Abstract
Bottled waters have been becoming increasingly popular in Korea over the last two decades due to the high demand for safe drinking water. Hydrochemical characterization of groundwater is essential for understanding quality properties of bottled waters. We investigated hydrochemistry of 60 manufacture factories for bottled waters in relation to geology. The mean EC value is highest in groundwaters of Ogcheon metamorphic rocks (213.6 μS/cm) > Precambrian gneiss (177.8 μS/cm) > Cretaceous granite (160.4 μS/cm) > Jurassic granite (131.3 μS/cm) > Quaternary Jeju Island volcanic rocks (99.2 μS/cm). The groundwater types are commonly classified as Ca-HCO3, Ca-Na-HCO3, or Ca-Mg-HCO3 types depending on bed rocks. Based on correlation matrix, the groundwater chemistry was controlled by water–rock interactions. We established relationships between groundwater compositions and bedrock geology in Korea.
1. Introduction
There has been increasing concern worldwide about the quality of drinking water. For instance, bottled water usually derived from groundwater is rapidly developing into the supplementary drinking water supply for the general population in large parts of Europe where 1916 mineral water brands are officially registered as of 2010 [1]. Likewise, bottled waters have been becoming increasingly popular in Korea over the last two decades due to the high demand for safe drinking water. For example, the nationwide consumption of bottled waters in Korea has been increased since the late 1990s. The production quantities of the domestic bottled groundwater market have increased manifold over the past 20 years: 0.89 million m3/d in 1995, 1.20 million m3/d in 2000, and 3,600,000 m3/d in 2014, respectively. The market size of the Korea bottled water has almost quadrupled in the same period: 129 million USD (US dollar) in 2000, 291 million USD in 2010, and 610 million USD in 2015, respectively. It is expected that the total production in the market amounts to 870 million USD in 2020. For comparison, the import price of Korea reached 9.11 million USD in 2010 [2]. Europe has the world’s highest rate of consumption of bottled water per capita, whereas Asia has lowest in the consumption [3]. The European bottled water industry is expected to be worth around USD 73.6 billion in 2017 and is expected to reach USD 116.2 billion by 2023, according to Mordor Intelligence [4].
Bottled waters worldwide are generally manufactured from groundwater sources. The hydrochemical quality of bottled water considerably relies on the geochemical features and the anthropogenic pollution [1,3,5,6,7,8,9]. In general, bottled waters may include mineral water, still bottled water, carbonated bottled water, sparkling mineral water, or medicinal mineral water, regardless of their sources and production processes. Bottled waters as a product of the food industry considerably change according to the characteristics of the parent water body, production processes, and storage conditions [10].
In Korea, bottled water refers to the natural mineral water that originates from groundwater with high quality to meet the standard for drinking water [11]. According to the Korea drinking water legislation 4908 set in 1995 by the Ministry of Environment of Korea, only groundwater is allowed as the water source for manufacturing bottled waters [12]. Therefore, the bottled water in Korea is the product of drinking water manufactured exclusively by physical treatments using groundwater originated from rocky aquifers which ensure high quality and stable conditions. Any chemical treatments for the original groundwater used for bottled waters are completely prohibited. In a strict sense, the bottled water in Korea means the low mineralization groundwater of high quality to meet the regulation standards for drinking water; that is, natural mineral water of high quality qualified for drinking water. Therefore, it is quite different from beverage products in definition.
In Korea, bottled waters have been regulated by the law for drinking water management by the Ministry of Environment since 1995. In 1997, the first nationwide investigation on the groundwater used for bottled mineral waters was taken by the Korea Institute of Geoscience and Mineral Resources (KIGAM) for ensuring groundwater of high quality and for better understanding hydrochemical properties of the groundwater [13]. In 2017, there were regulations that set maximum levels of 86 contaminants in drinking water, legally enforceable for bottled waters [14]. A total of 87 manufacturing companies for the bottled waters were officially registered in 2006, but 60 companies are in action as of June 2018, possibly due to economic problems related to the production and markets. Processing of original groundwater such as oxygen addition, some removal can significantly alter original water composition [7]. Some elements may show slight difference between source groundwater and the labels attached on bottled water products, which are resulted from storage duration and production processes. Therefore, understanding the original compositions of groundwater used for bottled waters is essential to provide insight into fundamental hydrochemistry of bottled waters.
For the present study, we investigated hydrochemistry of groundwater using major elements from 60 bottled water factories over the country, combined with geological classification. Correlation coefficients among various physicochemical parameters were determined to account for hydrochemical properties of groundwater used for bottled waters in Korea.
2. Materials and Methods
2.1. Geological Classification of Bottled Water Manufactures
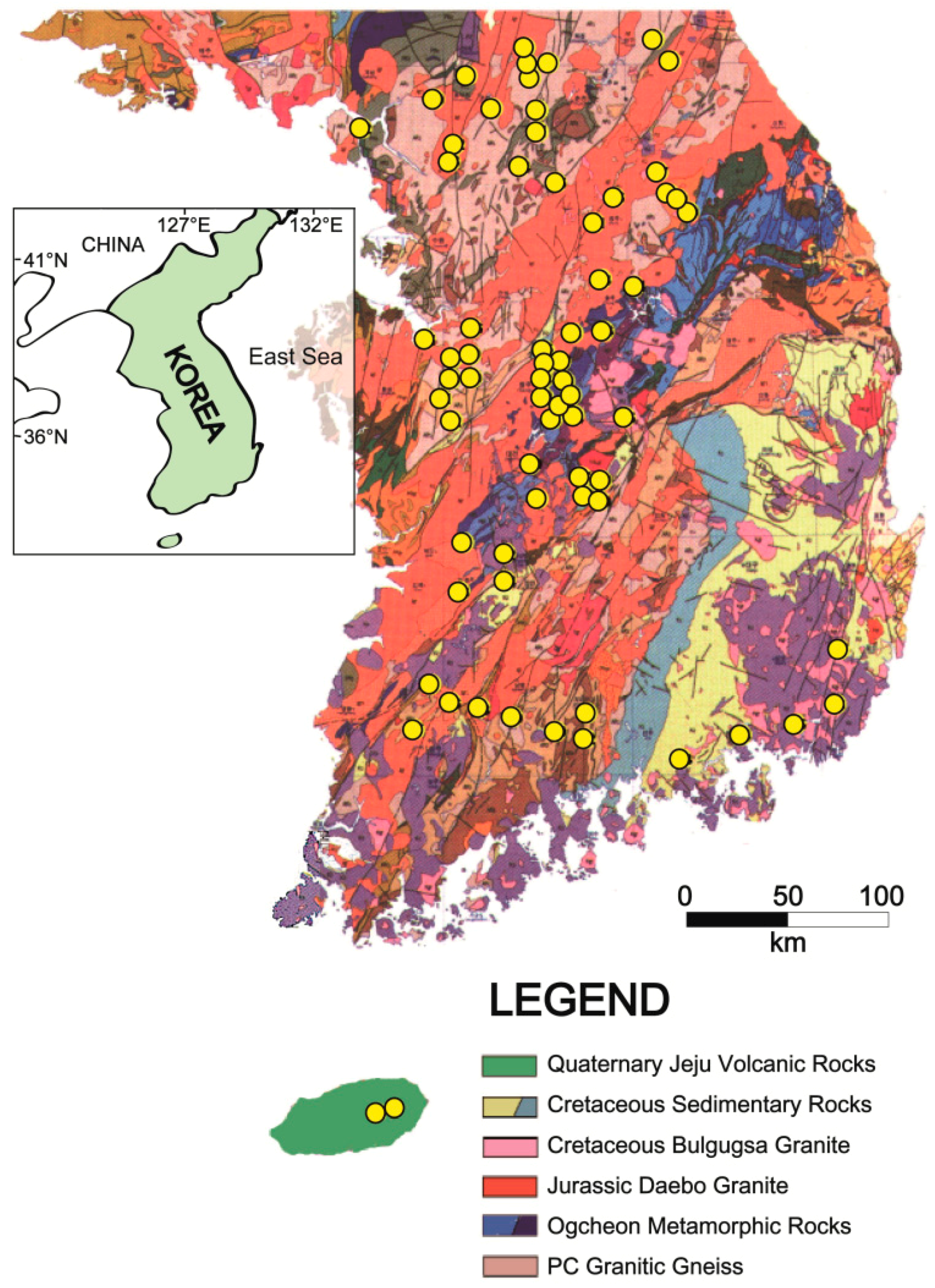
The geology of the Republic of Korea is quite complicated because of its complex tectonic events and wide ages ranging from Archean to Quaternary Holocene epoch (Figure 1). We classify the geology into six groups according to the geological characters of the areas where factories for bottled waters are located. It is noteworthy to mention that there is no bottled water plant in sedimentary rock areas such as the Cretaceous Gyeongsang basin located in the southeastern Korea. Basic information on main geologic units except sedimentary rocks is briefly described as follows.
