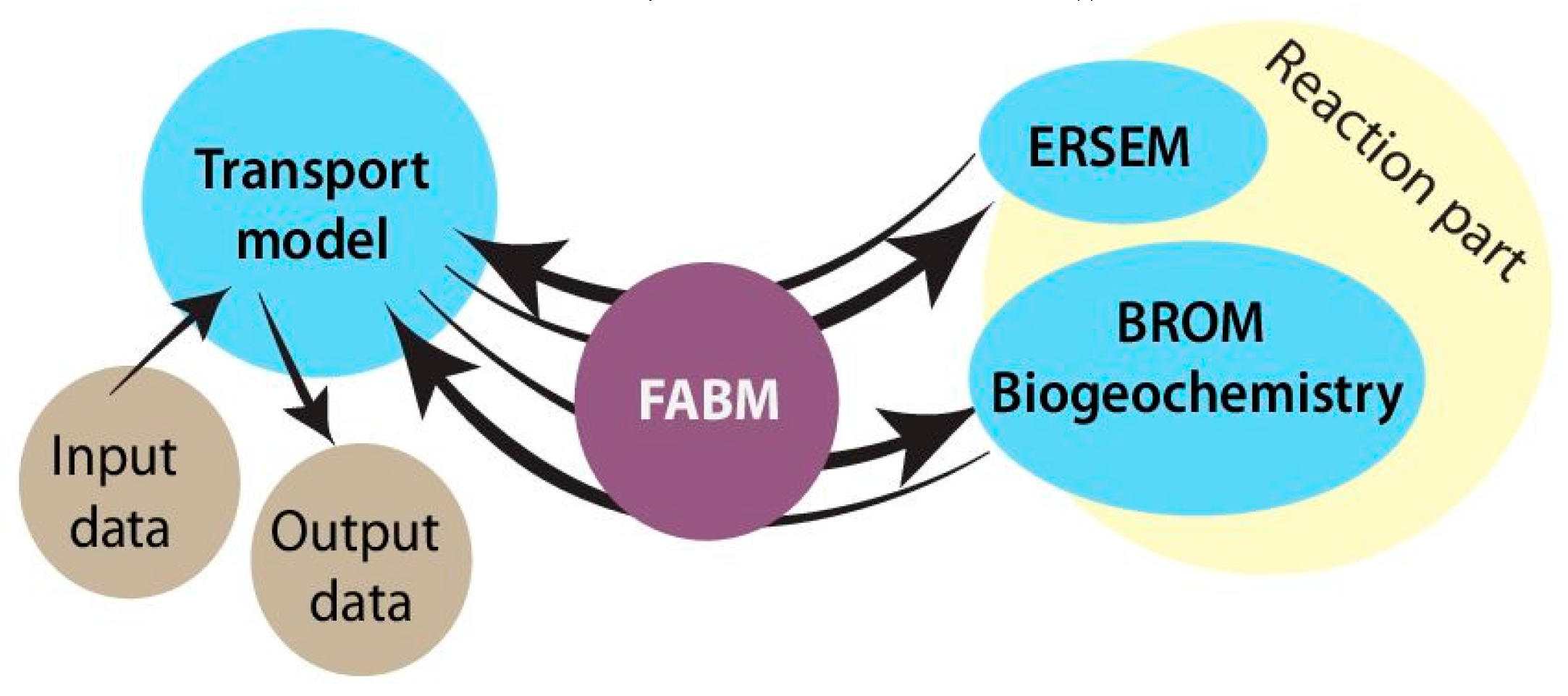
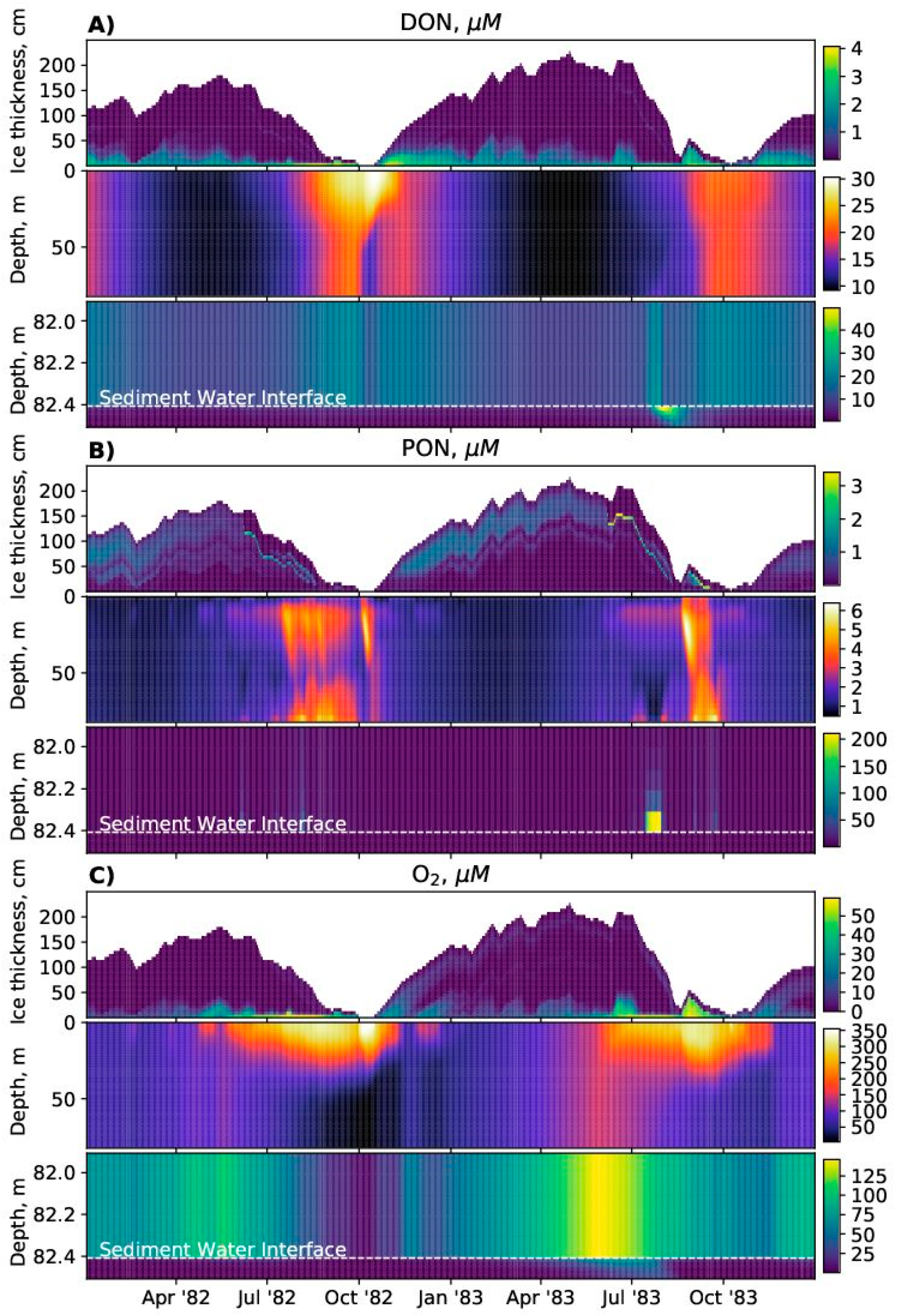
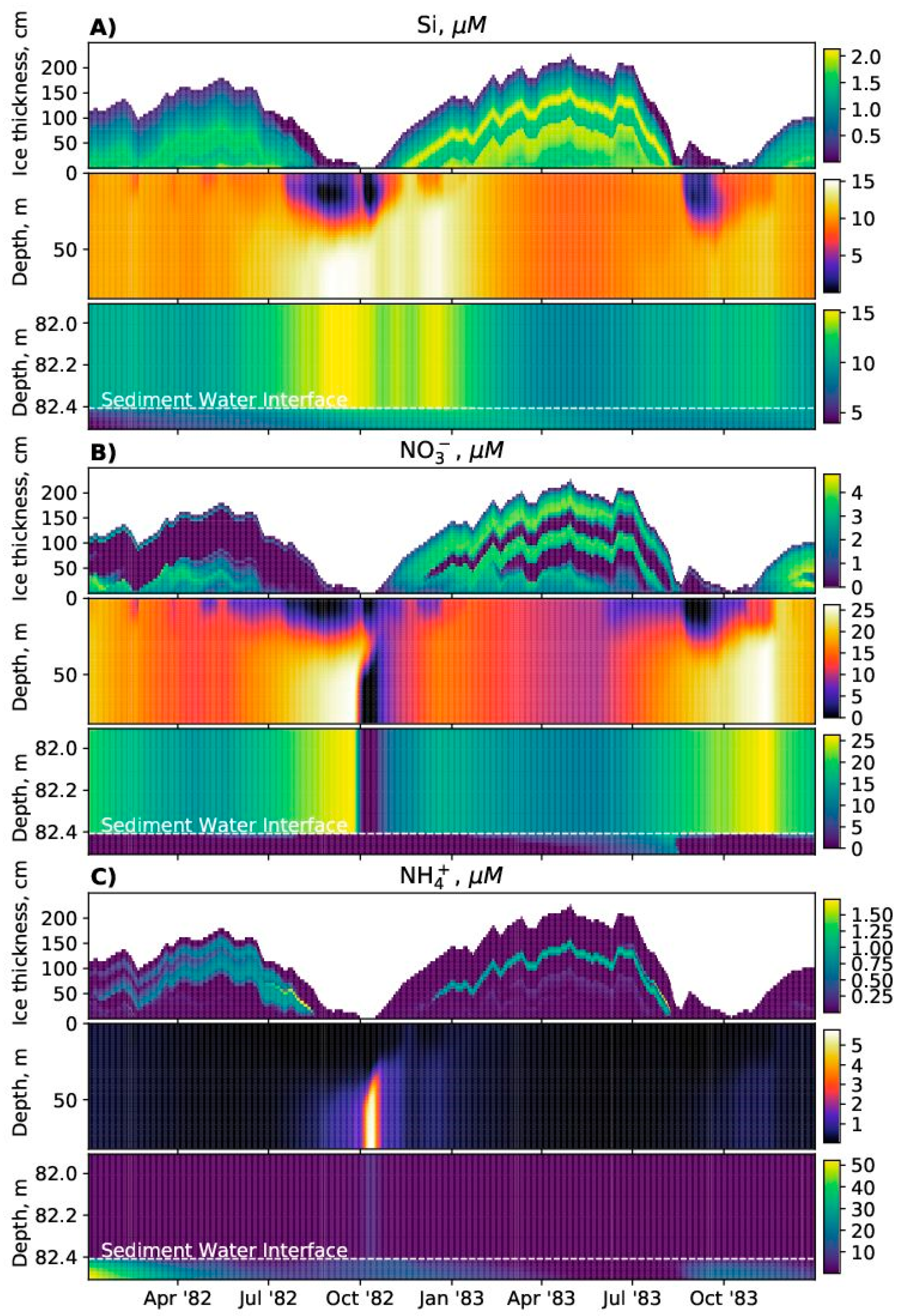
SPBM is a 1D advection–diffusion–reaction solver that uses FABM to define an arbitrary biogeochemical model structure and to calculate reaction terms, sinking speeds within the water domain, and various optional biogeochemical diagnostics. FABM distinguishes three types of model variables: state variables, diagnostic variables, and dependencies. State variables are the basic elements for which the rates of changes must be provided (e.g., nitrate, chlorophyll concentrations). Diagnostic variables are calculated within FABM according to the values of the state variables and dependencies at each time step (e.g., pH, nitrification rate). Dependencies are the physical environment variables and interconnections within FABM (e.g., temperature, salinity). SPBM sends dependencies to FABM and updates the state variables over each time step using various advection/diffusion algorithms and the FABM-calculated reaction terms. SPBM outputs all necessary state and diagnostic variables in NetCDF files. Within SPBM, state variables are considered as solute or particulate concentrations.
