Batch Study of Cadmium Biosorption by Carbon Dioxide Enriched Aphanothece sp. Dried Biomass
Abstract
1. Introduction
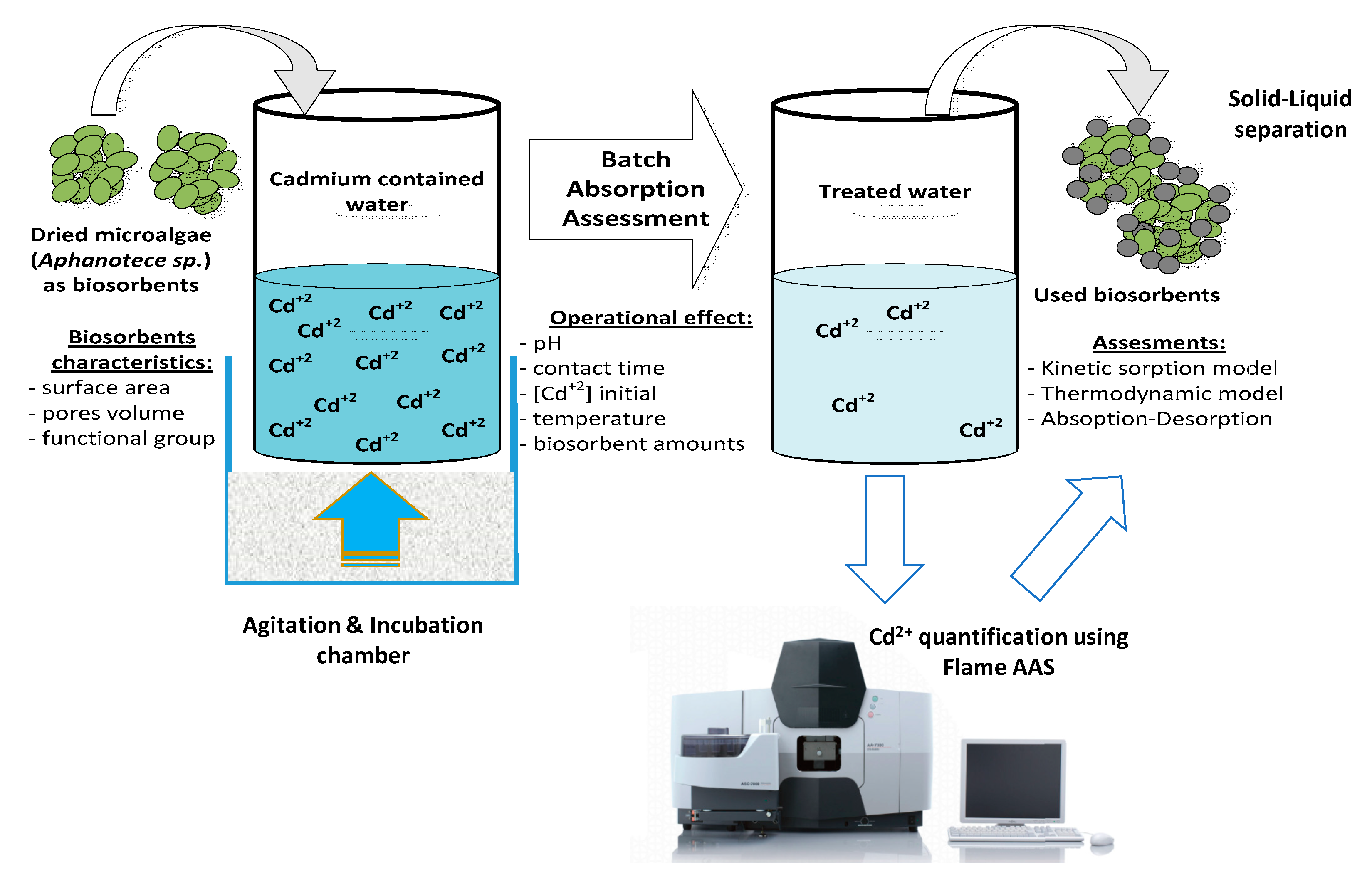
2. Materials and Methods
2.1. Dried Biosorbent
2.2. Reagent Preparation
2.3. Batch Sorption
2.4. Biosorbent Characterization
2.5. Desorption Study
2.6. Data Analysis
3. Results and Discussion
3.1. Characterization of Aphanothece sp. Dried Biosorbent
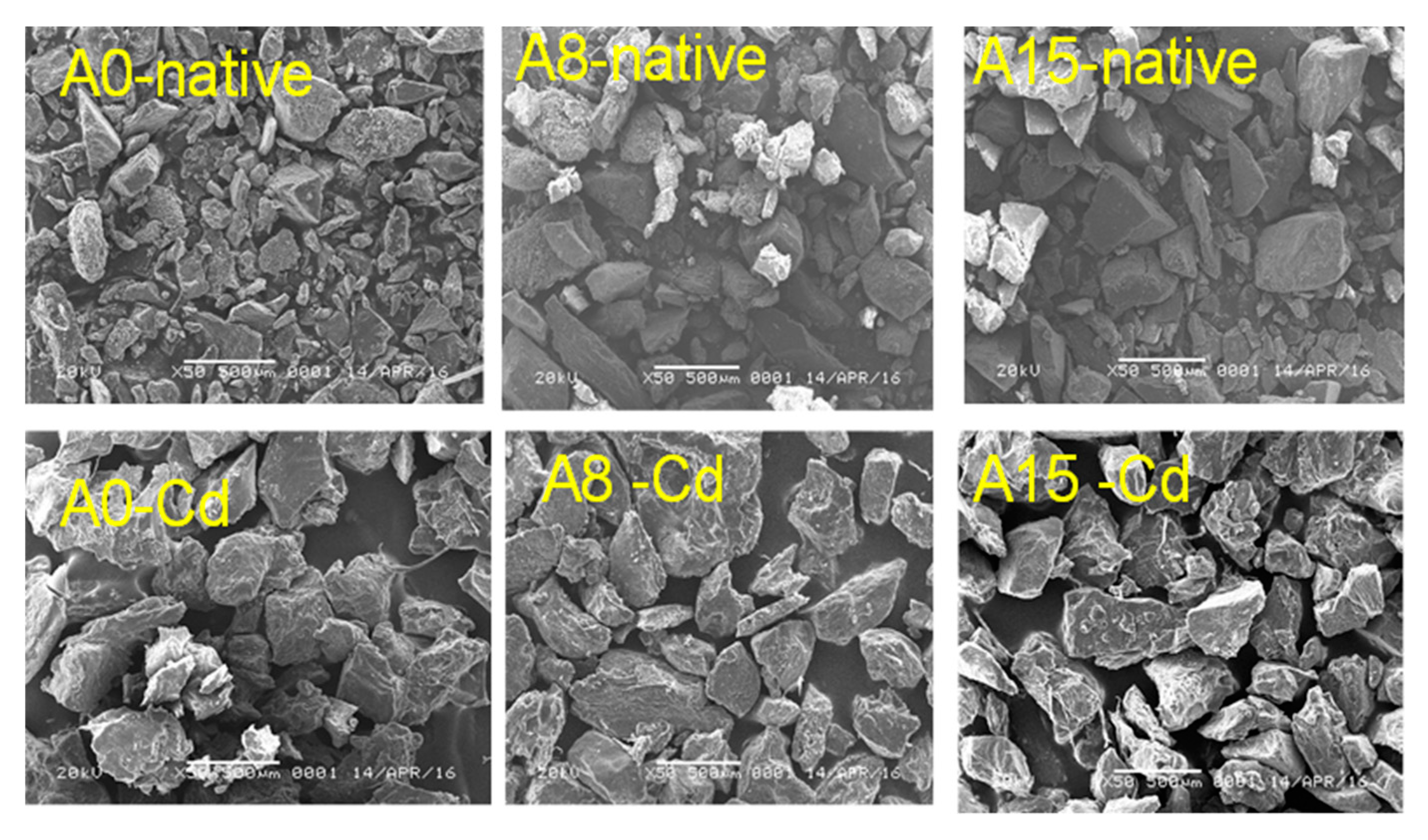
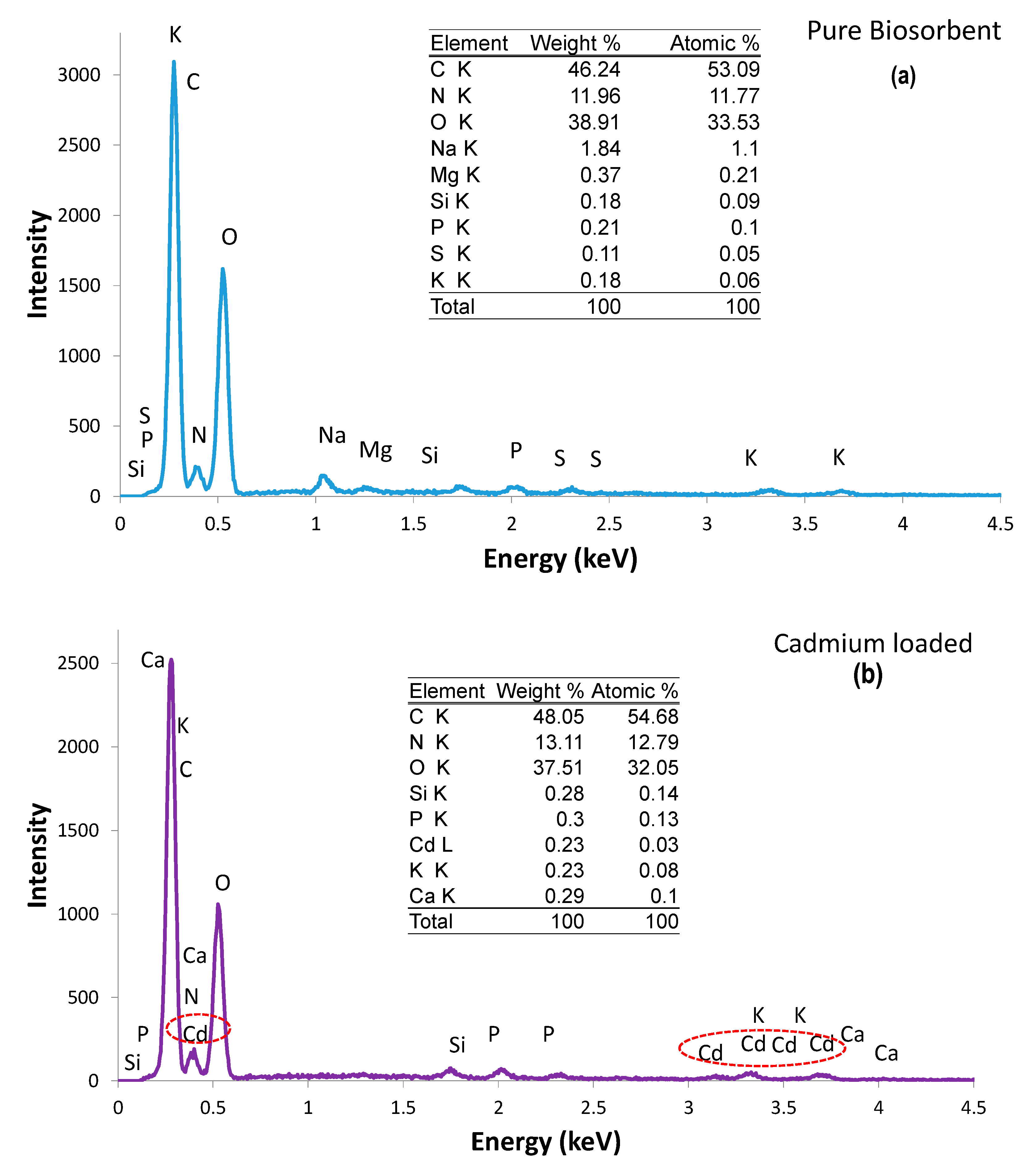
3.1.1. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray (EDX)
3.1.2. FTIR Analysis
3.1.3. BET Analysis
3.2. Biosorption Process Optimization
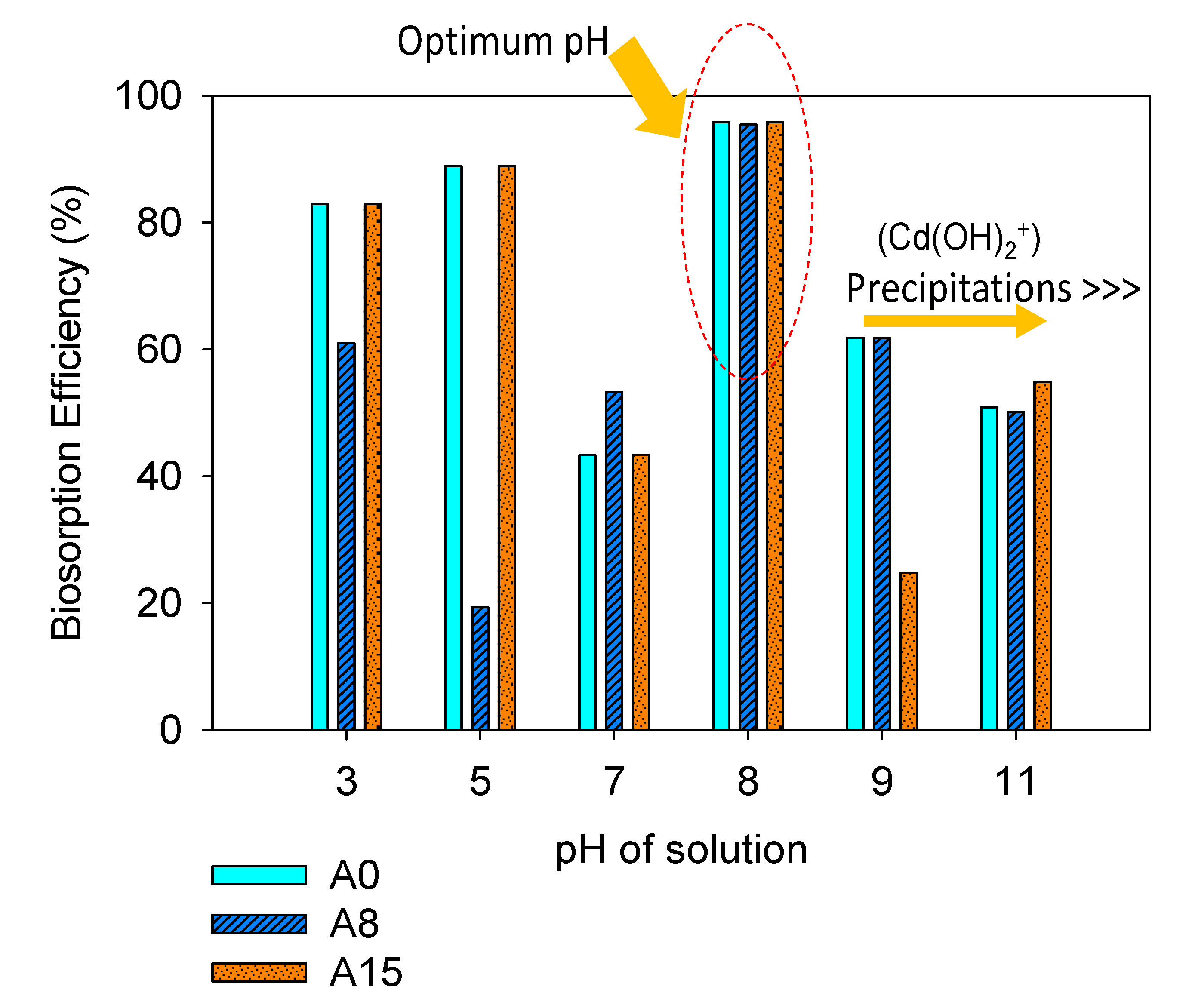
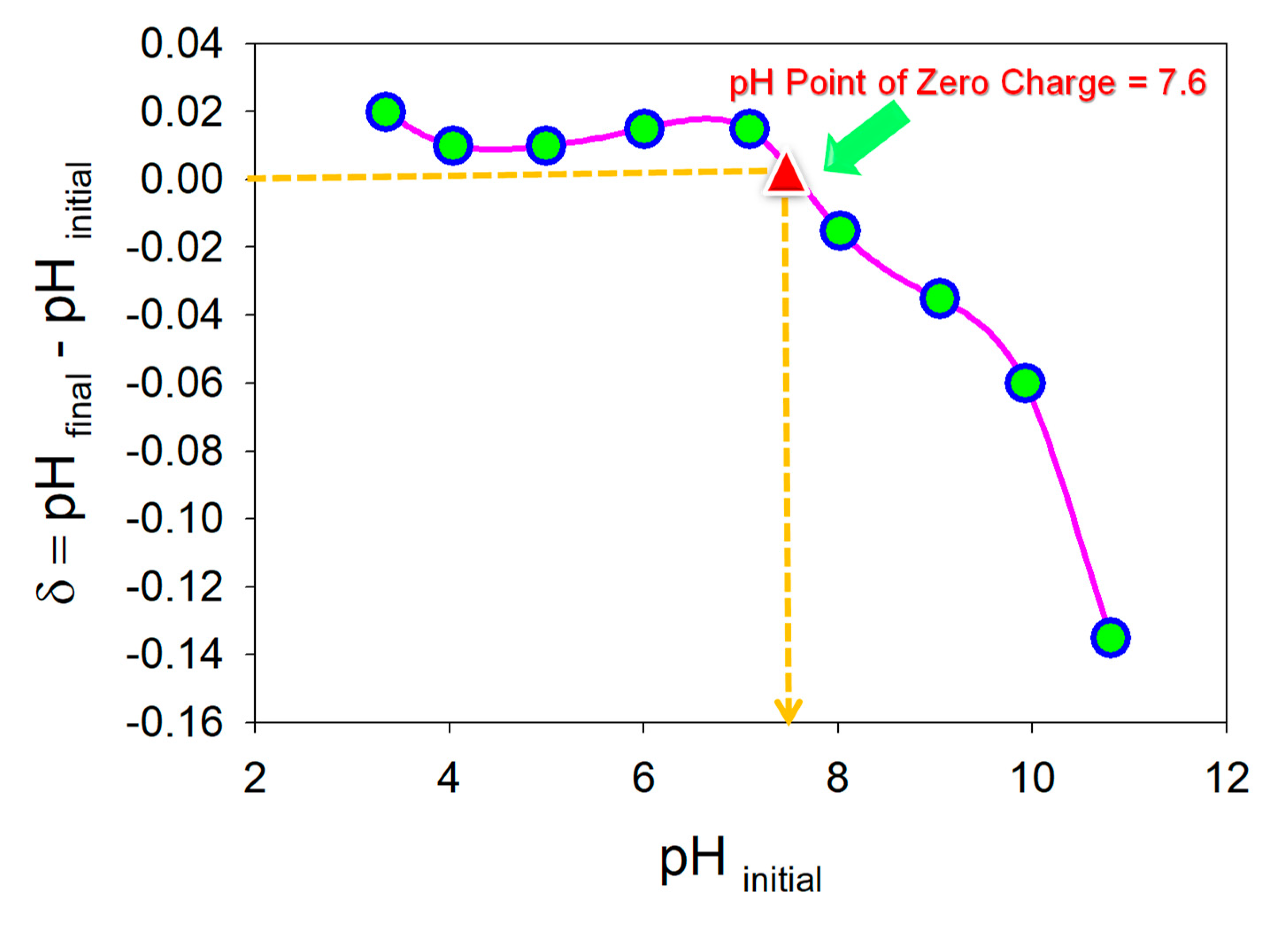
3.2.1. Effect of pH
3.2.2. Effect of Cd2+ Initial Concentration
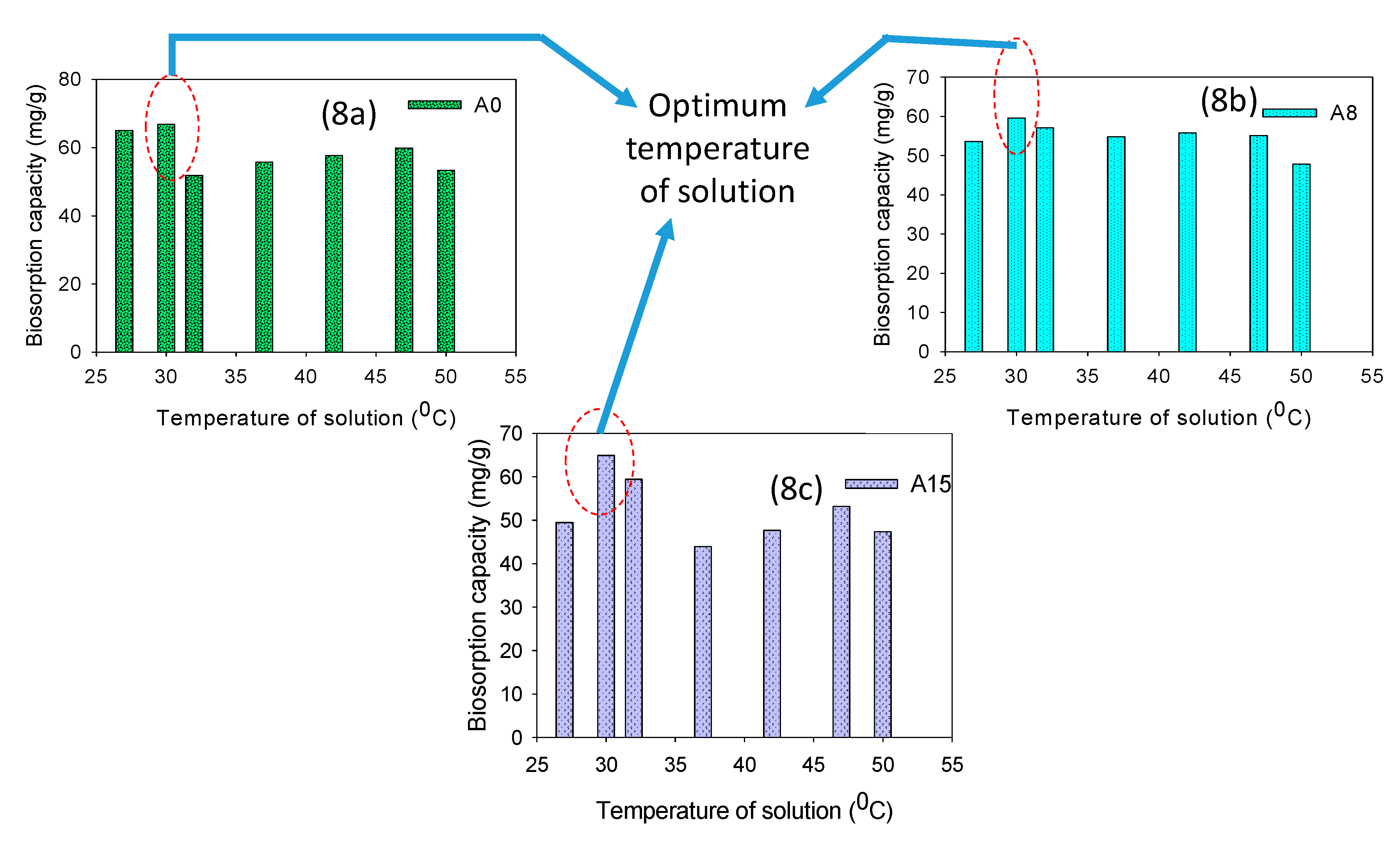
3.2.3. Effect of Temperature and Concentration of Biosorbent
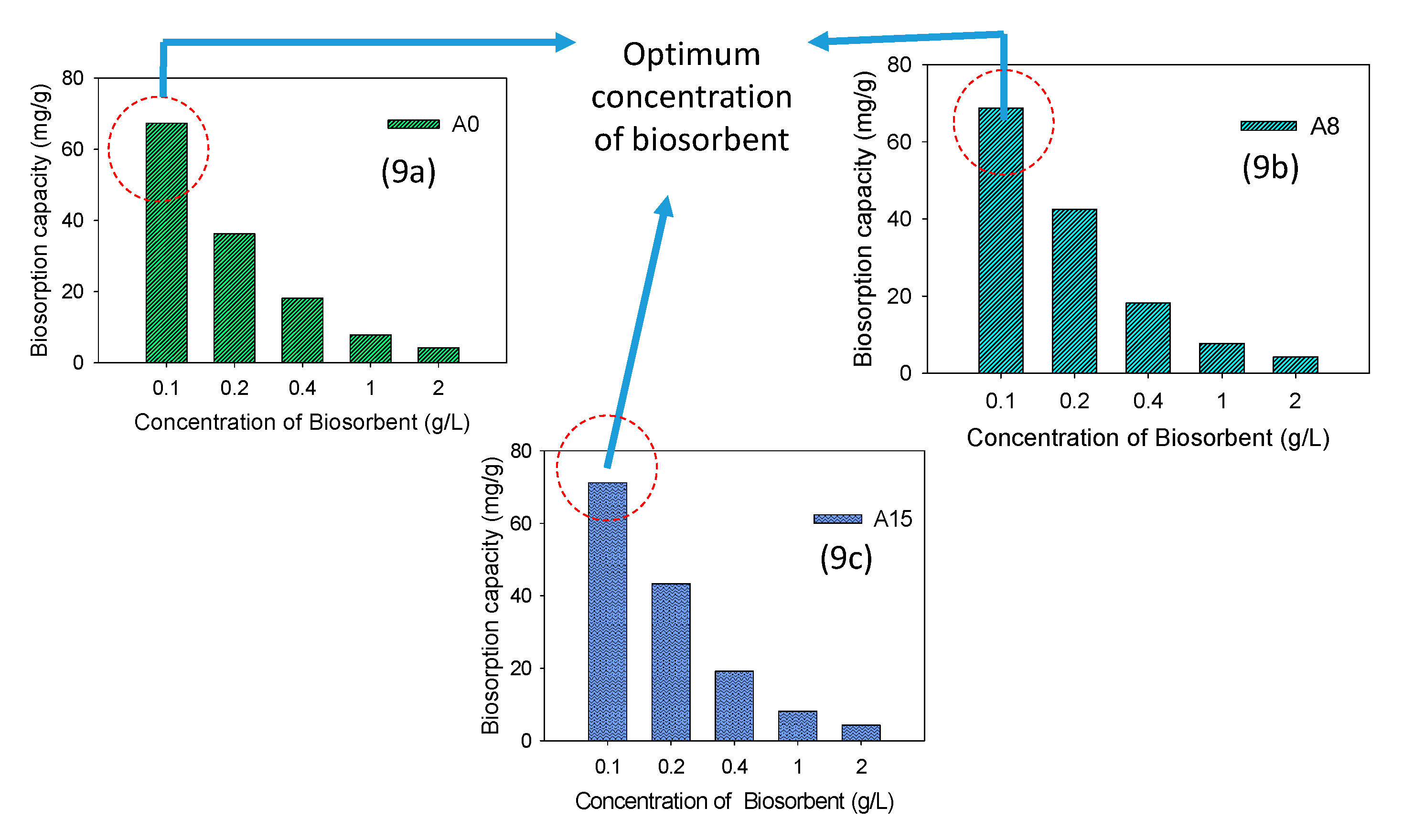
3.2.4. Effect of Biosorbent Concentration
3.3. Biosorption Properties of Aphanothece Dried Biosorbent
3.3.1. Sorption Isotherm Models
3.3.2. Adsorption Kinetic Models
3.3.3. Thermodynamic Model
3.4. Sorption–Desorption of Biosorbent in Batch Sorption of Cd2+
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naeem, M.A.; Imran, M.; Amjad, M.; Abbas, G.; Tahir, M.; Murtaza, B.; Zakir, A.; Shahid, M.; Bulgariu, L.; Ahmad, I. Batch and Column Scale Removal of Cadmium from Water Using Raw and Acid Activated Wheat Straw Biochar. Water 2019, 11, 1438. [Google Scholar] [CrossRef]
- Fernández-González, R.; Martín-Lara, M.Á.; Blázquez, G.; Pérez, A.; Calero, M. Recovering Metals from Aqueous Solutions by Biosorption onto Hydrolyzed Olive Cake. Water 2019, 11, 2519. [Google Scholar] [CrossRef]
- Zarei, S.; Niad, M. The isotherm, kinetic and thermodynamic studies of the cadmium uptake by colpomenia sinuosa. Sep. Sci. Technol. 2019, 1–9. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Parhi, P.K.; Pandey, S.; Bindhani, B.K.; Thatoi, H.; Panda, C.R. Active and passive biosorption of Pb(II) using live and dead biomass of marine bacterium Bacillus xiamenensis PbRPSD202: Kinetics and isotherm studies. J. Environ. Manag. 2019, 247, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Duy Nguyen, H.; Nguyen Tran, H.; Chao, H.-P.; Lin, C.-C. Activated Carbons Derived from Teak Sawdust-Hydrochars for Efficient Removal of Methylene Blue, Copper, and Cadmium from Aqueous Solution. Water 2019, 11, 2581. