Growth and Nutrient Removal Efficiency of Sweet Wormwood (Artemisia annua) in a Recirculating Aquaculture System for Nile Tilapia (Oreochromis niloticus)
Abstract
1. Introduction
2. Materials and Methods
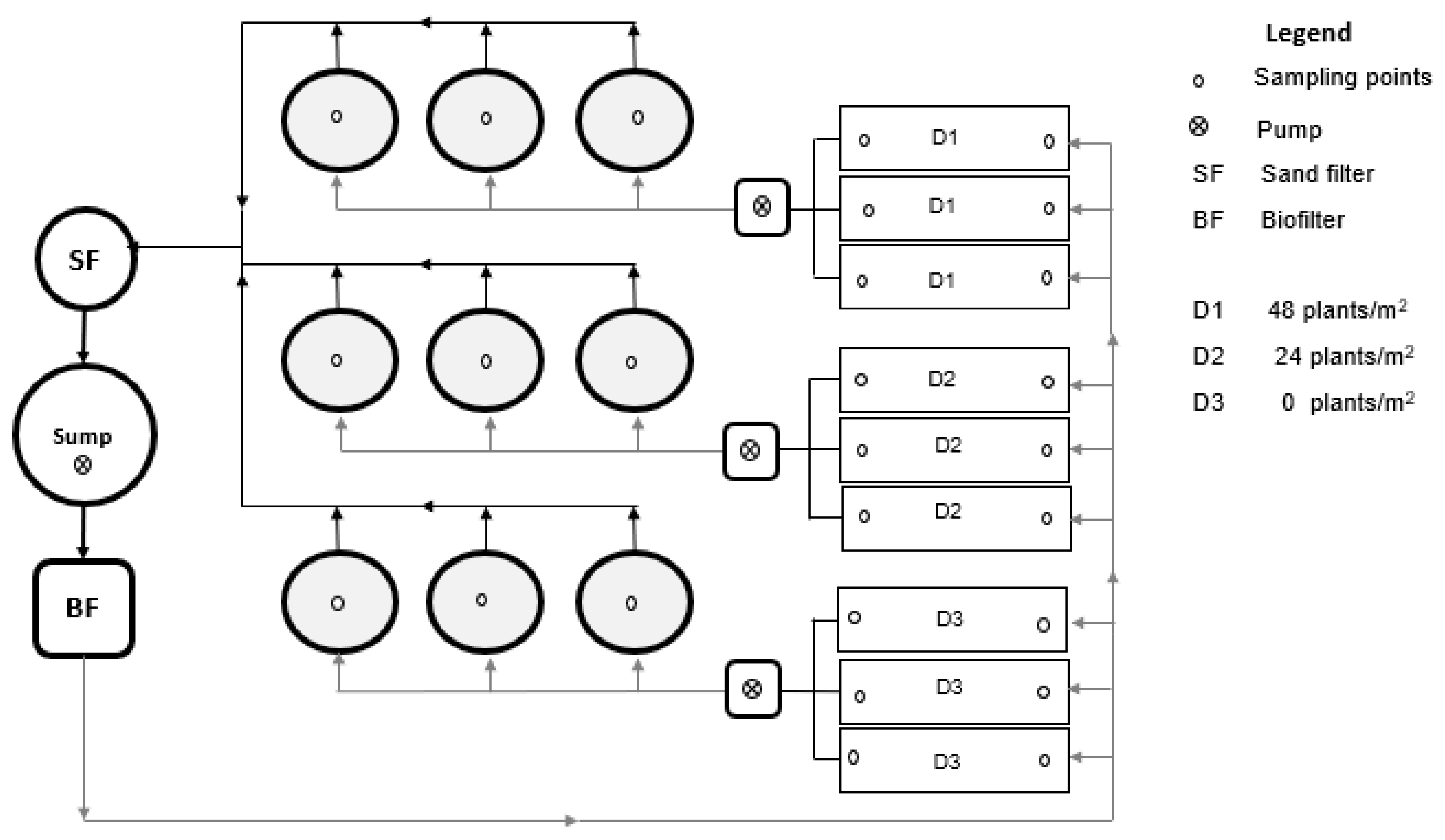
2.1. Experimental Design
2.2. Sampling and Analysis
2.3. Data Analysis
3. Results
3.1. Water Quality Parameters
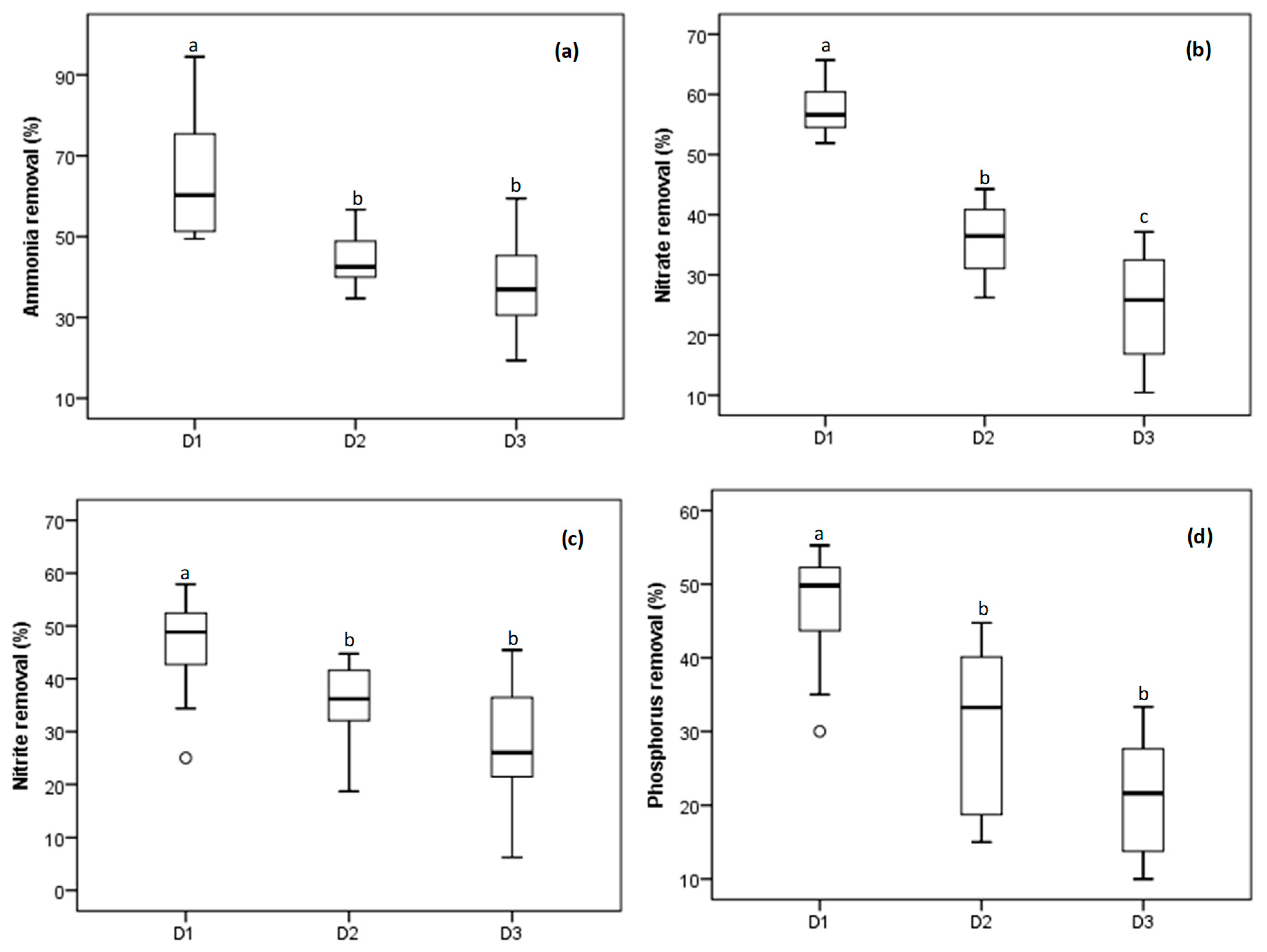
3.1.1. Nutrient Removal
3.1.2. Plant and Fish Growth
4. Discussion
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- FAO. The State of the World Fisheries and Aquaculture 2018. Meeting the Sustainable Development Goals; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; p. 194. [Google Scholar]
- Waite, R.; Beveridge, M.; Brummett, R.; Castine, S.; Chaiyawannakarn, N.; Kaushik, S.; Mungkung, R.; Nawapakpilai, S.; Phillips, M. Improving productivity and environmental performance of aquaculture. Create. Sustain. Food Future 2014, 1–60. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All; Food and Agricultural Organization of the United Nations: Rome, Italy, 2016; p. 204. [Google Scholar]
- Verdegem, M.C.J.; Bosma, R.H.; Verreth, J.A.J. Reducing Water Use for Animal Production through Aquaculture. Int. J. Water Resour. Dev. 2006, 22, 101–103. [Google Scholar] [CrossRef]
- Opiyo, M.A.; Marijanib, E.; Muendoc, P.; Odede, R.; Leschen, W.; Charo-Karisa, H. A review of aquaculture production and health management practices of farmed fish in Kenya. Int. J. Vet. Sci. Med. 2018, 6, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Funge-Smith, S.; Philips, M.J. Aquaculture systems and species. In Proceedings of the Aquaculture in the Third Millennium; NACA: Bangkok, Thailand; FAO: Rome, Italy, 2001; pp. 129–135. [Google Scholar]
- Trang, N.; Schierup, H.-H.; Brix, H. Leaf vegetables for use in integrated hydroponics and aquaculture systems: Effects of root flooding on growth, mineral composition and nutrient uptake. Afr. J. Biotechnol. 2010, 9, 4186–4196. [Google Scholar]
