Application of Poly-γ-Glutamic Acid Flocculant to Flocculation–Sedimentation Treatment of Ultrafine Cement Suspension
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
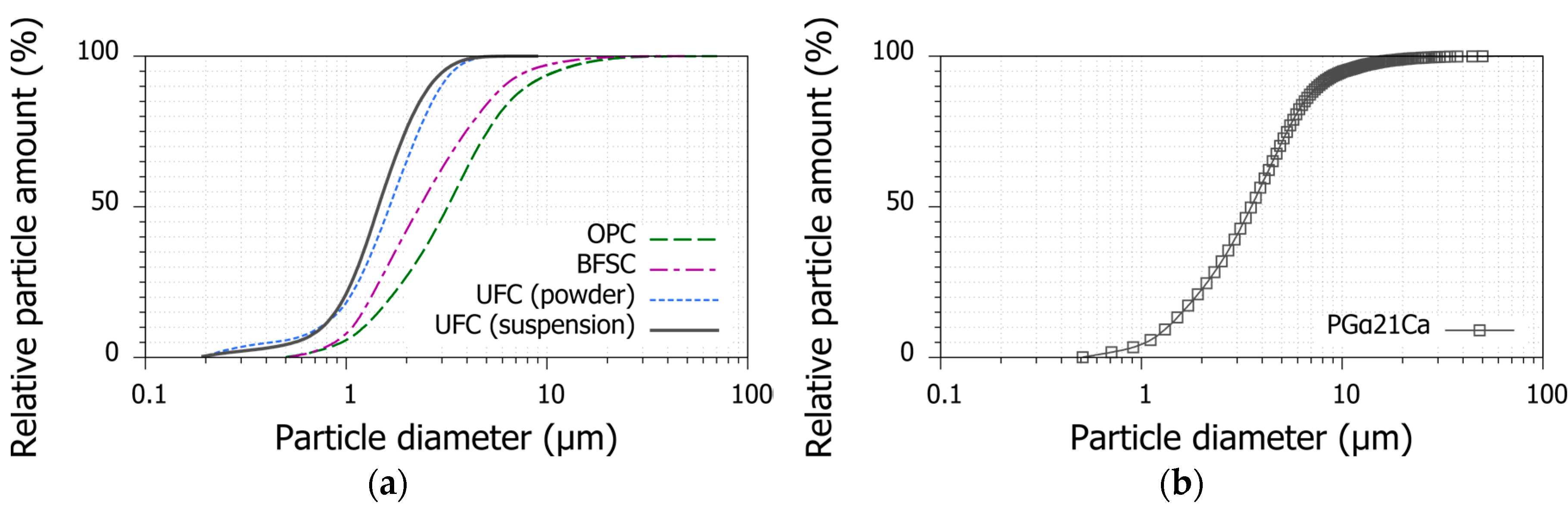
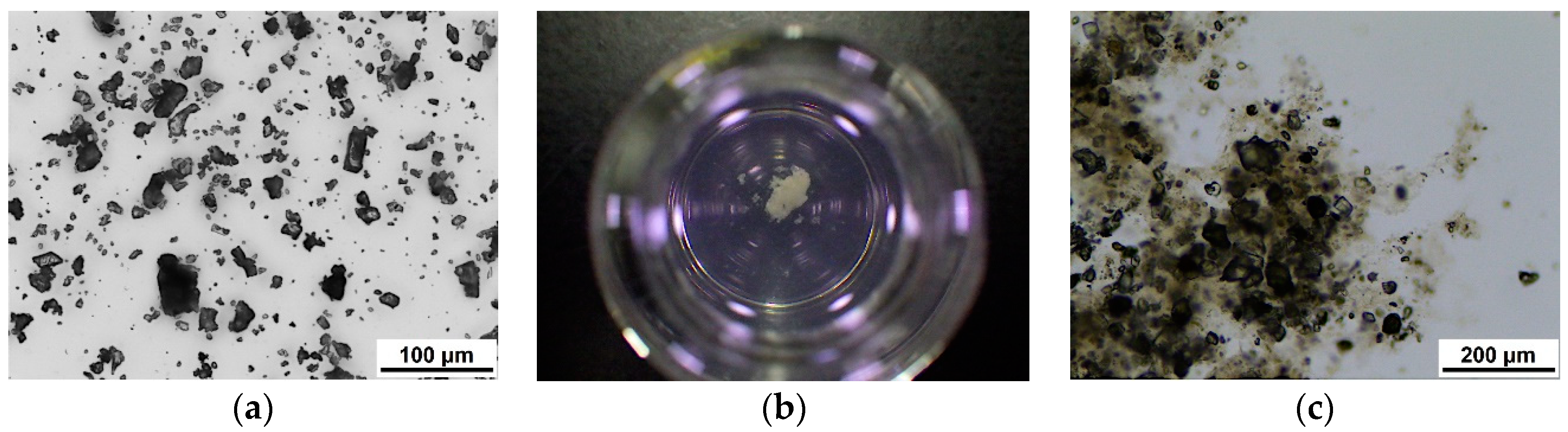
2.1.1. Ultrafine Cement
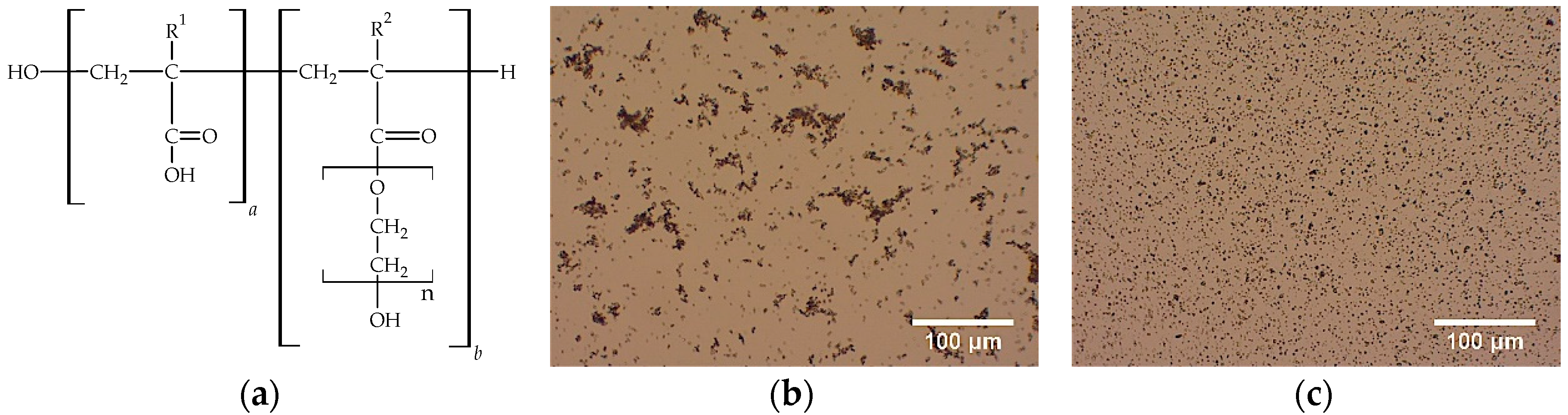
2.1.2. Polymeric Dispersant
2.1.3. Flocculant
2.2. Cement Suspension
2.3. Flocculation–Sedimentation Experiments
2.3.1. Preparation of Ultrafine Cement Suspension
2.3.2. Jar Test Procedure
2.3.3. Measurement of Zeta Potential
3. Results and Discussion
3.1. Ionic Composition of Cement Extract Solutions
3.2. Particle Removal by Poly-γ-Glutamic Acid Flocculant (PGAF)
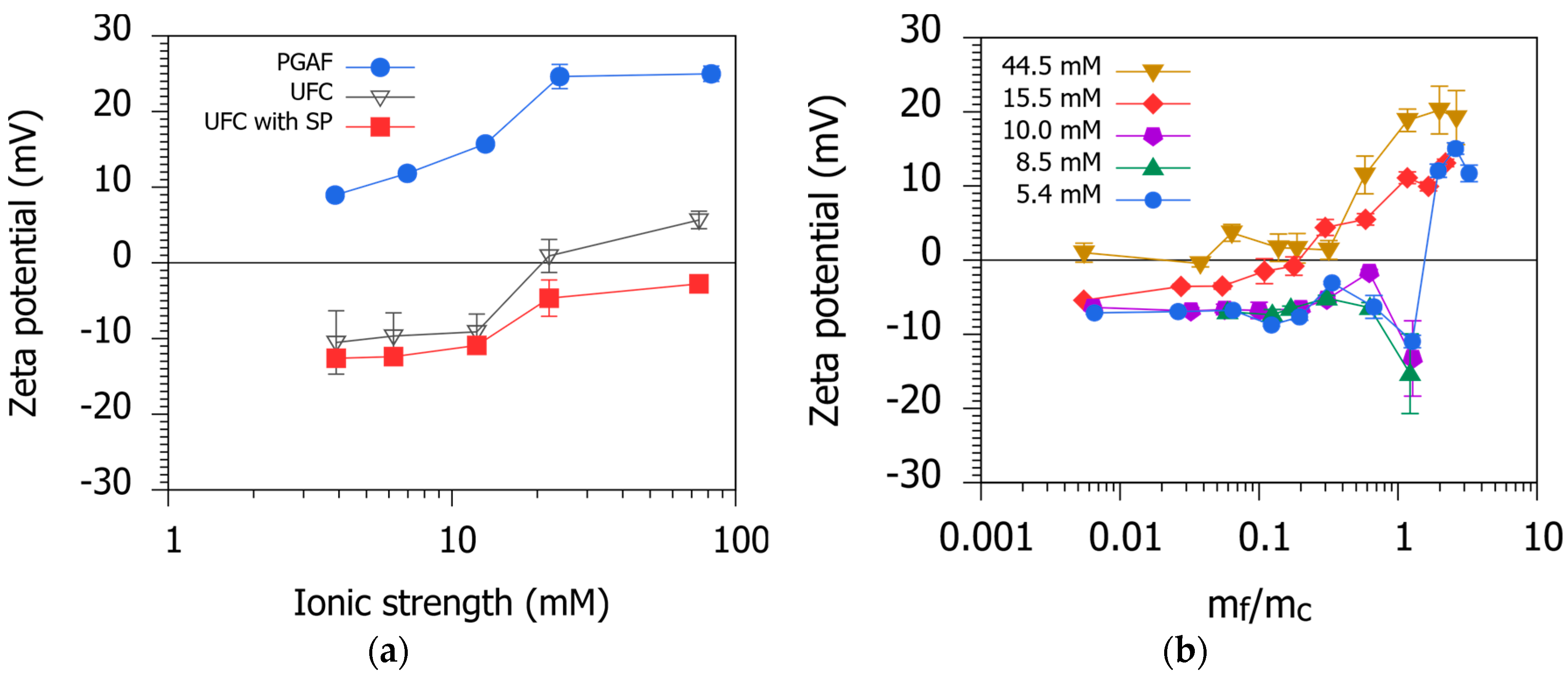
3.3. Zeta Potential
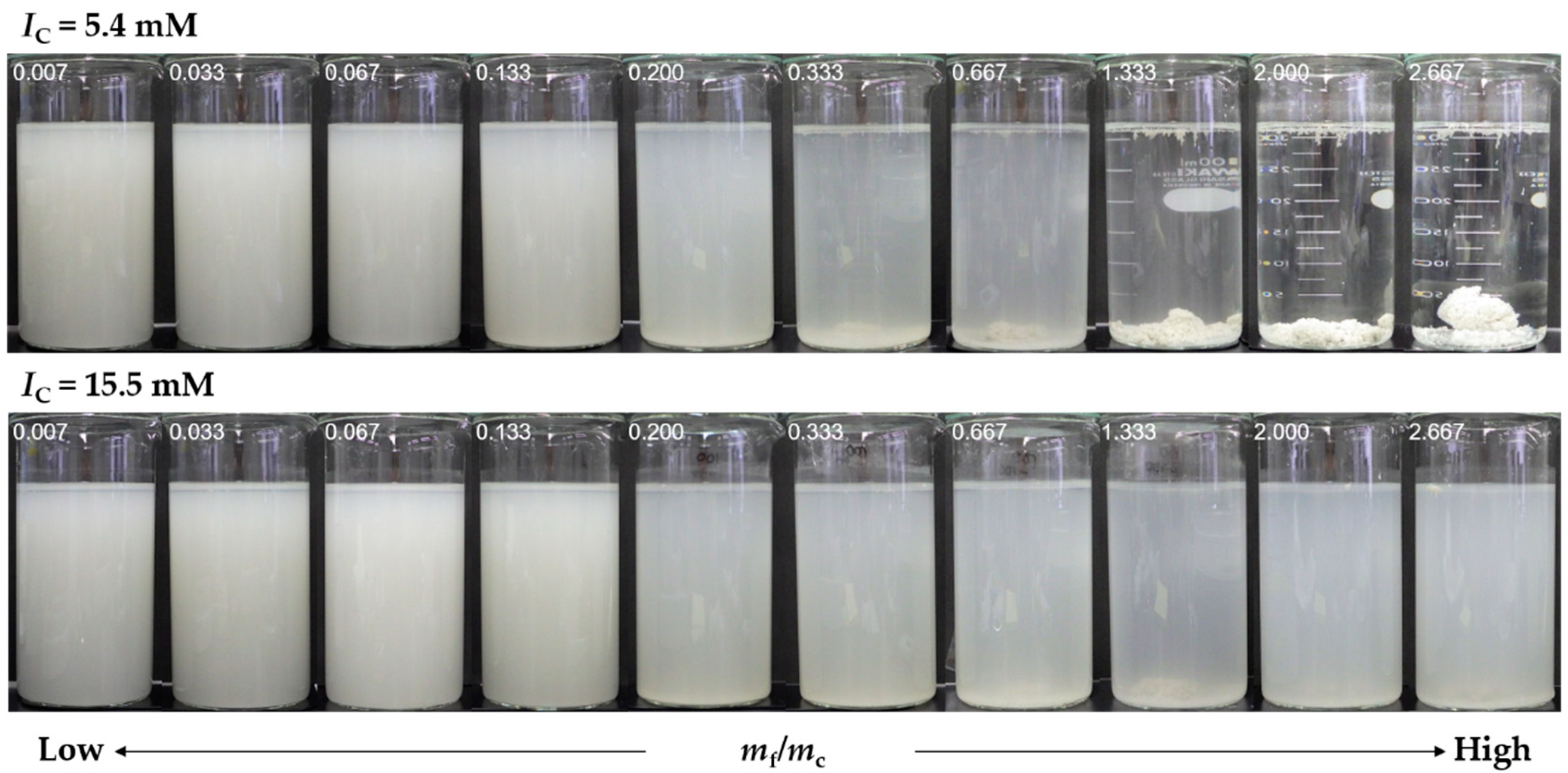
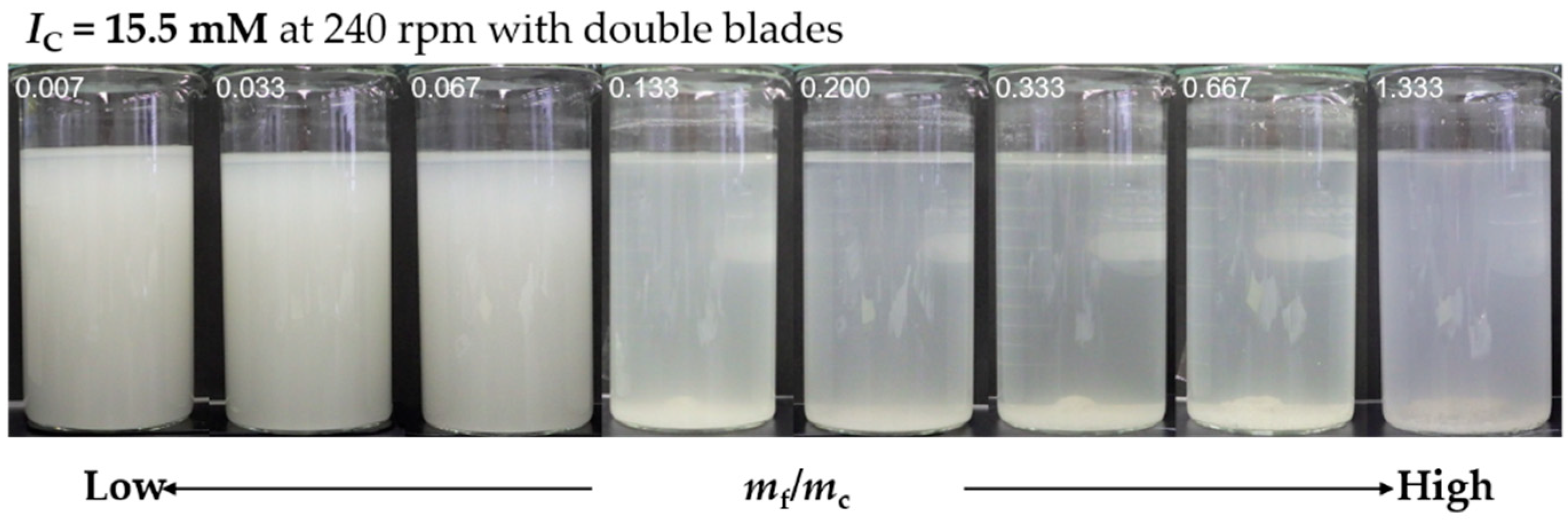
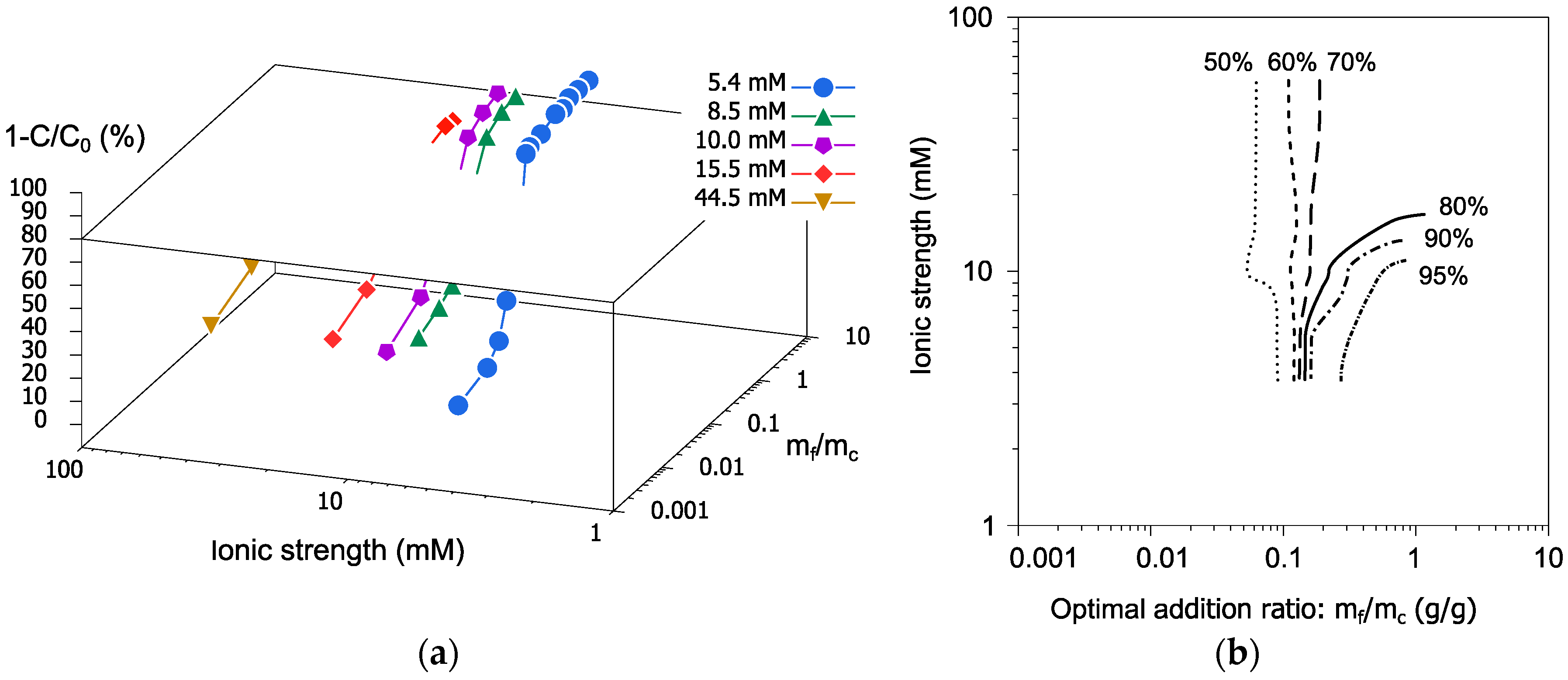
3.4. Influence of Ionic Strength on Flocculation–Sedimentation Characteristics of PGAF
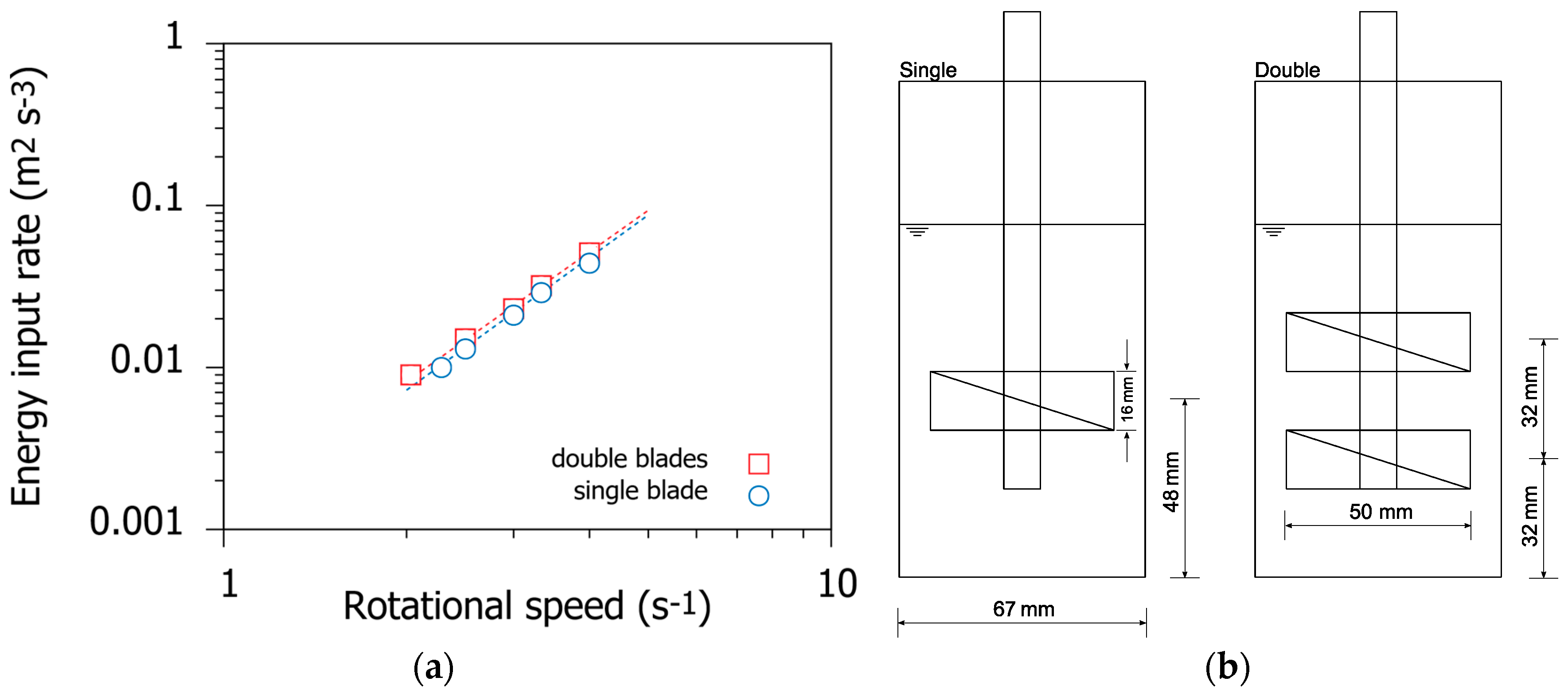
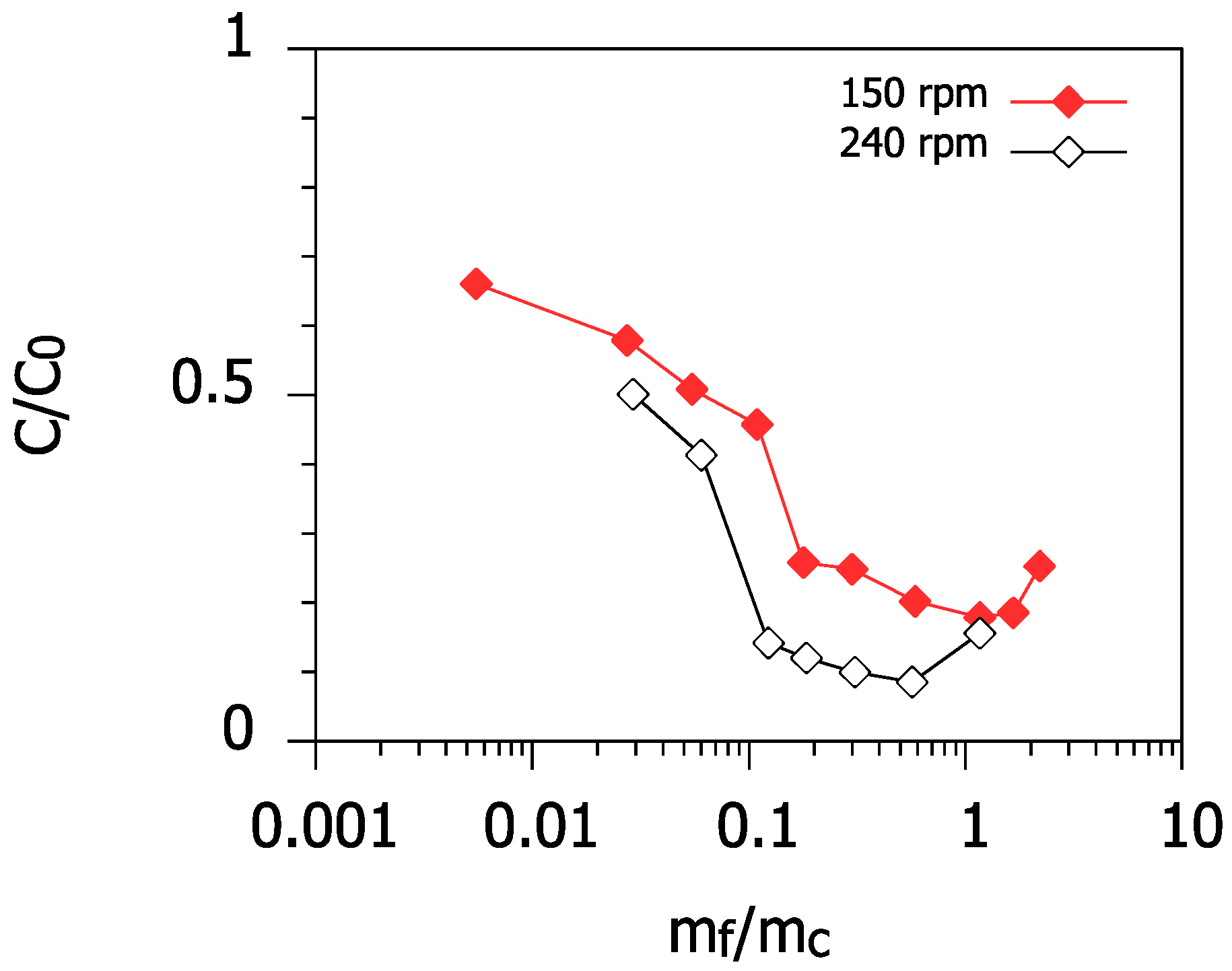
3.5. Effect of Mixing Intensity
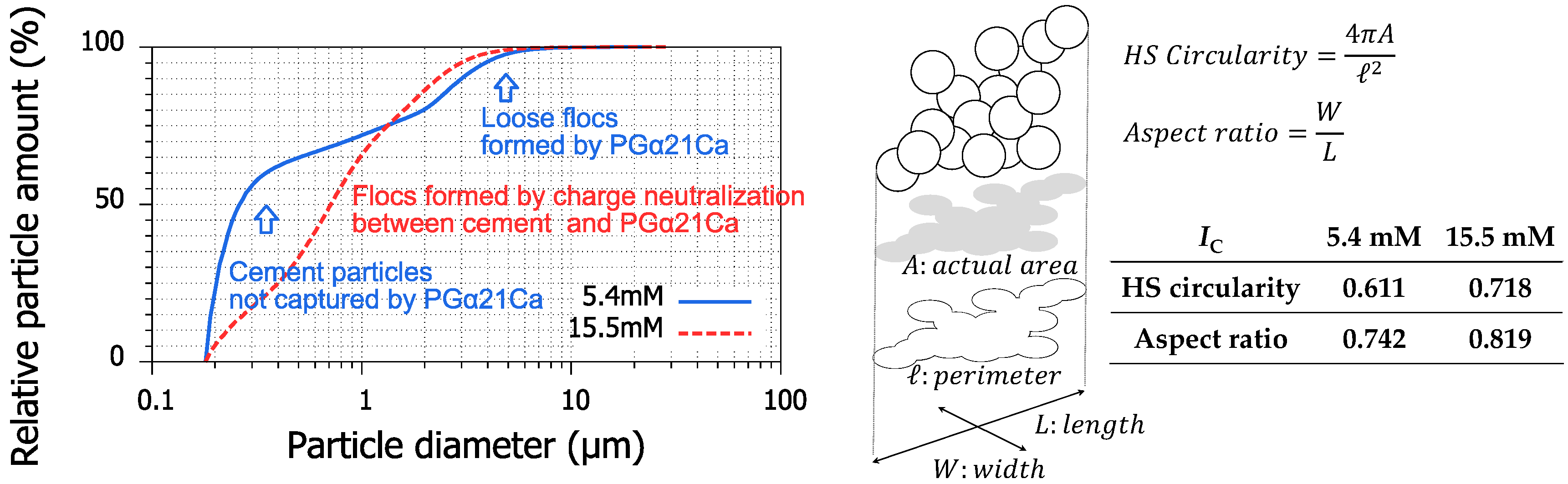
3.6. Applicability of PGα21Ca to Cement Suspension
4. Conclusions
- The flocculation–sedimentation treatment with PGAF successfully removed the SP-stabilized UFC particles through the gravitational settling of the formed flocs. However, the removal characteristics at ionic strengths of 10 and 15.5 mM were different.
- From the measurement of the zeta potential, the SP-stabilized UFC was found to be negatively charged. On the other hand, the PGAF was positively charged despite the presence of γ-PGA with carboxyl groups.
