Ordered Mesoporous Carbons for Adsorption of Paracetamol and Non-Steroidal Anti-Inflammatory Drugs: Ibuprofen and Naproxen from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Adsorbents
2.2.1. Synthesis Procedure
2.2.2. Activation of Mesoporous Carbon
2.3. Characterization of the Adsorbents
2.4. Adsorption Studies from Aqueous Solutions
3. Results and Discussion
3.1. Computational Calculation
Computational Methodology
Calculations
3.2. Characterization of Adsorbents
Functional Groups on the Adsorbents Surface
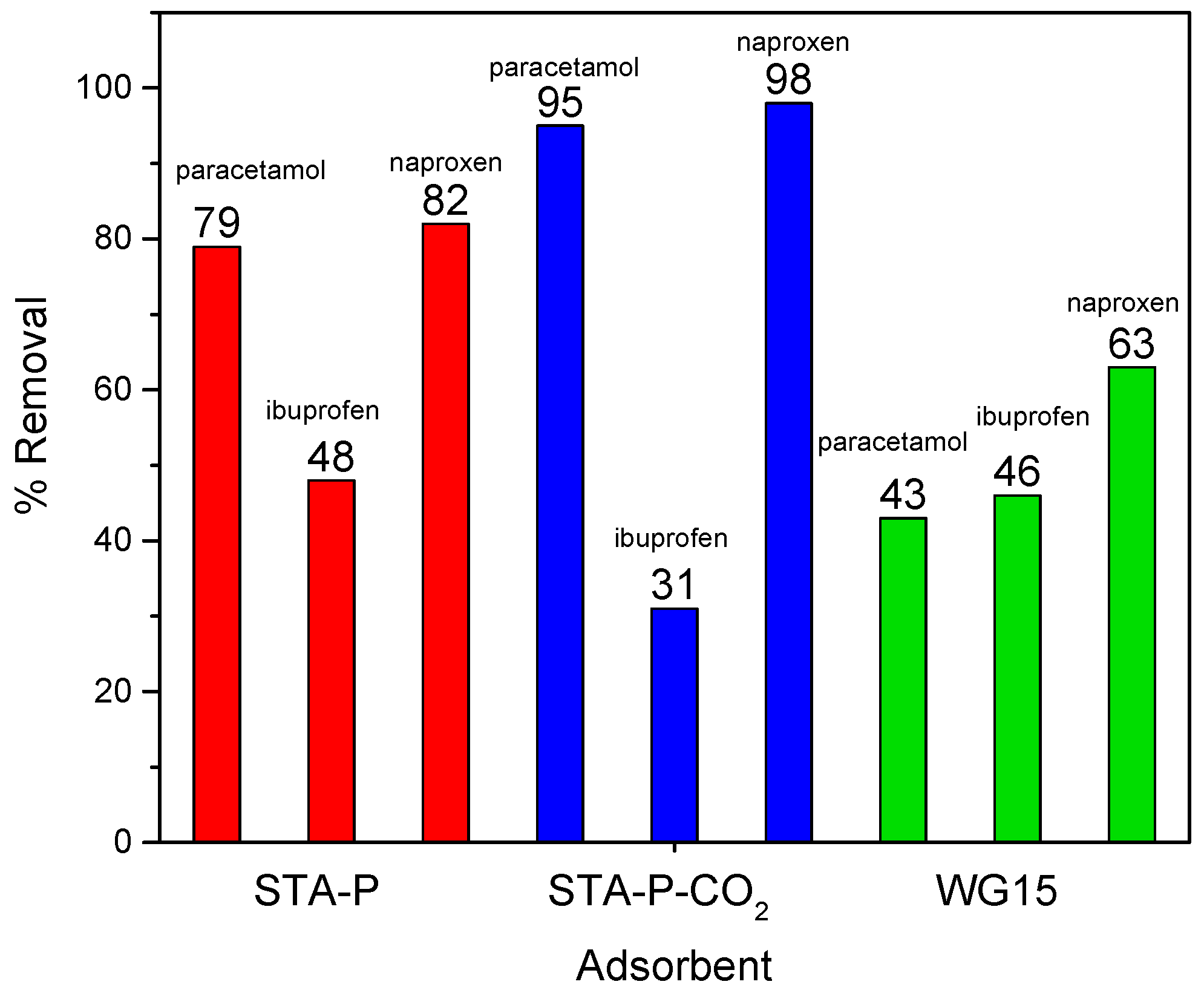
3.3. Adsorption Study
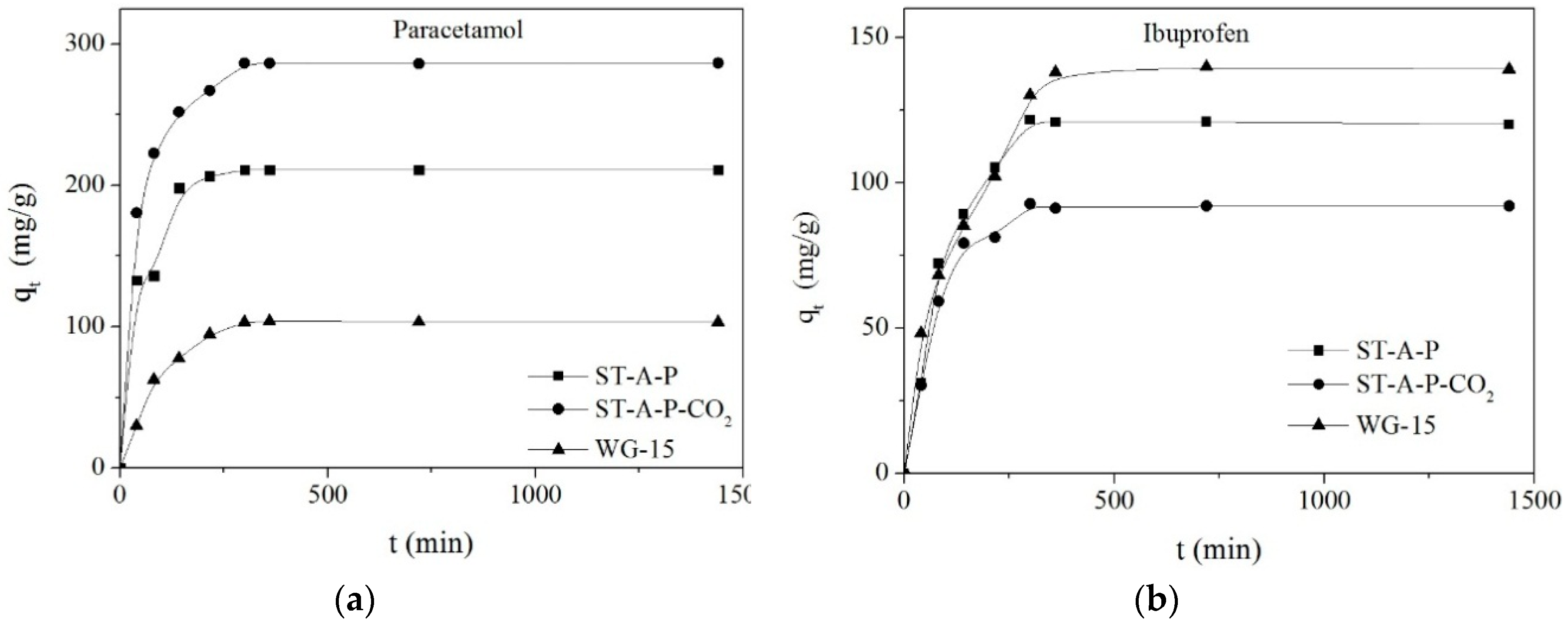
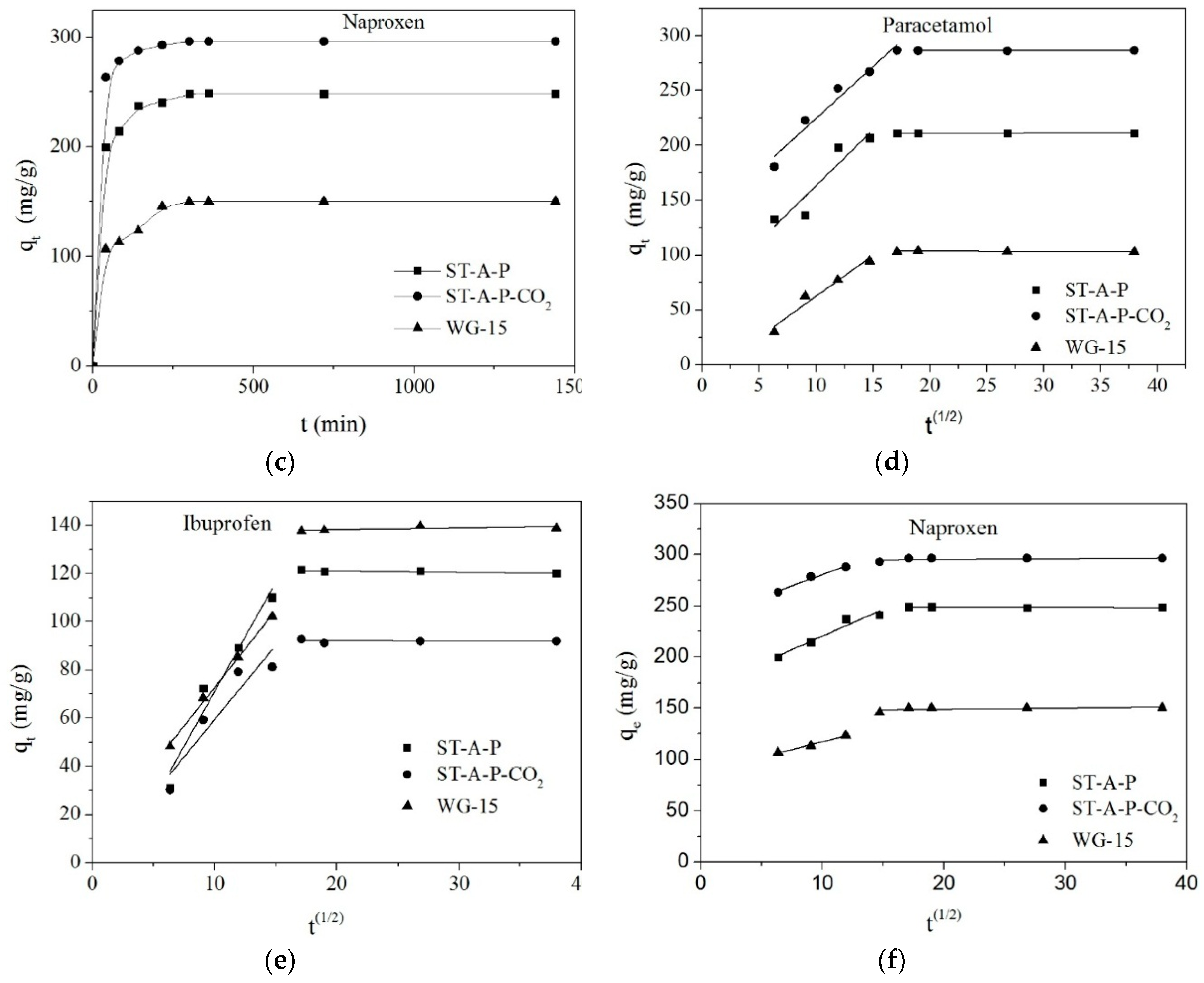
3.3.1. Kinetic Models
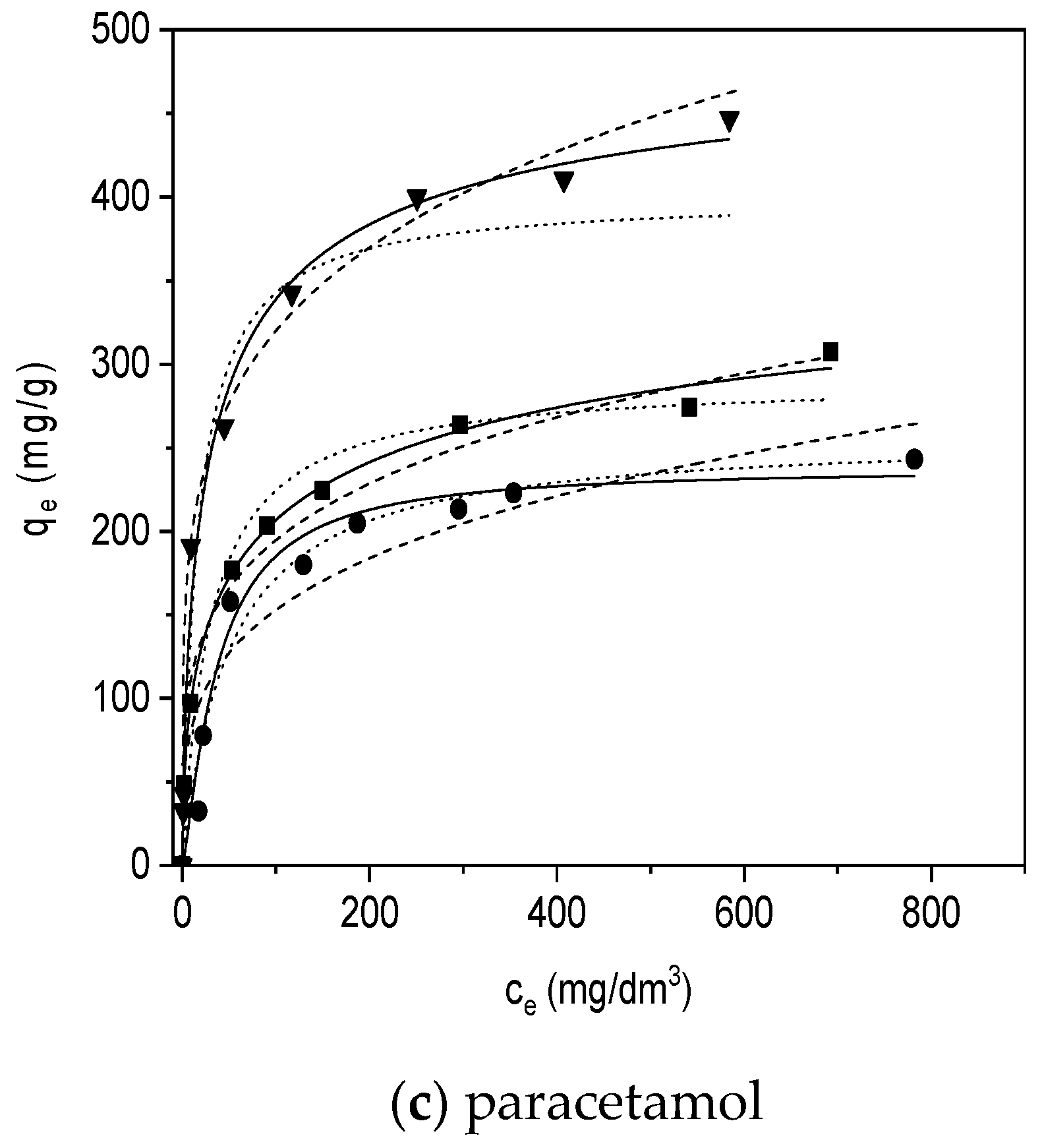
3.3.2. Adsorption Isotherms
Langmuir Model
Freundlich Model
Langmuir–Freundlich Model
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akhtar, J.; Aishah Saidina Amin, N.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Des. Water Treat. 2016, 57, 12842–12860. [Google Scholar] [CrossRef]
- Ahmed, M.J. Adsorption of non-steroidal anti-inflammatory drugs from aqueous solution using activated carbons. Review. J. Environ. Manag. 2017, 190, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Pluciennik-Koropczuk, E. Non-steroidal anti-infflamatory drugs in municipal wastewater and surface waters. Civ. Environ. Eng. Rep. 2014, 14, 63–74. [Google Scholar] [CrossRef]
- Dubey, S.P.; Dwivedi, A.D.; Sillanpää, M.; Gopal, K. Artemisia vulgaris-derived mesoporous honeycomb-shaped activated carbon for ibuprofen adsorption. Chem. Eng. J. 2010, 165, 537–544. [Google Scholar] [CrossRef]
- Cabrita, I.; Ruiz, B.; Mestre, A.S.; Fonseca, I.M.; Carvalho, A.P.; Ania, C.O. Removal of an analgesic using activated carbons prepared from urban and industrial residues. Chem. Eng. J. 2010, 163, 249–255. [Google Scholar] [CrossRef]
- Weigel, S.; Kuhlmann, J.; Hühnerfuss, H. Drugs and personal care products as ubiquitous pollutants: Occurrence and distribution of clofibric acid, caffeine and DEET in the North Sea. Sci. Total Environ. 2002, 295, 131–141. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Rodríguez, A.; Ovejero, G.; García, J. Comparative adsorption performance of ibuprofen and tetracycline from aqueous solution by carbonaceous materials. Chem. Eng. J. 2016, 283, 936–947. [Google Scholar] [CrossRef]
- Hernando, M.D.; Mezcua, M.; Fernandez-Alba, A.R.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar]
- Baccar, R.; Sarra, M.; Bouzid, J.; Feki, M.; Blánquez, P. Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem. Eng. J. 2012, 211, 310–317. [Google Scholar] [CrossRef]
- Iovino, P.; Chianese, S.; Canzano, S.; Prisciandaro, M.; Musmarra, D. Photodegradation of diclofenac in wastewaters. Desalin. Water Treat. 2017, 61, 293–297. [Google Scholar]