Figure 1.
Location map of bottled water manufactures marked as yellow points on the geological map of Korea.
2.1.1. Precambrian Gneiss
Twenty-six bottled water manufactures are located on Precambrian gneiss massifs. Precambrian gneiss, one of the most widespread geological units in Korea, is commonly distributed in Gyeonggi and Yeongam massifs, which are considered the Precambrian basements in Korea. The oldest rocks in both massifs belong to the Precambrian gneiss complex which is composed of migmatitic and other gneisses, and metasedimentary rocks [15]. High-grade metamorphic rocks in Precambrian massifs are primarily composed of Neoarchean to Paleoproterozoic gneisses of upper amphibolite to lower granulite facies, often reaching the anatectic condition [16]. Main constituent minerals are feldspars, quartz, amphibole, biotite, muscovite, chlorite, garnet, staurolite, cordierite, etc. Granitic gneiss is predominant in the Precambrian gneiss according to petrochemical properties and spatial distribution.
2.1.2. Ogcheon Metamorphic Rocks
Eleven bottled water manufactures are located in the Ogcheon metamorphic belt distributed mostly in the central part of Korea, called Chungcheong Provinces. The Ogcheon metamorphic belt, composed of Late Proterozoic to Paleozoic rocks, is situated between Gyeonggi and Yeongnam massifs, with NE–SW trend in the country. Ogcheon metamorphic rocks consist of metasedimentary and metavolcanic rocks of slate, phyllite, biotite schist, quartzite, quartz-sericite schist, calc-silicate, marble, dolomite, limestone, conglomerate, and amphibolite [17,18]. The constituent minerals include quartz, biotite, muscovite, calcite, dolomite, chlorite, magnetite, and various metamorphic minerals. The detrital zircon ages from the Ogcheon Supergroup showed two major events of Early Neoproterozoic and Paleozoic [19].
2.1.3. Jurassic Daebo Granite
Sixteen bottled water manufactures are located in areas of Jurassic Daebo granite in the Gyeonggi and Yeongnam massifs. The Daebo granite consists mainly of quartz, plagioclase, K-feldspar, and micas, associated with some accessory minerals including chlorite, apatite, sphene, hornblende, and pyroxene. Based on the petrochemical features, it is essentially of the ilmenite series or S-type granitic rocks. The Daebo granite is considered to have been formed by partial melting of lower continental crust, and solidified at relatively deep levels [20,21].
2.1.4. Cretaceous Bulguksa Granite
Five bottled water manufactures are located in Cretaceous Bulguksa granite terrain in the Gyeongsang province, southeastern Korea. Though the granitic rocks are diverse in composition, they are mostly granodiorite [21], belonging to the magnetite series and I-type granite of mantle origin. Major constituent minerals are quartz, plagioclase, K-feldspar, muscovite, and biotite, accompanied by hornblende, pyroxene, and trace opaque minerals. The Bulguksa graniteis considered to have been formed by partial melting of mantle and solidified at relatively shallow depths [20,21,22]. For comparison, the Bulguksa granitic rocks solidified at shallow depths, whereas the Daebo granitic rocks solidified at deeper levels [22].
2.1.5. Jeju Island Quaternary Volcanic Rocks
Jeju Island, the biggest island in Korea, preserves active quaternary volcanisms [23]. Two bottled water manufactures are situated at the central eastern part of the island, far away from the coast. Jeju Island consists of various volcanic rocks including predominantly alkali basalt in lowland areas and lesser trachybasalt, trachyandesite, and trachyte in highlands. Besides, at least 400 scoria cones, phreatomagmatic tuff rings, and tuff cones are widespread in the island, intercalated with shallow marine deposits [24]. Main constituent minerals of volcanic rocks are olivine, pyroxene, hornblende, biotite, feldspars, and volcanic glass. The water resources in the island rely entirely on groundwater because there is no actual surface water such as streams [25]. Groundwater levels in the northern and southern basins are generally higher than those in the eastern and western basins in the island [26].
2.2. Sampling and Analytical Methods
A total of 60 groundwater samples described herein were collected directly from the main production wells drilled in 60 manufacturing plants over the country. After temperature, pH, Reduction-Oxidation potential (Eh), and electrical conductivity (EC) had been stabilized, groundwater samples were taken after treatment processing with 0.45-μm membrane filter. Temperature, pH, EC, Eh, and alkalinity as bicarbonate (HCO3−) were measured in the field. Samples for cation analysis were acidified to pH 2 in the field immediately after sampling. Each water sample was analyzed to determine the concentration (as mg/L) of major and minor ions (K, Na, Ca, Mg, SiO2, F, Cl, SO42−, and NO3−) using Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES, Optima 4300 DV, PerkinElmer, Waltham, MA, USA), and Ion Chromatography (IC, DX-1500, Dionex, Sunnyvale, CA, USA) at KIGAM laboratory, respectively. Correlation analyses were performed to evaluate relationships amongst the variables in groundwater using SPSS software (IBM, Armonk, NY, USA). Correlation coefficients among various physicochemical parameters accounting for water quality were calculated using Pearson correlation analysis.
3. Results and Discussion
3.1. Wells in Manufactures of Bottled Waters
In Korea, all manufactures of bottled waters should operate the automated monitoring system installed in wells, which continuously records water level, water quantity pumped, and basic groundwater chemistry. The data measured are periodically transferred to the supervise office of local governments of environment to check if the hydrological systems in the manufacture remain constant. Each bottled water manufacture has mostly 3–7 production wells, with at least three monitoring wells.