- The removal efficiency reduced with the increase in the ionic strength. This decreased removal could have been due to the shrunk form of γ-PGA at high ionic strengths.
- Increasing the mixing intensity during rapid mixing improved the removal efficiency even at high ionic strengths.
- Based on a series of flocculation–sedimentation experiments, the removal ratios against ionic strengths and mf/mc was plotted in the three-dimensional diagram.
- Using this diagram as a control chart, we can determine the optimal addition amount of the PGAF for achieving the target removal rate for cement suspension under any ionic strengths.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Description |
| PGAF | poly-γ-glutamic acid flocculant |
| UFC | ultra-fine cement |
| SP | superplasticizer |
| γ-PGA | poly-γ-glutamic acid |
| PAC | poly-aluminum chloride |
| BFSC | blast furnace slag cement |
| OPC | ordinary Portland cement |
| AE | air-entrainment |
| EC | electrical conductivity |
| IC | ionic strength of cement suspension |
| C | absorbance of supernatant after flocculation–sedimentation |
| C0 | initial absorbance of cement suspension |
| C/C0 | the ratio of the absorbance of the supernatant to that of the initial suspension |
| w/c | water-to-cement mass ratio |
| mf | mass of PGAF |
| mc | mass of UFC |
| mf/mc | PGAF-to-UFC mass ratio |
References
- Ridi, F.; Fratini, E.; Baglioni, P. Cement: A two thousand year old nano-colloid. J. Colloid Interface Sci. 2011, 357, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Amirtharajah, A.; Mills, K.M. Rapid-mix design for mechanisms of alum coagulation. J. AWWA 1982, 74, 210–216. [Google Scholar] [CrossRef]
