
| 3 | 6 | 0.29 ± 0.01 | 38 ± 6 | 43 ± 7 | 5 ± 2 |
| Crayfish Group | Day Heart Rate, bpm | Max | Min | Night Heart Rate, bpm | Max | Min | Day Versus Night Heart Rate, p-Value |
|---|---|---|---|---|---|---|---|
| 1 | 53 ± 14 | 109 | 25 | 52 ± 14 | 117 | 20 | 0.54 |
| 2 | 50 ± 13 | 89 | 20 | 52 ± 13 | 91 | 26 | 0.37 |
| 3 | 47 ± 14 | 92 | 20 | 48 ± 15 | 92 | 23 | 0.68 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinovska, V.; Ložek, F.; Kuklina, I.; Císař, P.; Kozák, P. Crayfish as Bioindicators for Monitoring ClO2: A Case Study from a Brewery Water Treatment Facility. Water 2020, 12, 63. https://doi.org/10.3390/w12010063
Malinovska V, Ložek F, Kuklina I, Císař P, Kozák P. Crayfish as Bioindicators for Monitoring ClO2: A Case Study from a Brewery Water Treatment Facility. Water. 2020; 12(1):63. https://doi.org/10.3390/w12010063
Chicago/Turabian StyleMalinovska, Viktoriia, Filip Ložek, Iryna Kuklina, Petr Císař, and Pavel Kozák. 2020. "Crayfish as Bioindicators for Monitoring ClO2: A Case Study from a Brewery Water Treatment Facility" Water 12, no. 1: 63. https://doi.org/10.3390/w12010063
APA StyleMalinovska, V., Ložek, F., Kuklina, I., Císař, P., & Kozák, P. (2020). Crayfish as Bioindicators for Monitoring ClO2: A Case Study from a Brewery Water Treatment Facility. Water, 12(1), 63. https://doi.org/10.3390/w12010063