Permissible capacity of the groundwater ranged from 60 to 4600 m3/d, with an average of 751 m3/d, a median of 526 m3/d, and a standard deviation of 800 m3/d, which means that the well capacity is very different depending on aquifer conditions or manufacture’s market strategy.
Table 1 represents physicochemical properties of groundwater for all bottled waters. In all, depths of production wells varied from 100 to 420 m, with an average of 199.6 m, a median 193.1 m, and a standard deviation of 67.9 m. The deepest wells are located in bottled water manufactures in Jeju Island because of very low water level. The mean depth order was as follows: Jeju Island volcanic rocks (373 m) > Cretaceous granite (216 m) > Precambrian gneiss (204 m) > Jurassic granite (185 m) > Ogcheon metamorphic rocks (170 m). Except for Jeju Island, the median depth was in the range of 160 to 197 m.

Table 1.
Physicochemical compositions in groundwater for bottled waters. Concentration unit is mg/L unless otherwise noted. EC in μS/cm. PCG: Precambrian gneiss; OGM: Ogcheon metamorphic rocks; JG: Jurassic granite; CG: Cretaceous granite; QV: Quaternary Jeju Island volcanic rocks.
3.2. Hydrochemical Properties of Groundwater
3.2.1. Temperature
Groundwater temperatures showed a wide range of 10.2 to 20.9 °C, with an average of 14.9 °C, a median of 14.6 °C, and a standard deviation of 2.2 °C, which means that most temperatures are ~14–15 °C. It seems likely that a few wells were partly affected by atmospheric conditions, possibly due to unsatisfactory well casing or rapidly recharged aquifer. The temperature order was Cretaceous granite (17.7 °C) > Precambrian gneiss (15.2 °C) > Jeju Island volcanic rocks (15.0 °C) > Ogcheon metamorphic rocks (14.5 °C) > Jurassic granite (13.7 °C). Some groundwater wells installed in manufactures showed quite low or high temperatures. Highest temperature was recorded in a well with 400 m depth from Precambrian gneiss. It might be related to thermal gradient in an area with high heat flow.
3.2.2. pH
Physicochemical properties in groundwater were different depending on each geologic unit. pH values in groundwater varied considerably between 5.97 and 8.45, indicating weak acidic to weak alkaline conditions. The majority of water samples were neutral, considering an average of 7.17 and a standard deviation of 0.61.
The pH value was highest in groundwater from Jeju Island volcanic rocks, whereas it was lowest in groundwater from Precambrian gneiss areas. The mean pH values were in the following order; Jeju Island volcanic rocks (7.70) > Ogcheon metamorphic rocks (7.59) > Cretaceous granite (7.20) > Jurassic granite (6.95) > Precambrian gneiss (6.77).
3.2.3. EC
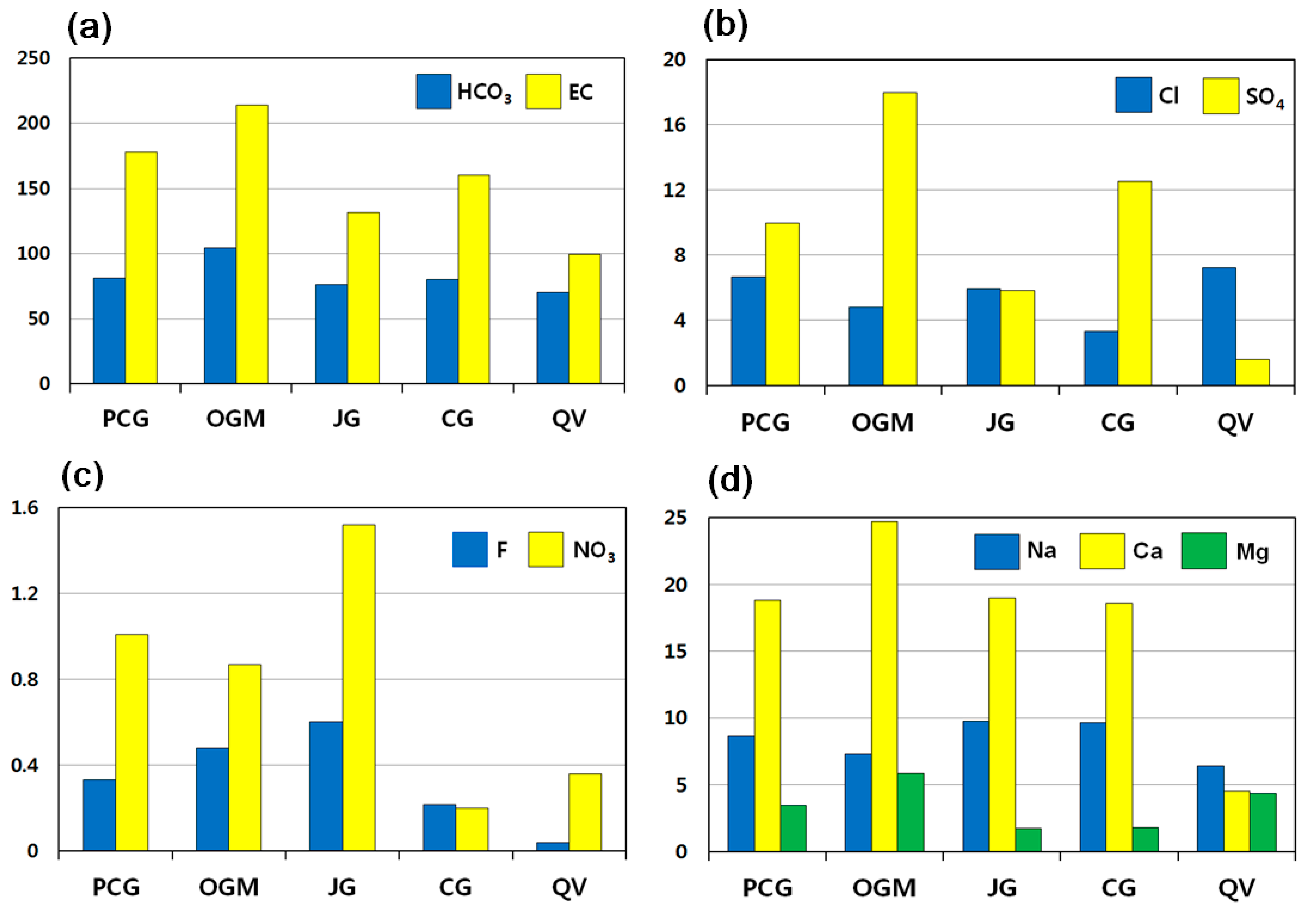
EC increased with increasing HCO3− in all samples. HCO3− served as the most significant contributor to EC, followed by SiO2 > Ca > SO4 > Na. The highest values of both EC and HCO3− were recorded in Ogcheon metamorphic rock area, followed by Precambrian gneiss > Cretaceous granite > Jurassic granite > Quaternary Jeju Island volcanic rocks in descending order (Figure 2a). The EC range of all samples was 37.5 to 344 μS/cm, with an average of 167.89 μS/cm and a standard deviation of 84.72 μS/cm. The mean EC value was highest in groundwater of Ogcheon metamorphic rocks (213.6 μS/cm), followed by Precambrian gneiss (177.8 μS/cm) > Cretaceous granite (160.4 μS/cm) > Jurassic granite (131.3 μS/cm) > Quaternary Jeju Island volcanic rocks (99.2 μS/cm). Groundwater in Jeju Island volcanic rocks has lowest EC because the island consists of permeable volcanic rocks such as basalt, scoria, and tuffaceous sedimentary rocks, which allow rapid percolation to prevent effective water–rock interactions. Therefore Jeju groundwater contains less mineralized water, compared to that of inland.