- Bhatti, Z.A.; Mahmood, Q.; Raja, I.A. Sewage water pollutants removal efficiency correlates to the concentration gradient of amendments. J. Chem. Soc. Pak. 2009, 31, 665–673. [Google Scholar]
- de Paula, H.M.; de Oliveira Ilha, M.S.; Andrade, L.S. Concrete plant wastewater treatment process by coagulation combining aluminum sulfate and Moringa oleifera powder. J. Clean. Prod. 2014, 76, 125–130. [Google Scholar] [CrossRef]
- Ogata, F.; Ueda, A.; Kawasaki, N. Removal of phosphate ions by PGAF (poly-γ-glutamic acid and flocculants). J. Water Environ. Technol. 2014, 12, 447–458. [Google Scholar] [CrossRef]
- Bajaj, I.B.; Singhal, R.S. Flocculation properties of poly (γ-glutamic acid) produced from Bacillus subtilis isolate. Food Bioprocess Technol. 2011, 4, 745–752. [Google Scholar] [CrossRef]
- Pan, Y.; Shi, B.; Zhang, Y. Research on flocculation property of bioflocculant PG·a21 Ca. Mod. Appl. Sci. 2009, 3, 106–112. [Google Scholar] [CrossRef]
- Taniguchi, M.; Kato, K.; Matsui, O.; Ping, X.; Nakayama, H.; Usuki, Y.; Ichimura, A.; Fujita, K.; Tanaka, T.; Tarui, Y.; et al. Flocculating activity of cross-linked poly-γ-glutamic acid against bentonite and Escherichia coli suspension pretreated with FeCl3 and its interaction with Fe3+. J. Biosci. Bioeng. 2005, 100, 207–211. [Google Scholar] [CrossRef]
- Yokoi, H.; Arima, T.; Hirose, J.; Hayashi, S.; Takasaki, Y. Flocculation properties of poly (γ-glutamic acid) produced by Bacillus subtilis. J. Ferment. Bioeng. 1996, 82, 84–87. [Google Scholar] [CrossRef]
- Campos, V.; Fernandes, A.R.A.C.; Medeiros, T.A.M.; Andrade, E.L. Physicochemical characterization and evaluation of PGA bioflocculant in coagulation-flocculation and sedimentation processes. J. Environ. Chem. Eng. 2016, 4, 3753–3760. [Google Scholar] [CrossRef]
