Treatment of Aquaculture Wastewater through Chitin/ZnO Composite Photocatalyst
Abstract
:1. Introduction
- broadening the responsive light zone to improve the utilization of solar light or UV;
- prohibiting the recombination of light-based electrons and catalyst holes (e−–h+), and enhancing the quantitative efficiency of the semiconductor photocatalyst;
- having an absorption capacity that plays a critical role in physical and chemical reactions.
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Photocatalyst
2.2.1. ZnO Photocatalyst Preparation
2.2.2. Chitin/ZnO Photocatalyst Preparation
2.3. Preparation of the Simulated Aquaculture Wastewater
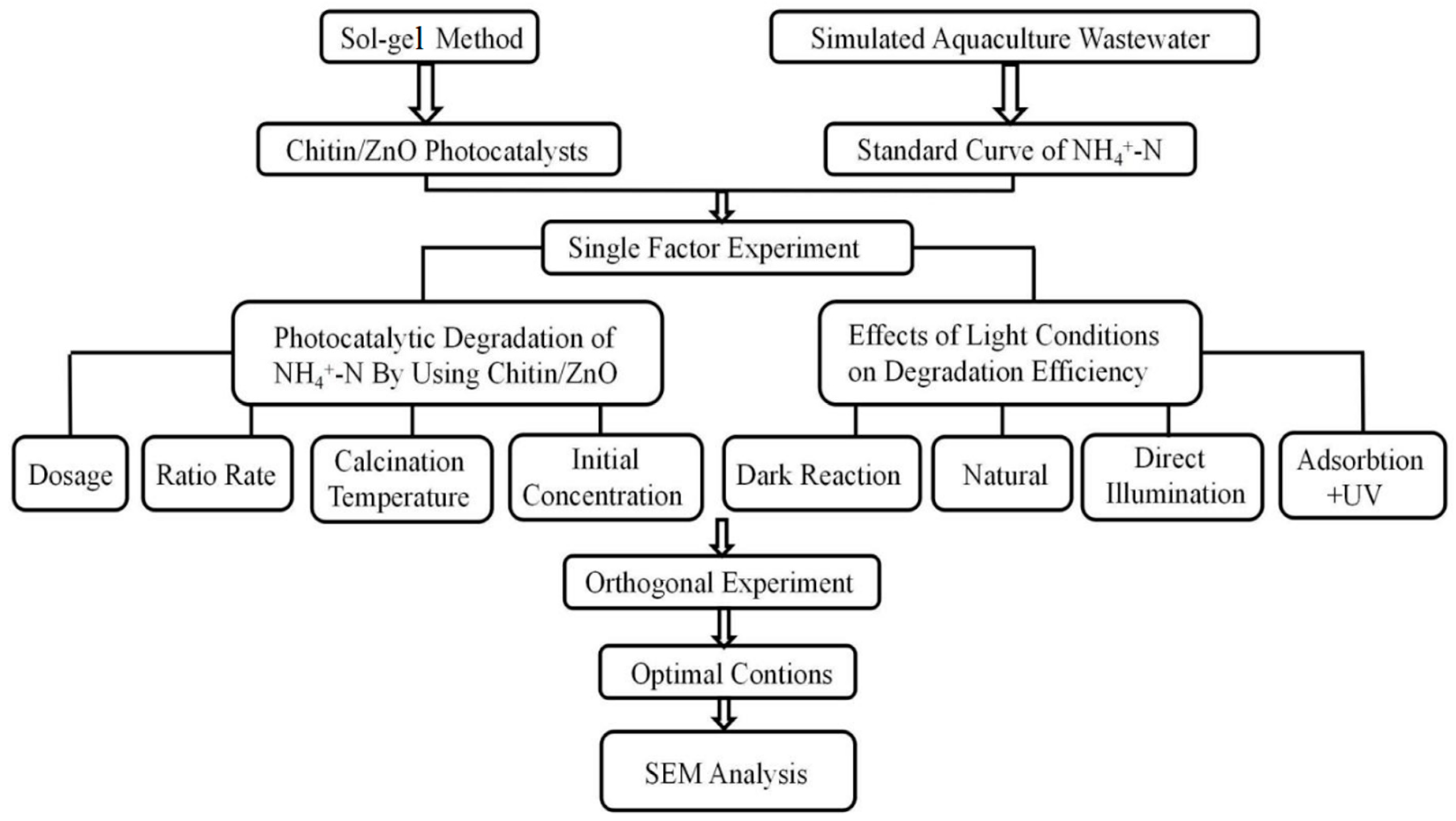
3. Results and Discussions
3.1. Degradation of Aquaculture Wastewater by the Chitin–ZnO Composite Photocatalyst
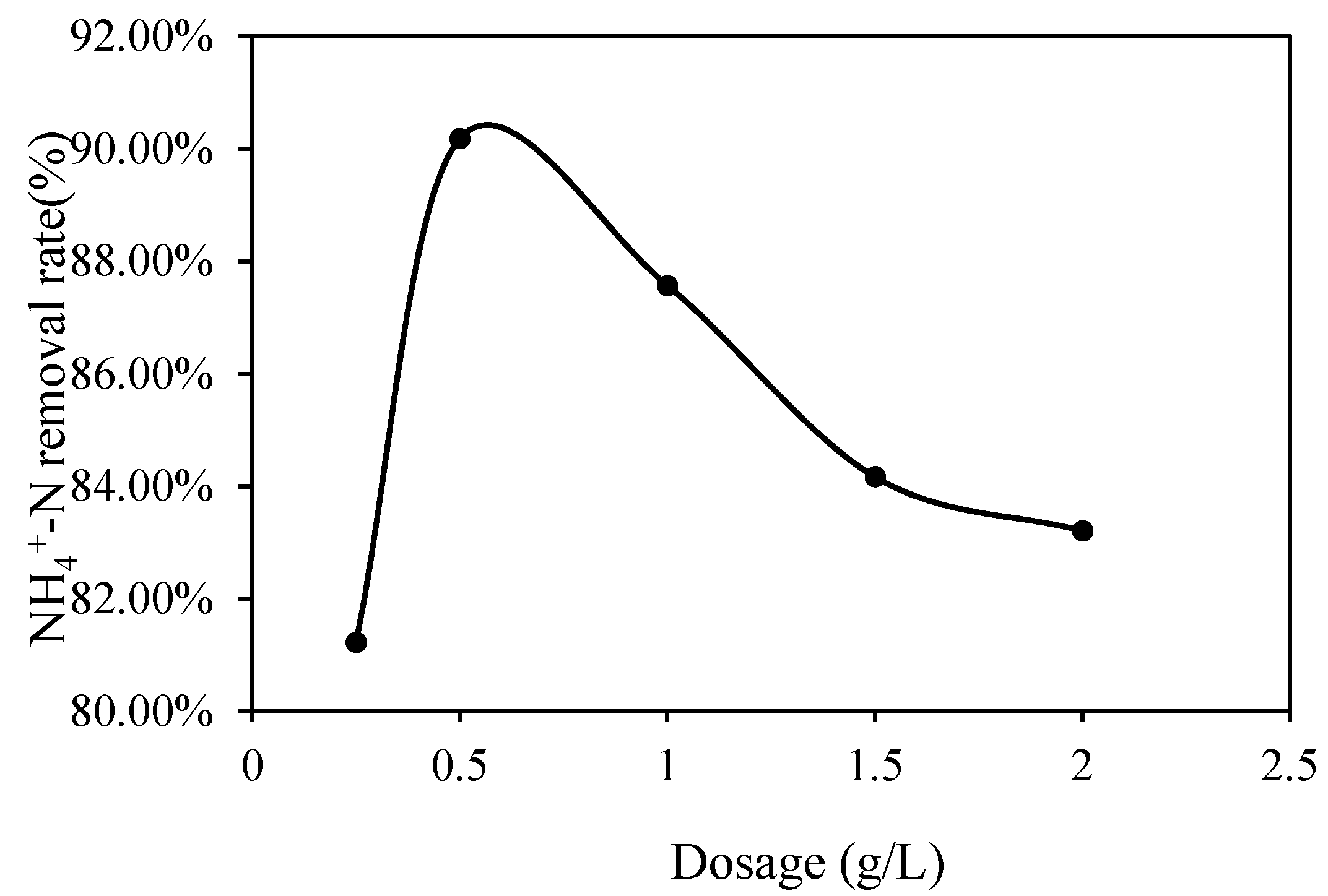
3.1.1. Effect of the Chitin–ZnO Dosage
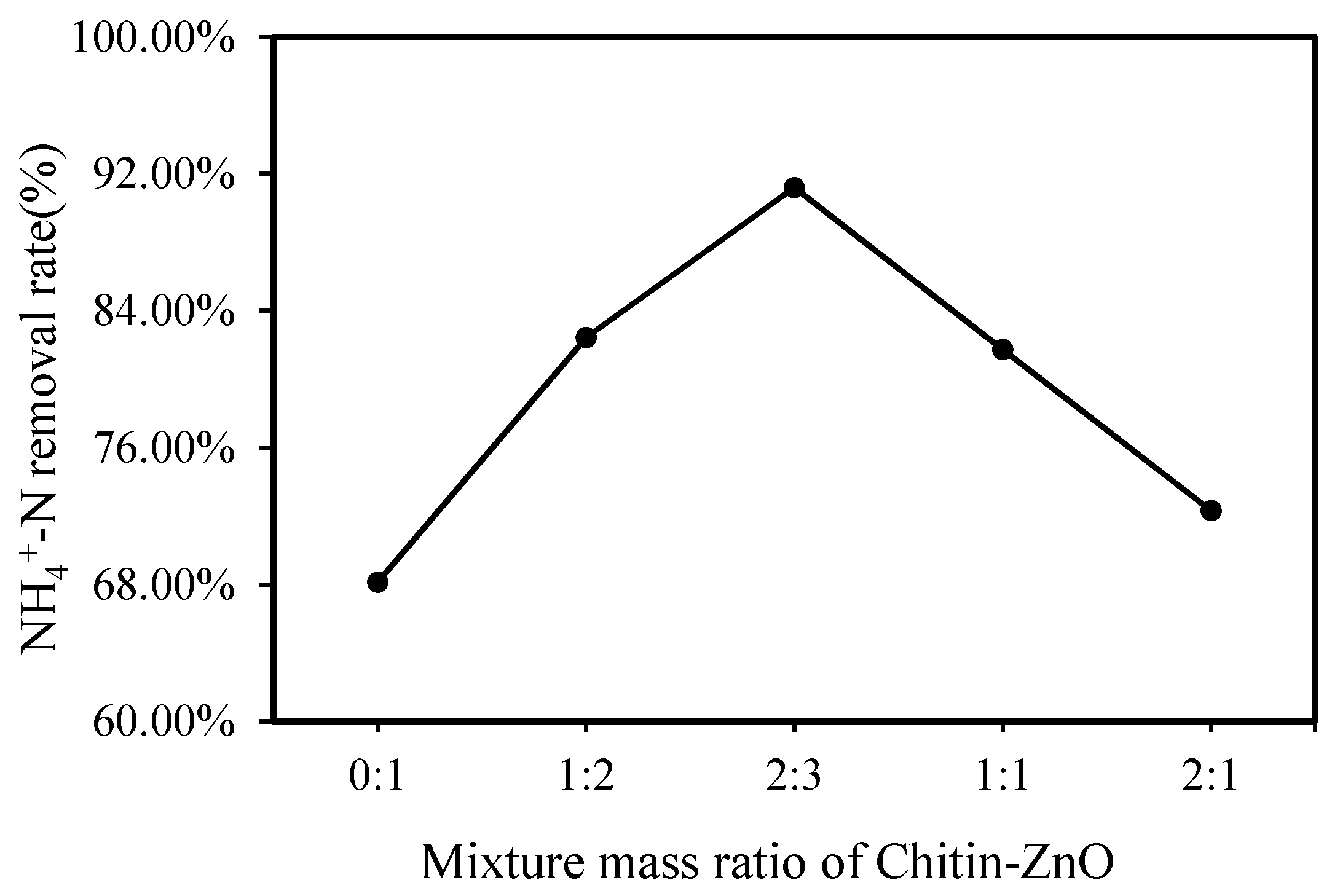
3.1.2. Effect of Mixture Mass Ratio
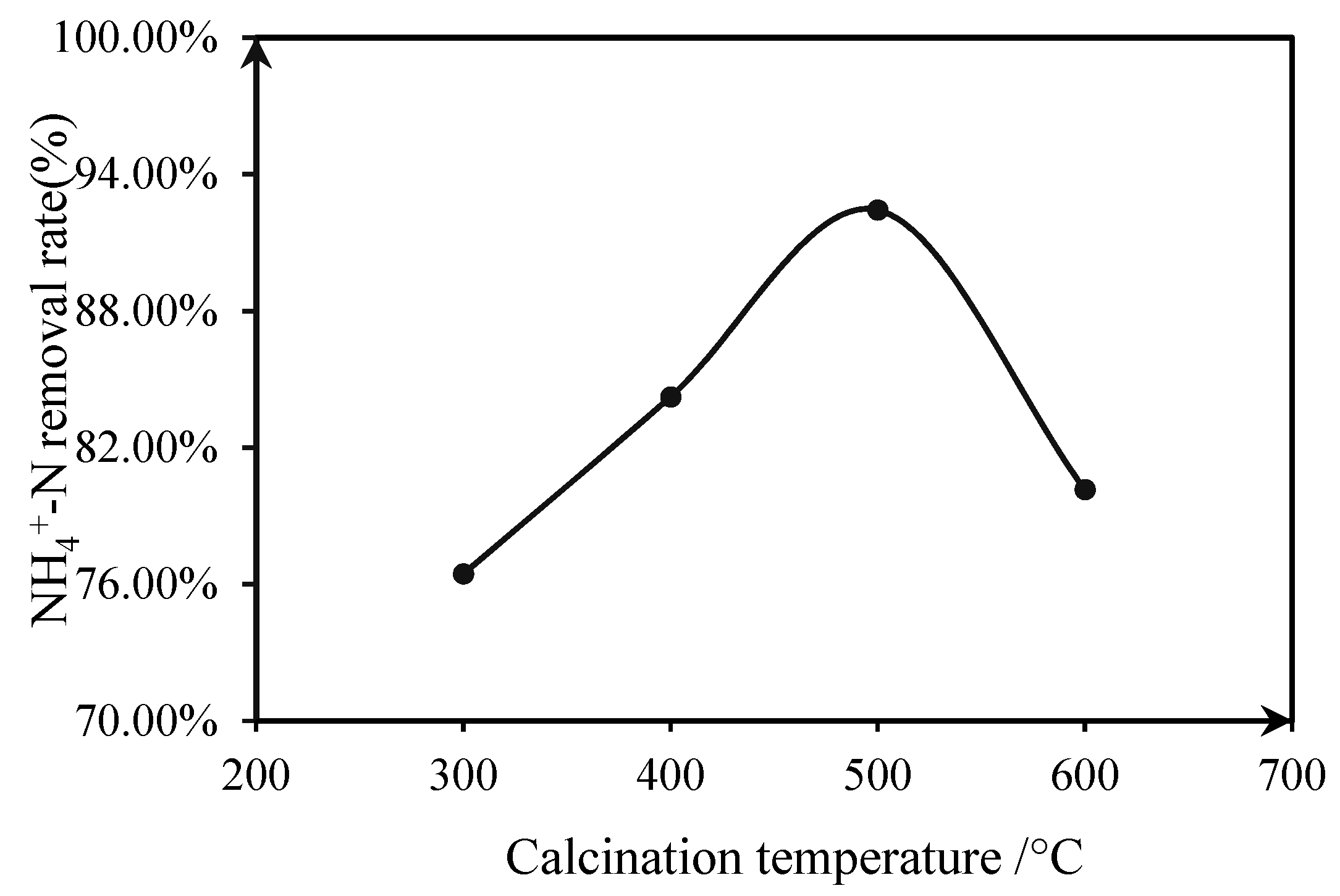
3.1.3. Effect of Calcination Temperature
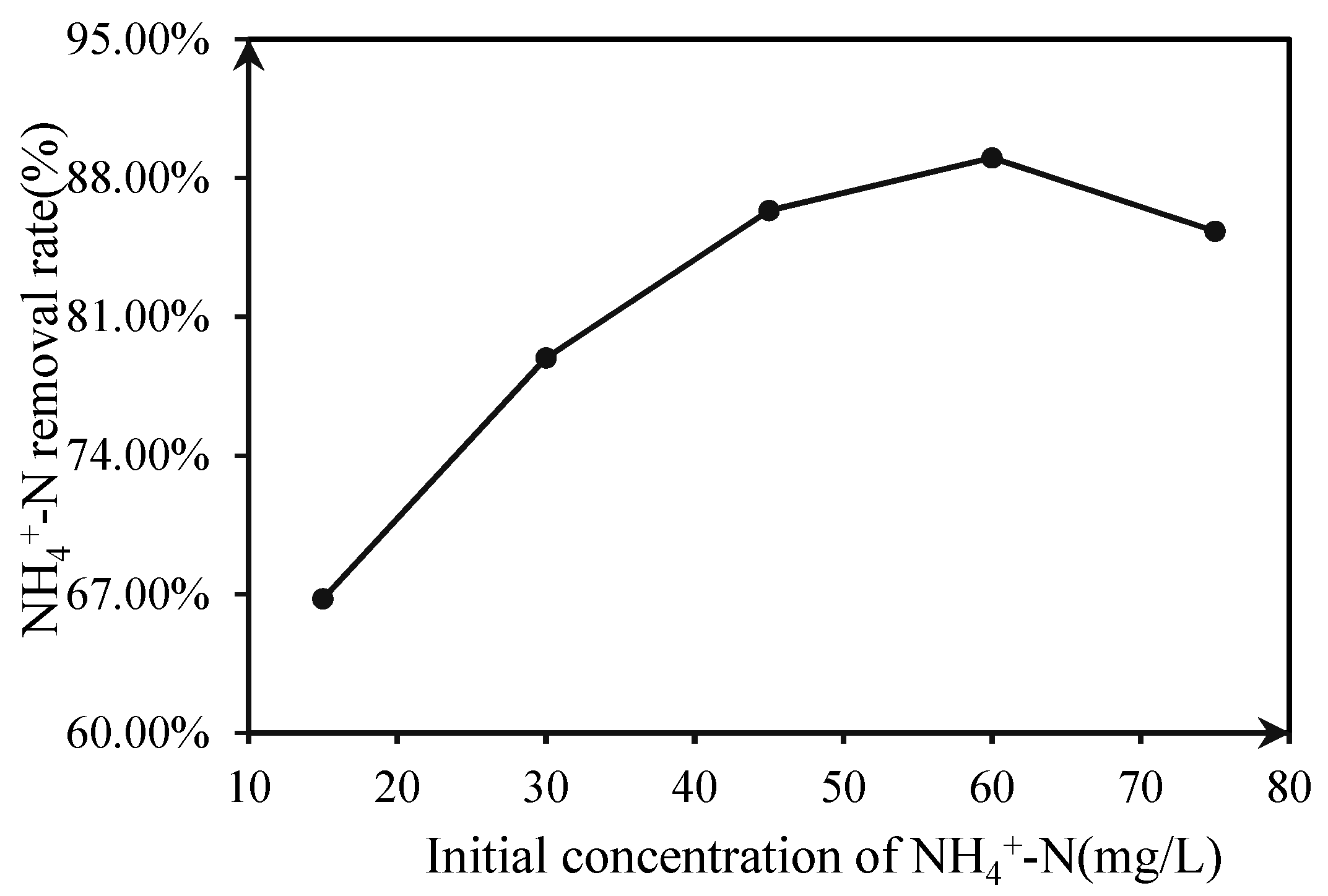
3.1.4. Effect of the Initial Concentration of NH4+–N
3.2. Effect of Light Conditions on Photocatalytic Degradation
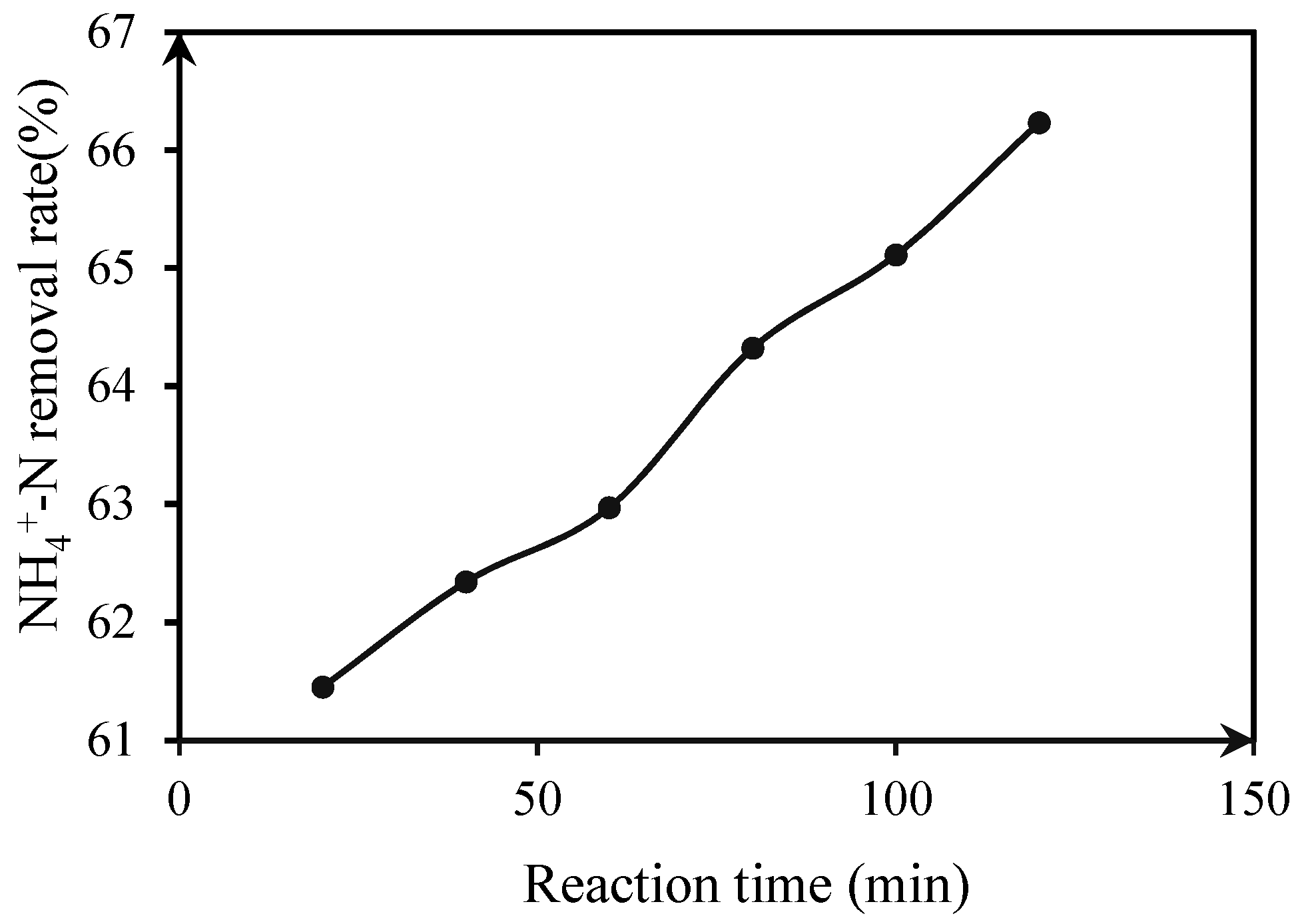
3.2.1. Under Dark Reaction Conditions
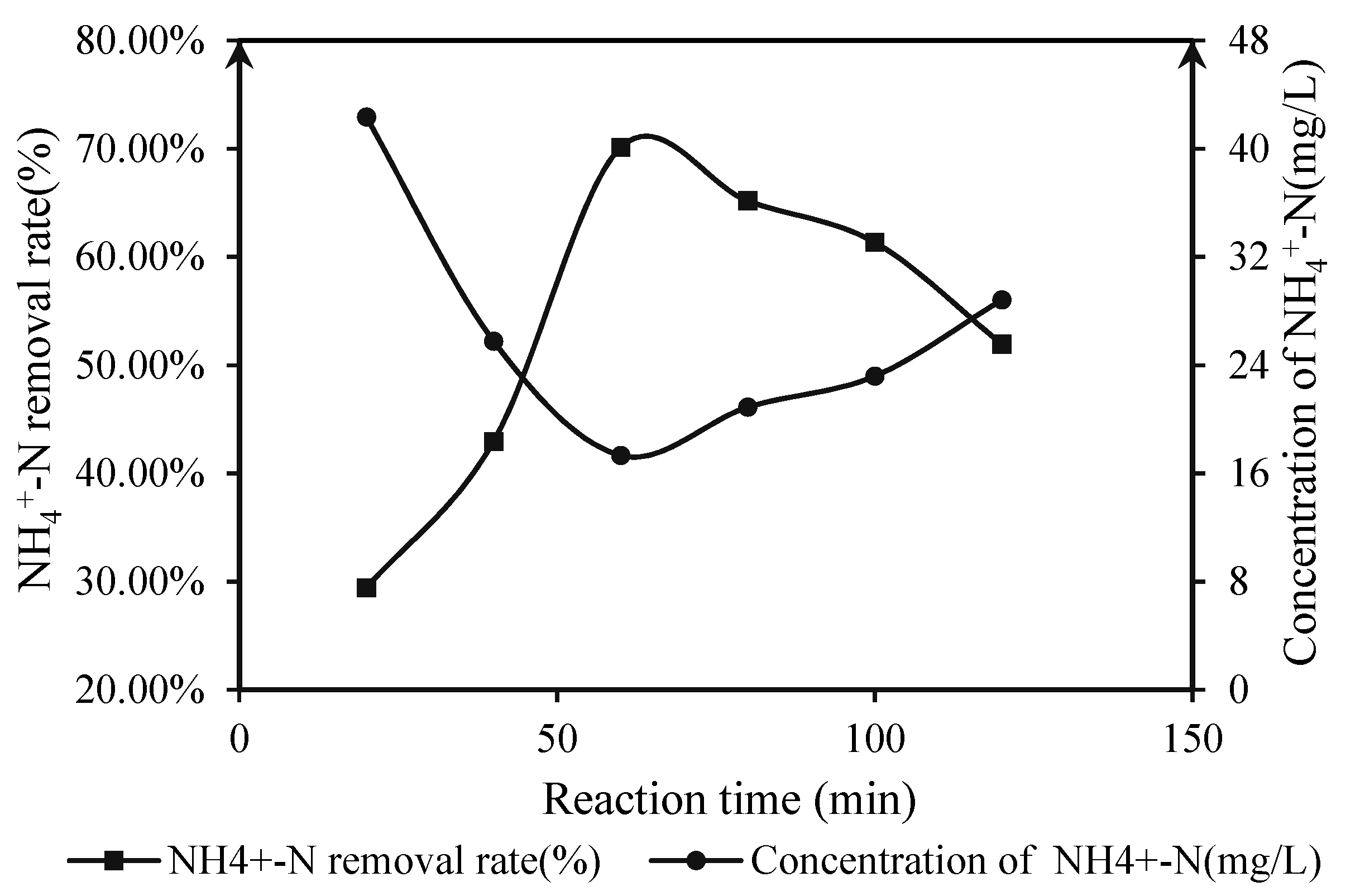
3.2.2. Under Natural Reaction Conditions
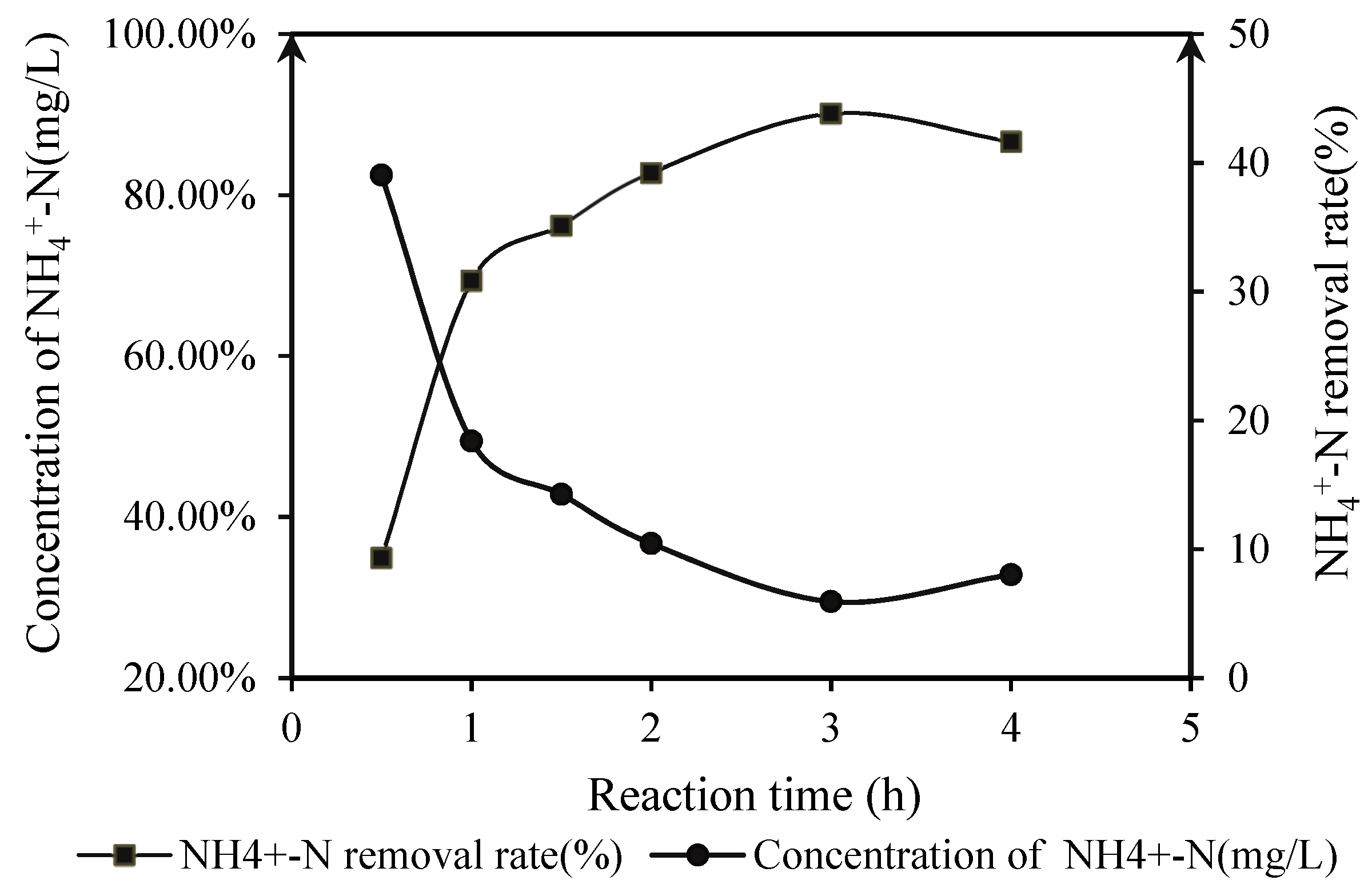
3.2.3. Under UV Direct Irradiation Conditions
- 1)
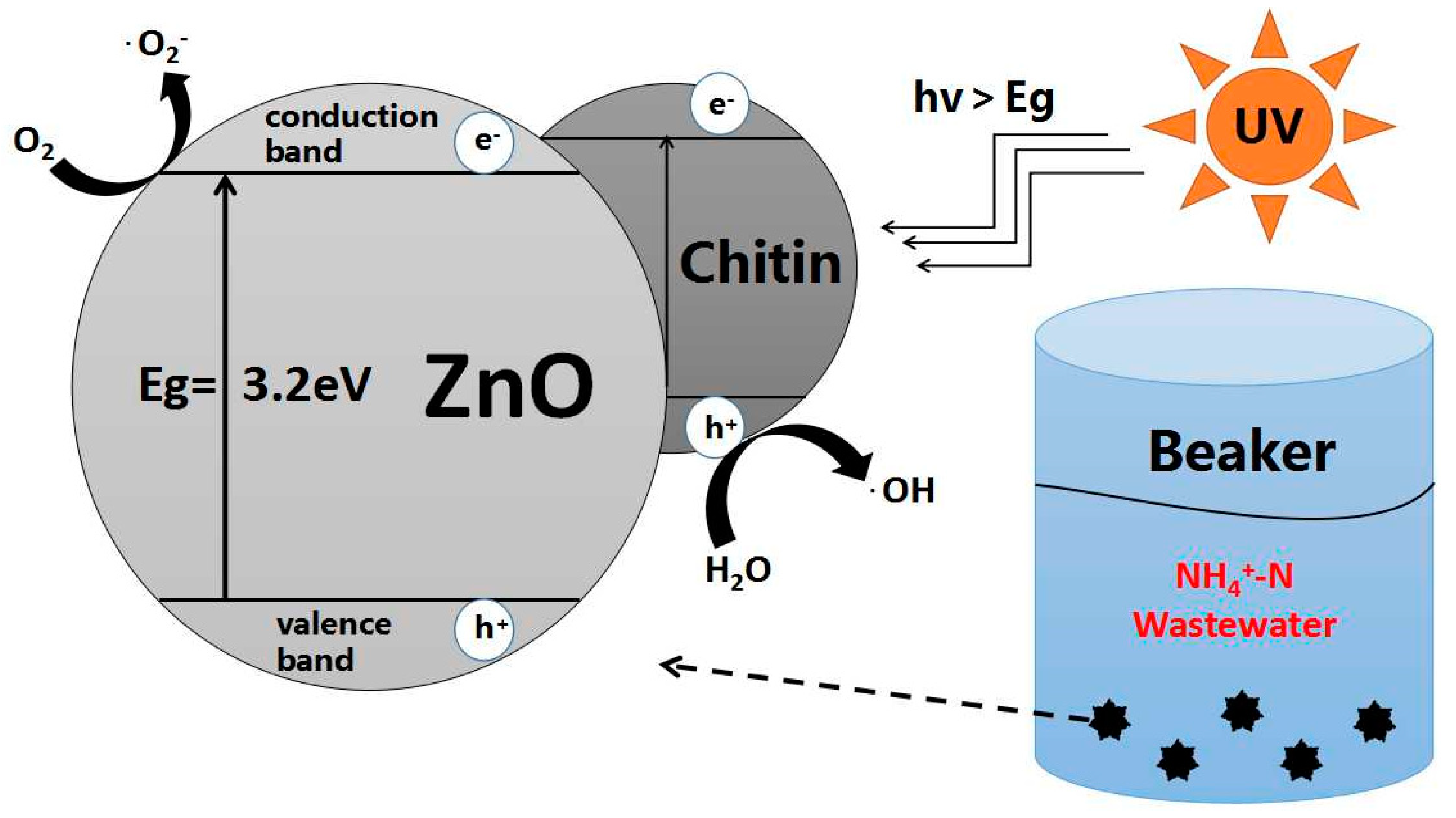