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.; Won, E.; Lee, J.; Shin, K. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Rodrigues, C.M.; Pires, J.C.M.; Simões, M. The effect of increasing CO2 concentrations on its capture, biomass production and wastewater bioremediation by microalgae and cyanobacteria. Algal Res. 2016, 14, 127–136. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, C.-Y.; Lee, D.-J.; Chang, J.-S. Perspectives on microalgal CO2-emission mitigation systems—A review. Biotechnol. Adv. 2011, 29, 189–198. [Google Scholar] [CrossRef]
- Sumada, K. Study on the efficiently industrial electroplating waste water treatment installation. J. Teknik Kimia 2006, 1, 26–36. (In Indonesian) [Google Scholar]
- Jacob-Lopes, E.; Gimenes Scoparo, C.H.; Queiroz, M.I.; Franco, T.T. Biotransformations of carbon dioxide in photobioreactors. Energy Convers. Manag. 2010, 51, 894–900. [Google Scholar] [CrossRef]
- Fernandes, B.D.; Mota, A.; Teixeira, J.A.; Vicente, A.A. Continuous cultivation of photosynthetic microorganisms: Approaches, applications and future trends. Biotechnol. Adv. 2015, 33, 1228–1245. [Google Scholar] [CrossRef]
- Markou, G.; Georgakakis, D. Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial wastes and wastewaters: A review. Appl. Energy 2011, 88, 3389–3401. [Google Scholar] [CrossRef]
- Apiratikul, R.; Pavasant, P. Batch and column studies of biosorption of heavy metals by Caulerpa lentillifera. Bioresour. Technol. 2008, 99, 2766–2777. [Google Scholar] [CrossRef] [PubMed]
- Luna, A.S.; Costa, A.L.H.; da Costa, A.C.A.; Henriques, C.A. Competitive biosorption of cadmium(II) and zinc(II) ions from binary systems by Sargassum filipendula. Bioresour. Technol. 2010, 101, 5104–5111. [Google Scholar] [CrossRef] [PubMed]
- Areco, M.M.; Hanela, S.; Duran, J.; dos Santos Afonso, M. Biosorption of Cu(II), Zn(II), Cd(II) and Pb(II) by dead biomasses of green alga Ulva lactuca and the development of a sustainable matrix for adsorption implementation. J. Hazard. Mater. 2012, 213–214, 123–132. [Google Scholar] [CrossRef]
- Dirbaz, M.; Roosta, A. Adsorption, kinetic and thermodynamic studies for the biosorption of cadmium onto microalgae Parachlorella sp. J. Environ. Chem. Eng. 2018, 6, 2302–2309. [Google Scholar] [CrossRef]
