Strategic Approach for Prioritising Local and Regional Sanitation Interventions for Reducing Global Antibiotic Resistance
Abstract
1. Introduction
1.1. Global Spread of Antibiotic Resistance
1.2. Sanitation Gaps, Infectious Disease and AR in LMICs
1.3. WASH Programs as a Platform for Reducing AR
2. Developing a Strategic Approach for Prioritising AR Mitigation Options
2.1. Philosophical Basis of the Approach: Relative Costs versus Relative Benefits
2.2. Relative Cost of Different AR Sanitation Interventions
2.3. Relative Benefit of Different AR Sanitation Interventions
2.4. Case Study: Cost–Benefits of Sanitation Interventions across SE Asia
3. Results and Discussion
3.1. Sanitation Options for Reducing AR: Relative Costs and Relative Benefits
3.2. Comparing AR Cost–Benefits of Different Sanitation Options
3.3. Case Study: Prioritising Sanitation Options to Mitigate AR in SE Asia
3.3.1. Using the Strategy for Prioritising AR Mitigation Sanitation Options for LMICs
3.3.2. Geographic, Economic and Sanitation Background for SE Asia
3.3.3. Cultural Considerations for AR Mitigation in SE Asia
3.3.4. Translating Cost–Benefit Analysis into Sanitation Priorities for Reducing AR Exposures
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allen, H.K.; Donato, J.; Wang, H.M.; Cloud-Hansen, C.A.; Davies, J.; Handelsman, J. Call of the wild: Antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 2010, 8, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, P.M.; Jones, A.M. The changing epidemiology of resistance. J. Antimicrob. Chemother. 2009, 64, i3–i10. [Google Scholar] [CrossRef] [PubMed]
- Knapp, C.W.; Dolfing, J.; Ehlert, F.A.I.; Graham, D.W. Evidence of increasing antibiotic resistance gene abundances in archived soils since 1940. Environ. Sci. Technol. 2010, 44, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Arcilla, M.S.; van Hattem, J.M.; Bootsma, M.C.; van Genderen, P.J.; Goorhuis, A.; Schultsz, C. The carriage of multiresistant bacteria after travel (COMBAT) prospective cohort study: Methodology and design. BMC Public Health 2014, 14, 410. [Google Scholar] [CrossRef] [PubMed]
- Von Wintersdorff, C.J.H.; Penders, J.; Stobberingh, E.E.; Lashof, A.M.L.O.; Hoebe, C.J.P.A.; Savelkoul, P.H.M.; Wolffs, P.F.G. High rates of antimicrobial drug resistance gene acquisition after international travel, The Netherlands. Emerg. Infect. Dis. 2014, 20, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Häsler, R.; Kautz, C.; Rehman, A.; Podschun, R.; Gassling, V.; Brzoska, P.; Sherlock, J.; Gräsner, J.-T.; Hoppenstedt, G.; Schubert, S.; et al. The antibiotic resistome and microbiota landscape of refugees from Syria, Iraq and Afghanistan in Germany. Microbiome 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S. Understanding the contribution of environmental factors in the spread of antimicrobial resistance. Environ. Health Prev. Med. 2015, 20, 243–252. [Google Scholar] [CrossRef]
- Williams, M.R.; Stedtfeld, R.D.; Guo, X.; Hashsham, S.A. Antimicrobial resistance in the environment. Water Environ. Res. 2016, 88, 1951–1967. [Google Scholar] [CrossRef]
- Quintela-Baluja, M.; Chan, W.C.; Alnakip, M.E.; Abouelnaga, M.; Graham, D.W. Sanitation, water quality, and antibiotic resistance dissemination. In The Battle Against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Méndez-Vilas, Ed.; Fomatex Research Center: Badajoz, Spain, 2015; pp. 965–975. ISBN 9788494213472. [Google Scholar]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet Health 2018, 2, e398–e405. [Google Scholar] [CrossRef]
- OECD. Stemming the Superbug Tide: Just a Few Dollars More; OECD Publishing: Paris, France, 2018. [Google Scholar]
- WHO and UNICEF Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines 2017. Available online: https://washdata.org/sites/default/files/documents/reports/2018-01/JMP-2017-report-final.pdf (accessed on 29 October 2018).
- Population, Total. Available online: https://data.worldbank.org/indicator/SP.POP.TOTL (accessed on 29 October 2018).
- WHO/UNICEF Joint Monitoring Programme (JMP) for Water Supply and Sanitation (wssinfo.org). People Practicing Open Defecation (% of Population). Available online: https://data.worldbank.org/indicator/SH.STA.ODFC.ZS?locations=BN-KH-TL-ID-LA-MY-MM-PH-SG-TH-VN&view=chart (accessed on 29 October 2018).
- World Bank National Accounts Data, and OECD National Accounts Data Files. GDP per Capita (current US$). Available online: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD (accessed on 29 October 2018).
- World Bank Staff Estimates Based on the United Nations Population Division’s World Urbanization Prospects: 2018 Revision. Rural population (% of total population). Available online: https://data.worldbank.org/indicator/SP.RUR.TOTL.ZS (accessed on 29 October 2018).
- Pew-Templeton Global Religious Futures Project Research Center. Available online: http://www.globalreligiousfutures.org/countries (accessed on 29 October 2018).
- Millennium Development Goals and Beyond 2015, End Open Defecation. Available online: http://www.un.org/millenniumgoals/endopendefecation.shtml (accessed on 29 October 2018).
- Bill and Melinda Gates Foundation. Reinvent the Toilet Challenge: Strategy Overview. Available online: https://www.gatesfoundation.org/What-We-Do/Global-Growth-and-Opportunity/Water-Sanitation-and-Hygiene/Reinvent-the-Toilet-Challenge (accessed on 18 September 2018).
- University of Science and Technology Beijing. Reinventing the Toilet. In Proceedings of the Reinvent the Toilet Challenge, Beijing, China, 6–8 November 2018; Available online: http://stepsforsanitation.org/wp-content/uploads/2018/11/The-Reinvented-Toilet-Exhibitor-Guide-FINAL.pdf (accessed on 21 November 2018).
- Zhou, X.; Li, Z.; Zheng, T.; Yan, Y.; Li, P.; Odey, E.A.; Mang, H.P.; Uddin, S.M.N. Review of global sanitation development. Environ. Int. 2018, 120, 246–261. [Google Scholar] [CrossRef]
- Graham, D.W.; Collignon, P.; Davies, J.; Larsson, D.G.J.; Snape, J.R. Underappreciated role of regionally poor water quality on globally increasing antibiotic resistance. Environ. Sci. Technol. 2014, 11746–11747. [Google Scholar] [CrossRef] [PubMed]
- The Economist. Beating the Bugs: How Bangladesh Vanquished Diarrhea; The Economist: London, UK, 2018; Available online: https://www.economist.com/asia/2018/03/22/how-bangladesh-vanquished-diarrhoea (accessed on 29 October 2018).