- When ZnO and other semiconductor particles are in close contact with the aqueous solution, the hydroxyl groups will form and adhere to the semiconductor surface. At the same time, owing to the valence band site of the semiconductor, ZnO is lower than the oxidation potential of the hydroxyl group [27]; the positively charged holes on the semiconductor surface will be first trapped by the surface hydroxyl radicals, producing hydroxyl radicals with strong oxidation via light irradiation:
- 2)
- When the oxygen molecule exists in ammonia nitrogen wastewater, the photogenerated electron will be trapped by the oxygen molecule adsorbed on the surface of the Chitin–ZnO composite photocatalyst [28], resulting in a superoxide anion group and the hydroxyl radical:
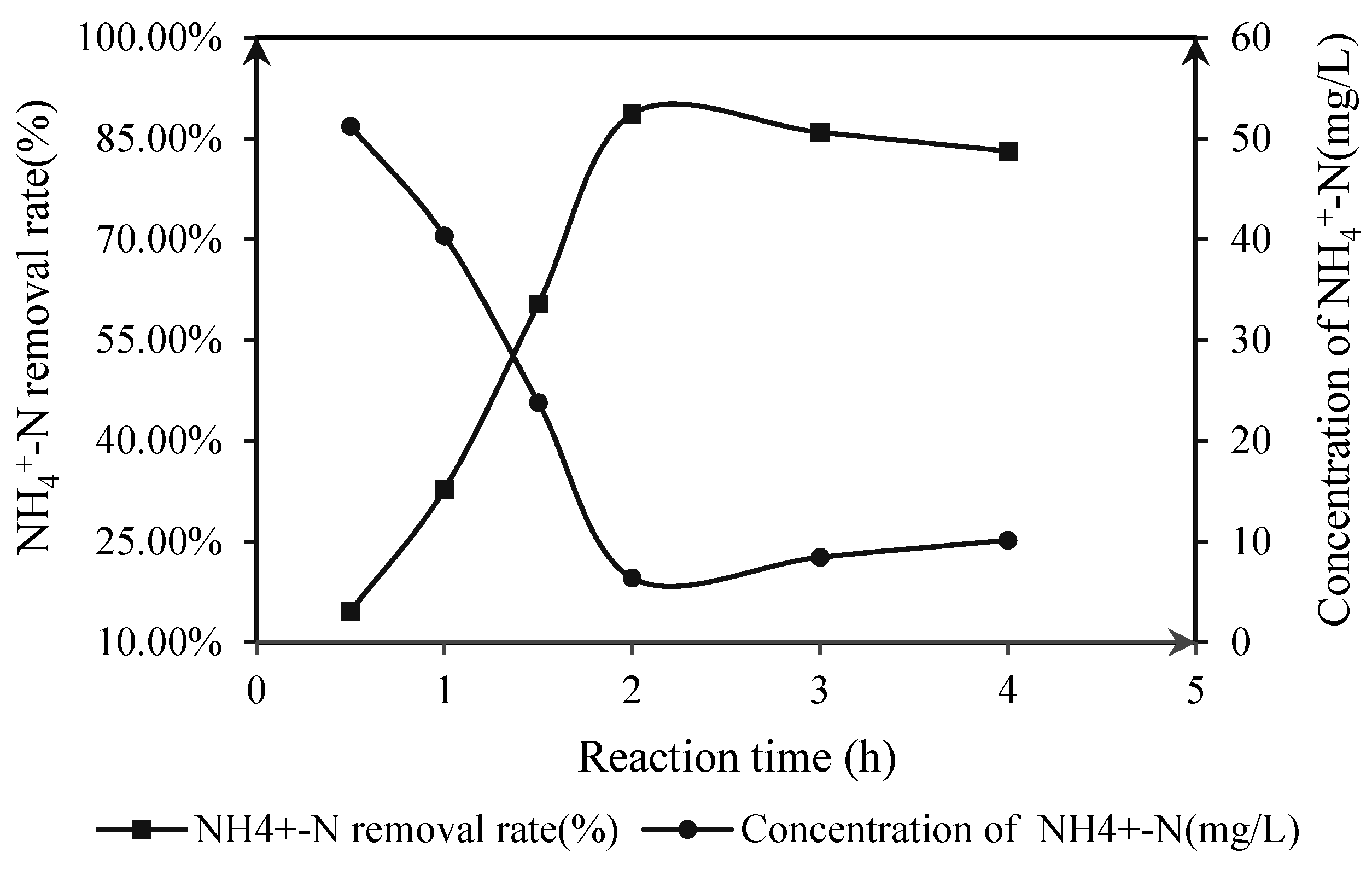
3.2.4. UV Irradiation after Adsorption
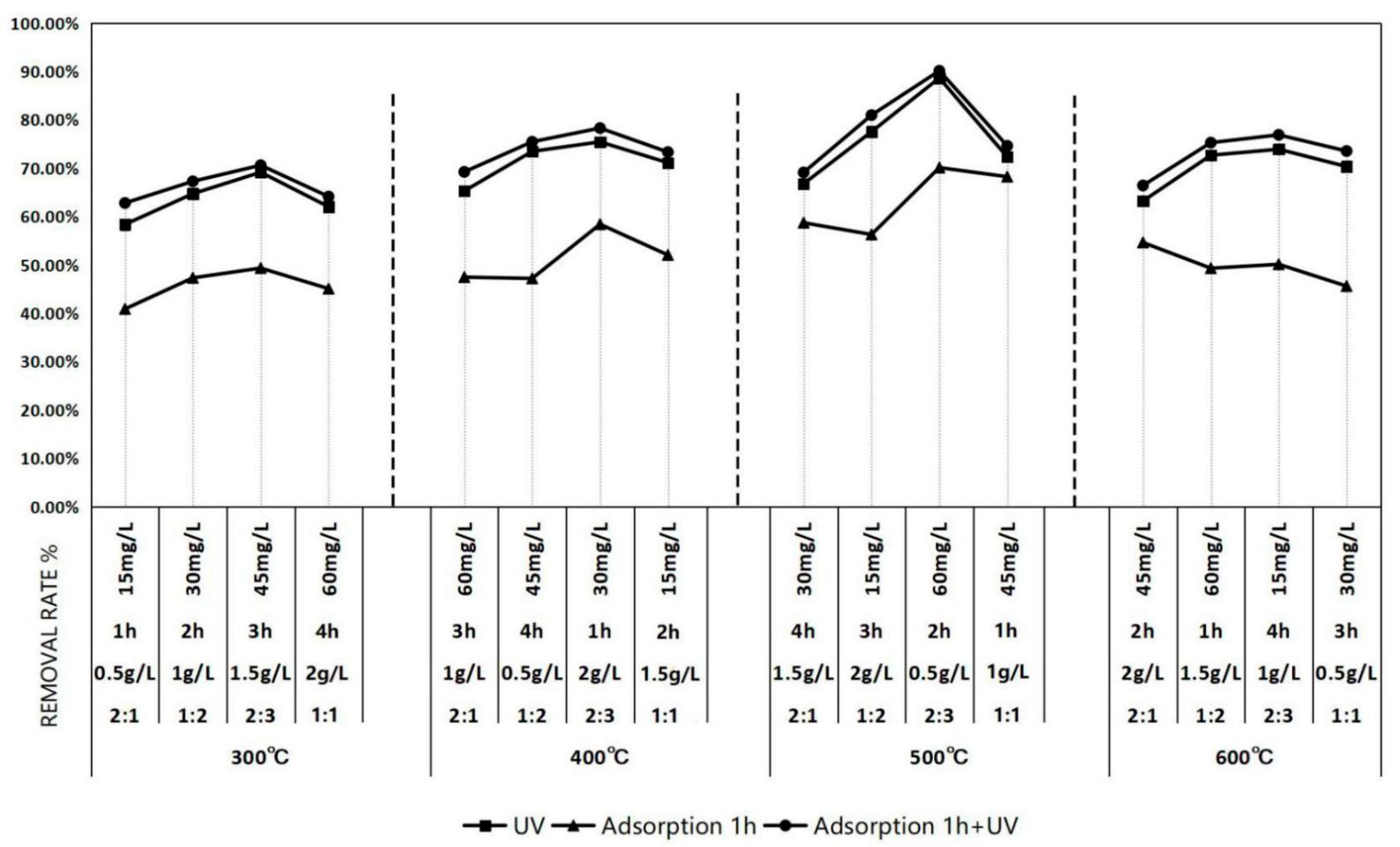
3.3. Orthogonal Test Under Ultraviolet Lamp Irradiation
3.3.1. Outline of the Orthogonal Test Scheme
3.3.2. Determination of the Optimal Reaction Conditions
3.4. Characterization of Chitin/ZnO
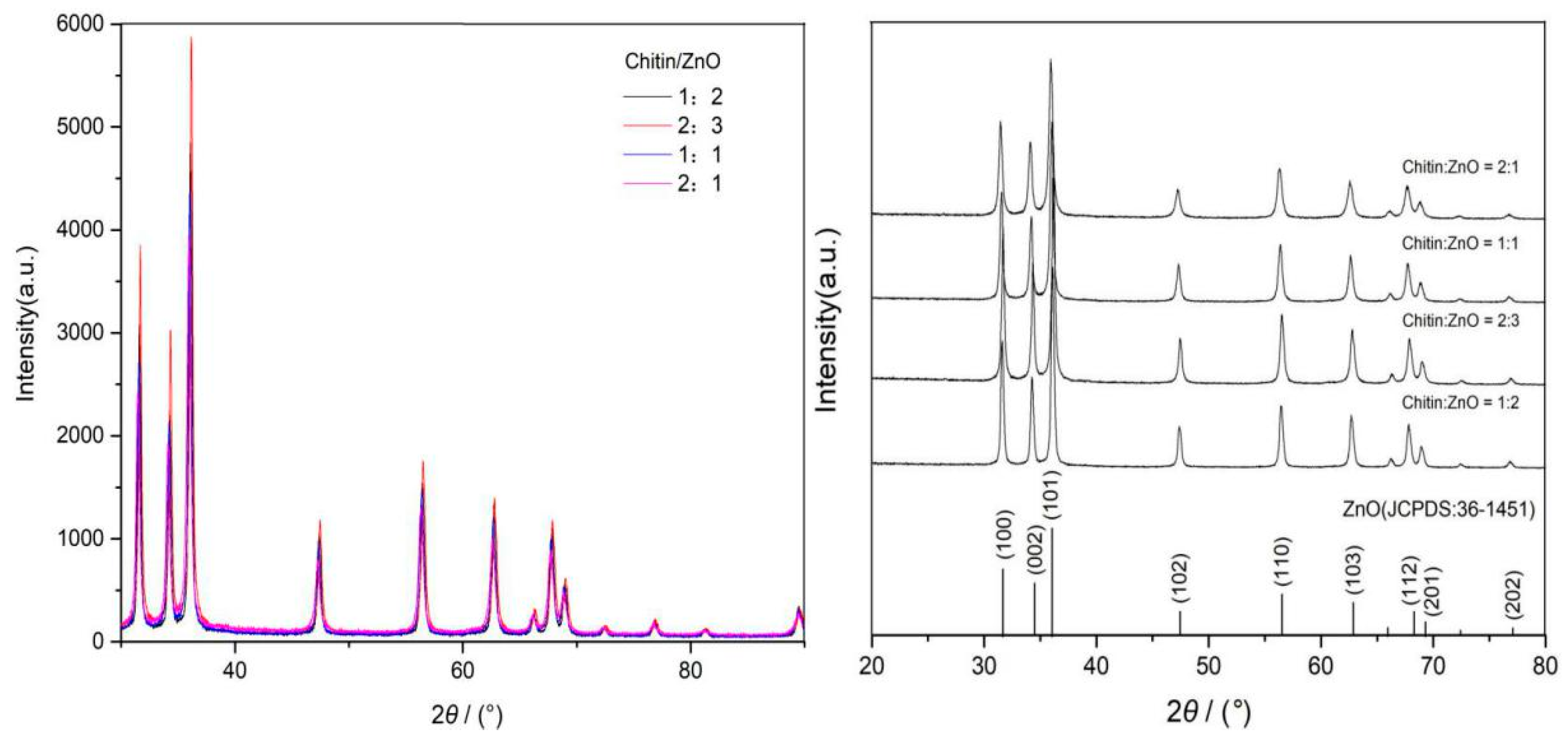
3.4.1. X-ray Diffraction Analysis of Chitin/ZnO
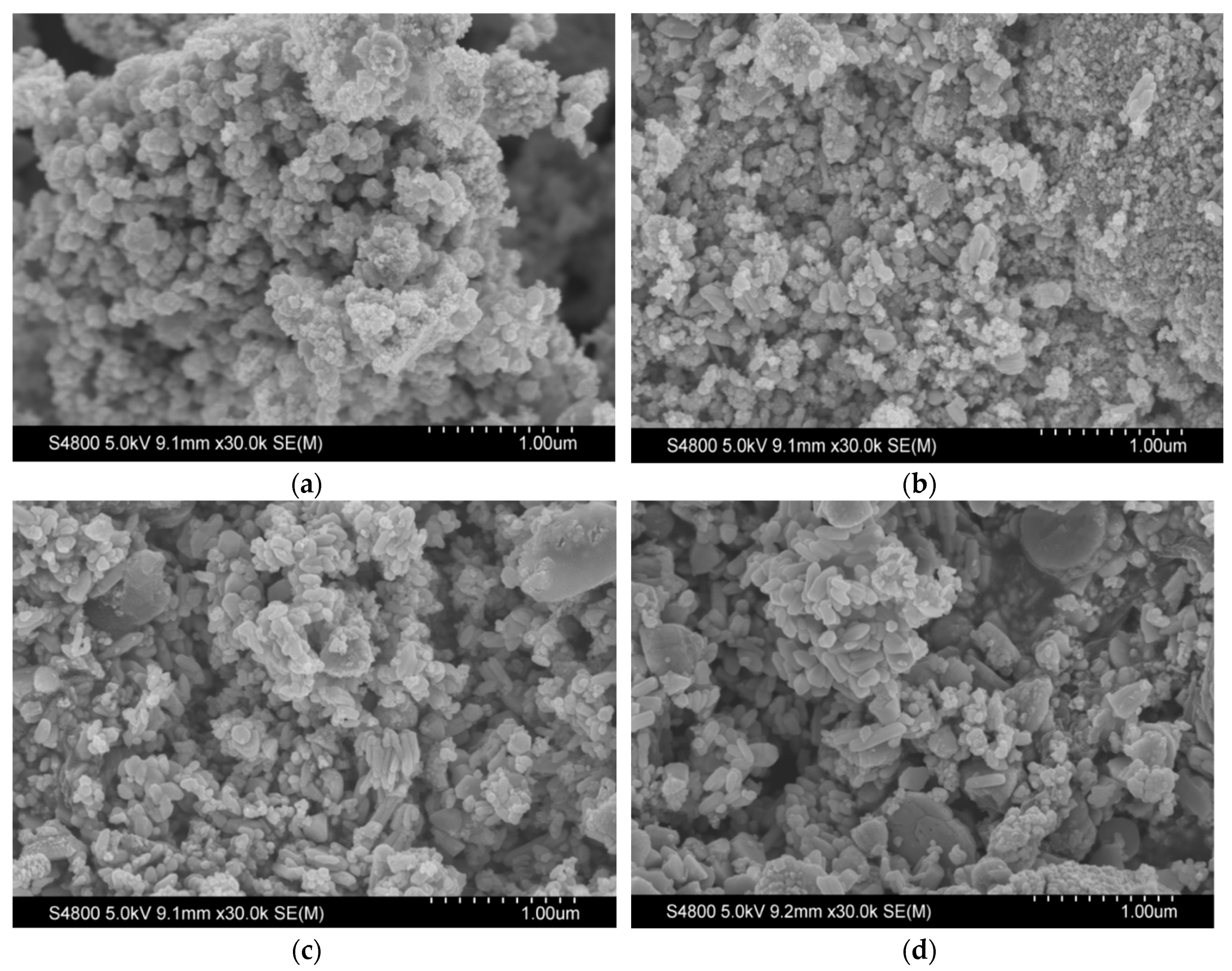
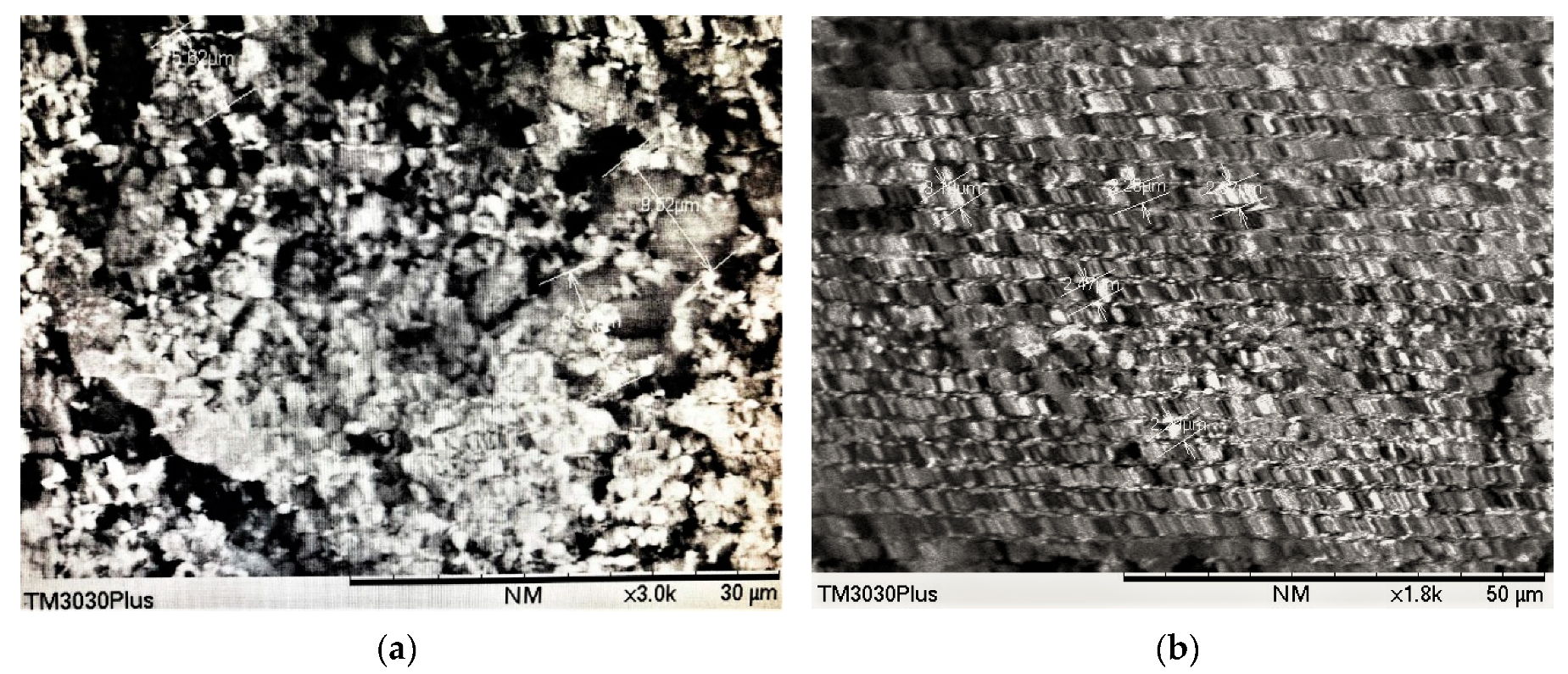
3.4.2. Scan Electron Microscope (SEM) Analysis of Chitin/ZnO
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, L.F.; Zhang, Y.; Yang, F.L.; Chen, G.; Jimmy, C.Y. Simultaneous photocatalytic removal of ammonium and nitrite in water using Ce3+–Ag+ modified TiO2. Sep. Purif. Technol. 2009, 67, 244–248. [Google Scholar] [CrossRef]
- Publicity and Education Center of the Ministry of Environmental Protection; Environmental Protection Foreign Cooperation Center of the Ministry of Environmental Protection. Persistent Organic Pollutants and Their Prevention and Control; China Institute of Environmental Management Cadres, Ed.; China Environment Press: Beijing, China, 2014; Volume 11, p. 152.
