Unraveling Flooding Dynamics and Nutrients’ Controls upon Phytoplankton Functional Dynamics in Amazonian Floodplain Lakes
Abstract
1. Introduction
2. Materials and Methods
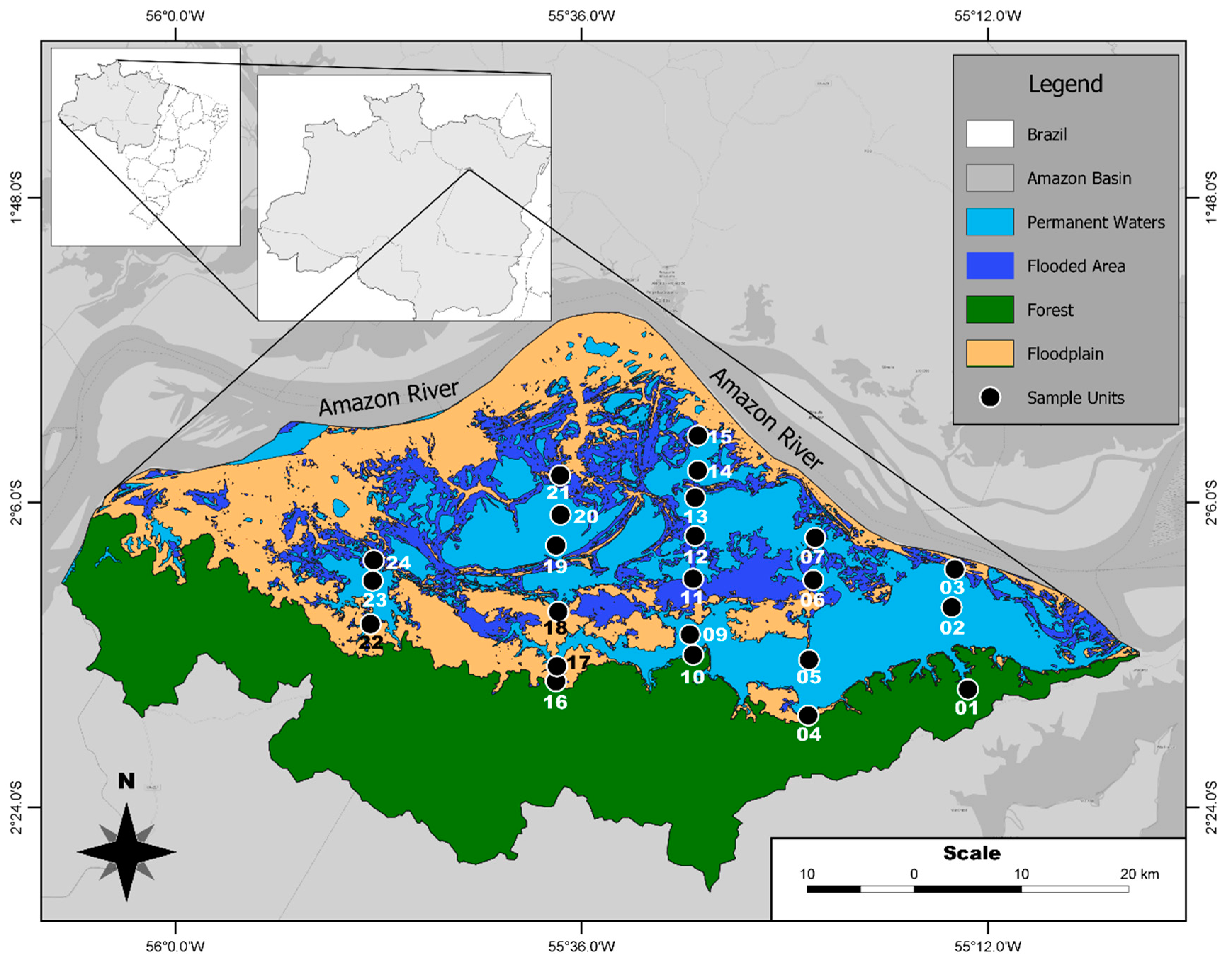
2.1. Environmental and Phytoplankton Data
2.2. Data Analysis
3. Results
3.1. Hydrological and Nutrients Data
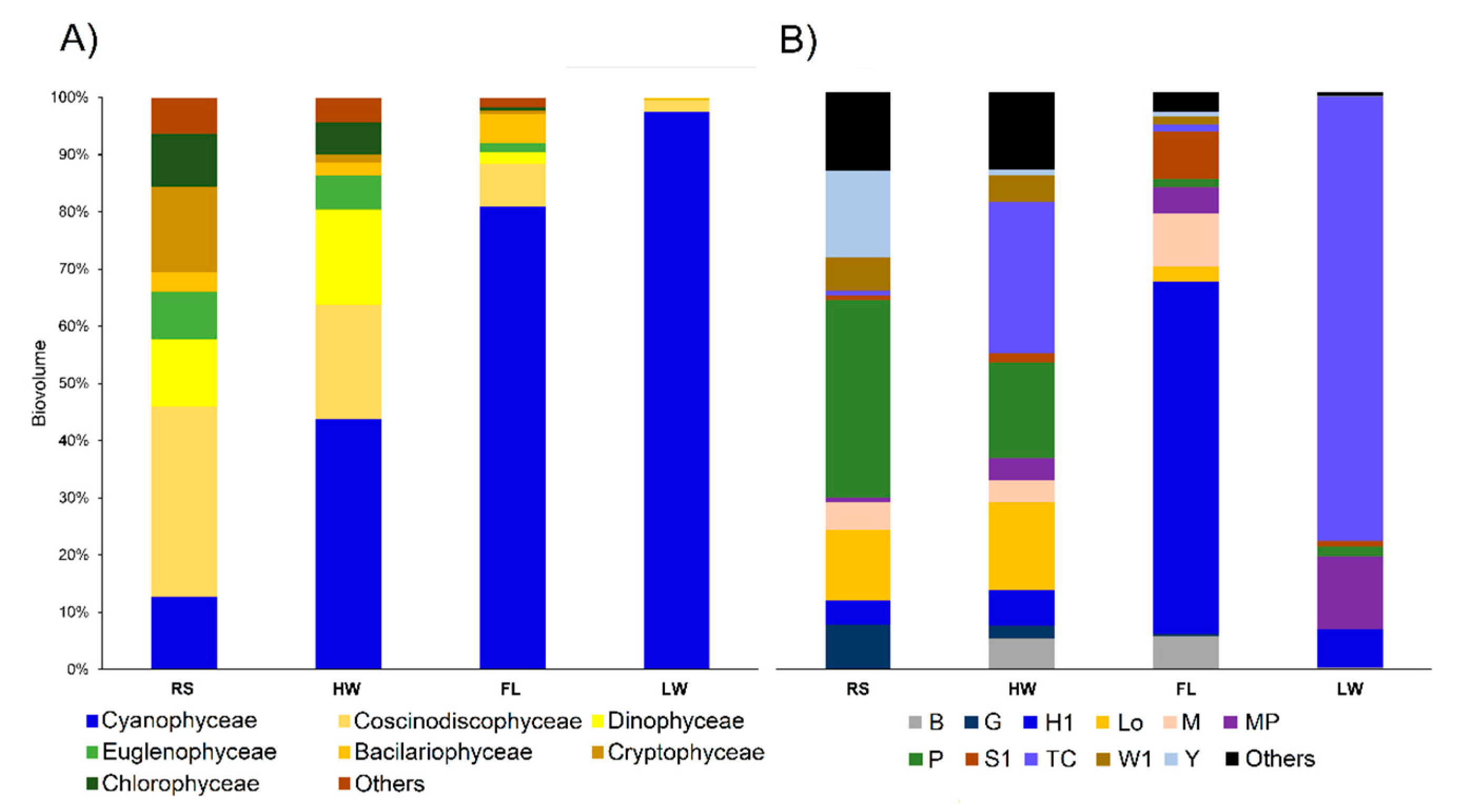
3.2. Biological Data
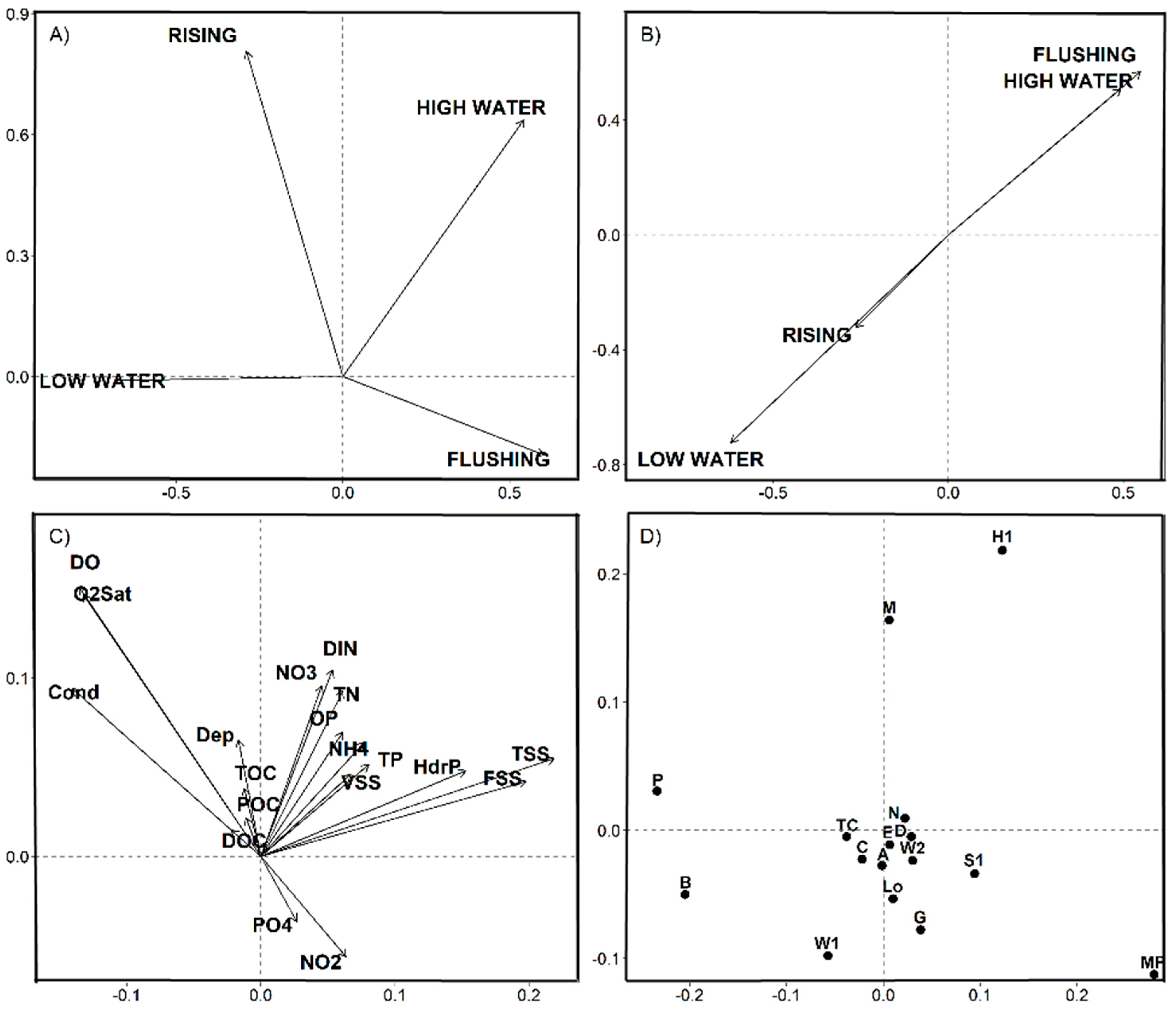
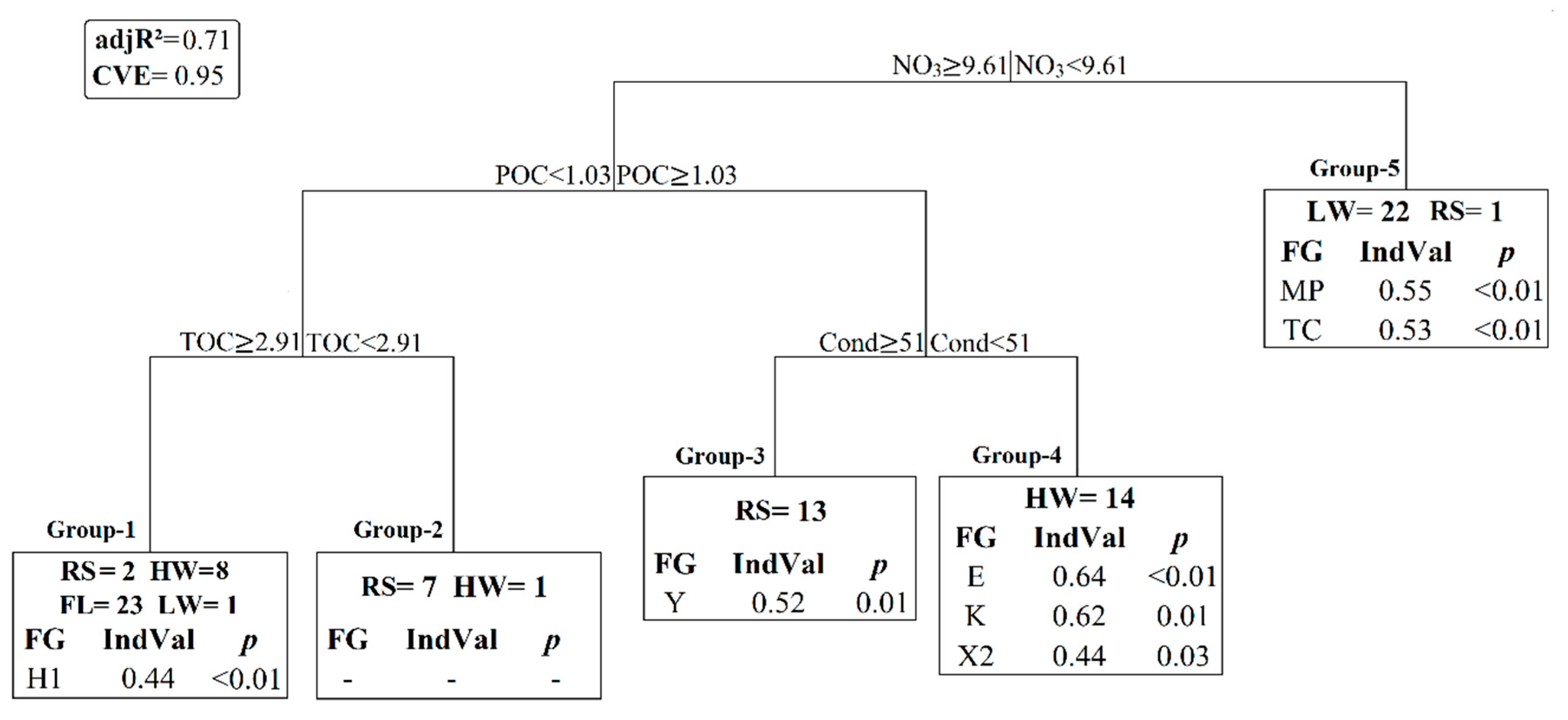
3.3. Statistical Results
4. Discussion
4.1. Space-Time Components and Environmental Partitions
4.2. Nutrients-Phytoplankton Relationships over Hydrological Cycle
4.3. Cyanobacteria Dynamics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fiore, M.D.F.; Neilan, B.A.; Copp, J.N.; Rodrigues, J.L.M.M.; Tsai, S.M.; Lee, H.; Trevors, J.T. Characterization of nitrogen-fixing cyanobacteria in the Brazilian Amazon floodplain. Water Res. 2005, 39, 5017–5026. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W. The dilemma of controlling cultural eutrophication of lakes. Proc. R. Soc. B Biol. Sci. 2012, 279, 4322–4333. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.K.; Litchman, E. Effects of temperature and nitrogen availability on the growth of invasive and native cyanobacteria. Hydrobiologia 2016, 763, 357–369. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [PubMed]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009. [Google Scholar] [CrossRef]
- Howarth, R.W. Nutrient Limitation of Net Primary Production in Marine Ecosystems. Annu. Rev. Ecol. Syst. 1988, 19, 89–110. [Google Scholar] [CrossRef]
- Lewis, W.M.; Wurtsbaugh, W.A.; Paerl, H.W. Rationale for Control of Anthropogenic Nitrogen and Phosphorus to Reduce Eutrophication of Inland Waters. Environ. Sci. Technol. 2011, 45, 10300–10305. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Abell, J.M.; Özkundakci, D.; Hamilton, D.P. Nitrogen and Phosphorus Limitation of Phytoplankton Growth in New Zealand Lakes: Implications for Eutrophication Control. Ecosystems 2010, 13, 966–977. [Google Scholar] [CrossRef]