- Rousham, E.K.; Unicomb, L.; Islam, M.A. Human, animal and environmental contributors to antibiotic resistance in low-resource settings: Integrating behavioural, epidemiological and One Health approaches. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29643217 (accessed on 29 October 2018).
- UNICEF. Water, Sanitation and Hygiene (WASH), Annual Report 2013; UNICEF: New York, NY, USA, 2014; Available online: https://www.unicef.org/wash/files/WASH_Annual_Report_Final_7_2_Low_Res.pdf (accessed on 29 October 2018).
- Lamba, M.; Gupta, C.; Shukla, R.; Graham, D.W.; Sreekrishnan, T.R.; Ahammad, Z.A. Carbapenem resistance exposures via wastewaters across New Delhi. Environ. Int. 2018, 119, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Milledge, D.G.; Gurjar, S.K.; Bunce, J.T.; Tare, V.; Sinha, R.; Carbonneau, P.E. Population density controls on microbial pollution across the Ganga catchment. Water Res. 2018, 128, 82–91. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Guidelines on Sanitation and Health; WHO: Geneva, Switzerland, 2018; Available online: http://www.who.int/water_sanitation_health/publications/guidelines-on-sanitation-and-health/en/ (accessed on 21 November 2018).
- O’Reilly, K.; Louis, E. The toilet tripod: Understanding successful sanitation in rural India. Health Place 2014, 29, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Hajra, G.; Dutta, A. The gap between construction and usage of toilets: An under identified problem. BMJ Glob. Health 2016, 1, A39. [Google Scholar] [CrossRef]
- Sinha, A.; Nagel, C.L.; Schmidt, W.P.; Torondel, B.; Boisson, S.; Routray, P.; Clasen, T.F. Assessing patterns and determinants of latrine use in rural settings: A longitudinal study in Odisha, India. Int. J. Hyg. Environ. Health 2017, 220, 906–915. [Google Scholar] [CrossRef]
- US EPA. Treatability Manual. Volume IV: Cost Estimating; EPA/600/8-80/042D (NTIS PB80223084); US EPA: Washington, DC, USA, 1980.
- Sustainable Development Goals. Goal 6: Ensure Access to Water and Sanitation for All. 2015. Available online: https://www.un.org/sustainabledevelopment/water-and-sanitation/ (accessed on 29 October 2018).
- U.S. Department of Health, Education, and Welfare. Modern Sewage Treatment Plants? How Much Do They Cost?: A Practical Guide to Estimating Municipal Sewage Treatment Plant Construction Cost; USPHS Publication No. 1229; U.S. Government Printing Office: Washington, DC, USA, 1964.
- Dodane, P.-H.; Mbéguéré, M.; Sow, O.; Strande, L. Capital and operating costs of full-scale fecal sludge management and wastewater treatment systems in Dakar, Senegal. Environ. Sci. Technol. 2012, 46, 3705–3711. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Miranda, J.P.; García-Ubaque, C.A.; Penagos-Londoño, J.C. Analysis of the investment costs in municipal wastewater treatment plants in Cundinamarca. Dyna 2015, 82, 230–238. [Google Scholar] [CrossRef]
- Hutton, G.; Varughese, M. Costs of meeting the 2030 Sustainable Development Goal Targets on Drinking Water, Sanitation, and Hygiene; Water and Sanitation Program; World Bank: Washington, DC, USA, 2016. [Google Scholar]
- Roefs, I.; Meulman, B.; Vreeburg, J.H.G.; Spiller, M. Centralised, decentralised or hybrid sanitation systems? Economic evaluation under urban development uncertainty and phased expansion. Water Res. 2017, 109, 274–286. [Google Scholar] [CrossRef]
- Metcalf, L.; Tchobanoglous, G. Wastewater Engineering Treatment and Reuse; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Eggimann, S.; Truffer, B.; Maurer, M. To connect or not to connect? Modelling the optimal degree of centralisation for wastewater infrastructures. Water Res. 2015, 84, 218–231. [Google Scholar] [CrossRef]
- Eggimann, S.; Truffer, B.; Feldmann, U.; Maurer, M. Screening European market potentials for small modular wastewater treatment systems—An inroad to sustainability transitions in urban water management? Land Use Policy 2018, 78, 711–725. [Google Scholar] [CrossRef]
- Galvin, S.; Boyle, F.; Hickey, P.; Vellinga, A.; Morris, D.; Cornican, M. Enumeration and characterization of antimicrobial-resistant Escherichia coli bacteria in effluent from municipal, hospital, and secondary treatment facility sources. Appl. Environ. Microbiol. 2010, 76, 4772–4779. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Wong, K.; Xagoraraki, I. Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res. 2011, 45, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Ahammad, Z.S.; Sreekrishnan, T.R.; Hands, C.L.; Knapp, C.W.; Graham, D.W. Increased waterborne blaNDM-1 resistance gene abundances associated with seasonal human pilgrimages to the Upper Ganges River. Environ. Sci. Technol. 2014, 48, 3014–3020. [Google Scholar] [CrossRef] [PubMed]
- Munck, C.; Albertsen, M.; Telke, A.; Ellabaan, M.; Nielsen, P.H.; Sommer, M.O. Limited dissemination of the wastewater treatment plant core resistome. Nat. Commun. 2015, 6, 8452. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.-B.; Guo, M.-T.; Yang, J. Fate of antibiotic resistant bacteria and genes during wastewater chlorination: Implications for antibiotic resistance control. PLoS ONE 2015, 10, e0119403. [Google Scholar] [CrossRef] [PubMed]
- DHI. Full Scale Advanced Wastewater Treatment at Herlev Hospital: Treatment Performance and Evaluation. Available online: https://www.dhigroup.com/-/media/shared%20content/global/news/2016/08/evaluation%20report.pdf?la=en (accessed on 29 October 2018).