- Fang, S.Q.; Hu, X.F.; Wu, H.Y. Technology and Application of Aquaculture Wastewater Treatment. Technol. Equip. Environ. Pollut. Control 2004, 5, 51–55. [Google Scholar]
- Zhou, S.L.; Huang, T.L.; Zhang, H.H. Screening of poor nutrition aerobic denitrifying bacteria and characteristics of nitrogen removal from source water. J. Environ. Eng. 2016, 10, 2955–2961. [Google Scholar]
- Su, B.T.; Hu, C.L.; Zuo, X.W. Preparation of nanometer zinc oxide and its photocatalytic activity in sunlight. Acta Inorgan. Chem. 2010, 26, 96–100. [Google Scholar]
- Jin, X.J.; Yu, X.C.; Wu, Y.Y.; Shang, X.L.; Yin, D.N.; Xue, G.H. Preparation of nano-SnO2 photocatalyst and its application in aquaculture wastewater treatment. Oceanography 2015, 33, 64–69. [Google Scholar]
- Yu, X.C.; Shang, X.L.; Ji, Q.Y.; Zhang, J.; Qi, X.Y.; Jin, X.J. Photocatalytic performance of nano-Ce/SnO2 in aquaculture wastewater treatment. Mar. Environ. Sci. 2016, 35, 501–506. [Google Scholar]
- Shavisi, Y.; Sharifnia, S.; Mohamadi, Z. Solar-light-harvesting degradation of aqueous ammonia by CuO/ZnO immobilized on pottery plate: Linear kinetic modeling for adsorption and photocatalysis process. J. Environ. Chem. Eng. 2016, 4, 2736–2744. [Google Scholar] [CrossRef]
- Zhang, X.H.; Lu, L.C.; Yu, J.S. Preparation of modified nano ZnO/chitosan composite membrane and its adsorption on methyl orange. J. Yibin Univ. 2016, 16, 103–107. [Google Scholar]
- Farzana, M.H.; Meenakshi, S. Visible light-driven photoactivity of zinc oxide impregnated chitosan beads for the detoxification of textile dyes. Appl. Catal. A Gen. 2015, 503, 124–134. [Google Scholar] [CrossRef]
- Mao, X.C. Application of Chitosan and Nano-ZnO Composites in Antibacterial Packaging. Printed J. 2017, 7, 54–56. [Google Scholar]