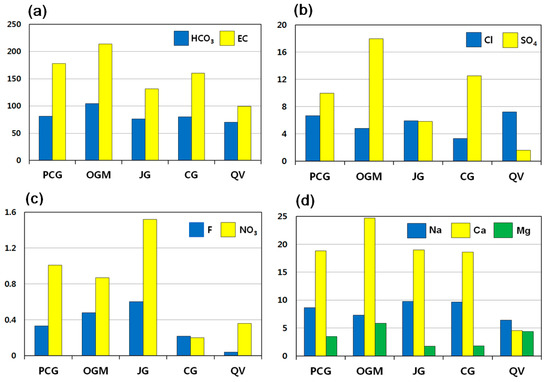
Figure 2.
Mean concentrations of chemical components in groundwater related to geology. (a) HCO3 and EC, (b) Cl and SO4, (c) F and NO3, and (d) Na, Ca, and Mg. PCG: Precambrian gneiss; OGM: Ogcheon metamorphic rocks; JG: Jurassic granite; CG: Cretaceous granite; QV: Quaternary Jeju Island volcanic rocks. EC as μS/cm. Unit in mg/L unless otherwise noted.
3.2.4. Anion Concentrations
Total anion concentrations are highest in groundwater from Ogcheon metamorphic rocks. Among anions, HCO3 is the most abundant species, followed by SO4 > Cl > F > NO3.
HCO3− values in all samples showed a wide range from 12.2 to 189.1 mg/L, with an average of 83.75 mg/L and a standard deviation of 44.96 mg/L. The mean HCO3− value is highest in groundwater of Ogcheon metamorphic rocks (104.5 mg/L), followed by Precambrian gneiss (81.40 mg/L) > Cretaceous granite (80.22 mg/L) > Jurassic granite (76.06 mg/L) > Quaternary Jeju Island volcanic rocks (70.15 mg/L). HCO3− is the most abundant species in groundwater with neutral pH conditions [27].
The Cl concentrations in all samples varied from 1.22 to 33.20 mg/L, with an average of 5.51 mg/L. As shown in Figure 2b, the mean values of Cl are highest in groundwater in Jeju Island volcanic rocks (7.24 mg/L), followed by Jurassic granite (5.93 mg/L) > Precambrian gneiss (5.84 mg/L) > Ogcheon metamorphic rocks (4.80 mg/L) > Cretaceous granite (3.33 mg/L). Chlorine is generally incorporated in (OH)-bearing minerals such as micas and amphibole. Otherwise, fluid inclusions trapped in hydrous rocks may contain Cl.
SO4 concentrations in groundwater ranged from 0.07 to 72.80 mg/L, with an average of 10.26 mg/L. SO4 was enriched in groundwater of Ogcheon metamorphic rocks, whereas it was depleted in the groundwater of Jeju Island volcanic rocks (Figure 2b). The mean values of SO4 were in the order of abundance as Ogcheon metamorphic rocks (17.95 mg/L) > Cretaceous granite (12.53 mg/L) > Precambrian gneiss (9.97 mg/L) > Jurassic granite (5.84 mg/L) > Jeju Island volcanic rocks (1.58 mg/L). Sulfate ions might be derived from dissolution of gypsum or sulfur minerals such as pyrite and chalcopyrite, or sedimentary rocks. Because gypsum rarely occurs in granitic bedrocks of bottled waters, gypsum cannot be a potential candidate for sulfate minerals in such crystalline rock aquifers.
The F concentrations in all samples ranged from 0.03 to 1.90 mg/L, with an average of 0.41 mg/L. It was enriched in groundwater from Jurassic granite, whereas it was depleted in groundwater from Jeju volcanic rocks (Figure 2c). The mean values of F were in the following order; Jurassic granite (0.60 mg/L) > Ogcheon metamorphic rocks (0.48 mg/L) > Precambrian gneiss (0.33 mg/L) > Cretaceous granite (0.22 mg/L) > Jeju Island volcanic rocks (0.04 mg/L). In a similar manner to Cl, F is also incorporated as replacement in (OH)-bearing minerals such as micas and amphibole. The concentration patterns show fairly good geological relations.
As shown in Figure 2c, NO3-N (nitrate nitrogen) concentration in groundwater was highest in Jurassic granite. Based on the Table 1 given above, 66.7% are below 1 mg/L, and 16.6% are higher than 2 mg/L. The nitrate concentrations in all samples averaged 1.03 mg/L. The mean values, in order of abundance are as follows; Jurassic granite (1.52 mg/L) > Precambrian gneiss (1.01 mg/L) > Ogcheon metamorphic rocks (0.87 mg/L) > Jeju Island volcanic rocks (0.36 mg/L) > Cretaceous granite (0.20 mg/L). It was found that all samples were quite safe, much lower than the guideline for drinking water of Korea (10 mg/L) [28]. Nevertheless, a few manufactures need improvement for protection of wells. Nitrate concentration in groundwater is attributed to non-lithological sources [9,29]. According to stable isotopic studies on groundwater for bottled waters [30], the nitrogen isotope data ranged from −11.8 to −5.1‰ δ15N, which suggested that NO3-N in the groundwater might be originated from atmospheric nitrate and ammonium fertilizers rather than manure or organic nitrate.
3.2.5. Cation Concentrations
The mean value of total cations was highest in groundwater from Ogcheon metamorphic rocks, which largely contribute to EC, together with anions to EC. Besides SiO2, cation concentrations were in order of abundance, Ca > Na > Mg > K.
The SiO2 concentrations in all samples ranged from 7.47 to 54.60 mg/L, with an average of 23.04 mg/L. Its mean value was highest in groundwater of Cretaceous granite (38.08 mg/L), followed by Jeju Island volcanic rocks (33.20 mg/L) > Jurassic granite (22.55 mg/L) > Precambrian gneiss (20.76 mg/L) > Ogcheon metamorphic rocks (20.45 mg/L). Silica solubility increases in alkaline solution, whereas it is low in acidic to neutral pH conditions [27,31]. The behavior of silica in solutions is strongly influenced by the solubility of quartz, amorphous silica and Si-bearing minerals [32,33,34]. As shown in Figure 2d, concentrations of some major cations such as Na, Ca, and Mg reflect geological differences. That is, Na is enriched in groundwater from granitic rocks such as Precambrian gneiss and Mesozoic granites, whereas Mg is enriched in Ogcheon metamorphic rocks and Jeju Island volcanic rocks. Characteristics of cation concentrations are described in more detail below.