- Waseem, H.; Williams, M.R.; Stedtfeld, R.D.; Hashsham, S.A. Antimicrobial resistance in the environment. Water Environ. Res. 2017, 89, 921–941. [Google Scholar] [CrossRef]
- Quach-Cu, J.; Herrera-Lynch, B.; Marciniak, C.; Adams, S.; Simmerman, A.; Reinke, R.A. The effect of primary, secondary, and tertiary wastewater treatment processes on antibiotic resistance gene (ARG) concentrations in solid and dissolved wastewater fractions. Water 2018, 10, 37. [Google Scholar] [CrossRef]
- Hong, P.-Y.; Julian, T.R.; Pype, M.-L.; Jiang, S.C.; Nelson, K.L.; Graham, D.W.; Pruden, A.; Manaia, C.M. Reusing treated wastewater: Consideration of the safety aspects associated with antibiotic-resistant bacteria and antibiotic resistance genes. Water 2018, 10, 244. [Google Scholar] [CrossRef]
- Melo, J. The Experience of Condominial Water and Sewerage Systems in Brazil; Report 34442; World Bank: Washington, DC, USA, 2007. [Google Scholar]
- Graham, D.W.; Giesen, M.J.; Jong, M.-C.; Bunce, J.T. Improving local-scale waste management: A solution for reducing global antibiotic resistance. In Proceedings of the 1st IWA Latin American & Caribbean Young Water Professionals Conference, Queretaro, Mexico, 5–9 November 2018. [Google Scholar]
- Manaia, C.M.; Macedo, G.; Fatta-Kassinos, D.; Nunes, O.C. Antibiotic resistance in urban aquatic environments: Can it be controlled? Appl. Microbiol. Biotechnol. 2016, 100, 1543–1557. [Google Scholar] [CrossRef]
- Cernea, M.M. Putting People First Sociological Variables in Rural Development; World Bank: Washington, DC, USA, 1985. [Google Scholar]