- Diao, W.W.; An, X.H.; Wang, X.W.; Li, L.T. Isolation and identification of ammonia-degrading bacteria from mixed culture ponds of shrimp and crab. Aquat. Sci. 2015, 34, 83–88. [Google Scholar]
- Yang, Y.; Guan, F.; Zhang, S.M.; Qiu, J.K. Aquaculture wastewater treatment technology and research progress. Contemp. Chem. Res. 2017, 8, 73–75. [Google Scholar]
- Ning, G.H.; Lin, H.J. Preparation and Characterization of Chitosan/ZnO Composites. J. Sichuan Norm. Univ. 2011, 34, 864–868. [Google Scholar]
- Zhang, W.Q.; Zhu, Y. Advances in the study on the harm of ammonia nitrogen in aquaculture water and its detection methods. J. Environ. Hyg. 2012, 2, 324–327. [Google Scholar]
- Shi, Z.F.; Shen, Z.G.; Wu, Y.D.; Wang, J.C.; Yang, L.L. Photocatalytic degradation of organic compounds in shrimp wastewater and their sterilization. J. Sun Yat-sen Univ. 2009, 48, 60–64. [Google Scholar]
- Song, K.S.; Yu, X.C.; Hu, D.D. Photocatalytic Degradation of Ammonia Nitrogen in Aquaculture Wastewater by Using Nano-ZnO. Adv. Mater. Res. 2013, 2115, 564–568. [Google Scholar] [CrossRef]
- Sadeghzadeh-Attar, A. Efficient photocatalytic degradation of methylene blue dye by SnO2 nanotubes synthesized at different calcination temperatures. Sol. Energy Mater. Sol. Cells 2018, 183, 16–24. [Google Scholar] [CrossRef]
- Nandi, P.; Das, D. Photocatalytic degradation of Rhodamine-B dye by stable ZnO nanostructures with different calcination temperature induced defects. Appl. Surf. Sci. 2019, 465, 546–556. [Google Scholar] [CrossRef]
- Nasirian, M.; Bustillo-Lecompte, C.F.; Mehrvar, M. Photocatalytic efficiency of Fe2O3/TiO2 for the degradation of typical dyes in textile industries: Effects of calcination temperature and UV-assisted thermal synthesis. J. Environ. Manag. 2017, 196, 487–498. [Google Scholar] [CrossRef]