- Thad Scott, J.; McCarthys, M.J. Nitrogen fixation may not balance the nitrogen pool in lakes over timescales relevant to eutrophication management. Limnol. Oceanogr. 2010, 55, 1265–1270. [Google Scholar] [CrossRef]
- Scheffer, M.; Van Nes, E.H. Shallow lakes theory revisited: Various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia 2007, 584, 455–466. [Google Scholar] [CrossRef]
- Janssen, A.B.G.; Teurlincx, S.; An, S.; Janse, J.H.; Paerl, H.W.; Mooij, W.M. Alternative stable states in large shallow lakes? J. Great Lakes Res. 2014, 40, 813–826. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Davidson, T.A.; Trolle, D.; Søndergaard, M.; Lauridsen, T.L.; Beklioglu, M.; Brucet, S.; Volta, P.; González-Bergonzoni, I.; et al. Climate change impacts on lakes: An integrated ecological perspective based on a multi-faceted approach, with special focus on shallow lakes. J. Limnol. 2014, 73, 88–111. [Google Scholar] [CrossRef]
- Lobo, M.T.M.P.S.; de Souza Nogueira, I.; Fabris Sgarbi, L.; Nunes Kraus, C.; de Oliveira Bomfim, E.; Garnier, J.; da Motta Marques, D.; Bonnet, M.-P. Morphology-based functional groups as the best tool to characterize shallow lake-dwelling phytoplankton on an Amazonian floodplain. Ecol. Indic. 2018, 95, 579–588. [Google Scholar] [CrossRef]
- Lampert, W.; Sommer, U. Limnoecology; Oxford University Press: Oxford, UK, 2007; ISBN 978-0-19-921392-4. [Google Scholar]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506–509, 135–145. [Google Scholar]
- Scheffer, M.; Rinaldi, S.; Gragnani, A.; Mur, L.R.; van Nes, E.H. On the Dominance of Filamentous Cyanobacteria in Shallow, Turbid Lakes. Ecology 1997, 78, 272. [Google Scholar] [CrossRef]
- Hays, S.G.; Ducat, D.C. Engineering cyanobacteria as photosynthetic feedstock factories. Photosynth. Res. 2014, 123, 1–11. [Google Scholar] [CrossRef]