- Mogane, B.S. Techniques for Gathering Socio-Cultural Data on Water Supply and Sanitation Programmes. 1990 Pretoria; CSIR, Division of Water Technology. Available online: https://www.ircwash.org/sites/default/files/202.5-90TE-9138.pdf (accessed on 19 November 2018).
- Aboud, F.E.; Singla, D.R. Challenges to changing health behaviours in developing countries: A critical overview. Soc. Sci. Med. 2012, 75, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Bisung, E.; Elliott, S.J.; Schuster-Wallace, C.J.; Karanja, D.M.; Bernard, A. Social capital, collective action and access to water in rural Kenya. Soc. Sci. Med. 2014, 119, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Coffey, D.; Spears, D.; Vyas, S. Switching to sanitation: Understanding latrine adoption in a representative panel of rural Indian households. Soc. Sci. Med. 2017, 188, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Mata, L. Sociocultural factors in the control and prevention of parasitic diseases. Rev. Infect. Dis. 1982, 4, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Routray, P.; Schmidt, W.P.; Boisson, S.; Clasen, T.; Jenkins, M.W. Socio-cultural and behavioural factors constraining latrine adoption in rural coastal Odisha: An exploratory qualitative study. BMC Public Health 2015, 15, 880. [Google Scholar] [CrossRef] [PubMed]
- Dwipayanti, N.; Rutherford, S.; Phung, D.; Chu, C. How important is culture to sanitation uptake? The influence of local values in Rural Bali. Adv. Sci. Lett. 2017, 23, 3537–3540. [Google Scholar] [CrossRef]
- Garn, J.V.; Sclar, G.D.; Freeman, M.C.; Penakalapati, G.; Alexander, K.T.; Brooks, P.; Rehfuess, E.A.; Boisson, S.; Medlicott, K.O.; Clasen, T.F. The impact of sanitation interventions on latrine coverage and latrine use: A systematic review and meta-analysis. Int. J. Hyg. Environ. Health 2017, 220, 329–340. [Google Scholar] [CrossRef]