- Abdel -Aty, A.M.; Ammar, N.S.; Abdel Ghafar, H.H.; Ali, R.K. Biosorption of cadmium and lead from aqueous solution by fresh water alga Anabaena sphaerica biomass. J. Adv. Res. 2013, 4, 367–374. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Kyzas, G.Z. Progress in batch biosorption of heavy metals onto algae. J. Mol. Liq. 2015, 209, 77–86. [Google Scholar] [CrossRef]
- Satya, A.; Sulawesty, F.; Harimawan, A.; Setiadi, T. Correlation of Aquatic Parameters to the Cadmium Bioaccumulation Capability onto Microalgae Biomass in an Urban Lake. J. Water Sustain. Univ. Technol. Syd. Xi’an Univ. Archit. Technol. 2018, 8, 59–72. [Google Scholar] [CrossRef]
- Ghaedi, M.; Hajati, S.; Karimi, F.; Barazesh, B.; Ghezelbash, G. Equilibrium, kinetic and isotherm of some metal ion biosorption. J. Ind. Eng. Chem. 2013, 19, 987–992. [Google Scholar] [CrossRef]
- Geetha, P.; Latha, M.S.; Pillai, S.S.; Koshy, M. Nanoalginate based biosorbent for the removal of lead ions from aqueous solutions: Equilibrium and kinetic studies. Ecotoxicol. Environ. Saf. 2015, 122, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Rastogi, A. Equilibrium and kinetic modelling of cadmium(II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J. Hazard. Mater. 2008, 153, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, V.; Kumar, A.S.K.; Rajesh, N. Biosorption of cadmium using a novel bacterium isolated from an electronic industry effluent. Chem. Eng. J. 2014, 235, 176–185. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Rodrigues, M.S.; de Carvalho, J.C.M.; Lodi, A.; Finocchio, E.; Perego, P.; Converti, A. Adsorption of Ni2+, Zn2+ and Pb2+ onto dry biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris. I. Single metal systems. Chem. Eng. J. 2011, 173, 326–333. [Google Scholar] [CrossRef]
- Fraile, A.; Penche, F.; Gonzalez, F.; Blazquez, M.; Munoz, J.; Ballester, A. Biosorption of Copper, zinc, cadmium and nickel by Chlorella vulgaris. Chem. Ecol. 2005, 21, 61–75. [Google Scholar] [CrossRef]
- Freitas, O.M.M.; Martins, R.J.E.; Delerue-Matos, C.M.; Boaventura, R.A.R. Removal of Cd(II), Zn(II) and Pb(II) from Aqueous Solutions by Brown Marine Macro Algae: Kinetic Modelling. J. Hazard. Mater. 2008, 153, 493–501. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef]