- Benoiston, A.-S.; Ibarbalz, F.M.; Bittner, L.; Guidi, L.; Jahn, O.; Dutkiewicz, S.; Bowler, C. The evolution of diatoms and their biogeochemical functions. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160397. [Google Scholar] [CrossRef]
- Peace, A. Effects of light, nutrients, and food chain length on trophic efficiencies in simple stoichiometric aquatic food chain models. Ecol. Model. 2015, 312, 125–135. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Catherine, Q.; Susanna, W.; Isidora, E.S.; Mark, H.; Aurélie, V.; Jean-François, H. A review of current knowledge on toxic benthic freshwater cyanobacteria—Ecology, toxin production and risk management. Water Res. 2013, 47, 5464–5479. [Google Scholar] [CrossRef]
- Boopathi, T.; Ki, J.-S. Impact of Environmental Factors on the Regulation of Cyanotoxin Production. Toxins 2014, 6, 1951–1978. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Madamwar, D.; Incharoensakdi, A. Bloom dynamics of cyanobacteria and their toxins: Environmental health impacts and mitigation strategies. Front. Microbiol. 2015, 6, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, A.; Quesada, A.; Salmaso, N. Global expansion of toxic and non-toxic cyanobacteria: Effect on ecosystem functioning. Biodivers. Conserv. 2015, 24, 889–908. [Google Scholar] [CrossRef]
- Vilmi, A.; Karjalainen, S.M.; Landeiro, V.L.; Heino, J. Freshwater diatoms as environmental indicators: Evaluating the effects of eutrophication using species morphology and biological indices. Environ. Monit. Assess. 2015, 187, 243. [Google Scholar] [CrossRef]
- Dolman, A.M.; Rücker, J.; Pick, F.R.; Fastner, J.; Rohrlack, T.; Mischke, U.; Wiedner, C. Cyanobacteria and cyanotoxins: The influence of nitrogen versus phosphorus. PLoS ONE 2012, 7, e38757. [Google Scholar] [CrossRef]
- LONGHI, M.L.; BEISNER, B.E. Patterns in taxonomic and functional diversity of lake phytoplankton. Freshw. Biol. 2010, 55, 1349–1366. [Google Scholar] [CrossRef]
- Colina, M.; Calliari, D.; Carballo, C.; Kruk, C. A trait-based approach to summarize zooplankton–phytoplankton interactions in freshwaters. Hydrobiologia 2015, 767, 221–233. [Google Scholar] [CrossRef]