- He, L.H.; Song, R.; Liu, F.J. Preparation and characterization of ZnO/Chitosan composite. J. Zhengzhou Light Ind. Inst. 2008, 23, 16–20. [Google Scholar]
- Yu, X.C.; Zhu, P.F.; Zhang, M.; Guo, J.Y.; Zheng, X. Study on the Photocatalytic Degradation of Ammonia Nitrogen in Aquaculture Wastewater by Sn4+-Doped Nano-TiO2 under UV Irradiation. Adv. Mater. Res. 2011, 347, 2351–2355. [Google Scholar] [CrossRef]
- Ali, W.; Ullah, H.; Zada, A.; Alamgir, M.K.; Muhammad, W.; Ahmad, M.J.; Nadhman, A. Effect of calcination temperature on the photoactivities of ZnO/SnO2 nanocomposites for the degradation of methyl orange. Mater. Chem. Phys. 2018, 213, 259–266. [Google Scholar] [CrossRef]
- Altomare, M.; Selli, E. Effects of metal nanoparticles deposition on the photocatalytic oxidation of ammonia in TiO2 aqueous suspensions. Catal. Today 2013, 209, 127–133. [Google Scholar] [CrossRef]
- Ou, H.H.; Hoffmann, M.R.; Liao, C.H.; Hong, J.H.; Lo, S.L. Photocatalytic oxidation of aqueous ammonia over platinized microwave-assisted titanate nanotubes. Appl. Catal. B 2010, 99, 74–80. [Google Scholar] [CrossRef]
- Jin, X.J.; Liu, J.C.; Zhang, T.; Li, Y.; Wang, P.; Cheng, R.C. Photocatalytic degradation of ammonia nitrogen in marine aquaculture wastewater by nano-Fe2O3-SnO2. J. Appl. Oceanogr. 2018, 37, 77–83. [Google Scholar]
- Colombo, E.; Li, W.; Bhangu, S.K.; Ashokkumar, M. Chitosan microspheres as a template for TiO2 and ZnO microparticles: Studies on mechanism, functionalization and applications in photocatalysis and H2S removal. RSC Adv. 2017, 7, 19373–19383. [Google Scholar] [CrossRef]