2.1. Formulation and Numerical Integration
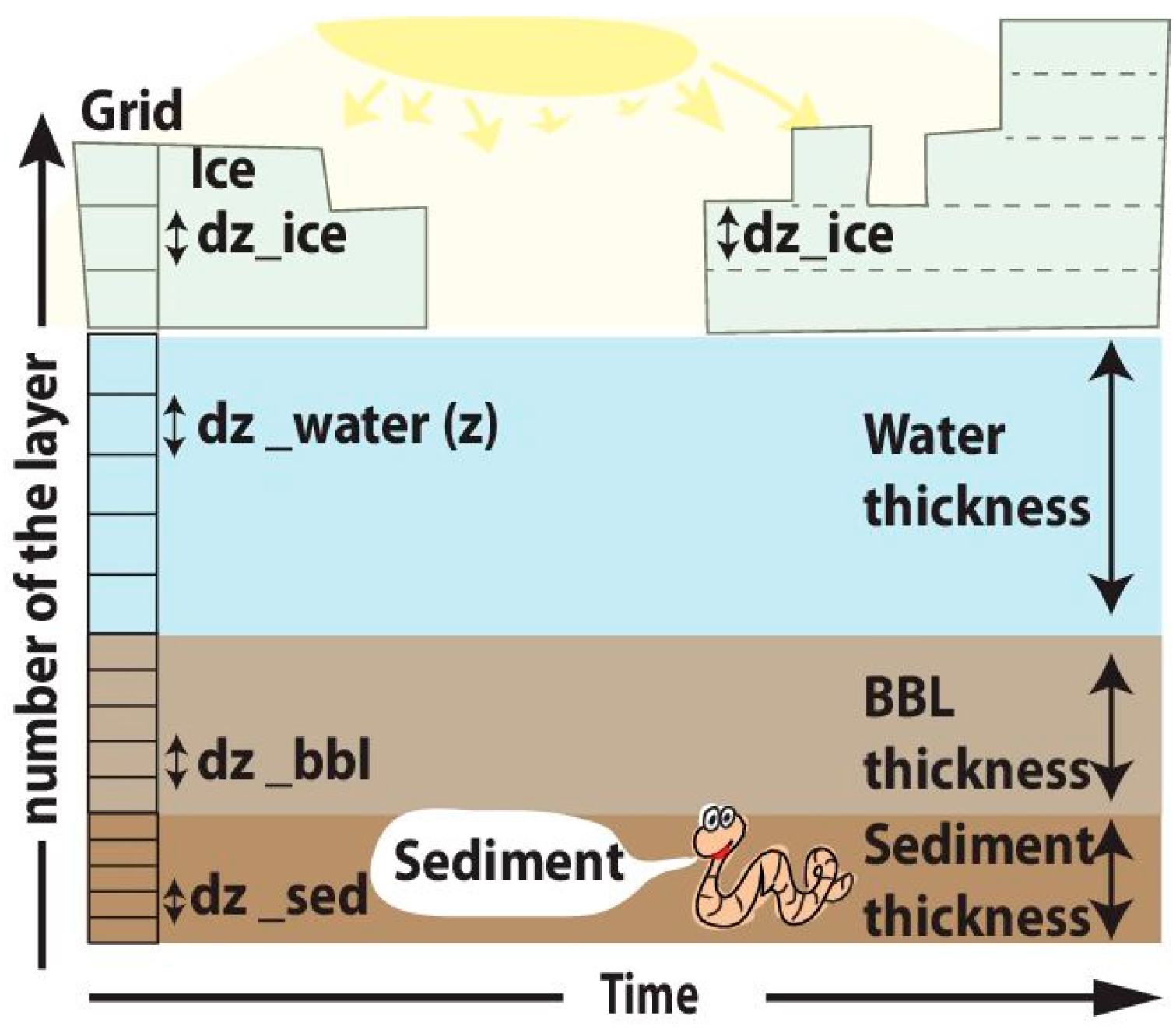
SPBM solves a system of 1-D transport equations in Cartesian coordinates for all three domains (ice, water column, and sediments). The dynamics are
where
is the
i-th state variable in units provided by the biogeochemical model through FABM, (
) or (
) (here total volume refers to a representative control volume including both liquid and solid);
is the time step, (
);
is the depth, (
);
is the porosity-related area restriction factor for fluxes, dimensionless;
is the total diffusivity,
;
is the porosity factor, dimensionless;
is the sinking velocity (advection/burial in the sediments), (
).