- Carvajal-Zarrabal, O.; Nolasco-Hipólito, C.; Barradas-Dermitz, D.M.; Hayward-Jones, P.M.; Aguilar-Uscanga, M.G.; Bujang, K. Treatment of vinasse from tequila production using polyglutamic acid. J. Environ. Manag. 2012, 95, S66–S70. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Nitanai, M.; Satta, N.; Adachi, Y. Coagulation and charging of latex particles in the presence of imogolite. Colloids Surf. A Physicochem. Eng. Asp. 2013, 435, 139–146. [Google Scholar] [CrossRef]
- Uchikawa, H.; Hanehara, S.; Sawaki, D. The role of steric repulsive force in the dispersion of cement particles in fresh paste prepared with organic admixture. Cem. Concr. Res. 1997, 27, 37–50. [Google Scholar] [CrossRef]
- Plank, J.; Pöllmann, K.; Zouaoui, N.; Andres, P.R.; Schaefer, C. Synthesis and performance of methacrylic ester based polycarboxylate superplasticizers possessing hydroxy terminated poly (ethylene glycol) side chains. Cem. Concr. Res. 2008, 38, 1210–1216. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X. Correlations of the dispersing capability of NSF and PCE types of superplasticizer and their impacts on cement hydration with the adsorption in fresh cement pastes. Cem. Concr. Res. 2015, 69, 1–9. [Google Scholar] [CrossRef]
- Hirata, T.; Ye, J.; Branicio, P.; Zheng, J.; Lange, A.; Plank, J.; Sullivan, M. Adsorbed conformations of PCE superplasticizers in cement pore solution unraveled by molecular dynamics simulations. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Silsbee, M.; Malek, R.I.A.; Roy, D.M. Composition of pore fluids extruded from slag-cement pastes. In Proceedings of the 8th International Congress on the Chemistry of Cement, Rio de Janeiro, Brazil, 22–27 September 1986; Volume IV, pp. 263–269. [Google Scholar]