- Li, J.; Ma, L.-Y.; Li, L.-S.; Xu, L. Photodegradation kinetics, transformation, and toxicity prediction of ketoprofen, carprofen, and diclofenac acid in aqueous solutions. Environ. Toxicol. Chem. 2017, 36, 3232–3239. [Google Scholar] [CrossRef]
- Vijay, M.; Vulava, W.; Cory, C.; Murphey, V.L.; Ulmer, C.Z. Sorption, photodegradation, and chemical transformation of naproxen and ibuprofen in soils and water. Sci. Total Environ. 2016, 565, 1063–1070. [Google Scholar]
- Zhang, H.; Zhang, P.; Ji, Y.; Tian, J.; Du, Z. Photocatalytic degradation of four non-steroidal anti-inflammatory drugs in water under visible light by P25-TiO2/tetraethyl orthosilicate film and determination via ultra performance liquid chromatography electrospray tandem mass spectrometry. Chem. Eng. J. 2015, 262, 1108–1115. [Google Scholar] [CrossRef]
- Kezzim, A.; Boudjemaa, A.; Belhadi, A.; Trari, M. Photo-catalytic degradation of ibuprofen over the new semiconducting catalyst α-(Cu,Fe)2O3 prepared by hydrothermal route. Res. Chem. Intermed. 2017, 43, 3727–3743. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mishra, S.K. Ferrate(VI) oxidation of ibuprofen: A kinetic study. Environ. Chem. Lett. 2006, 3, 182–185. [Google Scholar] [CrossRef]
- Molinari, R.; Pirillo, F.; Loddo, V.; Palmisano, L. Heterogeneous photocatalytic degradation of pharmaceuticals in water by using polycrystalline TiO2 and a nanofiltration membrane reactor. Catal. Today 2006, 118, 205–213. [Google Scholar] [CrossRef]
- Ciríaco, L.; Anjo, C.; Correia, J.; Pacheco, M.J.; Lopes, A. Electrochemical degradation of Ibuprofen on Ti/Pt/PbO2 and Si/BDD electrodes. Electrochim. Acta 2009, 54, 1464–1472. [Google Scholar] [CrossRef]
- Choina, J.; Kosslick, H.; Fischer, C.; Flechsig, G.-U.; Frunza, L.; Schulz, A. Photocatalytic decomposition of pharmaceutical ibuprofen pollutions in water over titania catalyst. Appl. Catal. B Environ. 2013, 129, 589–598. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Domínguez-Vargas, J.R.; Olivares-Marín, F.J.; de Heredia, J.B. On the use of carbon blacks as potential low-cost adsorbents for the removal of non-steroidal anti-inflammatory drugs from river water. J. Hazard. Mater. 2010, 177, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Rakic, V.; Rac, V.; Krmar, M.; Otman, O.; Auroux, A. The adsorption of pharmaceutically active compounds from aqueous solutions onto activated carbons. J. Hazard. Mater. 2015, 282, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, B.N.; Seo, P.W.; Jhung, S.H. Adsorption of diclofenac sodium from water using oxidized activated carbon. Chem. Eng. J. 2016, 301, 27–34. [Google Scholar] [CrossRef]
- Larous, S.; Meniai, A.-H. Adsorption of Diclofenac from aqueous solution using activated carbon prepared from olive stones. Int. J. Hydrogen Energy 2016, 41, 10380–10390. [Google Scholar] [CrossRef]