- Reynolds, R. Cleanliness and Godliness; Doubleday: New York, NY, USA, 1946. [Google Scholar]
- Warner, W.S. Cultural Influences that Affect the Acceptance of Compost Toilets: Psychology, Religion and Gender; Jordforsk Centre for Soil and Environmental Research: As, Norway, 1998. [Google Scholar]
- Greed, C. Inclusive Urban Design: Public Toilets; Routledge: London, UK, 2003; ISBN 978-0750653855. [Google Scholar]
- Gil, A.; Lanata, C.; Kleinau, E.; Penny, M. Children’s Feces Disposal Practices in Developing Countries and Interventions to Prevent Diarrheal Diseases: A Literature Review. In Environmental Health Project of USAID Strategic Report. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5217632/ (accessed on 24 November 2018).
- Dellström Rosenquist, L.E. A psychosocial analysis of the human-sanitation nexus. J. Environ. Psychol. 2005, 25, 335–346. [Google Scholar] [CrossRef]
- Anthony, K.; Dufresne, M. Potty Parity in Perspective: Gender and Family Issues in Planning and Designing Public Restrooms. J. Plan. Lit. 2007, 21, 267–294. [Google Scholar] [CrossRef]
- George, R. The Big Necessity: Adventures in the World of Human Waste; Portobello Books: London, UK, 2008. [Google Scholar]
- Shettar, S.C. Sociology of sanitation: Incorporating gender issues in sanitation. In Proceedings of the National Conference on Sociology of Sanitation, New Delhi, India, 28–29 January 2013; pp. 95–100. [Google Scholar]
- Coffey, D.; Gupta, A.; Hathi, P.; Khurana, N.; Spears, D.; Srivastav, N.; Vyas, S. Revealed preference for open defecation: Evidence from a new survey in rural North India. Econ. Polit. Wkly. 2014, 38, 43–55. [Google Scholar]