- Salmaso, N.; Naselli-Flores, L.; Padisák, J. Functional classifications and their application in phytoplankton ecology. Freshw. Biol. 2015, 60, 603–619. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S.S. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Padisák, J.; Crossetti, L.O.; Naselli-Flores, L. Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Kruk, C.; Huszar, V.L.M.; Peeters, E.T.H.M.; Bonilla, S.; Costa, L.; Lürling, M.; Reynolds, C.S.; Scheffer, M. A morphological classification capturing functional variation in phytoplankton. Freshw. Biol. 2010, 55, 614–627. [Google Scholar] [CrossRef]
- Machado, K.B.; Borges, P.P.; Carneiro, F.M.; de Santana, J.F.; Vieira, L.C.G.; de Moraes Huszar, V.L.; Nabout, J.C. Using lower taxonomic resolution and ecological approaches as a surrogate for plankton species. Hydrobiologia 2015, 743, 255–267. [Google Scholar] [CrossRef]
- Junk, W.J.; Bayley, P.B.; Sparks, R.E. The flood pulse concept in river-floodplain systems. Can. Spec. Publ. Fish. Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Junk, W.J.; Piedade, M.T.F.; Schöngart, J.; Wittmann, F. A classification of major natural habitats of Amazonian white-water river floodplains (várzeas). Wetl. Ecol. Manag. 2012, 20, 461–475. [Google Scholar] [CrossRef]
- Tockner, K.; Malard, F.; Ward, J.V. An extension of the flood pulse concept. Hydrol. Process. 2000, 14, 2861–2883. [Google Scholar] [CrossRef]
- Bonnet, M.P.P.; Barroux, G.; Martinez, J.M.M.; Seyler, F.; Moreira-Turcq, P.; Cochonneau, G.; Melack, J.M.M.; Boaventura, G.; Maurice-Bourgoin, L.; León, J.G.G.; et al. Floodplain hydrology in an Amazon floodplain lake (Lago Grande de Curuaí). J. Hydrol. 2008, 349, 18–30. [Google Scholar] [CrossRef]
- Junk, W.J.; Wantzen, K.M. The flood pulse concept: New aspects, approaches and applications—An update. In Proceedings of the Second International Symposium on the Management of Large Rivers for Fisheries, Rome, Italy, 11–14 February 2003; pp. 117–149. [Google Scholar]