- Bulgariu, D.; Bulgariu, L. Potential use of alkaline treated algae waste biomass as sustainable biosorbent for clean recovery of cadmium(II) from aqueous media: Batch and column studies. J. Clean. Prod. 2016, 112, 4525–4533. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M. Biosorption of Pb(II) and Cd(II) from aqueous solution using green alga (Ulva lactuta) biomass. J. Hazard. Mater. 2008, 152, 302–308. [Google Scholar] [CrossRef]
- Bakatula, E.N.; Cukrowska, E.M.; Weiersbye, I.M.; Mihaly-Cozmuta, L.; Peter, A.; Tutu, H. Biosorption of trace elements from aqueous systems in gold mining sites by the filamentous green algae (Oedogonium sp.). J. Geochem. Explor. 2014, 144, 492–503. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rastogi, A. Sorption and desorption studies of chromium(VI) from nonviable cyanobacterium Nostoc muscorum biomass. J. Hazard. Mater. 2008, 154, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rama, M.; Torres, E.; Suárez, C.; Herrero, C.; Abalde, J. Sorption isotherm studies of Cd(II) ions using living cells of the marine microalga Tetraselmis suecica (Kylin) Butch. J. Environ. Manag. 2010, 91, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Sarı, A.; Tuzen, M. Biosorption of cadmium(II) from aqueous solution by red algae (Ceramium virgatum): Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 157, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Sathvika, T.; Manasi; Rajesh, V.; Rajesh, N. Adsorption of chromium supported with various column modelling studies through the synergistic influence of Aspergillus and cellulose. J. Environ. Chem. Eng. 2016, 4, 3193–3204. [Google Scholar] [CrossRef]
- Sari, A.; Mendil, D.; Tuzen, M.; Soylak, M. Biosorption of Cd(II) and Cr(III) from aqueous solution by moss (Hylocomium splendens) biomass: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2008, 144, 1–9. [Google Scholar] [CrossRef]
- Sarada, B.; Prasad, M.K.; Kumar, K.K.; Ramachandra Murthy, C.V. Cadmium removal by macro algae Caulerpa fastigiata: Characterization, kinetic, isotherm and thermodynamic studies. J. Environ. Chem. Eng. 2014, 2, 1533–1542. [Google Scholar] [CrossRef]
- Şeker, A.; Shahwan, T.; Eroğlu, A.E.; Yılmaz, S.; Demirel, Z.; Dalay, M.C. Equilibrium, thermodynamic and kinetic studies for the biosorption of aqueous lead(II), cadmium(II) and nickel(II) ions on Spirulina platensis. J. Hazard. Mater. 2008, 154, 973–980. [Google Scholar] [CrossRef]