- Andersson, K.; Allard, B.; Bengtsson, M.; Magnusson, B. Chemical composition of cement pore solutions. Cem. Concr. Res. 1989, 19, 327–332. [Google Scholar] [CrossRef]
- Neubauer, C.M.; Yang, M.; Jennings, H.M. Interparticle potential and sedimentation behavior of cement suspensions: Effects of admixtures. Adv. Cem. Based Mater. 1998, 8, 17–27. [Google Scholar] [CrossRef]
- Lowke, D.; Gehlen, C. The zeta potential of cement and additions in cementitious suspensions with high solid fraction. Cem. Concr. Res. 2017, 95, 195–204. [Google Scholar] [CrossRef]
- Yang, M.; Neubauer, C.M.; Jennings, H.M. Interparticle potential and sedimentation behavior of cement suspensions review and results from paste. Adv. Cem. Based Mater. 1997, 5, 1–7. [Google Scholar] [CrossRef]
- Kobayashi, M.; Adachi, Y.; Ooi, S. Breakup of fractal flocs in a turbulent flow. Langmuir 2002, 15, 4351–4356. [Google Scholar] [CrossRef]
- Sugimoto, T.; Nishiya, M.; Kobayashi, M. Charge reversal of sulfate latex particles in the presence of lanthanum ion. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 18–26. [Google Scholar] [CrossRef]
- Takeshita, C.; Masuda, K.; Kobayashi, M. The effect of monovalent anion species on the aggregation and charging of allophane clay nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 103–109. [Google Scholar] [CrossRef]