- Bonnet, M.-P.; Pinel, S.; Garnier, J.; Bois, J.; Resende Boaventura, G.; Seyler, P.; Motta Marques, D. Amazonian floodplain water balance based on modelling and analyses of hydrologic and electrical conductivity data. Hydrol. Process. 2017, 31, 1702–1718. [Google Scholar] [CrossRef]
- Kraus, C.N.; Bonnet, M.P.; Miranda, C.A.; de Souza Nogueira, I.; Garnier, J.; Vieira, L.C.G. Interannual Hydrological Variation and Ecological Phytoplankton Patterns in Amazonian Floodplain Lakes. Hydrobiologia 2018. [Google Scholar] [CrossRef]
- Sioli, H. The Amazon and its main affluents: Hydrography, morphology of the river courses, and river types. In The Amazon: Limnology and Landscape Ecology of a Mighty Tropical River and Its Basin; Sioli, H., Ed.; Springer: Dordrecht, The Netherlands, 1984; pp. 127–165. ISBN 978-94-009-6542-3. [Google Scholar]
- Moquet, J.S.; Crave, A.; Viers, J.; Seyler, P.; Armijos, E.; Bourrel, L.; Chavarri, E.; Lagane, C.; Laraque, A.; Casimiro, W.S.L.; et al. Chemical weathering and atmospheric/soil CO(2) uptake in the Andean and Foreland Amazon basins. Chem. Geol. 2011, 287, 1–26. [Google Scholar] [CrossRef]
- Park, E.; Latrubesse, E.M. Water resources research. Water Resour. Res. 2015, 51, 9127–9140. [Google Scholar]
- Bonnet, M.P.; Garnier, J.; Barroux, G.; Boaventura, G.R.; Seyler, P.; Mse, C.C.; Bataillon, P.E. Biogeochemical functioning of amazonian floodplains: The case of lago Grande de Curuai. In Riparian Zones: Characteristics, Management Practices and Ecological Impacts, Environmental Research Advances; Pokrovsky, O.S., Viers, J., Eds.; Nova Science Publishers: New York, NY, USA, 2016; pp. 1–22. ISBN 978-1-63484-636-3. [Google Scholar]
- APHA Standard Methods for Examination of Water and Wastewater (Standard Methods for the Examination of Water and Wastewater); Standard Methods; American Public Health Association (APHA): Washington, DC, USA, 1998; pp. 5–16.