- Okomoda, V.T.; Tiamiyu, L.O.; Iortim, M. The effect of water renewal on growth of Clarias gariepinus fingerlings. Croat. J. Fish. 2016, 74, 25–29. [Google Scholar] [CrossRef]
- Trang, N.T.D.; Brix, H. Use of planted biofilters in integrated recirculating aquaculture-hydroponics systems in the Mekong Delta, Vietnam. Aquac. Res. 2014, 45, 460–469. [Google Scholar] [CrossRef]
- Silva, L.; Valdés-Lozano, D.; Escalante, E.; Gasca-Leyva, E. Dynamic root floating technique: An option to reduce electric power consumption in aquaponic systems. J. Clean. Prod. 2018, 183, 132–142. [Google Scholar] [CrossRef]
- Badiola, M.; Mendiola, D.; Bostock, J. Recirculating Aquaculture Systems (RAS) Analysis: Main Issues on Management and Future Challenges. Aquac. Eng. 2012, 51, 21–35. [Google Scholar]
- Piedrahita, R.H. Reducing the potential environmental impact of tank aquaculture effluents through intensification and recirculation. Aquaculture 2003, 226, 35–44. [Google Scholar] [CrossRef]
- Van Rijn, J.; Tal, Y.; Schreier, H.J. Denitrification in recirculating systems: Theory and applications. Aquac. Eng. 2006, 34, 364–376. [Google Scholar] [CrossRef]
- Boxman, S.E.; Nystrom, M.; Capodice, J.C.; Main, K.L.; Trotz, M.A. Effect of support medium, hydraulic loading rate and plant density on water quality and growth of halophytes in marine aquaponic systems. Aquac. Res. 2017, 48, 2463–2477. [Google Scholar] [CrossRef]
- Eck, M.; Sare, A.R.; Massart, S.; Schmautz, Z.; Junge, R.; Smits, T.H.M.; Jijakli, M.H. Exploring Bacterial Communities in Aquaponic Systems. Water 2019, 11, 260. [Google Scholar] [CrossRef]
- Bartelme, R.P.; Oyserman, B.O.; Sepulveda-Villet, O.J.; Newton, R.J. Stripping Away the Soil: Plant Growth Promoting Microbiology Opportunities in Aquaponics. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulou, P.; Berillis, P.; Levizou, E.; Sakellariou-Makrantonaki, M.; Kormas, A.K.; Aggelaki, A.; Kapsis, P.; Vlahos, N.; Mente, E. Aquaponics: A mutually beneficial relationship of fish, plants and bacteria. In Proceedings of the 3rd International Congress on Applied Ichthyology & Aquatic Environment, Volos, Greece, 8–11 November 2018. [Google Scholar]