- Thys, S.; Mwape, K.E.; Lefèvre, P.; Dorny, P.; Marcotty, T.; Phiri, A.M.; Phiri, I.K.; Gabriël, S. Why latrines are not used: Communities’ perceptions and practices regarding latrines in a Taenia solium endemic rural area in Eastern Zambia. PLoS Negl. Trop. Dis. 2015, 9, e0003570. [Google Scholar] [CrossRef]
- Coffey, D.; Gupta, A.; Hathi, P.; Spears, D.; Srivastav, N.; Vyas, S. Understanding open defecation in rural India: Untouchability, pollution, and latrine pits. Econ. Polit. Wkly. 2017, 52, 59–66. [Google Scholar]
- Capodaglio, A.G. Integrated, decentralized wastewater management for resource recovery in rural and peri-urban areas. Resources 2017, 6, 22. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A.; Cecconet, D.; Molognoni, D. Sustainability of decentralized wastewater treatment technologies. Water Pract. Technol. 2017, 12, 463–477. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tjandraatmadja, G.; Cook, S.; Gardner, T. Decentralised systems—Definition and drivers in the current context. In Proceedings of the IWA Regional Conference on Wastewater Purification and Reuse, Heraklion, Greece, 28–30 March 2012. [Google Scholar]
- Tandukar, M.; Uemura, S.; Ohashi, A.; Harada, H. Combining UASB and the “fourth generation” down-flow hanging sponge reactor for municipal wastewater treatment. Water Sci. Technol. 2006, 53, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Bundy, C.A.; Wu, D.; Jong, M.-C.; Edwards, S.R.; Ahammad, Z.S.; Graham, D.W. Enhanced denitrification in downflow hanging sponge reactors for decentralised domestic wastewater treatment. Bioresour. Technol. 2017, 226, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jong, M.-C.; Su, J.-Q.; Bunce, J.T.; Harwood, C.R.; Snape, J.R.; Zhu, Y.-G.; Graham, D.W. Co-optimization of sponge-core bioreactors for removing total nitrogen and antibiotic resistance genes from domestic wastewater. Sci. Total Environ. 2018, 634, 1417–1423. [Google Scholar] [CrossRef]