The concentration range of Ca in all samples was 10.20 to 51.90 mg/L, with an average of 19.44 mg/L. Ca was enriched in groundwater from Ogcheon metamorphic rocks, whereas it was depleted in Jeju volcanic rocks. The mean values, in order of abundance, are as follows; Ogcheon metamorphic rocks (24.71 mg/L) > Jurassic granite (18.97 mg/L) > Precambrian gneiss (18.81 mg/L) > Cretaceous granite (18.57 mg/L) > Jeju Island volcanic rocks (4.58 mg/L). Ca is commonly enriched in calcite, dolomite, gypsum, apatite, and Ca-bearing silicate minerals. The former three minerals have quite high Ca concentrations, occurring in carbonates or evaporites as sedimentary rocks. Ca-bearing silicate minerals include plagioclase, Ca-amphibole such as tremolite and actinolite, and Ca-pyroxene such as diopside and hedenbergite, which are easily found in granites and metamorphic rocks. It is well known that the dissolution rate of calcite is fastest among minerals [35]. In this study for bottled waters, sedimentary or evaporate aquifers cannot be the candidate for Ca source in groundwater because there is no bottled water manufactures in those bedrocks in Korea.
The Na concentrations in all samples ranged from 1.69 to 23.60 mg/L, with an average of 8.71 mg/L. Na is enriched in granitic aquifers, whereas it is depleted in Jeju volcanic rocks: Jurassic granite (9.77 mg/L) > Cretaceous granite (9.64 mg/L) > Precambrian gneiss (8.65 mg/L) > Ogcheon metamorphic rocks (7.28 mg/L) > Jeju Island volcanic rocks (6.41 mg/L). Na is readily leached from albite plagioclase because it is one of the most common constituents in granites. The rate of dissolution of albite is influenced by pH, dissolved Al concentration, and reaction time between feldspar and the aqueous solution [36].
The Mg concentrations in all samples ranged from 0.24 to 19.30 mg/L, with an average of 3.35 mg/L. The mean values, in order of abundance, are as follows; Ogcheon metamorphic rocks (5.85 mg/L) > Jeju Island volcanic rocks (4.41 mg/L) > Precambrian gneiss (3.47 mg/L) > Cretaceous granite (1.83 mg/L) > Jurassic granite (1.76 mg/L). Mg is enriched in some minerals, such as dolomite, high Mg-calcite, biotite, amphibole, and olivine, which are relatively common in the Ogcheon metamorphic terrain.
The K concentrations in all samples ranged from 0.10 to 2.56 mg/L, with an average of 1.02 mg/L. The mean values, in order of abundance, are as follows; Jeju Island volcanic rocks (2.34 mg/L) > Precambrian gneiss (1.21 mg/L) > Ogcheon metamorphic rocks (1.18 mg/L) > Jurassic granite (0.64 mg/L) > Cretaceous granite (0.42 mg/L). Potassium is enriched in K-bearing minerals, such as muscovite and K-feldspar, which are commonly incorporated in granitic rocks.
3.2.6. Water Types of Groundwater
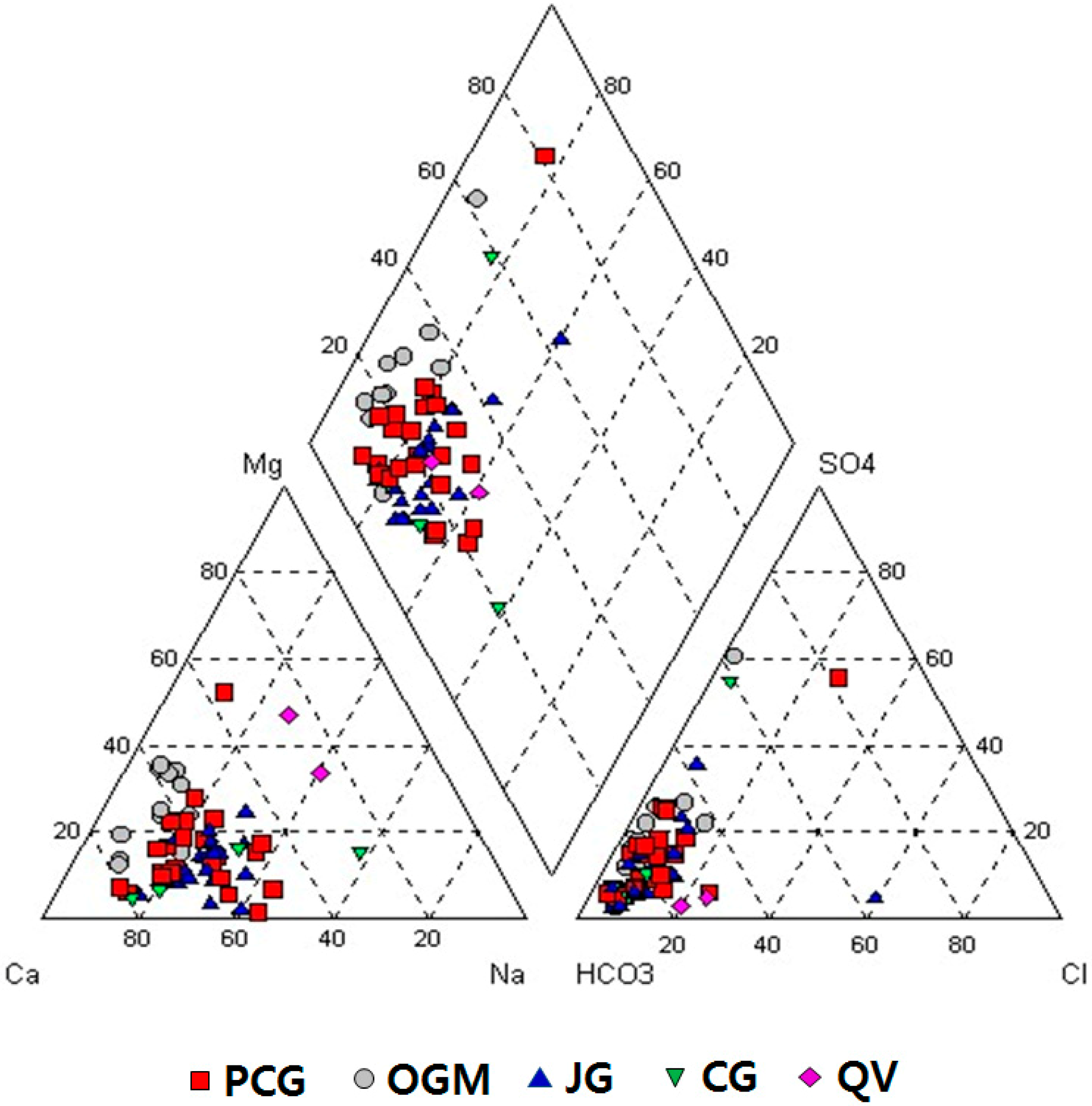
Chemical differences are found among the groundwater of the various rock types. Groundwater types, classified by the Piper plot, showed that the Ca-HCO3 type is predominant, though other types such as Ca-Na-HCO3, Ca-Mg-HCO3, Na-HCO3, and Ca-SO4(Cl) can be recognized (Figure 3).