- Goddek, S.; Espinal, C.A.; Delaide, B.; Jijakli, M.H.; Schmautz, Z.; Wuertz, S.; Keesman, K.J. Navigating towards decoupled aquaponic systems: A system dynamics design approach. Water 2016, 8, 303. [Google Scholar] [CrossRef]
- Gichana, Z.M.; Liti, D.; Waidbacher, H.; Zollitsch, W.; Drexler, S.; Waikibia, J. Waste management in recirculating aquaculture system through bacteria dissimilation and plant assimilation. Aquac. Int. 2018, 26, 1541–1572. [Google Scholar] [CrossRef]
- FAO. FAO Technical Workshop on Advancing Aquaponics: An Efficient Use of Limited Resources. Saint John’s, Antigua and Barbuda, 14–18 August 2017; FAO Fisheries and Aquaculture Report. No. 1214; FAO Subregional Office for the Caribbean: Bridgetown, Barbados, 2017. [Google Scholar]
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.V.; Jijakli, H.; Thorarinsdottir, R. Challenges of sustainable and commercial aquaponics. Sustainability 2015, 7, 4199–4224. [Google Scholar] [CrossRef]
- Endut, A.; Jusoh, A.; Ali, N.; Wan Nik, W.B.; Hassan, A. A study on the optimal hydraulic loading rate and plant ratios in recirculation aquaponic system. Bioresour. Technol. 2010, 101, 1511–1517. [Google Scholar] [CrossRef]
- Rakocy, J.E. Integrating fish and plant culture. In Aquaculture Production Systems; John Wiley & Sons, Inc.: Oxford, UK, 2012. [Google Scholar]
- Lam, S.S.; Ma, N.L.; Jusoh, A.; Ambak, M.A. Biological nutrient removal by recirculating aquaponic system: Optimization of the dimension ratio between the hydroponic & rearing tank components. Int. Biodeterior. Biodegrad. 2015, 102, 107–115. [Google Scholar]
- Maboko, M.; Du Plooy, C. Effects of plant spacing and harvesting frequency on the yield of Swiss chard cultivars (Beta vulgaris L.) in a closed hydroponic system. Afr. J. Agric. Res. 2013, 8, 936–942. [Google Scholar]
- Søberg, E.-E. The Growth and Development of Lettuce, Coriander and Swiss Chard in a Cold Water Aquaponic System Optimized for Lettuce Production. Master’s Thesis, Norwegian University of Life Sciences, Oslo, Norway, 2016. [Google Scholar]