- Viallis-Terrisse, H.; Nonat, A.; Petit, J.C. Zeta-potential study of calcium silicate hydrates interacting with alkaline cations. J. Colloid Interface Sci. 2001, 244, 58–65. [Google Scholar] [CrossRef]
- Pointeau, I.; Reiller, P.; Macé, N.; Landesman, C.; Coreau, N. Measurement and modeling of the surface potential evolution of hydrated cement pastes as a function of degradation. J. Colloid Interface Sci. 2006, 300, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Lowke, D.; Gehlen, C. The effect of pore solution composition on zeta potential and superplasticizer adsorption. In Proceedings of the 11th International Conference on Superplasticizers and Other Chemical Admixtures in Concrete, Ottawa, ON, Canada, 10–13 July 2015; pp. 253–264. [Google Scholar]
- Plank, J.; Hirsch, C. Impact of zeta potential of early cement hydration phases on superplasticizer adsorption. Cem. Concr. Res. 2007, 37, 537–542. [Google Scholar] [CrossRef]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Gauckler, L. Adsorption of polyelectrolytes and its influence on the rheology, zeta potential, and microstructure of various cement and hydrate phases. J. Colloid Interface Sci. 2008, 323, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Kaufmann, J.; Winnefeld, F.; Plank, J. Interaction of cement model systems with superplasticizers investigated by atomic force microscopy, zeta potential, and adsorption measurements. J. Colloid Interface Sci. 2010, 347, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Kong, X.; Su, T.; Wang, D. Comparative study of two PCE superplasticizers with varied charge density in Portland cement and sulfoaluminate cement systems. Cem. Concr. Res. 2019, 115, 43–58. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Kimura, J.; Takeuchi, Y.; Uyama, H.; Park, C.; Sung, M.H. Chelation of calcium ions by poly (γ-glutamic acid) from bacillus subtilis. J. Microbiol. Biotechnol. 2010, 20, 1436–1439. [Google Scholar] [CrossRef]