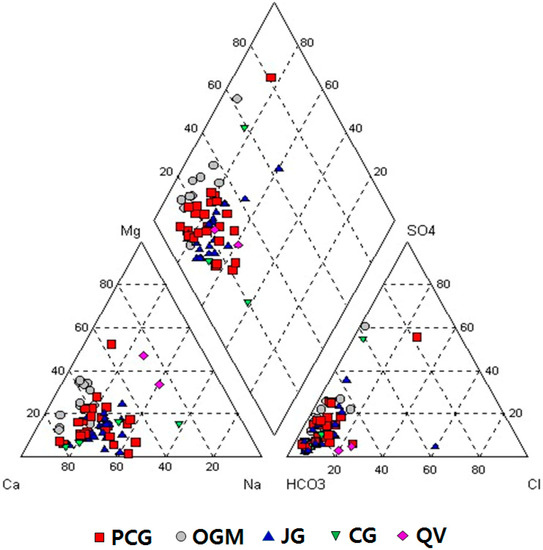
Figure 3.
Water types plotted on the Piper diagram. PCG: Precambrian gneiss, OGM: Ogcheon metamorphic rocks, JG: Jurassic granite, CG: Cretaceous granite, QV: Quaternary Jeju Island volcanic rocks.
Groundwater in Precambrian gneiss has mostly Ca-HCO3 type, but some show Ca-Mg-HCO3, Ca-Na-HCO3 types. One belongs to Ca-SO4(Cl) type due to dissolution of sulfate that is the dominant anion in the intermediate zone of aquifers.
Groundwater in Ogcheon metamorphic rocks showed quite different water types from other geologic units, that is, Ca-Mg-HCO3 type is common, though Ca-HCO3-SO4 type is rarely found. This result is can be explained by the fact that Ogcheon metamorphic rocks have some calcareous compositions, so that HCO3, Ca, and Mg concentrations in groundwater are also high. The Ca and Mg could be easily released from calc-mafic silicate minerals and carbonates such as dolomite.
Groundwater in Jurassic granite showed mostly Ca-HCO3 type, but other types, such as Ca-Na-HCO3 and Ca-Na-HCO3-SO4(Cl), were also found. Groundwater in Cretaceous granite had mostly Ca-Na-HCO3 type and Na-Ca-HCO3, but Ca-HCO3-SO4(Cl) types were also identified. Ca and Na might be related to dissolution of feldspar enriched in granite.
Groundwater in Jeju Island volcanic rocks showed different water types, though only two manufactures are present in the island, where both are ~4 km apart from each other: Na-Mg-HCO3 at the one site with a shallower water level and Mg-Ca-Na-HCO3 at the other site with a deeper water level.
Groundwater tends to evolve with increasing flow path, age, and water–rock interaction as hydrochemical facies, therefore, dissolved solids concentration in groundwater also increase along flow paths, through the aquifer because of dissolution of minerals [37,38]. The Ca-Na-HCO3 type commonly observed in this study may be due to dissolution of feldspars in granitic rocks. The Na-HCO3 type is generally related to silicate dissolution in granitic rocks [9,39,40]. The Ca-Mg-HCO3 type found in Ogcheon metamorphic rocks might be related to recharge aquifer or dissolution effect of calc-silicate minerals [41,42]. The majority of European bottled waters are classified as of Ca-HCO3 type, because of the widespread carbonate lithologies [6,40]. Water containing high bicarbonate is generally present in the upper aquifer zone where active groundwater flushing through relatively well-leached rocks, water containing high sulfate is present where groundwater circulation is less active and higher total dissolved solids, and old water containing high Cl is generally present where groundwater flow is very sluggish and flushing of the aquifer is minimal [37,38,43]. NaCl facies in crystalline rocks including gneiss and metamorphic rocks indicates prolonged water–rock interaction and matured stage of water condition [44].
3.3. Correlation Coefficients
Table 2 illustrates that correlation coefficients between chemical species are variable depending on geology. Groundwater in the Precambrian aquifer shows that the correlation coefficient between EC-HCO3 (0.94) is highest among the elements studied. EC-Ca (0.90), Ca-Cl (0.85), Ca-HCO3 (0.84), Cl-SO4 (0.80), Na-F (0.73), EC-Na (0.73), Mg-HCO3 (0.78), EC-Cl (0.79), and EC-SO4 (0.71) have high correlation coefficients. The relations between EC-Mg (0.69), Cl-HCO3 (0.64), Na-Ca (0.63), Na-Cl (0.65), Na-HCO3 (0.62) also are high.

Table 2.
Correlation matrix between physicochemical components in groundwater for bottled waters. PCG: Precambrian gneiss; OGM: Ogcheon metamorphic rocks; JG: Jurassic granite.
Groundwater in Ogcheon metamorphic aquifer shows high correlations. The relation between Ca-SO4 (0.89) is the highest among the elements studied. EC-Ca (0.88), EC-HCO3 (0.85), EC-Mg (0.82), Mg-HCO3 (0.81), EC-K (0.73), EC-Na (0.73), EC-SO4 (0.74), K-SiO4 (0.70), Na-Mg (0.69), Na-Cl (0.65), and Ca-HCO3 (0.61) also show relatively good relationships. These factors that affect hardness or water types may be attributed to geochemical sources such as Ca-bearing minerals and anhydrite or gypsum. These elements are enriched in metamorphic rocks and granite. In reality, there is no possibility that evaporate minerals such as anhydrite or gypsum play a main role in water chemistry of granitic rocks in Korea because these are very rare minerals in geologic units mentioned earlier. NO3 is inversely related with pH, with correlation coefficient of −0.85.
The groundwater in the Daebo granite area shows that Mg-SO4 (0.81) is the highest among elements studied. Mg-SO4 (0.81), Na-SO4 (0.71), Na-HCO3 (0.64), EC-F (0.62), SO4-NO3 (0.63), and pH-SiO2 (−0.61) also show relatively good correlations. This suggests the results from water–rock interaction in silicate-rich rocks. The association suggests the entry of possible anthropogenic or organic matters into groundwater. However, as explained above, the origin of nitrogen into groundwater needs further to be sought, not by simple contaminant sources such as manure or organic nitrate [30]. The groundwater nitrate concentrations were greater in the moderately sloped parts of the areas where agriculture was intensive and denitrification limited [29].
Groundwater in Cretaceous granite aquifer showed some high correlations (not shown in Table 2 because of small numbers in dataset samples). High correlations were found between some components: EC-Ca (0.98), pH-SO4 (0.97), Mg-F (0.95), K-Cl (0.90), Na-Mg (0.84), Na-F (0.76), pH-Cl (−0.90), Cl-SO4 (−0.83), Ca-SiO2 (−0.81), and pH-Na (−0.77). Highly positive correlations for alkali and alkaline earth elements such as K, Na, Ca, Mg with HCO3 are mostly related to water–rock interaction of silicate minerals and carbonate minerals [42]. Especially, sodium concentration is attributed to dissolution of albite plagioclase [43]. These lithological properties are highly attributed to water–rock interactions in granitic rocks or metamorphic rocks in Korea. Na, F, and Cl may be attributed to geochemical processes by dissolution of minerals such as micas and albite plagioclase in granitic rocks, rather than salinity or contaminants. F and Cl are commonly incorporated in micas as structural components and Na is enriched in albite plagioclase.