- Pulice, G.; Pelaz, S.; Matías-Hernández, L. Molecular Farming in Artemisia annua, a Promising Approach to Improve Anti-malarial Drug Production. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.F.S. Nutrient Deficiency in the Production of Artemisinin, Dihydroartemisinic Acid, and Artemisinic Acid in Artemisia annua L. J. Agric. Food Chem. 2007, 55, 1686–1694. [Google Scholar] [CrossRef]
- WHO. WHO Monograph on Good Agricultural and Collection Practices (GACP) for Artemisia annua L.; World Health Organization: Geneva, Switzerland, 2006; p. 49. [Google Scholar]
- Namuli, A.; Bazira, J.; Casim, T.U.; Engeu, P.O. A review of various efforts to increase artemisinin and other antimalarial compounds in Artemisia Annua L plant. Cog. Biol. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Delong, D.P.; Losordo, T.M. How to start a Biofilter. SRAC Publ. South. Reg. Aquac. Cent. 2012, 3, 1–4. [Google Scholar]
- Bruno, R.; Chen, W.; Cottle, P.; Lai, V.; Loc, H.; Delson, N. Aquaponics Ebb and Flow Mechanisms ECOLIFE Foundation. MAE 156B: Fundamental Principles of Mechanical Design II; University of California: Oakland, CA, USA, 2011; p. 85. [Google Scholar]
- American Public Health Association (APHA). Standard Method for the Examination of Water and Wastewater; Water Pollution Control Federation: Baltimore, MD, USA, 2008. [Google Scholar]
- Tylova-Munzarova, E.; Lorenzen, B.; Brix, H.; Votrubova, O. The effects of NH4+ and NO3− on growth, resource allocation and nitrogen uptake kinetics of Phragmites australis and Glyceria maxima. Aquat. Bot. 2005, 81, 326–342. [Google Scholar] [CrossRef]
- Ngo Thuy Diem, T.; Konnerup, D.; Brix, H. Effects of recirculation rates on water quality and Oreochromis niloticus growth in aquaponic systems. Aquac. Eng. 2017, 78, 95–104. [Google Scholar] [CrossRef]
- Hargreaves, J.A.; Tucker, C.S. Managing ammonia in fish pond. SRAC Publ. South. Reg. Aquac. Cent. 2004, 8. Available online: http://fisheries.tamu.edu/files/2013/09/SRAC-Publication-No.-4603-Managing-Ammonia-in-Fish-Ponds.pdf (accessed on 2 March 2019).