- Alvarez-Torrellas, S.; Munoz, M.; Zazo, J.A.; Casas, J.A.; García, J. Synthesis of high surface area carbon adsorbents prepared from pine sawdust-Onopordum acanthium L. for nonsteroidal anti-inflammatory drugs adsorption. J. Environ. Manag. 2016, 183, 294–305. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, J.; Nogueira, J.M.F.; Carvalho, A.P. Activated carbons for the adsorption of ibuprofen. Carbon 2007, 45, 1979–1988. [Google Scholar] [CrossRef]
- Guedidi, H.; Reinert, L.; Leveque, J.-M.; Soneda, Y.; Bellakhal, N.; Duclaux, L. The effects of the surface oxidation of activated carbon, the solution pH and the temperature on adsorption of ibuprofen. Carbon 2013, 54, 432–443. [Google Scholar] [CrossRef]
- Guedidi, H.; Reinert, L.; Soneda, Y.; Bellakhal, N.; Duclaux, L. Adsorption of ibuprofen from aqueous solution on chemically surface-modified activated carbon cloths. Arab. J. Chem. 2017, 10, 3584–3594. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, L.; Yang, S.; Schott, J.A.; Liu, X.; Mahurin, S.M.; Huang, C.; Zhang, Y.; Fulvio, P.F.; Chisholm, M.F.; et al. Solid-state synthesis of ordered mesoporous carbon catalysts via a mechanochemical assembly through coordination cross-linking. Nat. Commun. 2017, 8, 15020. [Google Scholar] [CrossRef]
- Choma, J.; Jedynak, K.; Marszewski, M.; Jaroniec, M. Organic acid-assisted soft-templating synthesis of ordered mesoporous carbons. Adsorption 2013, 19, 563–569. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.; Shi, Y.; Cheng, L.; Feng, D.; Wu, Z.; Chen, Z.; Wan, Y.; Stein, A.; et al. A family of highly ordered mesoporous polymer resin and carbon structures from organic–organic self-assembly. Chem. Mater. 2006, 18, 4447–4464. [Google Scholar] [CrossRef]
- Wang, X.; Liang, C.D.; Dai, S. Facile synthesis of ordered mesoporous carbons with high thermal stability by self-assembly of resorcinol−formaldehyde and block copolymers under highly acidic conditions. Langmiur 2008, 24, 7500–7505. [Google Scholar] [CrossRef] [PubMed]
- ChemIDplus A Toxnet Data Base. Available online: http//chem.nlm.nih.gov/chemidplus/ (accessed on 17 April 2019).
- Choma, J.; Kalinowska, A.; Jedynak, K.; Marszewski, M.; Jaroniec, M. Reproducibility of the synthesis and adsorption properties of ordered mesoporous carbons obtained by the soft-templating method. Ochrona Srodowiska 2012, 34, 3–10. [Google Scholar]
- Wickramaratne, N.P.; Jaroniec, M. Activated Carbon Spheres for CO2 Adsorption. ACS Appl. Mater. Interfaces 2013, 5, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption. Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Jaroniec, M.; Kaneko, K. Physicochemical foundations for characterization of adsorbents by using high-resolution comparative plots. Langmuir 1997, 13, 6589–6596. [Google Scholar] [CrossRef]