- Jang, J.S.; Kim, H.K.; Lee, J.S. Heterojunction semiconductors: A strategy to develop efficient photocatalytic materials for visible light water splitting. Catal. Today 2012, 185, 270–277. [Google Scholar] [CrossRef]
- Nithya, A.; Jothivenkatachalam, K. Chitosan assisted synthesis of ZnO nanoparticles: An efficient solar light driven photocatalyst and evaluation of antibacterial activity. J. Mater. Sci. Mater. Electron. 2015, 26, 10207–10216. [Google Scholar] [CrossRef]
- Kumar, H.; Rani, R. Structural and Optical Characterization of ZnO Nanoparticles Synthesized by Microemulsion Route. Int. Lett. Chem. Phys. Astron. 2013, 19, 26–36. [Google Scholar] [CrossRef]
- Zhong, L.W. Nanostructures of zinc oxide. Mater. Today 2004, 7, 26–33. [Google Scholar]
- Guo, M.; Diao, P.; Cai, S. Hydrothermal growth of well-aligned ZnO nanorod arrays: Dependence of morphology and alignment ordering upon preparing conditions. J. Solid State Chem. 2005, 178, 1864–1873. [Google Scholar] [CrossRef]
- Li, L.H.; Deng, J.C.; Deng, H.R.; Liu, Z.L.; Xin, L. Synthesis and characterization of chitosan/ZnO nanoparticle composite membranes. Carbohydr. Res. 2010, 345, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Roy, A.; Silberschmidt, V.V. Temperature-dependent crystal-plasticity model for magnesium: A bottom-up approach. Mech. Mater. 2017, 113, 44–56. [Google Scholar] [CrossRef]
| Drug Reagent Name | Molecular Formula | Purity |
|---|---|---|
| Zinc nitrate hexahydrate | ZnNO3·6H2O | AR |
| Polyethylene glycol | HO(CH2CH2O)nH | AR |
| Sodium hydroxide | NaOH | AR |
| Deacetyl chitin | (C8H13NO5)N | AR |
| Acetic acid | CH3COOH | AR |
| Zinc sulfate | ZnSO4 | AR |
| Ammonium chloride | NH4Cl | GR |
| Sulfuric acid solution | H2SO4 | AR |
| Nessler’s reagent | - | AR |
| Potassium sodium tartrate | C4H4KNaO6-4H2O | AR |