- Ross, L. Environmental physiology and energetics. In Tilapias: Biology and exploitation; Springer: Dordrecht, The Netherlands, 2000; pp. 89–128. [Google Scholar]
- Delong, D.P.; Losordo, T.M.; Rakocy, J.E. Tank Culture of Tilapia; Southern Regional Aquaculture Center: toneville, MS, USA, 2009. [Google Scholar]
- Knaus, U.; Palm, H.W. Effects of the fish species choice on vegetables in aquaponics under spring-summer conditions in northern Germany (Mecklenburg Western Pomerania). Aquaculture 2017, 473, 62–73. [Google Scholar] [CrossRef]
- Pinho, S.M.; de Mello, G.L.; Fitzsimmons, K.M.; Emerenciano, M.G.C. Integrated production of fish (pacu Piaractus mesopotamicus and red tilapia Oreochromis sp.) with two varieties of garnish (scallion and parsley) in aquaponics system. Aquac. Int. 2018, 26, 99–112. [Google Scholar] [CrossRef]
- Wongkiew, S.; Hu, Z.; Chandran, K.; Lee, J.W.; Khanal, S.K. Nitrogen transformations in aquaponic systems: A review. Aquac. Eng 2017, 76, 9–19. [Google Scholar] [CrossRef]
- Espinosa-Moya, A.; Álvarez-gonzález, A.; Albertos-alpuche, P. Growth and development of herbaceous plants in aquaponic systems. Acta Univ. Multidisp. Sci. J. 2018, 28, 1–8. [Google Scholar]
- Masser, M.P.; Rakocy, J.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems Management of Recirculating Systems. South. Reg. Aquac. Cent. 1992, 1–12. Available online: https://www.academia.edu/26860809/Southern_Regional_Aquaculture_Center_Recirculating_Aquaculture_Tank_Production_Systems_Management_of_Recirculating_Systems. (accessed on 22 January 2019).
- Pinho, S.M.; Molinari, D.; de Mello, G.L.; Fitzsimmons, K.M.; Coelho Emerenciano, M.G. Effluent from a biofloc technology (BFT) tilapia culture on the aquaponics production of different lettuce varieties. Ecol. Eng. 2017, 103, 146–153. [Google Scholar] [CrossRef]
- Estim, A.; Saufie, S.; Mustafa, S. Water quality remediation using aquaponics sub-systems as biological and mechanical filters in aquaculture. J. Water Process. Eng. 2018. [CrossRef]
- Buzby, K.M.; Lin, L.S. Scaling aquaponic systems: Balancing plant uptake with fish output. Aquac. Eng. 2014, 63, 39–44. [Google Scholar] [CrossRef]
- Jones, C.; Olson-rutz, K.; Dinkins, C. Nutrient Uptake Timing by Crops, to Assist with Fertilizing Decisions; Montana State University: Bozeman, MT, USA, 2015; p. 8. [Google Scholar]
- Endut, A.; Lananan, F.; Jusoh, A.; Cik, W.N.W.; Nora’aini, A. Aquaponics Recirculation System: A Sustainable Food Source for the Future Water Conserves and Resources. Malays. J. Appl. Sci. 2016, 1, 1–12. [Google Scholar]
- Timmons, M.B.; Ebeling, J.M.; Wheaton, F.W.; Summerfelt, S.T.; Vinci, B.J. Recirculating Aquaculture Systems, 2nd ed.; Cayuga Aqua Ventures Llc: New York, NY, USA, 2002. [Google Scholar]
- Webb, J.M.; Quintã, R.; Papadimitriou, S.; Rigby, N.L.; Norman, M.; Thomas, D.N.; Le Vay, L. The effect of halophyte planting density on the efficiency of constructed wetlands for the treatment of wastewater from marine aquaculture. Ecol. Eng. 2013, 61, 145–153. [Google Scholar] [CrossRef]