- Choma, J.; Jaroniec, M. New methods enabling description of the porous structure of active carbons on the basis of adsorption data. Ochrona Srodowiska. 1999, 21, 13–17. [Google Scholar]
- Kruk, M.; Jaroniec, M.; Gadkaree, K.P. Nitrogen adsorption studies of novel synthetic active carbons. J. Colloid Interface Sci. 1997, 192, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distribution in porous substances. 1. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Lim, C.K.; Bay, H.H.; Noeh, C.H.; Aris, A.; Majid, Z.A.; Ibrahim, Z. Application of zeolite-activated carbon macrocomposite for the adsorption of Acid Orange 7: Isotherm. kinetic and thermodynamic studies. Environ. Sci. Pollut. Res. 2013, 20, 7243–7255. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-García, M.A.; Moreno-Castilla, C. Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef]
- Jedynak, K.; Wideł, D.; Rędzia, N. Removal of rhodamine b (a basic dye) and acid yellow 17 (an acidic dye) from aqueous solutions by ordered mesoporous carbon and commercial activated carbon. Colloid Interface 2019, 3, 30. [Google Scholar] [CrossRef]
- Tamijani, A.A.; Salam, A.; de Lara-Castells, M.P. Adsorption of noble-gas atoms on the TiO2(110) surface: An ab initio-assisted study with van der waals-corrected DFT. J. Phys. Chem. C 2016, 120, 18126–18139. [Google Scholar] [CrossRef]
- Berland, K.; Cooper, V.R.; Lee, K.; Schröder, E.; Thonhauser, T.; Hyldgaard, P.; Lundqvist, B.I. Van der waals forces in density functional theory: A review of the vdW-DF method. Rep. Prog. Phys. 2015, 78, 066501. [Google Scholar] [CrossRef] [PubMed]
- Brauer, B.; Kesharwani, M.K.; Kozuchb, S.; Martin, J.M.L. The S66 × 8 benchmark for noncovalent interactions revisited: Explicitly correlated ab initio methods and density functional theory. Phys. Chem. Chem. Phys. 2016, 18, 20905–20925. [Google Scholar] [CrossRef] [PubMed]
- Vydrov, O.A.; Voorhis, T.V. Nonlocal van der Waals density functional: The simpler the better. J. Chem. Phys. 2010, 133, 244103. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Choma, J. Characterization of nanoporous active carbons by using gas adsorption isotherms. Wegiel Aktywny w Ochronie Srodowiska i Przemysle 2006, 9, 37. [Google Scholar]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Bernal, V.; Erto, A.; Giraldo, L.; Moreno-Piraján, J.C. Effect of solution pH on the adsorption of paracetamol on chemically modified activated carbons. Molecules 2017, 22, 1032. [Google Scholar] [CrossRef]
- Lagregren, S. About the theory of so-called adsorption of soluble substances. Kungl. Sven. Veten. Akad. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second-order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Kopelman, R. Fractal Reaction Kinetics. Science 1988, 241, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Balsamo, M.; Montagnaro, F. Fractal-like vermeulen kinetic equation for the description of diffusion-controlled adsorption dynamics. J. Phys. Chem. C 2015, 119, 8781–8785. [Google Scholar] [CrossRef]