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton-methodik. Mitt. Int. Ver. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Lund, J.W.G.; Kipling, C.; Le Cren, E.D. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication. Available online: http//www.algaebase.org (accessed on 20 October 2018).
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume Calculation for Pelagic and Benthic Microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Kruk, C.; Mazzeo, N.; Lacerot, G.; Reynolds, C.S. Classification schemes for phytoplankton: A local validation of a functional approach to the analysis of species temporal replacement. J. Plankton Res. 2002, 24, 901–912. [Google Scholar] [CrossRef]
- Thioulouse, J. Simultaneous analysis of a sequence of paired ecological tables: A comparison of several methods. Ann. Appl. Stat. 2012, 5, 2300–2325. [Google Scholar] [CrossRef]
- Dray, S.; Chessel, D.; Thioulouse, J. Co-Inertia Analysis and the Linking of Ecological Data Tables. Ecology 2003, 84, 3078–3089. [Google Scholar] [CrossRef]
- Blanchet, F.G.; Legendre, P.; Borcard, D. Forward selection of explanatory variables. Ecology 2008, 89, 2623–2632. [Google Scholar] [CrossRef]
- De’ath, G. Multivariate Regression Tree: A New Technique for Modeling Species–Environment Relationships. Ecology 2002, 83, 1105–1117. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Community Diversity. In Numerical Ecology with R; Springer: Berlin/Heidelberg, Germany, 2018; pp. 369–412. [Google Scholar]
- De’Ath, G.; Fabricius, K.E. Classification and regression trees: A powerful yet simple technique for ecological data analysis. Ecology 2000, 81, 3178–3192. [Google Scholar] [CrossRef]
- Therneau, T.M.; Atkinson, B.; Ripley, B.; Oksanen, J.; De’ath, G. MVpart. A Package for Running Multivariate Regression Trees in R Software. Available online: https://cran.r-project.org/src/contrib/Archive/mvpart/ (accessed on 30 November 2018).
- Ouellette, M.H.; Legendre, P. MVPARTwrap: Additional Features for Package Mvpart. R Package, Version 0.1-9.2. Available online: https://cran.r-project.org/src/contrib/Archive/MVPARTwrap/ (accessed on 30 November 2018).
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, J.; Li, R. Comparative studies on photosynthesis and phosphate metabolism of Cylindrospermopsis raciborskii with Microcystis aeruginosa and Aphanizomenon flos-aquae. Harmful Algae 2009, 8, 910–915. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, S.; Wang, Y. Characteristics of bioavailable organic phosphorus in sediment and its contribution to lake eutrophication in China. Environ. Pollut. 2016, 219, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Junk, W.J. The flood pulse concept of large rivers: Learning from the tropics. Large Rivers 1999, 11, 261–280. [Google Scholar] [CrossRef]
- Zhou, J.; Lau, K.M. Principal modes of interannual and decadal variability of summer rainfall over South America. Int. J. Clim. 2001, 21, 1623–1644. [Google Scholar] [CrossRef]
- Osborne, P.L. Tropical Ecosystems and Ecological Concepts; Cambridge University Press: Cambridge, UK, 2000; ISBN 0521645239. [Google Scholar]
- Silva, T.S.F.; Melack, J.M.; Novo, E.M.L.M. Responses of aquatic macrophyte cover and productivity to flooding variability on the Amazon floodplain. Glob. Chang. Biol. 2013, 19, 3379–3389. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, W.H.; Reckhow, K.H.; Bernhardt, E.S. Global change: The nitrogen cycle and rivers. Water Resour. Res. 2006, 42, 5–6. [Google Scholar] [CrossRef]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. The Nitrogen Cascade. Bioscience 2003, 53, 341. [Google Scholar] [CrossRef]