- Thorarinsdottir, R.I. Aquaponics Guidelines. In Lifelong learning Programme; European Commission: Reykjavik, Iceland, 2015; p. 69. [Google Scholar]
- Cerozi, B.S.; Fitzsimmons, K. Phosphorus dynamics modeling and mass balance in an aquaponics system. Agric. Syst. 2017, 153, 94–100. [Google Scholar] [CrossRef]
- Snow, A.M.; Ghaly, A.E. A Comparative Study of the Purification of Aquaculture Wastewater Using Water Hyacinth, Water Lettuce and Parrot’s Feather. Am. J. Appl. Sci. 2008, 5, 440–453. [Google Scholar]
- Endut, A.; Jusoh, A.; Ali, N.; Wan Nik, W.B. Nutrient removal from aquaculture wastewater by vegetable production in aquaponics recirculation system. Desalin. Water Treat. 2011, 32, 422–430. [Google Scholar] [CrossRef]
- Silva, L.; Escalante, E.; Valdés-Lozano, D.; Hernández, M.; Gasca-Leyva, E. Evaluation of a semi-intensive aquaponics system, with and without bacterial biofilter in a tropical location. Sustainability 2017, 9, 592. [Google Scholar] [CrossRef]
- Stickney, R.R. Aquaculture: an introduction text; CABI Publication: Cambridge, MA, USA, 2005. [Google Scholar]
- El-Sherif, M.S.; El-Feky, A.M. Effect of Ammonia on Nile Tilapia (O. niloticus) Performance and some Hematological and Histological Measures. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008; pp. 513–530. [Google Scholar]
- Davidson, J.; Good, C.; Welsh, C.; Brazil, B.; Summerfelt, S. Heavy metal and waste metabolite accumulation and their potential effect on rainbow trout performance in a replicated water reuse system operated at low or high flushing rates. Aquac. Eng. 2009, 41, 136–145. [Google Scholar] [CrossRef]
- Colt, J. Water quality requirements for reuse systems. Aquac. Eng. 2006, 34, 143–156. [Google Scholar] [CrossRef]
- Martins, C.I.M.; Ochola, D.; Ende, S.S.W.; Eding, E.H.; Verreth, J.A.J. Is growth retardation present in Nile tilapia Oreochromis niloticus cultured in low water exchange recirculating aquaculture systems? Aquaculture 2009, 298, 43–50. [Google Scholar] [CrossRef]
- Mota, V.C.; Limbua, P.; Martins, C.I.M.; Eding, E.; Verreth, A.J. The effect of nearly closed RAS on the feed intake and growth of Nile tilapia (Oreochromis niloticus), African catfish (Clarias gariepinus) and European eel (Anguilla anguilla). Aquac. Eng. 2015, 68, 1–5. [Google Scholar] [CrossRef]
- Bakiu, R.; Shehu, J. Aquaponic systems as excellent agricultural research instruments in Abania. Albanian J. Agric. Sci 2014, 2014. [Google Scholar]
- Kim, W.S.; Choi, W.J.; Lee, S.; Kim, W.J.; Lee, D.C.; Sohn, U.D.; Shin, H.S.; Kim, W. Anti-inflammatory, Antioxidant and Antimicrobial Effects of Artemisinin Extracts from Artemisia annua L. Korean J. Physiol. Pharmacol. 2015, 19, 21–27. [Google Scholar] [CrossRef] [PubMed]

| Treatments | |||||
|---|---|---|---|---|---|
| Parameters | Units | D1 | D2 | D3 | p |
| Temperature | (°C) | 23.34 ± 0.53 | 23.02 ± 0.4 | 23.13 ± 0.44 | 0.24 |
| pH | 7.93 ± 0.14 | 7.97 ± 0.17 | 7.97 ± 0.17 | 0.77 | |