- Montagnaro, F.; Balsamo, M. Modelling CO2 Adsorption Dynamics onto Amine-Functionalised Sorbents: A Fractal-like Kinetic Perspective. Chem. Eng. Sci. 2018, 192, 603–612. [Google Scholar] [CrossRef]
- Haerifar, M.; Azizian, S. Fractal-like Adsorption Kinetics at the Solid/Solution Interface. J. Phys. Chem. C 2012, 116, 13111–13119. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–59. [Google Scholar]
- Kusmierek, K.; Swiątkowski, A.; Kaminski, W. Adsorption of 4-chlorophenol from aqueous solutions on mixed adsorbents: Activated carbon and carbon nanotubes. Inzynieria i Ochrona Srodowiska 2015, 18, 373–383. [Google Scholar]
- Langmuir, I. Chemical reactions at low pressures. J. Am. Chem. Soc. 1915, 27, 1139–1143. [Google Scholar] [CrossRef]
- Jeppu, G.P.; Clement, T.P. A modified Langmuir-Freundlich isotherm model for simulating pH-dependent adsorption effects. J. Contam. Hydrol. 2012, 129, 46–53. [Google Scholar] [CrossRef]
- Thirunavukkarasu, O.S.; Viraraghavan, T.; Subramanian, K.S. Arsenic removal from drinking water using iron oxide-coated sand. Water Air Soil Pollut. 2003, 142, 95–111. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Uber die Adsorption in Losungen. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Lin, T.F.; Wu, J.K. Adsorption of arsenite and arsenatewithin activated alumina grains: Equilibrium and kinetics. Water Res. 2001, 35, 2049–2057. [Google Scholar] [CrossRef]
- Sips, R. Combined form of Langmuir and Freundlich equations. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Vadi, M.; Omidi, A. Comparative study of adsorption isotherms two nonsteroidal anti-inflammatory drugs(nsaids), acetaminophen and diclofenac on carbon nanotube. Oriental J. Chem. 2012, 28, 1325–1330. [Google Scholar] [CrossRef][Green Version]
- Vadi, M. Adsorption isotherms of some non-steroidal drugs on single wall carbon nanotube. Asian J. Chem. 2013, 25, 3431–3433. [Google Scholar] [CrossRef]
- Shahbeig, H.; Bagheri, N.; Ghorbanian, S.A.; Hallajisani, A.; Poorkarimi, S. A new adsorption isotherm model of aqueous solutions on granular activated carbon. World J. Model. Simul. 2013, 9, 243–254. [Google Scholar]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, freundlich, temkin and dubinin–radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Tempkin, M.I.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys. Chim. USSR 1940, 12, 327–356. [Google Scholar]
- Balhachemi, M.; Addoun, F. Comparative adsorption isotherms and modeling of methylene blue onto activated carbons. Appl. Water Sci. 2011, 1, 111–117. [Google Scholar] [CrossRef]
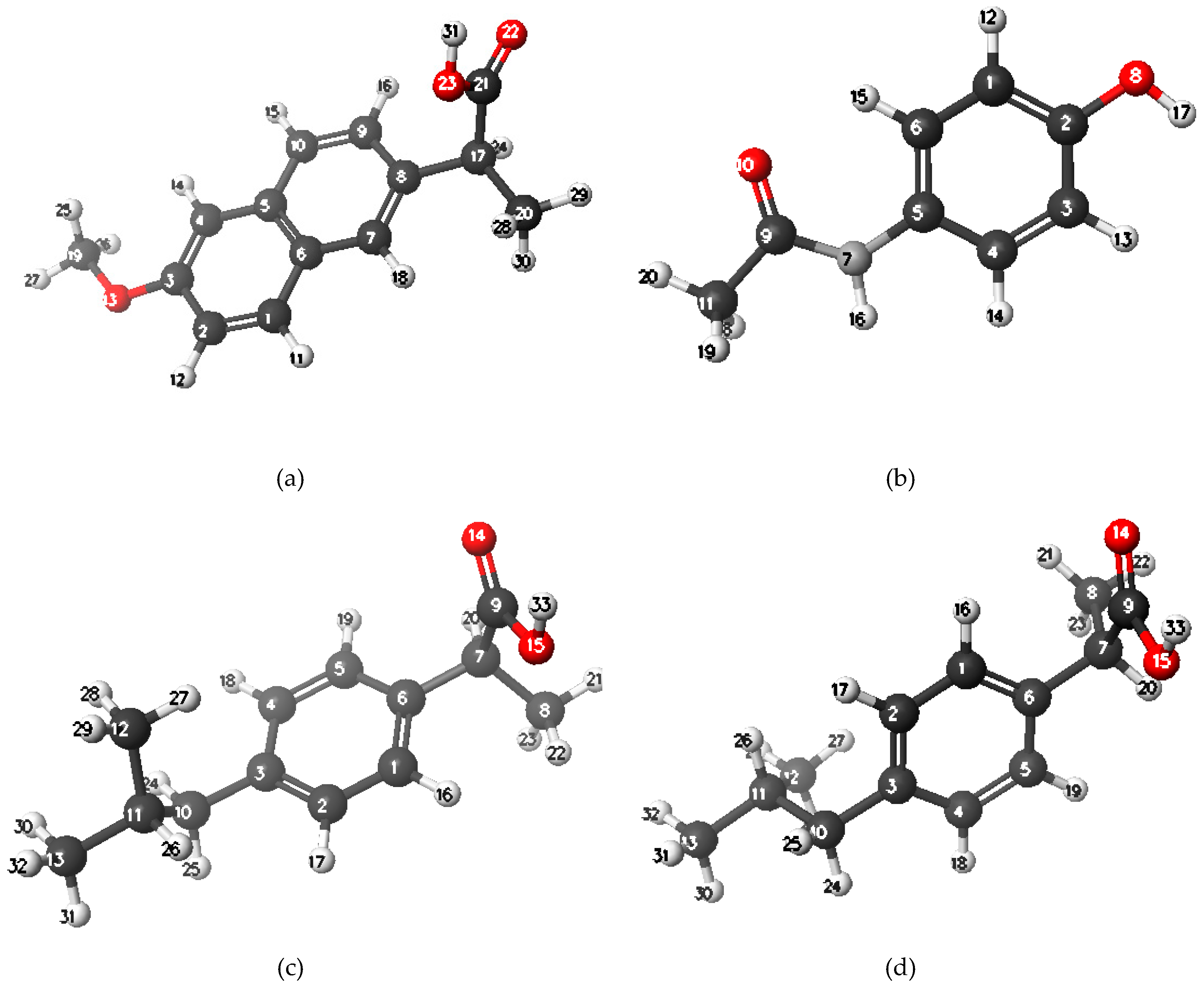