- Blanes, P.S.; Bordoni, M.E.; González, J.C.; García, S.I.; Atria, A.M.; Sala, L.F.; Bellú, S.E. Application of soy hull biomass in removal of Cr(VI) from contaminated waters. Kinetic, thermodynamic and continuous sorption studies. J. Environ. Chem. Eng. 2016, 4, 516–526. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Coconut-based biosorbents for water treatment—A review of the recent literature. Adv. Colloid Interface Sci. 2010, 160, 1–15. [Google Scholar] [CrossRef]
- Chojnacka, K.; Chojnacki, A.; Górecka, H. Trace element removal by Spirulina sp. from copper smelter and refinery effluents. Hydrometallurgy 2004, 73, 147–153. [Google Scholar] [CrossRef]
- Chojnacka, K. Biosorption and bioaccumulation—the prospects for practical applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Sandau, E.; Sandau, P.; Pulz, O.; Zimmermannn, M. Heavy metal sorption by marine algae and algal by product. Acta Biotechnol. 1996, 16, 103–119. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.; Malcata, F.X. Cadmium Removal by Two Strains of Desmodesmus pleimorphus cells. Water Air Soil Pollut. 2010, 208, 17–27. [Google Scholar] [CrossRef]
- Tüzün, İ.; Bayramoğlu, G.; Yalçın, E.; Başaran, G.; Çelik, G.; Arıca, M.Y. Equilibrium and kinetic studies on biosorption of Hg(II), Cd(II) and Pb(II) ions onto microalgae Chlamydomonas reinhardtii. J. Environ. Manag. 2005, 77, 85–92. [Google Scholar] [CrossRef]
- Schmitt, D.; Muller, A.; Csogor, Z.; Frimmel, F.; Posten, C. The adsorption kinetics of metal ions onto different microalgae and siliceous earth. Water Resour. 2001, 35, 779–785. [Google Scholar] [CrossRef]
- Singh, A.; Mehta, S.; Gaur, J. Removal of heavy metals from aqueous solution by common freshwater filamentous algae. World J. Microbiol. Biotechnol. 2007, 23, 1115–1120. [Google Scholar] [CrossRef]
- Pérez-Rama, M.; Abalde Alonso, J.; Herrero López, C.; Torres Vaamonde, E. Cadmium removal by living cells of the marine microalga Tetraselmis suecica. Bioresour. Technol. 2002, 84, 265–270. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chang, H.-W.; Kao, P.-C.; Pan, J.-L.; Chang, J.-S. Biosorption of cadmium by CO2-fixing microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 105, 74–80. [Google Scholar] [CrossRef]
- Katırcıoğlu, H.; Aslım, B.; Rehber Türker, A.; Atıcı, T.; Beyatlı, Y. Removal of cadmium(II) ion from aqueous system by dry biomass, immobilized live and heat-inactivated Oscillatoria sp. H1 isolated from freshwater (Mogan Lake). Bioresour. Technol. 2008, 99, 4185–4191. [Google Scholar] [CrossRef]