- Ulusoy, U.; Kursun, I. Comparison of different 2D image analysis measurement techniques for the shape of talc particles produced by different media milling. Miner. Eng. 2011, 24, 91–97. [Google Scholar] [CrossRef]
- Nap, R.J.; Szleifer, I. Effect of calcium ions on the interactions between surfaces end-grafted with weak polyelectrolytes. J. Chem. Phys. 2018, 149, 163309. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, I.; Trefalt, G.; Tiraferri, A.; Maroni, P.; Borkovec, M. Polyelectrolyte adsorption, interparticle forces, and colloidal aggregation. Soft Matter 2014, 10, 2479–2502. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Kobayashi, A.; Kobayashi, M. Structure of colloidal flocs in relation to the dynamic properties of unstable suspension. Int. J. Polym. Sci. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Ratnaweera, H.; Fettig, J. State of the art of online monitoring and control of the coagulation process. Water 2015, 7, 6574–6597. [Google Scholar] [CrossRef]
- Hägg, K.; Cimbritz, M.; Persson, K.M. Combining chemical flocculation and disc filtration with managed aquifer recharge. Water 2018, 10, 1854. [Google Scholar] [CrossRef]
- Bu, F.; Gao, B.; Yue, Q.; Liu, C.; Wang, W.; Shen, X. The combination of coagulation and adsorption for controlling ultra-filtration membrane fouling in water treatment. Water 2019, 11, 90. [Google Scholar] [CrossRef]



| Cement Concentration | 0.5, 1, 2, 10, and 100 (g/L) |