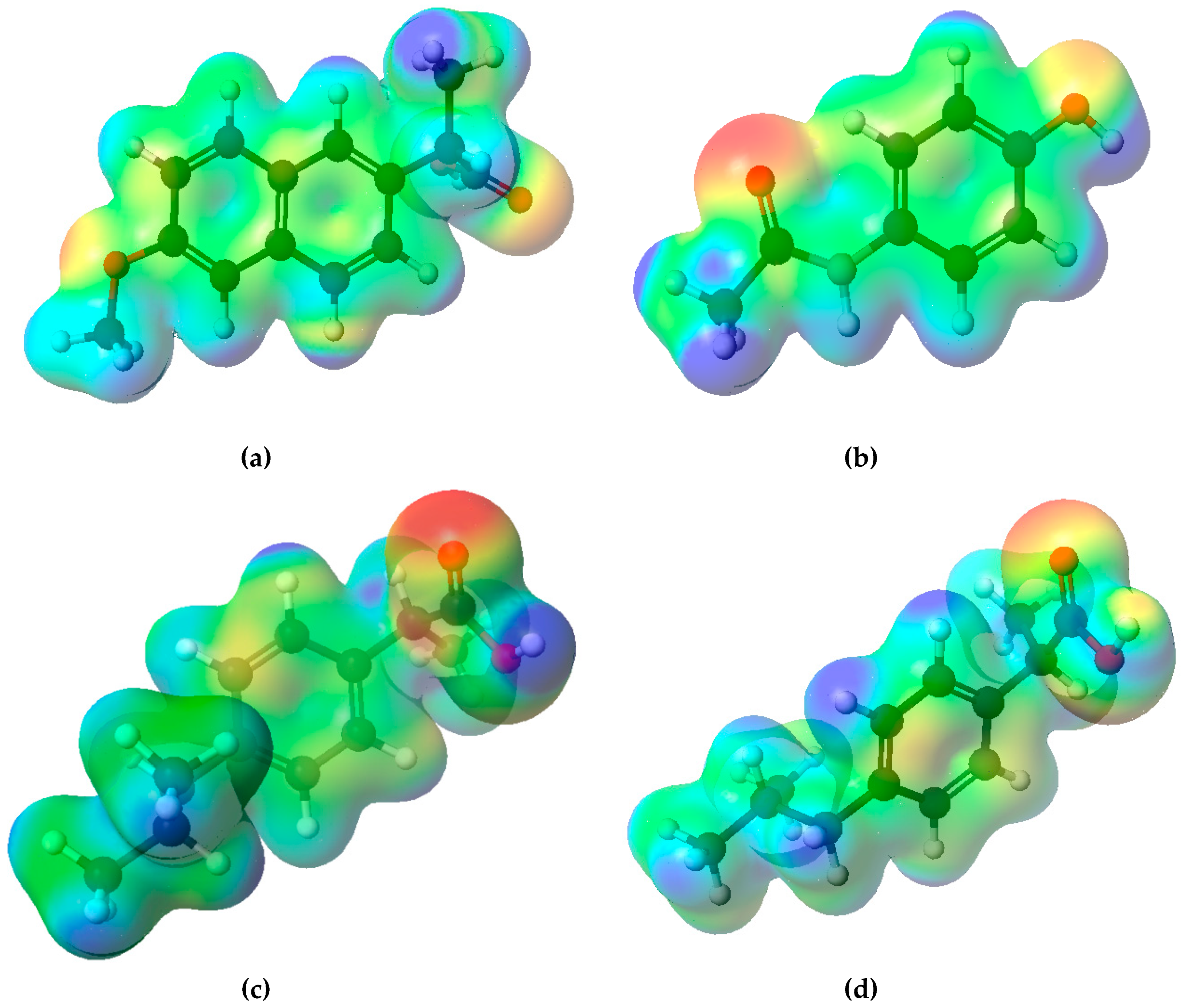

| Compound | Paracetamol | Ibuprofen | Naproxen |
|---|---|---|---|
| Molecular structure | 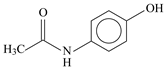 | 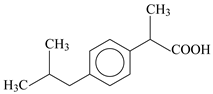 | 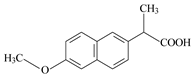 |
| IUPAC name | N-(4-hydroxyphenyl)-acetamide | (RS)-2-(4-(2-methylpropyl)phenyl)-propanoic acid | (+)-(S)-2-(6-methoxy-naphthalen-2-yl)propanoic acid |
| Chemical formula | C8H9NO2 | C13H18O2 | C14H14O3 |
| Molecular weight | 151.16 g/mol | 206.28 g/mol | 230.26 g/mol |
| Water solubility | 1.4 mg/L (25 °C) | 21 mg/L (25 °C) | 15.9 mg/L (25 °C) |
| pKa * | 9.38 | 4.91 | 4.15 |
| λ (nm) ** | 243 | 221 | 273 |
| (S)-naproxen | Paracetamol | (S)-ibuprofen | (R)-ibuprofen | ||||
|---|---|---|---|---|---|---|---|
| partial charge | partial charge | partial charge | partial charge | ||||
| C1 | −0.350 | C1 | −0.309 | C1 | −0.315 | C1 | −0.337 |
| C2 | −0.325 | C2 | 0.317 | C2 | −0.329 | C2 | −0.322 |
| C3 | 0.341 | C3 | −0.352 | C3 | 0.305 | C3 | 0.303 |
| C4 | −0.533 | C4 | −0.372 | C4 | −0.346 | C4 | −0.351 |
| C5 | 0.283 | C5 | 0.364 | C5 | −0.352 | C5 | −0.366 |
| C6 | 0.293 | C6 | −0.325 | C6 | 0.273 | C6 | 0.303 |
| C7 | −0.533 | N7 | −0.428 | C7 | −0.267 | C7 | −0.254 |
| C8 | 0.312 | O8 | −0.478 | C8 | −0.625 | C8 | −0.608 |
| C9 | −0.34 | C9 | 0.259 | C9 | 0.267 | C9 | 0.233 |
| C10 | −0.364 | O10 | −0.344 | C10 | −0.486 | C10 | −0.481 |
| H11 | 0.225 | C11 | −0.678 | C11 | −0.133 | C11 | −0.133 |
| H12 | 0.235 | H12 | 0.234 | C12 | −0.618 | C12 | −0.618 |
| O13 | −0.271 | H13 | 0.201 | C13 | −0.614 | C13 | −0.615 |
| H14 | 0.23 | H14 | 0.203 | O14 | −0.308 | O14 | −0.31 |
| H15 | 0.218 | H15 | 0.306 | O15 | −0.441 | O15 | −0.428 |
| H16 | 0.235 | H16 | 0.329 | H16 | 0.221 | H16 | 0.24 |
| C17 | −0.241 | H17 | 0.399 | H17 | 0.216 | H17 | 0.218 |
| H18 | 0.237 | H18 | 0.212 | H18 | 0.219 | H18 | 0.218 |
| C19 | −0.464 | H19 | 0.212 | H19 | 0.222 | H19 | 0.214 |
| C20 | −0.672 | H20 | 0.251 | H20 | 0.231 | H20 | 0.222 |
| C21 | 0.247 | H21 | 0.216 | H21 | 0.224 | ||
| O22 | −0.308 | H22 | 0.22 | H22 | 0.213 | ||
| O23 | −0.43 | H23 | 0.217 | H23 | 0.213 | ||
| H24 | 0.241 | H24 | 0.2 | H24 | 0.199 | ||
| H25 | 0.201 | H25 | 0.207 | H25 | 0.208 | ||
| H26 | 0.2 | H26 | 0.194 | H26 | 0.193 | ||
| H27 | 0.244 | H27 | 0.227 | H27 | 0.225 | ||
| H28 | 0.223 | H28 | 0.184 | H28 | 0.184 | ||
| H29 | 0.237 | H29 | 0.203 | H29 | 0.202 | ||
| H30 | 0.219 | H30 | 0.191 | H30 | 0.191 | ||
| H31 | 0.408 | H31 | 0.2 | H31 | 0.201 | ||
| H32 | 0.212 | H32 | 0.212 | ||||
| H33 | 0.409 | H33 | 0.408 | ||||
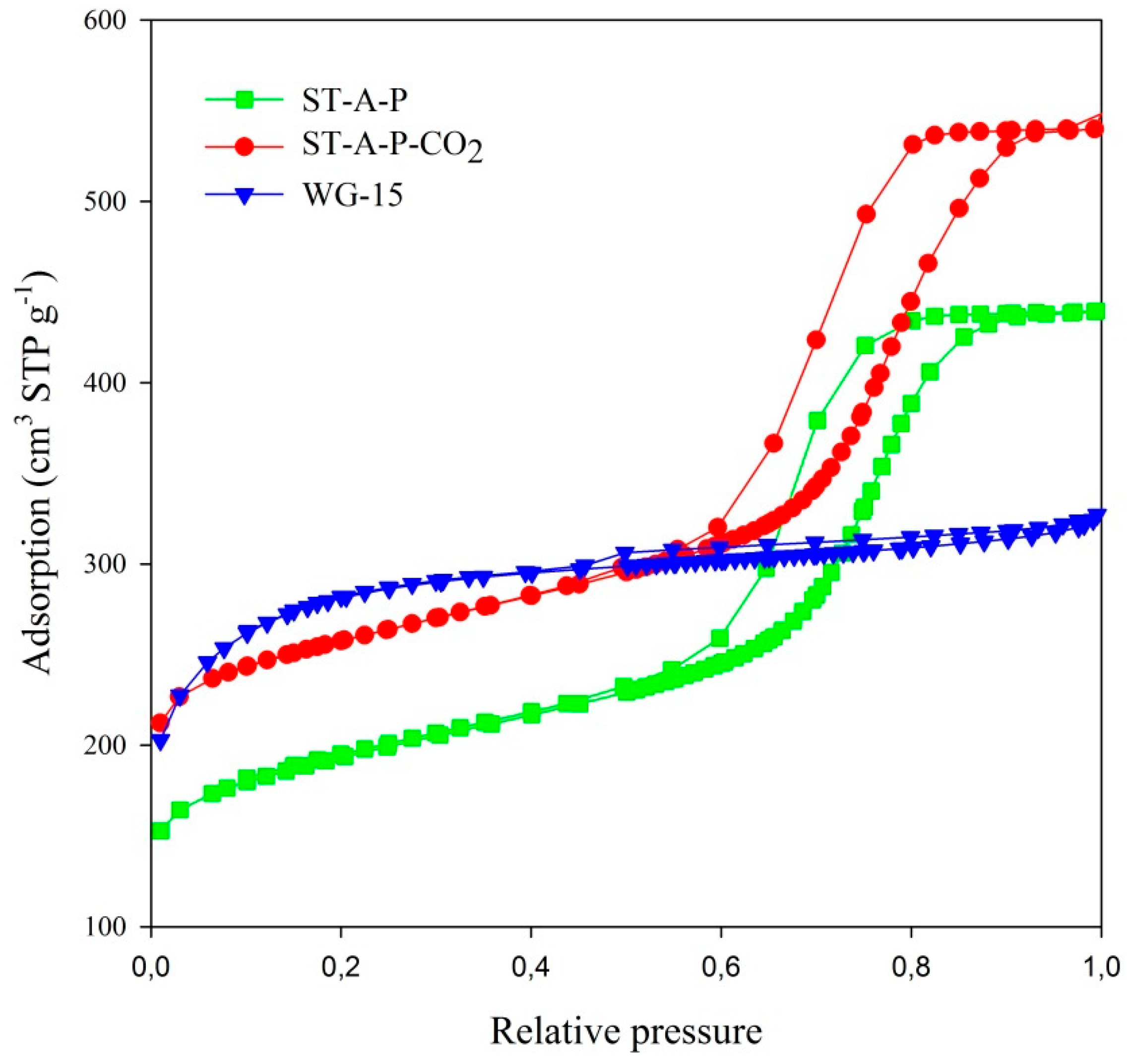
| Carbon Materials | SBET m2/g | Vt cm3/g | Vme cm3/g | Vmi cm3/g | Mesoporosity % |
|---|---|---|---|---|---|
| ST-A-P | 670 | 0.68 | 0.51 | 0.17 | 75 |
| ST-A-P-CO2 | 886 | 0.84 | 0.58 | 0.26 | 69 |