- Peterson, B.J.; Wollheim, W.M.; Mulholland, P.J.; Webster, J.R.; Meyer, J.L.; Tank, J.L.; Marti, E.; Bowden, W.B.; Valett, H.M.; Hershey, A.E.; et al. Control of nitrogen export from watersheds by headwater streams. Science 2001, 292, 86–90. [Google Scholar] [CrossRef]
- Burkart, M.R.; Stoner, J.D. Nitrogen in Groundwater Associated with Agricultural Systems. Nitrogen Environ. 2008, 177–202. [Google Scholar] [CrossRef]
- Xiao, M.; Willis, A.; Burford, M.A. Differences in cyanobacterial strain responses to light and temperature reflect species plasticity. Harmful Algae 2017, 62, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Cottingham, K.L.; Ewing, H.A.; Greer, M.L.; Carey, C.C.; Weathers, K.C. Cyanobacteria as biological drivers of lake nitrogen and phosphorus cycling. Ecosphere 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Dokulil, M.T.; Teubner, K. Cyanobacterial dominance in lakes. Hydrobiologia 2000, 438, 1–12. [Google Scholar] [CrossRef]
- Johnston, C.A. Sediment and nutrient retention by freshwater wetlands: Effects on surface water quality. Crit. Rev. Environ. Control 1991, 21, 491–565. [Google Scholar] [CrossRef]
- Nogueira, I.D.S.; Nabout, J.C.; Ibañez, M.D.S.R.; Bourgoin, L.M.; Rodrigues, S. Determinants of beta diversity: The relative importance of environmental and spatial processes in structuring phytoplankton communities in an Amazonian floodplain. Acta Limnol. Bras. 2010, 22, 247–256. [Google Scholar] [CrossRef]
- Affonso, A.; Barbosa, C.; Novo, E. Water quality changes in floodplain lakes due to the Amazon River flood pulse: Lago Grande de Curuaí (Pará). Braz. J. Biol. 2011, 71, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.S.; Descy, J.-P.; Padisàk, J. Are phytoplankton dynamics in rivers so different from those in shallow lakes? Hydrobiologia 1994, 289, 1–7. [Google Scholar] [CrossRef]
- Shan, K.; Li, L.; Wang, X.; Wu, Y.; Hu, L.; Yu, G.; Song, L. Modelling ecosystem structure and trophic interactions in a typical cyanobacterial bloom-dominated shallow Lake Dianchi, China. Ecol. Model. 2014, 291, 82–95. [Google Scholar] [CrossRef]
- Barbosa, C.C.F.; Moraes Novo, E.M.L.; Melack, J.M.; Gastil-Buhl, M.; Filho, W.P. Geospatial analysis of spatiotemporal patterns of pH, total suspended sediment and chlorophyll-a on the Amazon floodplain. Limnology 2009, 11, 155–166. [Google Scholar] [CrossRef]
- Bourgoin, L.M.; Bonnet, M.-P.; Martinez, J.-M.; Kosuth, P.; Cochonneau, G.; Moreira-Turcq, P.; Guyot, J.-L.; Vauchel, P.; Filizola, N.; Seyler, P. Temporal dynamics of water and sediment exchanges between the Curuaí floodplain and the Amazon River, Brazil. J. Hydrol. 2007, 335, 140–156. [Google Scholar] [CrossRef]
- Mulholland, P.J.; Helton, A.M.; Poole, G.C.; Hall, R.O.; Hamilton, S.K.; Peterson, B.J.; Tank, J.L.; Ashkenas, L.R.; Cooper, L.W.; Dahm, C.N.; et al. Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 2008, 452, 202–205. [Google Scholar] [CrossRef] [PubMed]
| Dep m | DO mg L−1 | O2Sat % | Cond µS/cm | TP μg L−1 | PO4 μg L−1 | HdrP μg L−1 | OP μg L−1 | TN μg L−1 | DIN μg L−1 | NH4 μg L−1 | NO3 μg L−1 | NO2 μg L−1 | TOC mg L−1 | DOC mg L−1 | POC mg L−1 | SST mg L−1 | SSF mg L−1 | SSV mg L−1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RS | |||||||||||||||||||
| Min | 1.70 | 4.5 | 61.9 | 38.0 | 22.1 | 0.1 | 2.2 | 0.1 | 225.4 | 86.0 | 0.4 | 5.0 | 5.0 | 1.9 | 1.6 | 0.0 | 32.0 | 0.0 | 0.0 |
| Max | 5.70 | 7.6 | 107.2 | 82.0 | 186.4 | 75.0 | 74.3 | 136.7 | 629.6 | 422.4 | 187.9 | 148.0 | 17.0 | 8.9 | 5.4 | 5.6 | 108.0 | 98.0 | 40.0 |