is the combined sources minus sinks of the
i-th state variable provided by the biogeochemical model through FABM, (
or (
). The porosity factor
is used to calculate the volume concentration in brine (in the ice column) or in pore water/solid matrix in the sediments. Exchange within the ice and sediment layers occurs through brine channels and through pores or solid matrix, so the area restriction factor
is included to limit fluxes within the respective phases (intraphase mixing). The values of
,
,
and
depend on whether these parameters are calculated in ice, water column, or sediment domains and whether the state variable is solute or particulate.
In the ice domain:
For particulates, it is assumed that the concentration is the same in both the brine channels and ice matrix, hence
. However, vertical fluxes are assumed to be restricted to the brine channels where the particulates are mobilised in suspension, hence
. Here, the dimensionless porosity
is equal to the relative volume of the brine channels in the ice [
26], which can be obtained from an ice thermodynamic model or using empirical relationships (see
Appendix A). Solutes are assumed to be excluded from the ice matrix, hence
, and fluxes are again restricted to the brine channels, hence
. The total diffusivity
in the ice brine channels is a sum of the molecular diffusivity
(
) on the ice–water interface (applied only to solutes), the gravity drainage diffusivity
(
at depths
within the ice, and the diffusivity caused by convection that occurs in the bottom layer of the growing ice
(
[
26]:
where
means that the value of the parameter is determined only on the interface between the bottom (skeletal) layer of ice and surface water layer;
is a constant mean flux volume rate from the brine channels, (
);
is the vertical distance over which the ice column is influenced by brine tube convection (depths where
), (
);
is the total ice growth rate (
);
is the thickness of the ice layer, (
).
is not equal to zero only during the period of ice build-up and only on the interface between water and ice. Alternatively, the total diffusivity
can be read from an input file generated by e.g., an ice thermodynamic model.
The sinking velocity
is non-zero only for particulate variables in the layers where
(if
sea ice brine pockets are not interconnected) and is generally determined at each time step by the biogeochemical model through FABM. For all diatoms living in the ice column, to represent their ability to maintain their vertical position relative to the skeletal layer [
26],
is set to a constant but possibly layer-dependent value within the ice column and zero on the ice–water interface between ice and water domains, while the total diffusivity
is set to zero.
In the water column domain:
Here and at all depths for both solutes and particulates, since there is only one phase to consider.
The total diffusivity
is composed of the molecular diffusivity
(
(applied only to solutes) and the turbulence diffusivity
(
:
where
is taken from the hydrophysical model as input data. The water column domain contains the structure that could be called the BBL. It is located in the lower part of the water column. Turbulent diffusivity for each layer
within the BBL is linearly decreasing from the deepest non-zero value of the diffusivity
as follows:
where
(
) is the deepest depth with non-zero value of
and
(
) is the bottom depth.
The sinking velocity is taken from the biogeochemical model through FABM for all particulates and is zero for all solutes.
In the sediment domain:
Within the sediments, particulate variables are confined to the sediment matrix and solutes are confined to the pore water. So, for solid particulates the porosity factor and the area restriction factor at depths . For solutes and . There is no adsorption in the present version.
A time-independent porosity
at depths
through the entire sediment domain is described following [
27]:
where
is the deep porosity, dimensionless;
is the porosity at the sediment–water interface (SWI), dimensionless;
is the depth of the SWI, (
);
is the coefficient for exponential porosity change, (
).