The groundwater evolved toward the lower zone of aquifers, or the groundwater moved sluggish became saline with increasing age during the late stage of groundwater facies [39]. Furthermore, it should be noted that there is no bottled water manufacture in areas of sedimentary rocks or coastal regions. Therefore, it is interpreted that Na, F, and Cl elements in groundwater were derived from the water–rock interaction in granitic rocks. Statistical results allow the determination of the relationships between the chemical components in groundwater and lithology [6,8,45].
3.4. Groundwater Quality in Relation to Lithology
Highly positive correlations for alkali and alkaline earth elements, such as K, Na, Ca, and Mg, with HCO3 are mostly related to water–rock interaction of silicate minerals and carbonate minerals. Since bottled mineral waters are derived from groundwater aquifers, their chemical composition is originally defined by geochemical water–rock interaction processes [7,9,45].
The geochemical properties of groundwater for bottled waters in Korea show different types in relation to lithology. Different lithological features naturally control the hydrochemical properties of bottled waters in many countries due to geological environments [40,46]. As mentioned earlier, there is no bottled water manufacture in areas of sedimentary rocks because of unfavorable water taste, high SO4, and EC. Most manufactures for bottled waters in Korea are located in areas of granitic rocks and metamorphic rocks, except for Jeju Island. In general, the water chemistry of groundwater for bottled waters is characterized by low mineralization. It is worth emphasizing that one of the most popular bottled water brands in Korea is located in Jeju volcanic island. It takes more than 30% of the total market in Korea, possibly due to the notion that the island composed of volcanic rocks may preserve remarkably clean groundwater with a pollution-free environment.
The quality of groundwater used for bottled waters should be strictly monitored in order to ensure safe drinking water, although hydrochemical contaminant levels in groundwater are far below the standards for drinking water quality regulated by the Korea legislation [28]. For the several manufacturers with high nitrogen concentrations, special care should be taken to prevent aquifers from the potential entry of nitrogen and trace contaminants into groundwater to ensure groundwater of high quality. Further geochemical studies on groundwater source using stable isotopes and trace elements are needed to better understand the water–rock interactions and potential inflow of contaminants into aquifer for bottled waters.
4. Conclusions
The geochemical properties of groundwater for bottled waters in Korea show different types in relation to lithology. Most groundwater types classified by the Piper plot are Ca-HCO3 and Ca-Na-HCO3, and Ca-Mg-HCO3 because the majority of bed rocks are composed of granite, granitic gneiss, and calcareous metasedimentary rocks. The groundwater chemistry was controlled by water–rock interaction, except nitrate. High correlations for alkali and alkaline earth elements such as K, Na, Ca, and Mg with HCO3 are mostly related to water–rock interactions of silicate minerals and carbonate minerals. We established relationships between groundwater compositions and bedrock geology in Korea.
Author Contributions
C.O.C. and B.D.L. prepared the article. Y.H.O., B.W.C., and U.Y. were responsible for field works, sample preparation, and data collection.
Funding
This research was financially supported by the Basic Research Project (193411), the Korea Institute of Geoscience and Mineral Resources (KIGAM), funded by the Ministry of Science and ICT of Korea.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Reimann, C.; Birke, M. Geochemistry of European Bottled Water; Gebr. Borntraeger Science Publishers: Stuttgart, Germany, 2010. [Google Scholar]
- Ministry of Environment (MOE). Management Legislation on Natural Mineral Water; Ministry of Environment: Sejong, Korea, 2011.
- Kończyk, J.; Muntean, E.; Gega, J.; Frymus, A.; Michalski, R. Major inorganic anions and cations in selected European bottled waters. J. Geochem. Expl. 2019, 197, 27–36. [Google Scholar] [CrossRef]
- Mordor Intelligence. Available online: https://www.mordorintelligence.com/industry-reports/europe-bottled-water-market (accessed on 1 April 2019).
- Ikem, A.; Odueyungbo, S.; Egiebor, N.O.; Nyavor, K. Chemical quality of bottled waters from three cities in eastern Alabama. Sci. Total Env. 2002, 285, 165–175. [Google Scholar] [CrossRef]
- Dinelli, E.; Lima, A.; De Vivo, B.; Albanese, S.; Cicchella, D.; Valera, P. Hydrogeochemical analysis on Italian bottled mineral waters: Effects of geology. J. Geochem. Expl. 2010, 107, 317–335. [Google Scholar] [CrossRef]
- Fugedi, U.; Kuti, L.; Jordan, G.; Kerek, B. Investigation of the hydrogeochemistry of some bottled mineral waters in Hungary. J. Geochem. Expl. 2010, 107, 305–316. [Google Scholar] [CrossRef]
- Lourenço, C.; Ribeiro, L.; Cruz, J. Classification of natural mineral and spring bottled waters of Portugal using principal component analysis. J. Geochem. Expl. 2010, 107, 362–372. [Google Scholar] [CrossRef]
- Bulia, I.L.; Enzweiler, J. The hydrogeochemistry of bottled mineral water in São Paulo state, Brazil. J. Geochem. Expl. 2018, 188, 43–54. [Google Scholar] [CrossRef]
- Brenčič, M.; Ferjan, T.; Gosar, M. Geochemical survey of Slovenian bottled waters. J. Geochem. Expl. 2010, 107, 400–409. [Google Scholar] [CrossRef]
- Lee, B.D. Integrated management system of natural mineral water information. In Proceedings of the Korea Society of Engineering Geology Conference, Gyeongju, Korea, 8–9 April 2010. [Google Scholar]
- Ministry of Environment (MOE). A Study on Characterization Plan of Natural Mineral Water; Ministry of Environment: Sejong, Korea, 2011.
- Ministry of Environment (MOE). Management System of Natural Mineral Water (III); Ministry of Environment: Seoul, Korea, 2000.
- Ministry of Environment (MOE). Regulations and Manual of Drinking Water; Ministry of Environment: Sejong, Korea, 2017.