- Rathinam, A.; Maharshi, B.; Janardhanan, S.K.; Jonnalagadda, R.R.; Nair, B.U. Biosorption of cadmium metal ion from simulated wastewaters using Hypnea valentiae biomass: A kinetic and thermodynamic study. Bioresour. Technol. 2010, 101, 1466–1470. [Google Scholar] [CrossRef]
- Sarı, A.; Tuzen, M. Biosorption of total chromium from aqueous solution by red algae (Ceramium virgatum): Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 160, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Akar, T.; Celik, S.; Gorgulu Ari, A.; Tunali Akar, S. Removal of Pb2+ ions from contaminated solutions by microbial composite: Combined action of a soilborne fungus Mucor plumbeus and alunite matrix. Chem. Eng. J. 2013, 215–216, 626–634. [Google Scholar] [CrossRef]
- Kumar, D.; Gaur, J.P. Metal biosorption by two cyanobacterial mats in relation to pH, biomass concentration, pretreatment and reuse. Bioresour. Technol. 2011, 102, 2529–2535. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-M.; Xiao, X.; Luo, S.-L. Biosorption of cadmium(II) from aqueous solutions by industrial fungus Rhizopus cohnii. Trans. Nonferrous Metals Soc. China 2010, 20, 1104–1111. [Google Scholar] [CrossRef]
- Rangsayatorn, N.; Upatham, E.S.; Kruatrachue, M.; Pokethitiyook, P.; Lanza, G.R. Phytoremediation potential of Spirulina (Arthrospira) platensis: Biosorption and toxicity studies of cadmium. Environ. Pollut. 2002, 119, 45–53. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Ferreira, L.S.; Carvalho, J.C.M.d.; Lodi, A.; Finocchio, E.; Converti, A. Metal biosorption onto dry biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris: Multi-metal systems. J. Hazard. Mater. 2012, 217–218, 246–255. [Google Scholar] [CrossRef] [PubMed]
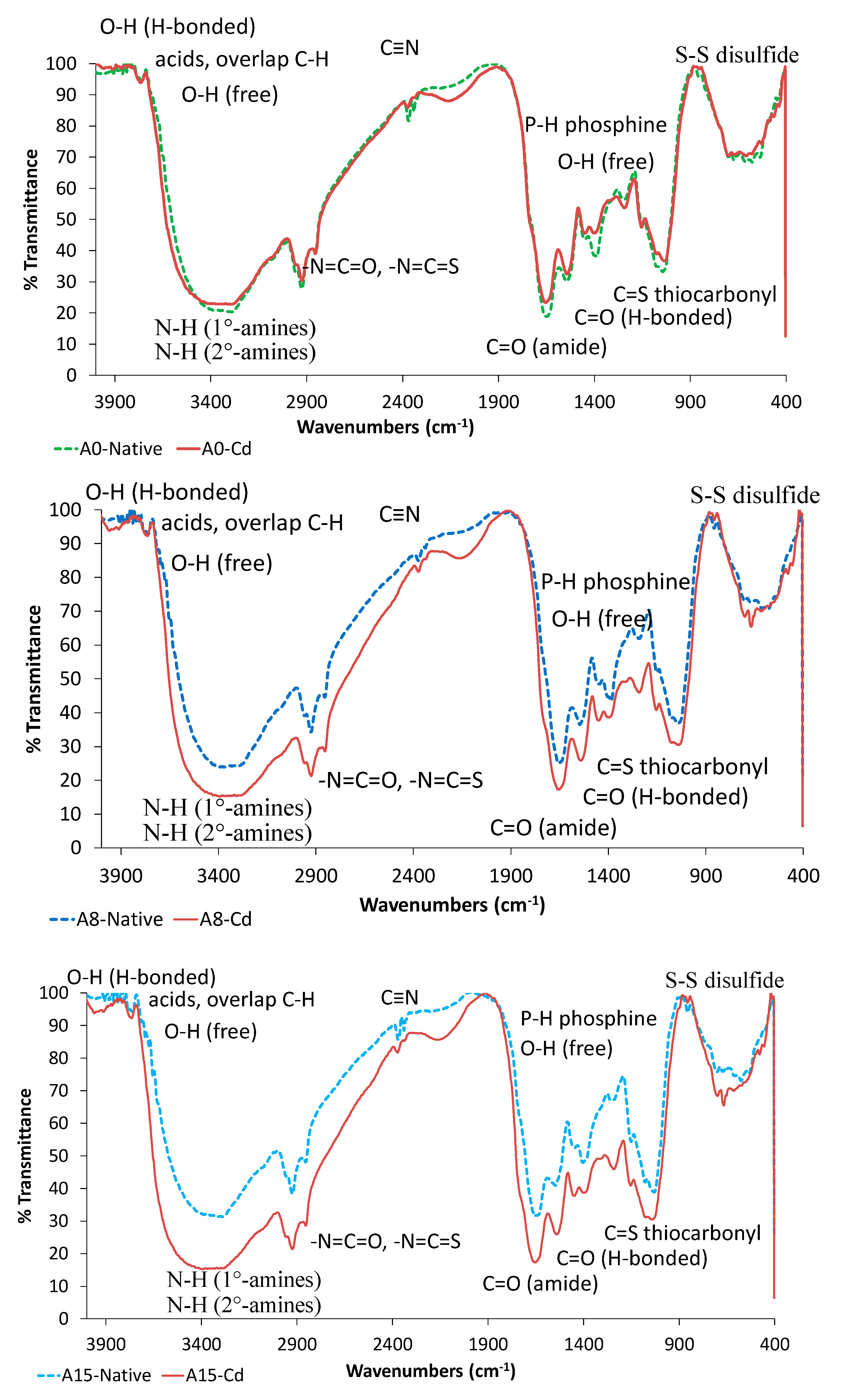

| Functional Groups | The Peak at Wavenumber (cm−1) | |||||
|---|---|---|---|---|---|---|
| Pure A0 | A0-Cd | Pure A8 | A8-Cd | Pure A15 | A15-Cd | |
| Disulfides (S–S), Sulfur ether (SOR) | 671.23 | 667.37 | 536.21 | 526.57 | 536.21 | 524.64 |
| Thio carbonyl (C=S), Sulfoxide (S=O) | 1043.49 | 1039.63 | 1078.21 | 1076.28 | 1076.28 | 1072.42 |
| Carbonyl (C–O) on Alcohols and Carboxylates | 1544.98 | 1539.20 | 1548.84 | 1541.12 | 1544.98 | 1539.20 |
| Amines (tertiary, secondary, and primary) | 2926.01 | 2924.09 | 2924.09 | 2921.09 | 2924.09 | 2921.09 |