- Finley, R.L.; Collignon, P.; Larsson, D.G.J.; McEwen, S.A.; Li, X.-Z.; Gaze, W.H.; Reid-Smith, R.; Timinouni, M.; Graham, D.W.; Topp, E. The scourge of antibiotic resistance: The important role of the environment. Clin. Inf. Dis. 2013, 57, 704–710. [Google Scholar] [CrossRef] [PubMed]
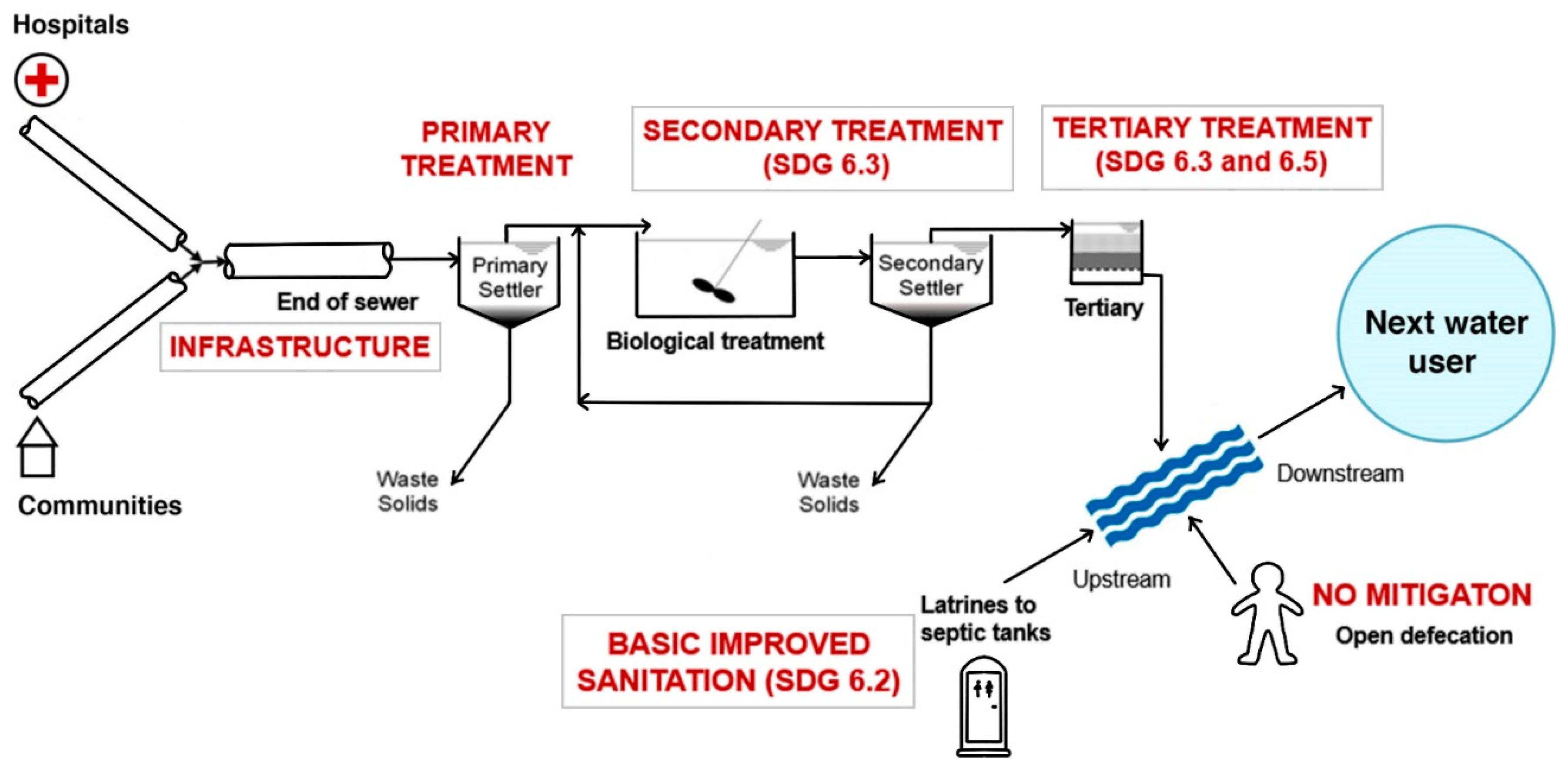
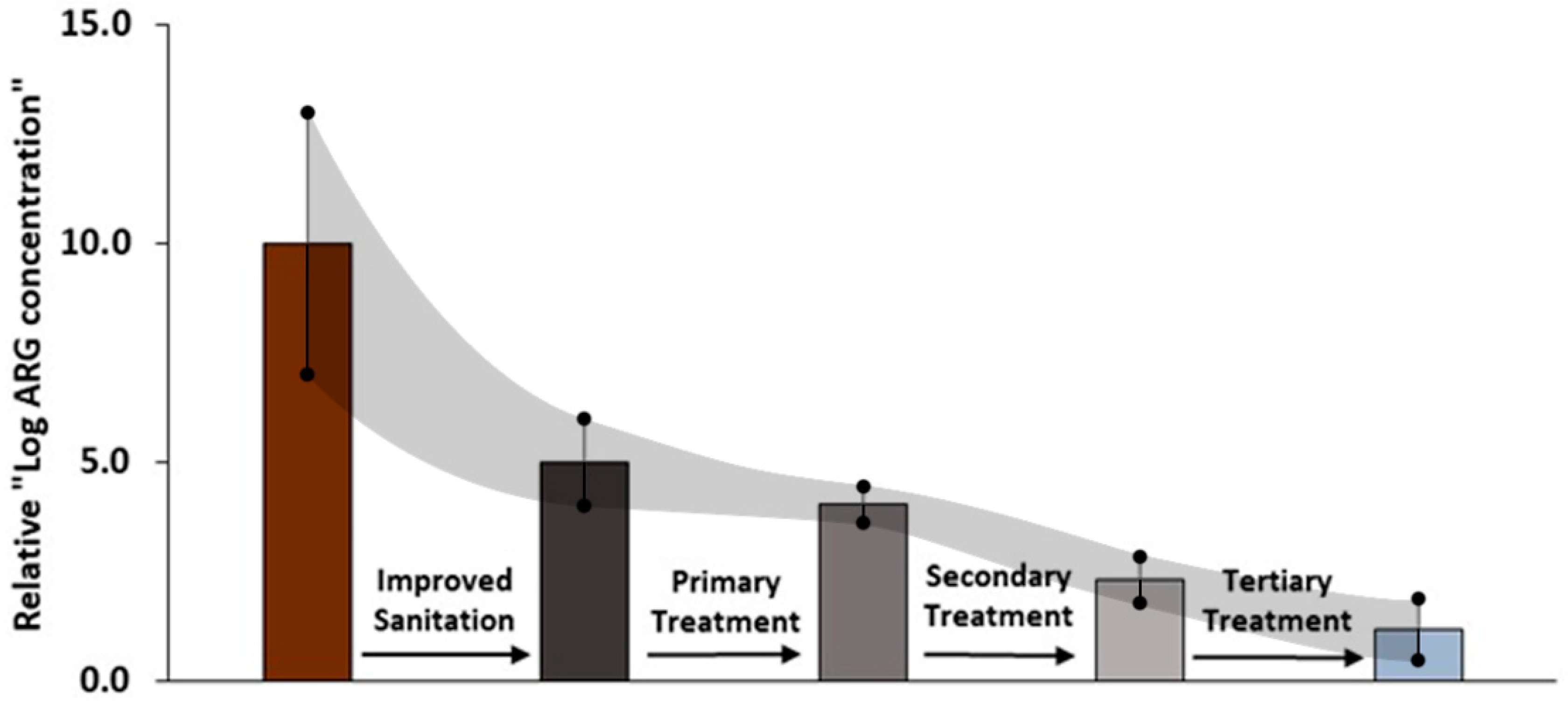
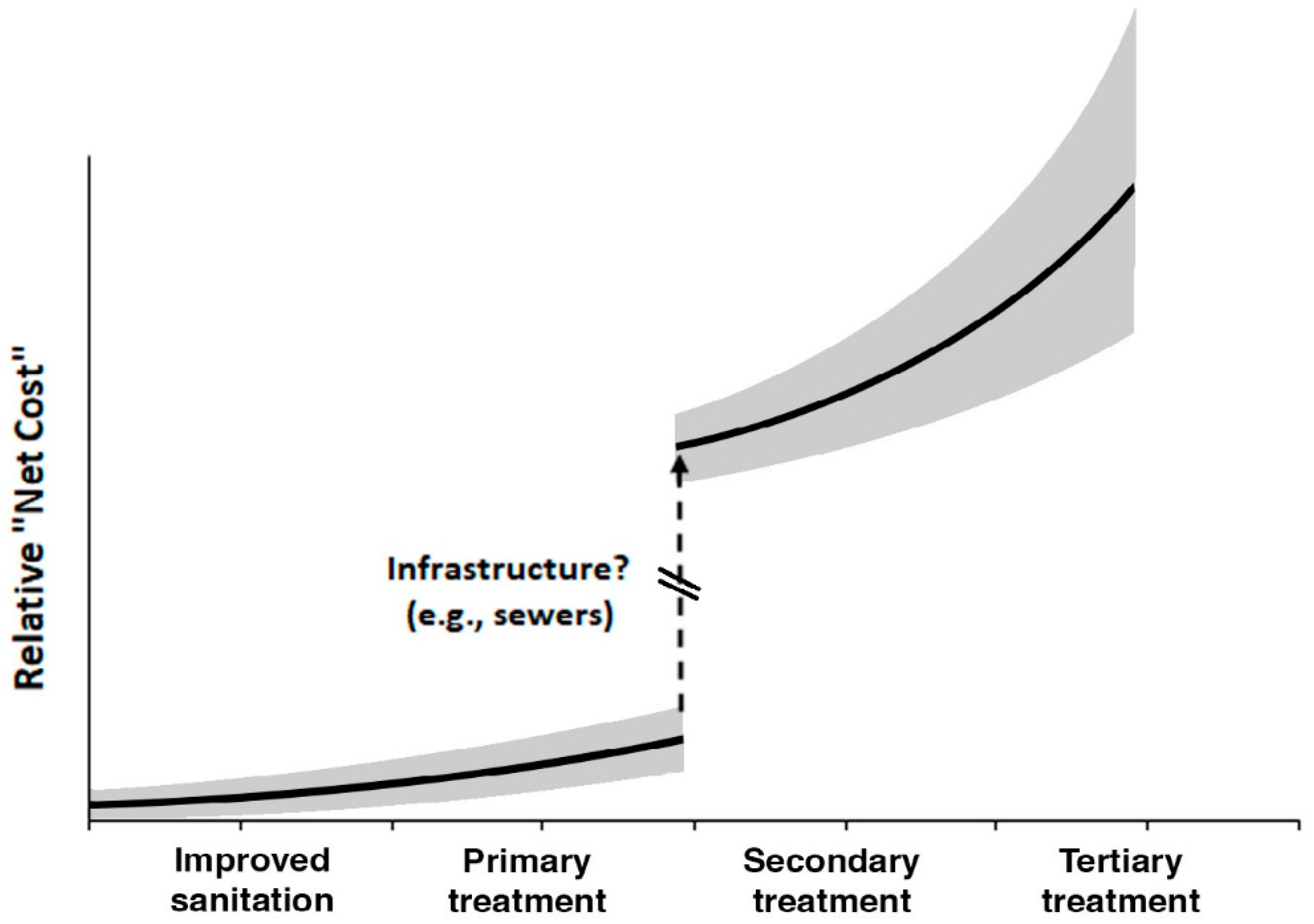
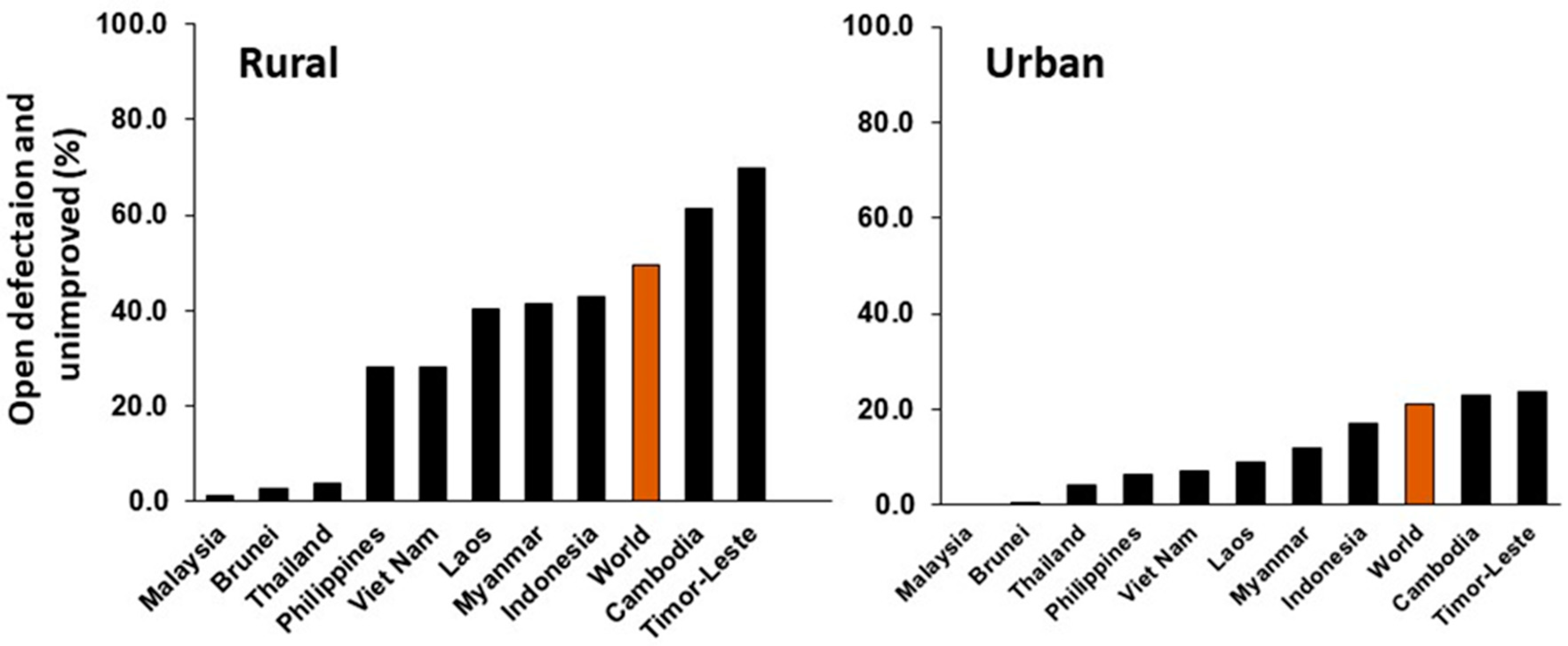
 = Investment should prioritize decentralized secondary waste treatment. The difference between the latter two categories is the availability of existing plumbing with septic tanks or similar.
= Investment should prioritize decentralized secondary waste treatment. The difference between the latter two categories is the availability of existing plumbing with septic tanks or similar.
 = Investment should prioritize decentralized secondary waste treatment. The difference between the latter two categories is the availability of existing plumbing with septic tanks or similar.
= Investment should prioritize decentralized secondary waste treatment. The difference between the latter two categories is the availability of existing plumbing with septic tanks or similar.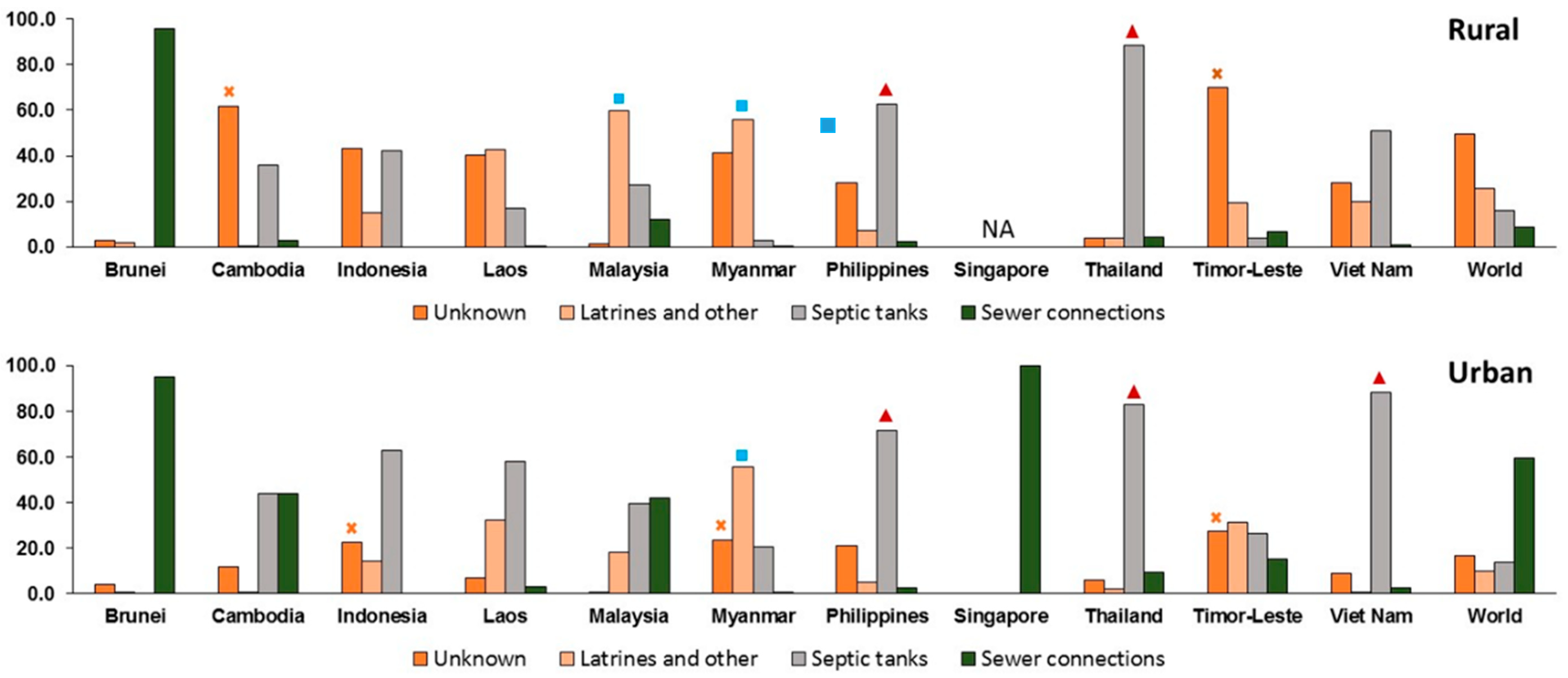
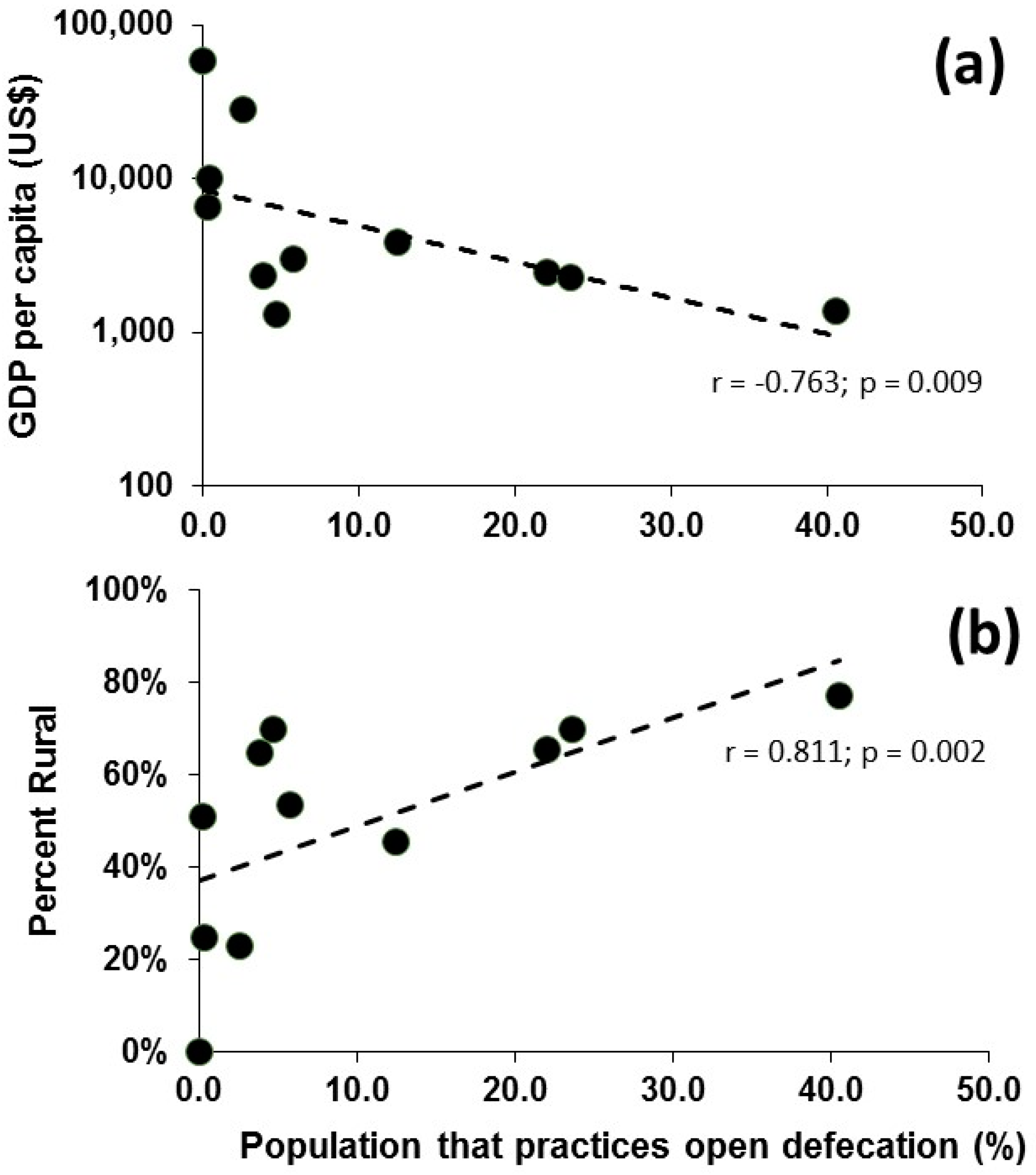
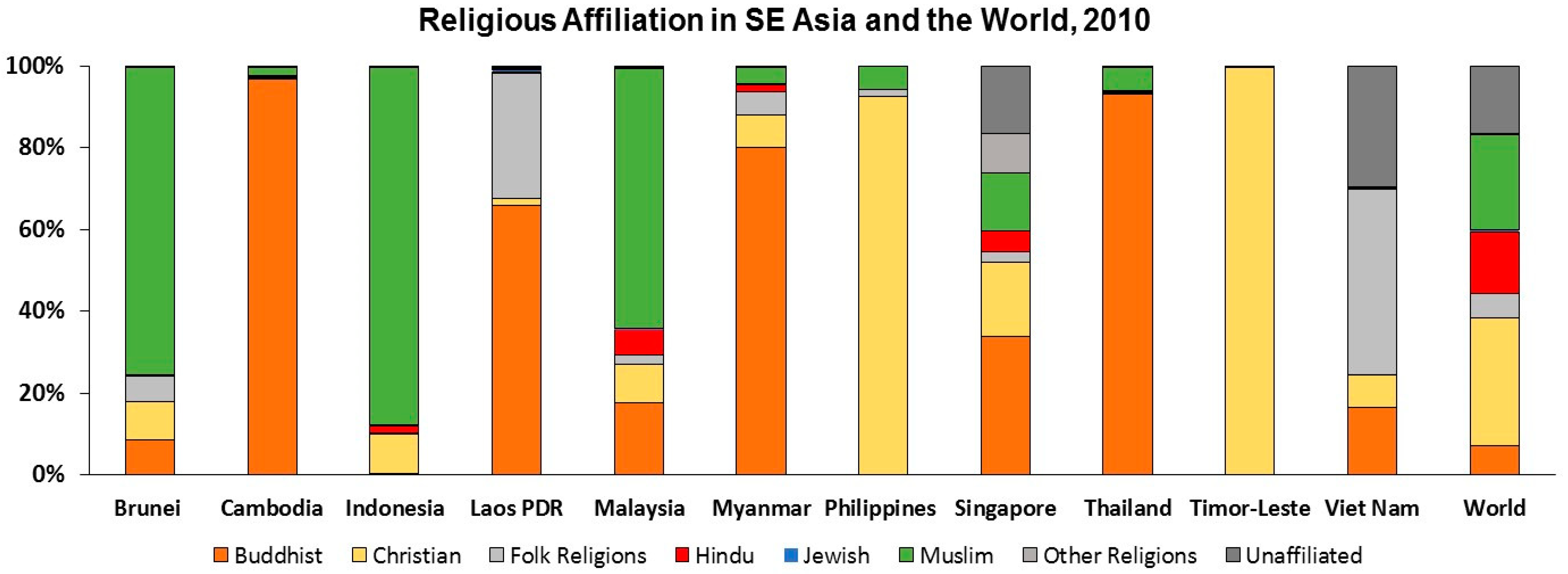
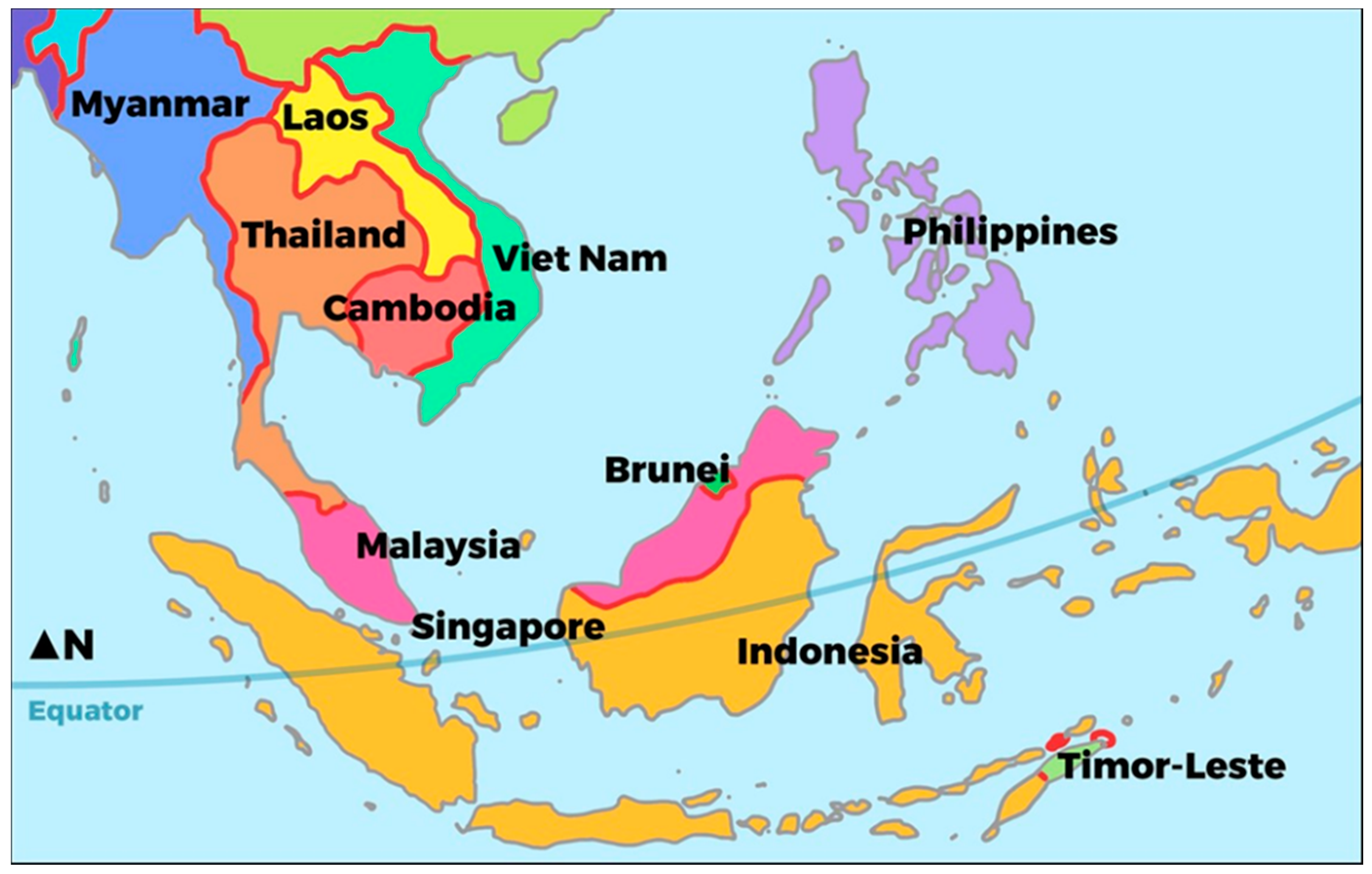
| Population | Area (km2) | Population Density (#/km2) | Urban Population (% of Total Population) | GDP Per Capita ($US) | |