| Instrument Name | Model |
|---|---|
| Electronic balance | TE124S |
| Magnetic stirring apparatus | CJ78-1 |
| Chamber electric furnace | SX2-2.5-10A |
| DHG Series Heating and Drying Oven | DHG-9146A |
| Vacuum pump | SHZ-D (III) |
| pH meter | PHS-3C |
| Multiparameter water quality analyzer | 5B-3B (V8) |
| Ultraviolet visible photometer | V-1100D |
| Medium pressure pump lamp | M-169H |
| SEM electron microscope | HitachiS4800 TM3030plus |
| X’Pert Powder | PANalytical EPSILON5 |
| Level | Calcination Temperature/°C | m(Chitin)/m(ZnO) | Dosage (g/L) | Irradiation Time/h | Initial Concentration of NH4+–N (mg/L) | UV % | Adsorption 1 h + UV % | Adsorption 1 h % | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 300 °C | 2:1 | 0.5 | 1 | 15 | 58.37% | 62.80% | 40.90% | |||||
| 2 | 1:2 | 1 | 2 | 30 | 64.72% | 67.28% | 47.33% | ||||||
| 3 | 2:3 | 1.5 | 3 | 45 | 69.15% | 70.60% | 49.35% | ||||||
| 4 | 1:1 | 2 | 4 | 60 | 62.03% | 64.13% | 45.10% | ||||||
| 5 | 400 °C | 2:1 | 1 | 3 | 60 | 65.29% | 69.23% | 47.48% | |||||
| 6 | 1:2 | 0.5 | 4 | 45 | 73.46% | 75.45% | 47.21% | ||||||
| 7 | 2:3 | 2 | 1 | 30 | 75.43% | 78.28% | 58.41% | ||||||
| 8 | 1:1 | 1.5 | 2 | 15 | 71.12% | 73.32% | 52.05% | ||||||
| 9 | 500 °C | 2:1 | 1.5 | 4 | 30 | 66.78% | 69.11% | 58.72% | |||||
| 10 | 1:2 | 2 | 3 | 15 | 77.53% | 80.96% | 56.29% | ||||||
| 11 | 2:3 | 0.5 | 2 | 60 | 88.64% | 90.12% | 70.11% | ||||||
| 12 | 1:1 | 1 | 1 | 45 | 72.33% | 74.61% | 68.23% | ||||||
| 13 | 600 °C | 2:1 | 2 | 2 | 45 | 63.21% | 66.42% | 54.64% | |||||
| 14 | 1:2 | 1.5 | 1 | 60 | 72.67% | 75.27% | 49.32% | ||||||
| 15 | 2:3 | 1 | 4 | 15 | 73.91% | 76.88% | 50.13% | ||||||
| 16 | 1:1 | 0.5 | 3 | 30 | 70.33% | 73.54% | 45.63% | ||||||