The total diffusivity
is a sum of the molecular diffusivity
(
(applied only to solutes) and the bioturbation diffusivity
(
[
28]:
where
is the infinite-dilution molecular diffusivity, (
is the relative dynamic viscosity, dimensionless;
is the oxygen concentration in the bottom layer of the water column, (
);
is the half-saturation constant, (
). The oxygen-saturated bioturbation diffusivity [
22]
(
depends on the distance
(
) between the interface depth
and the depth with a constant bioturbation activity as follows:
where
is the depth at the SWI, (
);
is the constant bioturbation activity layer thickness, (
);
is the maximum bioturbation diffusivity, (
;
is the bioturbation decay scale, (
).
On the SWI it is assumed that the bioturbation diffusivity mixes concentrations in units (
) instead of (
) (interphase mixing). Therefore, special values of
are needed for the layers immediately above and below the SWI (see
Appendix B):
where the subscripts
,
and
determine a location of the corresponding variables:
means the layer above,
the layer below,
on the SWI.
The advection/burial velocities
are described following [
22]:
where
is the deep burial velocity, (
);
is the interphase component of the total bioturbation diffusivity
, and
is nonzero only on the SWI where
(
). Note that although a non-zero
beyond the SWI would alter the computed advection/burial velocities
via Equations (2) and (3), the net transport of biogeochemical tracers would not be affected because corresponding interphase components
and
would need to be added to the diffusive fluxes in Equation (1), and these will exactly cancel the contributions of
to advection/burial. In other words, when the porosity profile is specified and used to compute advection/burial velocities under steady state compaction, the tracer advection/diffusion depends only on the total bioturbation diffusivity, and the intraphase form assumed by SPBM for diffusion inside the sediments is correct irrespective of the relative contribution of inter- vs. intraphase mixing, see [
29]. However, there can be no intraphase component of a Fickian particulate diffusion across the SWI because by definition there is no solid matrix above the SWI [
22].
Equation 1 is integrated numerically over a single combined (ice, water column, sediments) grid, using a constant model time step. The coupling method follows an operator splitting approach [
30]: concentrations are successively updated by contributions over one time step of diffusion, reaction, and sinking/advection/burial, in that order. Diffusive updates are calculated by a semi-implicit central-space algorithm adapted from a routine in BROM-transport [
22] which in turn was adapted from the general ocean turbulence model (GOTM) [
31]. Sinking/advection/burial updates are calculated using a first-order upwind differencing scheme. Reaction updates are calculated from forward Euler time steps.
2.3. Irradiance Formulation
FABM biogeochemical models generally need to know the photosynthetically active radiation (PAR), e.g., (), in each layer of the model grid. Some FABM models compute water column PAR given only surface PAR, but they do not assume the existence of the ice column and consider all grid points to be located within the water column. SPBM therefore provides the following simple approach to calculate PAR in both ice and water column domains.
PAR on the surface of water or ice
can be calculated from the surface shortwave radiative flux
(
), depending on the solar declination
(
):
where
is the theoretical maximum of 24-h average surface downwelling shortwave irradiance in air, (
);
is the factor to convert downwelling shortwave irradiance in air to scalar PAR in water, (
) [
32]. Alternatively,
(or
) can be read from an input file.
In the presence of ice, PAR after considering albedo influence
becomes [
33]:
where
is the fraction of radiation transmitted through the highly scattering surface of the ice, dimensionless;
is the ice albedo for visible light, dimensionless;
is the snow albedo for visible light, dimensionless;
is the snow light extinction coefficient, (
);
is the snow depth, (
).
PAR at any depth in the ice
is given by:
where
is the ice light extinction coefficient, (
);
is the ice depth, (
).
PAR in the water column
is calculated according to the Beer–Lambert formulation:
where
is the PAR at the ice bottom layer;
is the vertically varying water light extinction coefficient provided by the FABM models, describing attenuation due to living and non-living optically-active substances, (
);
is the water layer depth, (
).
In the sediment domain, PAR equals zero in all layers.