| Carboxylates (C=O) | 1654.92 | 1651.07 | 1654.92 | 1652.92 | 1654.92 | 1651.07 |
| Hydroxyls (O–H) | 3292.49 | 3282.84 | 3292.49 | 3289.43 | 3292.49 | 3289.42 |
| Biosorbent | Enrichment of CO2 During Cultivation | BET Surface Area (m2/g) | Average Pore Diameter (Å) | Pore Volume (cm3/gram) |
|---|---|---|---|---|
| A0 | 0.04% | 0.571 | 271.55 | 3.88 × 10−3 |
| A8 | 8.00% | 1.846 | 102.70 | 4.74 × 10−3 |
| A15 | 15.00% | 1.735 | 111.29 | 4.74 × 10−3 |
| Biosorbent | Langmuir Constants | Freundlich Constants | Dubinin–Radushkevich Constants | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qmax (mg/g) | b (L/mg) | R2 | KF (mg/g(L/g)1/n) | n | R2 | qmax (mol/g) | E (kJ/mol) | R2 | |
| 1. A0 | 12.24 | 43.00 | 0.992 | 0.031 | 1.195 | 0.904 | 9.74 × 10−4 | 8.45 | 1.000 |
| 2. A8 | 36.90 | 17.13 | 0.994 | 0.094 | 0.696 | 0.731 | 4.79 × 10−3 | 11.18 | 0.998 |
| 3. A15 | 60.24 | 1.66 | 0.992 | 0.174 | 0.868 | 0.868 | 9.12 × 10−3 | 11.18 | 0.934 |
| Biosorbent | qe(experiment) | Pseudo-First-Order Parameters | Pseudo-Second-Order Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (mg/g) | k1 (min−1) | qe(cal) (mg/g) | RSD | R2 | k2 (g/mg.min) | qe(cal) (mg/g) | RSD | R2 | |
| A0 | 9.30 | 3.69 | 5.20 | 0.83 | 0.823 | 2.79 × 10−2 | 9.23 | 0.32 | 0.992 |
| A8 | 11.11 | 4.14 | 8.85 | 0.73 | 0.923 | 3.96 × 10−2 | 11.16 | 0.42 | 0.972 |
| A15 | 13.62 | 4.57 | 2.78 | 0.21 | 0.412 | 4.54 × 10−2 | 13.39 | 0.12 | 0.997 |
| Temperature (K) | Thermodynamic Parameters | ||
|---|---|---|---|
| ∆G0 (kJ/mol) | ∆H0 (kJ/mol) | ∆S0 (kJ/mol K) | |
| Biosorbent A0 | |||
| 310 | −10.23 | −41.85 | −0.102 |
| 315 | −9.58 | ||
| 320 | −9.15 | ||
| 323 | −8.89 | ||
| Biosorbent A8 | |||
| 305 | −10.36 | −42.76 | −0.106 |
| 310 | −9.97 | ||
| 323 | −8.48 | ||
| Biosorbent A15 | |||
| 303 | −10.88 | −49.16 | −0.126 |
| 315 | −9.17 | ||
| 323 | −8.39 |
| No. | Biosorbent | pH | C0 (mg/L) | qmax (mg/g) | References |
|---|---|---|---|---|---|
| 1 | Aphanothece A0 | 8 | 1–7 | 12.24 | This study |
| 2 | Aphanothece A8 | 8 | 1–7 | 36.90 | This study |
| 3 | Aphanothece A15 | 8 | 1–7 | 60.24 | This study |
| 4 | Spirulina spp. | 7.5 | 3.8 | 0.46 | Chojnacka et al. [40,41] |
| 6 | AER Chlorella | 3–7 | - | 7.74 | Sandau et al. [42] |
| 8 | Desmodesmus pleimorphus | 4 | 0.5–5 | 47.1 | Monteiro et al. [43] |
| 9 | Chlorella vulgaris | 6 | - | 12.45 | Sandau, Sandau, Pulz, and Zimmermannn [42] |