| Dissolved oxygen | (mg/L) | 2.85 ± 0.77 | 3.29 ± 0.82 | 3.24 ± 0.74 | 0.33 |
| Conductivity | (µS/cm) | 1388.6 ± 38.1 | 1385.1 ± 40.6 | 1384.1 ± 43.1 | 0.96 |
| Ammonia | (mg/L) | 1.89 ± 0.8 | 2.16 ± 0.87 | 2.4 ± 1.00 | 0.39 |
| Nitrates | (mg/L) | 2.65 ± 0.44 | 2.74 ± 0.47 | 2.73 ± 0.33 | 0.88 |
| Nitrites | (mg/L) | 0.58 ± 0.21 | 0.55 ± 0.27 | 0.6 ± 0.30 | 0.88 |
| Phosphorus | (mg/L) | 2.88 ± 0.78 | 2.82 ± 0.52 | 2.65 ± 0.46 | 0.63 |
| Parameter | Units | D1 | D2 | D3 | p |
|---|---|---|---|---|---|
| Temperature | (°C) | 25.21 ± 0.53 | 25.53 ± 0.53 | 25.39 ± 0.41 | 0.3 |
| pH | 7.89 ± 0.13 | 7.82 ± 0.32 | 7.92 ± 0.08 | 0.44 | |
| Dissolved oxygen | (mg/L) | 2.66 ± 0.35 | 2.32 ± 0.45 | 2.35 ± 0.36 | 0.07 |
| Electrical conductivity | (µS/cm) | 1425.1 ± 15.8 | 1432.7 ± 19.2 | 1430.0 ± 19.6 | 0.59 |
| Parameters | Units | D1 | D2 |
|---|---|---|---|
| Initial height | (cm) | 4.75 ± 0.43 a | 5.37 ± 1.87 a |
| Final height | (cm) | 52.42 ± 2.89 a | 55. 78 ± 4.57 a |
| Final weight (fresh weight) | (g) | 423.3 ± 25.2 a | 223.3 ± 25.2 b |
| Weight gain (fresh weight) | (g) | 390.7 ± 26.8 a | 209 ± 24.3 b |
| Yield (fresh weight) | (kg/m2) | 0.56 ± 0.03 a | 0.3 ± 0.03 b |
| Relative growth rate | (g/d) | 0.05 ± 0.003 a | 0.05 ± 0.001 a |
| Parameters | Units | D1 | D2 | D3 |
|---|---|---|---|---|
| Weight gain | (g) | 21.6 ± 1.57 a | 19.2 ± 2.38 a | 12.9 ± 3.15 b |
| Specific growth rate | (%) | 0.35 ± 0.03 a | 0.32 ± 0.02 a | 0.22 ± 0.04 b |
| Feed conversion ratio | 1.9 ± 0.20 a | 2.0 ± 0.25 a | 2.3 ± 0.20 a | |
| Survival rate | (%) | 96.7 ± 3.06 a | 98.0 ± 3.46 a | 95.3 ± 6.43 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gichana, Z.; Meulenbroek, P.; Ogello, E.; Drexler, S.; Zollitsch, W.; Liti, D.; Akoll, P.; Waidbacher, H. Growth and Nutrient Removal Efficiency of Sweet Wormwood (Artemisia annua) in a Recirculating Aquaculture System for Nile Tilapia (Oreochromis niloticus). Water 2019, 11, 923. https://doi.org/10.3390/w11050923
Gichana Z, Meulenbroek P, Ogello E, Drexler S, Zollitsch W, Liti D, Akoll P, Waidbacher H. Growth and Nutrient Removal Efficiency of Sweet Wormwood (Artemisia annua) in a Recirculating Aquaculture System for Nile Tilapia (Oreochromis niloticus). Water. 2019; 11(5):923. https://doi.org/10.3390/w11050923
Chicago/Turabian StyleGichana, Zipporah, Paul Meulenbroek, Erick Ogello, Silke Drexler, Werner Zollitsch, David Liti, Peter Akoll, and Herwig Waidbacher. 2019. "Growth and Nutrient Removal Efficiency of Sweet Wormwood (Artemisia annua) in a Recirculating Aquaculture System for Nile Tilapia (Oreochromis niloticus)" Water 11, no. 5: 923. https://doi.org/10.3390/w11050923
APA StyleGichana, Z., Meulenbroek, P., Ogello, E., Drexler, S., Zollitsch, W., Liti, D., Akoll, P., & Waidbacher, H. (2019). Growth and Nutrient Removal Efficiency of Sweet Wormwood (Artemisia annua) in a Recirculating Aquaculture System for Nile Tilapia (Oreochromis niloticus). Water, 11(5), 923. https://doi.org/10.3390/w11050923