|---|---|
| Measurement items | Na+, K+, Ca2+, Cl−, SO42−, pH, EC |
| Items | 100 g/L (w/c = 10) | 10 g/L (w/c = 100) | 2 g/L (w/c = 500) | 1 g/L (w/c = 1000) | 0.5 g/L (w/c = 2000) | |
|---|---|---|---|---|---|---|
| Data of ion chromatography | Na+ | 3.65 | 0.23 | 0.14 | 0.21 | 0.28 |
| K+ | 2.39 | 0.27 | 0.13 | 0.04 | 0.00 | |
| Ca2+ | 21.80 | 6.09 | 4.44 | 2.10 | 1.28 | |
| Cl− | 0.73 | 0.08 | 0.04 | 0.01 | 0.00 | |
| SO42− | 9.47 | 1.52 | 0.90 | 0.34 | 0.12 | |
| Data of pH | pH | 12.51 | 12.23 | 11.66 | 11.46 | 11.27 |
| OH− | 32.36 | 16.98 | 4.57 | 2.88 | 1.86 | |
| Calculations | IC | 82.11 | 24.00 | 13.12 | 6.45 | 3.87 |
| 1 | 1.07 | 1.98 | 2.68 | 3.83 | 4.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanagibashi, T.; Kobayashi, M.; Omori, K. Application of Poly-γ-Glutamic Acid Flocculant to Flocculation–Sedimentation Treatment of Ultrafine Cement Suspension. Water 2019, 11, 1748. https://doi.org/10.3390/w11091748
Yanagibashi T, Kobayashi M, Omori K. Application of Poly-γ-Glutamic Acid Flocculant to Flocculation–Sedimentation Treatment of Ultrafine Cement Suspension. Water. 2019; 11(9):1748. https://doi.org/10.3390/w11091748
Chicago/Turabian StyleYanagibashi, Tomokazu, Motoyoshi Kobayashi, and Keisuke Omori. 2019. "Application of Poly-γ-Glutamic Acid Flocculant to Flocculation–Sedimentation Treatment of Ultrafine Cement Suspension" Water 11, no. 9: 1748. https://doi.org/10.3390/w11091748
APA StyleYanagibashi, T., Kobayashi, M., & Omori, K. (2019). Application of Poly-γ-Glutamic Acid Flocculant to Flocculation–Sedimentation Treatment of Ultrafine Cement Suspension. Water, 11(9), 1748. https://doi.org/10.3390/w11091748