| k1j | 2.54 | 2.65 | 2.54 | 2.68 | 2.91 | 3.02 | 2.79 | 2.91 | 2.81 | 2.94 | T = 11.25 CT = T2/n = 7.9101 | T = 11.68 CT = T2/n = 8.5264 | Average = 52.56% |
| k2j | 2.85 | 2.96 | 2.88 | 2.99 | 2.76 | 2.88 | 2.88 | 2.97 | 2.77 | 2.88 | |||
| k3j | 3.05 | 3.15 | 3.07 | 3.16 | 2.80 | 2.88 | 2.82 | 2.94 | 2.78 | 2.87 | |||
| k4j | 2.80 | 2.92 | 2.76 | 2.86 | 2.78 | 2.90 | 2.76 | 2.86 | 2.89 | 2.99 | |||
| k1j2 | 6.47 | 7.01 | 6.43 | 7.16 | 8.46 | 9.11 | 7.77 | 8.47 | 7.89 | 8.64 | |||
| k2j2 | 8.14 | 8.78 | 8.32 | 8.94 | 7.63 | 8.29 | 8.28 | 8.83 | 7.69 | 8.31 | |||
| k3j2 | 9.32 | 9.91 | 9.43 | 9.98 | 7.82 | 8.31 | 7.97 | 8.66 | 7.74 | 8.24 | |||
| k4j2 | 7.85 | 8.53 | 7.61 | 8.16 | 7.74 | 8.40 | 7.63 | 8.16 | 8.33 | 8.93 | |||
| SSj | 0.033 | 0.032 | 0.037 | 0.032 | 0.003 | 0.003 | 0.001 | 0.002 | 0.002 | 0.002 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Yang, A.; Huang, G.; Zhou, X.; Zhai, Y.; Chen, X.; McBean, E. Treatment of Aquaculture Wastewater through Chitin/ZnO Composite Photocatalyst. Water 2019, 11, 310. https://doi.org/10.3390/w11020310
Lin X, Yang A, Huang G, Zhou X, Zhai Y, Chen X, McBean E. Treatment of Aquaculture Wastewater through Chitin/ZnO Composite Photocatalyst. Water. 2019; 11(2):310. https://doi.org/10.3390/w11020310
Chicago/Turabian StyleLin, Xiajing, Aili Yang, Guohe Huang, Xiong Zhou, Yuanyuan Zhai, Xiujuan Chen, and Ed McBean. 2019. "Treatment of Aquaculture Wastewater through Chitin/ZnO Composite Photocatalyst" Water 11, no. 2: 310. https://doi.org/10.3390/w11020310
APA StyleLin, X., Yang, A., Huang, G., Zhou, X., Zhai, Y., Chen, X., & McBean, E. (2019). Treatment of Aquaculture Wastewater through Chitin/ZnO Composite Photocatalyst. Water, 11(2), 310. https://doi.org/10.3390/w11020310