|---|---|---|---|---|---|
| Brunei | 428,697 | 5765 | 74 | 77.3 | 28,291 |
| Cambodia | 16,005,373 | 181,035 | 88 | 23.0 | 1384 |
| Indonesia | 263,991,379 | 1,904,569 | 139 | 54.7 | 3847 |
| Laos PDR | 6,858,160 | 236,800 | 29 | 34.4 | 2457 |
| Malaysia | 31,624,264 | 329,847 | 96 | 75.4 | 9945 |
| Myanmar | 53,370,609 | 676,000 | 79 | 30.3 | 1299 |
| Philippines | 104,918,090 | 343,448 | 305 | 46.7 | 2989 |
| Singapore | 5,612,253 | 724 | 7752 | 100 | 57,714 |
| Thailand | 69,037,513 | 513,120 | 135 | 49.2 | 6594 |
| Timor-Leste | 1,296,311 | 14,874 | 87 | 30.2 | 2279 |
| Viet Nam | 95,540,800 | 331,210 | 288 | 35.2 | 2343 |
| Cultural Factors | |
| |
| Personal Considerations | |
|
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graham, D.W.; Giesen, M.J.; Bunce, J.T. Strategic Approach for Prioritising Local and Regional Sanitation Interventions for Reducing Global Antibiotic Resistance. Water 2019, 11, 27. https://doi.org/10.3390/w11010027
Graham DW, Giesen MJ, Bunce JT. Strategic Approach for Prioritising Local and Regional Sanitation Interventions for Reducing Global Antibiotic Resistance. Water. 2019; 11(1):27. https://doi.org/10.3390/w11010027
Chicago/Turabian StyleGraham, David W., Myra J. Giesen, and Joshua T. Bunce. 2019. "Strategic Approach for Prioritising Local and Regional Sanitation Interventions for Reducing Global Antibiotic Resistance" Water 11, no. 1: 27. https://doi.org/10.3390/w11010027
APA StyleGraham, D. W., Giesen, M. J., & Bunce, J. T. (2019). Strategic Approach for Prioritising Local and Regional Sanitation Interventions for Reducing Global Antibiotic Resistance. Water, 11(1), 27. https://doi.org/10.3390/w11010027