| WG-15 | 987 | 0.50 | 0.23 | 0.27 | 46 |
| Adsorbents | Total Basic Groups (mmol g−1) | Total Acidic Groups (mmol g−1) | Phenolic Groups (mmol g−1) | Carbonyl Groups (mmol g−1) |
|---|---|---|---|---|
| ST-A-P [35] | 0.24 | 0.69 | 0.35 | 0.34 |
| ST-A-P-CO2 | 0.56 | 0.82 | 0.44 | 0.38 |
| WG-15 | 2.35 | 0.66 | - | 0.66 |
| Adsorbate | Adsorbent | qe (exp) (mg g−1) | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe (mg g−1) | R2 | k2 (g mg−1 min−1) | qe (mg g−1) | R2 | |||
| Paracetamol | ST-A-P | 211.2 | 0.0177 | 204.7 | 0.9467 | 0.00008 | 256.4 | 0.9616 |
| ST-A-P-CO2 | 286.6 | 0.0139 | 154.3 | 0.8774 | 0.00010 | 303.0 | 0.9999 | |
| WG-15 | 103.4 | 0.0118 | 172.4 | 0.9332 | 0.00007 | 117.6 | 0.9847 | |
| Ibuprofen | ST-A-P | 120.1 | 0.0247 | 240.0 | 0.9392 | 0.00005 | 166.7 | 0.9924 |
| ST-A-P-CO2 | 92.0 | 0.0347 | 212.3 | 0.9513 | 0.00010 | 114.9 | 0.9909 | |
| WG-15 | 139.0 | 0.0166 | 210.7 | 09817 | 0.00009 | 137.0 | 0.9933 | |
| Naproxen | ST-A-P | 248.8 | 0.0110 | 74.0 | 0.9402 | 0.00030 | 256.4 | 0.9999 |
| ST-A-P-CO2 | 296.3 | 0.0128 | 53.0 | 0.9986 | 0.00050 | 303.0 | 1.0000 | |
| WG-15 | 150.3 | 0.0126 | 95.5 | 0.8613 | 0.00020 | 158.7 | 0.9819 | |
| Adsorbate | Adsorbent | Fractal-Like Pseudo-First-Order Kinetic Model | Fractal-Like Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| k0,1′ (min-n) | α | R2 | k0,2′ (g mg−1 min−n) | α | R2 | ||
| Paracetamol | ST-A-P | 0.01011 | 0.85 | 0.9853 | 0.00018 | 1 | 0.7183 |
| ST-A-P-CO2 | 0.00443 | 0.82 | 0.9339 | 0.00016 | 1 | 0.8405 | |
| WG-15 | 0.03042 | 0.83 | 0.9867 | 0.00015 | 1 | 0.9767 | |
| Ibuprofen | ST-A-P | 0.00452 | 0.85 | 0.9817 | 0.00014 | 1 | 0.9055 |
| ST-A-P-CO2 | 0.00773 | 0.98 | 0.9551 | 0.00023 | 1 | 0.9083 | |
| WG-15 | 0.00268 | 0.76 | 0.9449 | 0.00011 | 0.95 | 0.8906 | |
| Naproxen | ST-A-P | 0.17076 | 0.56 | 0.9554 | 0.00041 | 1 | 0.9275 |
| ST-A-P-CO2 | 0.35772 | 0.46 | 0.9942 | 0.00056 | 1 | 0.6862 | |
| WG-15 | 0.00666 | 0.51 | 0.8894 | 0.00066 | 0.83 | 0.7627 | |
| Adsorbate | Adsorbent | kid1 (mg g−1 min−1/2) | c1 (mg g−1) | R12 | kid2 (mg g−1 min−1/2) | c2 (mg g−1) | R22 |
|---|---|---|---|---|---|---|---|
| Paracetamol | ST-A-P | 28.32 | 97.62 | 0.8646 | 0.19 | 97.25 | 0.9999 |
| ST-A-P-CO2 | 25.63 | 164.92 | 0.9579 | 0.11 | 207.72 | 0.9998 | |
| WG-15 | 17.91 | 19.92 | 0.9483 | 0.002 | 284.41 | 0.9999 | |
| Ibuprofen | ST-A-P | 21.88 | 19.21 | 0.9474 | 0.21 | 113.92 | 1.0000 |
| ST-A-P-CO2 | 14.71 | 24.44 | 0.8951 | 0.25 | 84.11 | 0.9997 | |
| WG-15 | 18.00 | 31.26 | 0.9966 | 0.91 | 110.39 | 0.9999 | |
| Naproxen | ST-A-P | 12.44 | 190.87 | 0.9302 | 0.18 | 242.93 | 0.9999 |
| ST-A-P-CO2 | 9.79 | 256.27 | 0.9500 | 0.22 | 289.77 | 1.0000 | |
| WG-15 | 12.71 | 90.65 | 0.9269 | 0.61 | 132.20 | 0.9998 |
| Isotherms | Parameters | Adsorbent | ||
|---|---|---|---|---|