- Turek, A.; Kim, C.-B. U-Pb zircon ages for Precambrian rocks in southwestern Ryeongnam and southwestern Gyeonggi massifs, Korea. Geochem. J. 1996, 30, 231–249. [Google Scholar] [CrossRef]
- Cho, D.L.; Kwan, S.T. Hornblende geobarometer of the Mesozoic granitoids in South Korea and the evolution of crustal thickness. J. Geol. Soc. Korea 1994, 30, 41–61. [Google Scholar]
- Oh, C.W.; Kim, S.W.; Ryu, I.C.; Okada, T.; Hyodo, H.; Itaya, T. Tectono-metamorphic evolution of the Okcheon metamorphic belt, South Korea: tectonic implications in East Asia. Islan. Arc 2004, 123, 387–402. [Google Scholar] [CrossRef]
- Cho, M.; Cheong, W.; Ernst, W.G.; Yi, K.; Kim, J. SHRIMP U-Pb ages of detrital zircons in metasedimentary rocks of the central Ogcheon fold-thrust belt, Korea: Evidence for tectonic assembly of Paleozoic sedimentary protoliths. J. Asian Ear. Sci. 2013, 63, 234–249. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, S.-I.; Jang, Y.; Kwon, S.; Kim, S.J.; Santosh, M. Tracking Paleozoic evolution of the south Korean peninsula from detrital zircon records: Implications for the tectonic history of East Asia. Gondwana Res. 2017, 50, 195–215. [Google Scholar] [CrossRef]
- Uchida, E.; Choi, S.-G.; Baba, D.; Wakisaka, Y. Petrogenesis and solidification depth of the Jurassic Daebo and Cretaceous Bulguksa granitic rocks in South Korea. Resour. Geol. 2012, 62, 281–295. [Google Scholar] [CrossRef]
- Jwa, Y.J. Possible source rocks of Mesozoic granites in South Korea: Implications for crustal evolution in NE Asia. Trans. R. Soc. Edinb. Earth Sci. 2004, 95, 181–198. [Google Scholar]
- Tsusue, A.; Mizuta, T.; Watanabe, M.; Min, K.G. Jurassic and Cretaceous granitic rocks in South Korea. Min. Geol. 1981, 31, 261–280. [Google Scholar]
- Brenna, M.; Cronin, S.J.; Smith, I.E.M.; Sohn, Y.K. Spatio-temporal evolution of a dispersed magmatic system and its implications for volcano growth, Jeju Island Volcanic Field, Korea. Lithos 2012, 148, 337–352. [Google Scholar] [CrossRef]
- Sohn, Y.K.; Park, K.H. Early-stage volcanism and sedimentation of Jeju Island revealed by the Sagye borehole, SW Jeju Island, Korea. Geosci. J. 2004, 8, 73–84. [Google Scholar] [CrossRef]
- Won, J.H.; Lee, J.Y.; Kim, J.-W.; Koh, G.-W. Groundwater occurrence on Jeju Island, Korea. Hydrogeol. J. 2006, 14, 532–547. [Google Scholar] [CrossRef]
- Won, J.H.; Kim, J.W.; Koh, G.W.; Lee, J.Y. Evaluation of hydrogeological characteristics in Jeju Island, Korea. Geosci. J. 2005, 9, 33–46. [Google Scholar] [CrossRef]
- Drever, J.I. The Geochemisty of Natural Waters, 2nd ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1988. [Google Scholar]
- Ministry of Environment (MOE). Standards for Drinking Water Quality. Ministry of Environment of Korea. Legislation 792 (acted on 1 January 2019). Available online: http://www.law.go.kr/%20lsInfoP.do?%20urlMode=lsInfoP&lsId=007134#0000. (accessed on 20 April 2019).
- Akale, A.T.; Moges, M.A.; Dagnew, D.C.; Tilahun, S.A.; Steenhuis, T.S. Assessment of nitrate in wells and springs in the north central Ethiopian highlands. Water 2018, 10, 476. [Google Scholar] [CrossRef]
- Choo, C.O.; Lee, B.D.; Cho, B.W.; Sung, I.H.; Chi, S.J. Nitrate contamination of confined groundwaters: Application of nitrogen, oxygen, and hydrogen isotopes. J. Eng. Geol. 2002, 12, 285–294. [Google Scholar]
- White, A.F.; Brantley, S.L. Chemical weathering rates of silicate minerals: An overview. In Chemical Weathering Rates of Silicate Minerals; White, A.F., Brantley, S.L., Eds.; Mineralogical Society of America: Washington, DC, USA, 1995; Reviews in Mineralogy; Volume 31, pp. 1–22. [Google Scholar]
- Fournier, R.O.; Rowe, J.J. The solubility of amorphous silica in water at high temperatures and high pressures. Am. Miner. 1977, 62, 1052–1056. [Google Scholar]
- Rimstidt, J.D.; Barnes, H.L. The kinetics of silica-water reactions. Geochim. Cosmochim. Acta 1980, 44, 1683–1699. [Google Scholar] [CrossRef]
- Rimstidt, J.D. Quartz solubility at low temperatures. Geochim. Cosmochim. Acta 1997, 61, 2553–2558. [Google Scholar] [CrossRef]
- Lasaga, A.C. Chemical kinetics of water-rock interactions. J. Geophy. Res. 1984, 89, 4009–4025. [Google Scholar] [CrossRef]
- Chou, L.; Wollast, R. Steady-state kinetics and dissolution mechanisms of albite. Am. J. Sci. 1985, 285, 963–993. [Google Scholar] [CrossRef]
- Chebotarev, I.I. Metamorphism of natural waters in the crust of weathering-1. Geochim. Cosmochim. Acta 1955, 8, 22–48. [Google Scholar] [CrossRef]
- Freeze, R.A.; Cherry, J.A. Groundwater; Prentice Hall Inc.: Englewood Cliffs, NJ, USA, 1979. [Google Scholar]
- Frengstad, B.; Banks, D.; Skrede, A.M.; Krog, J.R.; Siewers, U.; Strand, T. The hydrochemistry of crystalline bedrock groundwater in Norway. NGU Bull. 2002, 439, 87–98. [Google Scholar]
- Demetriades, A.; Reimann, C.; Birke, M. The geological atlas of European ground water with emphasis on Hellas. Bull. Geol. Soc. Greece 2012, 46, 39–80. [Google Scholar] [CrossRef]
- Ishaku, J.M. Hydrochemical Evolution of Groundwater in Jimeta-Yola Area, Northeastern Nigeria. Global J. Geol. Sci. 2011, 9, 99–121. [Google Scholar]
- Ravikumar, P.; Somashekar, R.K. Principal component analysis and hydrochemical facies characterization to evaluate groundwater quality in Varahi river basin, Karnataka state, India. Appl. Water Sci. 2017, 7, 745–755. [Google Scholar] [CrossRef]
- Ray, R.K.; Mukherjee, R. Hydrochemical evolution of groundwater in the phreatic aquifers of Chhattisgarh. J. Geol. Soc. India 2008, 72, 405–414. [Google Scholar]
- Saravanan, K.; Srinivasamoorthy, K.; Prakash, R.; Gopinath, S.; Suma, C.S. An evaluation of hydrogeochemistry of groundwater in upper Vellar sub–basin using mineral stability and solute transport modelling. Aqua. Procedia 2015, 4, 1119–1125. [Google Scholar] [CrossRef]
- Oyebog, S.A.; Ako, A.A.; Nkeng, G.E.; Suh, E.C. Hydrogeochemical characteristics of some Cameroon bottled waters, investigated by multivariate statistical analyses. J. Geochem. Expl. 2012, 112, 118–130. [Google Scholar] [CrossRef]
- Misund, A.; Frengstad, B.; Sewersd, U.; Reimann, C. Variation of 66 elements in European bottled mineral waters. Sci. Total Envion. 1999, 243–244, 21–41. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).