| 10 | Chlamydomonas reinhardtii | 6 | 100 | 42.6 | Tüzün et al. [44] |
| 11 | Phaeodactylum tricornutum | 6 | - | 1.24 | Schmitt et al. [45] |
| 12 | Hydrodictyon reticulatum | 5 | - | 7.2 | Singh et al. [46] |
| 13 | Tetraselmis suecica (Kylin) Butch | 7.8 | 0.6–45 | 30.13 | Pérez-Rama et al. [47] |
| 14 | Scenedesmus obliquus CNW-N | 5 | 25–200 | 24.4 | Chen et al. [48] |
| 15 | Ulva lactuta | 5 | 10 | 28.1 | Sari and Tuzen [29] |
| 16 | Oscillatoria sp. | 2–8 | 25–100 | 30.1 | Katırcıoğlu et al. [49] |
| 17 | Hypnea valentiae | 6 | 25–500 | 15.79 | Rathinam et al. [50] |
| 18 | Ceramium virgatum | 5 | 10 | 39.7 | Sarı and Tuzen [51] |
| 19 | Colpomenia sinuosa | - | 5 and 100 | 64.9 | Zarei and Niad [3] |
| Sorption Capacity (mg/g) | |||
|---|---|---|---|
| 1. Biosorbent of A0 | Cycle 1 | Cycle 2 | Cycle 3 |
| Sorption (B) | 59.93 | 63.51 | 58.23 |
| Desorption (D) | 48.71 | 44.77 | 15.62 |
| The ratio of (D/B) | 0.81 | 0.70 | 0.27 |
| 2. Biosorbent A8 | |||
| Sorption (B) | 5.61 | 2.53 | 2.02 |
| Desorption (D) | 5.24 | 2.01 | 1.13 |
| The ratio of (D/B) | 0.93 | 0.77 | 0.56 |
| 3. Biosorbent A15 | |||
| Sorption (B) | 5.86 | 5.91 | 5.89 |
| Desorption (D) | 5.63 | 5.81 | 4.05 |
| Ratio of (D/B) | 0.96 | 0.98 | 0.69 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satya, A.; Harimawan, A.; Haryani, G.S.; Johir, M.A.H.; Vigneswaran, S.; Ngo, H.H.; Setiadi, T. Batch Study of Cadmium Biosorption by Carbon Dioxide Enriched Aphanothece sp. Dried Biomass. Water 2020, 12, 264. https://doi.org/10.3390/w12010264
Satya A, Harimawan A, Haryani GS, Johir MAH, Vigneswaran S, Ngo HH, Setiadi T. Batch Study of Cadmium Biosorption by Carbon Dioxide Enriched Aphanothece sp. Dried Biomass. Water. 2020; 12(1):264. https://doi.org/10.3390/w12010264
Chicago/Turabian StyleSatya, Awalina, Ardiyan Harimawan, Gadis Sri Haryani, Md. Abu Hasan Johir, Saravanamuthu Vigneswaran, Huu Hao Ngo, and Tjandra Setiadi. 2020. "Batch Study of Cadmium Biosorption by Carbon Dioxide Enriched Aphanothece sp. Dried Biomass" Water 12, no. 1: 264. https://doi.org/10.3390/w12010264
APA StyleSatya, A., Harimawan, A., Haryani, G. S., Johir, M. A. H., Vigneswaran, S., Ngo, H. H., & Setiadi, T. (2020). Batch Study of Cadmium Biosorption by Carbon Dioxide Enriched Aphanothece sp. Dried Biomass. Water, 12(1), 264. https://doi.org/10.3390/w12010264