| ST-A-P | ST-A-P-CO2 | WG-15 | ||
| Freundlich | KF (mg g−1) (dm−3 mg−1)1/n | 67.26 | 122.64 | 44.97 |
| 1/n | 0.23 | 0.21 | 0.26 | |
| R2 | 0.9594 | 0.8373 | 0.8170 | |
| Langmuir | KL (dm3 mg−1) | 0.034 | 0.06 | 0.02 |
| qm (mg g−1) | 290.52 | 400.00 | 257.82 | |
| R2 | 0.9562 | 0.9668 | 0.9637 | |
| Langmuir- Freundlich | KLF (dm3 mg−1)1/n | 0.106 | 0.108 | 0.01 |
| qm (mg g−1) | 413.53 | 501.91 | 238.00 | |
| n | 2.05 | 1.56 | 0.79 | |
| R2 | 0.9968 | 0.9901 | 0.9709 | |
| Isotherms | Parameters | Adsorbent | ||
|---|---|---|---|---|
| ST-A-P | ST-A-P-CO2 | WG-15 | ||
| Freundlich | KF (mg g−1) (dm−3 mg−1)1/n | 83.42 | 106.16 | 21.04 |
| 1/n | 0.23 | 0.29 | 0.40 | |
| R2 | 0.9646 | 0.8461 | 0.9578 | |
| Langmuir | KL (dm3 mg−1) | 0.077 | 0.108 | 0.02 |
| qm (mg g−1) | 286.04 | 395.54 | 226.74 | |
| R2 | 0.9612 | 0.8576 | 0.9851 | |
| Langmuir- Freundlich | KLF (dm3 mg−1)1/n | 0.068 | 0.729 | 0.0326 |
| qm (mg g−1) | 300.00 | 301.00 | 51.35 | |
| n | 1.47 | 1.38 | 1.45 | |
| R2 | 0.9773 | 0.9836 | 0.9939 | |
| Isotherms | Parameters | Adsorbent | ||
|---|---|---|---|---|
| ST-A-P | ST-A-P-CO2 | WG-15 | ||
| Freundlich | KF (mg g−1) (dm−3 mg−1)1/n | 15.40 | 19.66 | 27.71 |
| 1/n | 0.38 | 0.30 | 0.29 | |
| R2 | 0.9516 | 0.8744 | 0.9089 | |
| Langmuir | KL (dm3 mg−1) | 0.02 | 0.02 | 0.02 |
| qm (mg g−1) | 162.23 | 129.93 | 193.03 | |
| R2 | 0.9075 | 0.9064 | 0.9436 | |
| Langmuir–Freundlich | KLF (dm3 mg−1)1/n | 0.01 | 0.01 | 0.02 |
| qm (mg g−1) | 193.61 | 148.05 | 210.96 | |
| n | 1.03 | 1.03 | 1.16 | |
| R2 | 0.9926 | 0.9898 | 0.9813 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jedynak, K.; Szczepanik, B.; Rędzia, N.; Słomkiewicz, P.; Kolbus, A.; Rogala, P. Ordered Mesoporous Carbons for Adsorption of Paracetamol and Non-Steroidal Anti-Inflammatory Drugs: Ibuprofen and Naproxen from Aqueous Solutions. Water 2019, 11, 1099. https://doi.org/10.3390/w11051099
Jedynak K, Szczepanik B, Rędzia N, Słomkiewicz P, Kolbus A, Rogala P. Ordered Mesoporous Carbons for Adsorption of Paracetamol and Non-Steroidal Anti-Inflammatory Drugs: Ibuprofen and Naproxen from Aqueous Solutions. Water. 2019; 11(5):1099. https://doi.org/10.3390/w11051099
Chicago/Turabian StyleJedynak, Katarzyna, Beata Szczepanik, Nina Rędzia, Piotr Słomkiewicz, Anna Kolbus, and Paweł Rogala. 2019. "Ordered Mesoporous Carbons for Adsorption of Paracetamol and Non-Steroidal Anti-Inflammatory Drugs: Ibuprofen and Naproxen from Aqueous Solutions" Water 11, no. 5: 1099. https://doi.org/10.3390/w11051099
APA StyleJedynak, K., Szczepanik, B., Rędzia, N., Słomkiewicz, P., Kolbus, A., & Rogala, P. (2019). Ordered Mesoporous Carbons for Adsorption of Paracetamol and Non-Steroidal Anti-Inflammatory Drugs: Ibuprofen and Naproxen from Aqueous Solutions. Water, 11(5), 1099. https://doi.org/10.3390/w11051099