| Mean | 4.00 | 6.2 | 83.6 | 70.0 | 85.8 | 5.0 | 11.7 | 69.3 | 379.0 | 225.9 | 37.2 | 63.9 | 8.8 | 5.1 | 3.6 | 1.9 | 56.7 | 37.0 | 19.7 |
| SD | 1.43 | 0.9 | 13.1 | 12.0 | 38.9 | 16.3 | 14.8 | 32.8 | 93.9 | 76.9 | 39.7 | 41.9 | 2.6 | 2.3 | 1.0 | 1.8 | 21.3 | 30.6 | 14.6 |
| CV | 0.36 | 0.15 | 0.16 | 0.17 | 0.45 | 3.24 | 1.27 | 0.47 | 0.25 | 0.34 | 1.07 | 0.66 | 0.29 | 0.45 | 0.29 | 0.96 | 0.38 | 0.83 | 0.74 |
| HW | |||||||||||||||||||
| Min | 4.11 | 0.4 | 6.0 | 35.0 | 34.2 | 0.1 | 1.3 | 5.3 | 277.4 | 187.9 | 8.0 | 36.2 | 1.0 | 2.9 | 2.6 | 0.2 | 4.0 | 1.0 | 0.5 |
| Max | 7.53 | 9.6 | 131.2 | 50.0 | 105.4 | 306.6 | 173.1 | 136.7 | 519.4 | 415.8 | 306.6 | 136.8 | 68.6 | 5.9 | 4.5 | 3.4 | 24.0 | 16.8 | 13.4 |
| Mean | 6.30 | 4.4 | 58.5 | 44.1 | 62.4 | 24.9 | 41.4 | 53.4 | 362.5 | 275.3 | 66.6 | 80.6 | 8.3 | 4.5 | 3.6 | 1.2 | 14.6 | 8.3 | 6.3 |
| SD | 1.03 | 1.9 | 26.2 | 3.7 | 18.4 | 64.3 | 37.5 | 28.8 | 68.6 | 56.2 | 70.7 | 31.9 | 14.0 | 0.7 | 0.6 | 0.7 | 5.2 | 4.6 | 3.5 |
| CV | 0.16 | 0.44 | 0.45 | 0.08 | 0.30 | 2.33 | 0.90 | 0.54 | 0.19 | 0.20 | 1.06 | 0.40 | 1.69 | 0.16 | 0.16 | 0.57 | 0.36 | 0.55 | 0.57 |
| FL | |||||||||||||||||||
| Min | 2.50 | 0.5 | 6.8 | 39.0 | 7.1 | 0.1 | 0.1 | 0.1 | 187.1 | 175.2 | 7.0 | 10.0 | 10.0 | 2.9 | 2.8 | 0.0 | 6.5 | 3.0 | 1.5 |
| Max | 4.30 | 12.5 | 172.4 | 81.0 | 111.3 | 25.0 | 79.7 | 77.9 | 570.0 | 608.9 | 183.0 | 246.2 | 10.0 | 7.1 | 6.8 | 0.8 | 66.5 | 62.0 | 12.5 |
| Mean | 3.77 | 6.5 | 86.9 | 51.1 | 52.1 | 1.2 | 26.4 | 25.2 | 314.0 | 288.7 | 30.0 | 84.0 | 10.0 | 4.0 | 3.8 | 0.3 | 29.0 | 23.9 | 5.2 |
| SD | 0.71 | 3.1 | 42.4 | 11.4 | 26.7 | 5.2 | 23.0 | 21.3 | 105.9 | 101.0 | 41.9 | 68.8 | 0.0 | 1.0 | 0.9 | 0.2 | 15.5 | 15.1 | 3.0 |
| CV | 0.19 | 0.48 | 0.49 | 0.22 | 0.51 | 4.39 | 0.87 | 0.84 | 0.34 | 0.35 | 1.39 | 0.82 | 0.00 | 0.25 | 0.25 | 0.76 | 0.53 | 0.63 | 0.58 |
| LW | |||||||||||||||||||
| Min | 0.45 | 6.2 | 83.0 | 19.0 | 9.9 | 0.0 | 22.2 | 0.1 | 125.6 | 106.8 | 6.9 | 3.6 | 0.1 | 2.8 | 2.6 | 0.1 | 20.0 | 14.0 | 2.0 |
| Max | 2.40 | 11.0 | 150.9 | 69.0 | 119.2 | 306.6 | 268.3 | 20.0 | 756.0 | 732.3 | 450.5 | 12.5 | 381.5 | 7.0 | 6.0 | 1.3 | 284.0 | 263.0 | 21.0 |
| Mean | 1.24 | 7.8 | 106.1 | 50.9 | 49.9 | 39.1 | 98.7 | 1.0 | 475.0 | 362.5 | 195.1 | 5.9 | 80.1 | 4.1 | 3.5 | 0.5 | 67.0 | 58.0 | 9.0 |
| SD | 0.54 | 1.0 | 14.4 | 13.5 | 28.1 | 78.3 | 51.6 | 4.1 | 141.6 | 121.4 | 114.9 | 2.2 | 90.1 | 1.1 | 0.8 | 0.3 | 53.3 | 49.9 | 4.5 |
| CV | 0.44 | 0.13 | 0.14 | 0.27 | 0.56 | 2.01 | 0.52 | 4.30 | 0.30 | 0.33 | 0.59 | 0.38 | 1.13 | 0.26 | 0.23 | 0.57 | 0.80 | 0.86 | 0.50 |
| Space-Time Test | Partition Test | ||||||
|---|---|---|---|---|---|---|---|
| R2 | F | p | Adj.R2 | F | p | ||
| Space-time | 0.060 | 1.18 | 0.221 | Nutr | 0.128 | 1.89 | 0.001 |
| Time | 0.530 | 35.09 | 0.001 | Hydr | 0.068 | 2.00 | 0.001 |
| Space | 0.128 | 1.15 | 0.114 | Nutr + Hydr | 0.126 | - | - |
| Residuals | 0.679 | - | - | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraus, C.N.; Bonnet, M.-P.; de Souza Nogueira, I.; Morais Pereira Souza Lobo, M.T.; da Motta Marques, D.; Garnier, J.; Cardoso Galli Vieira, L. Unraveling Flooding Dynamics and Nutrients’ Controls upon Phytoplankton Functional Dynamics in Amazonian Floodplain Lakes. Water 2019, 11, 154. https://doi.org/10.3390/w11010154
Kraus CN, Bonnet M-P, de Souza Nogueira I, Morais Pereira Souza Lobo MT, da Motta Marques D, Garnier J, Cardoso Galli Vieira L. Unraveling Flooding Dynamics and Nutrients’ Controls upon Phytoplankton Functional Dynamics in Amazonian Floodplain Lakes. Water. 2019; 11(1):154. https://doi.org/10.3390/w11010154
Chicago/Turabian StyleKraus, Cleber Nunes, Marie-Paule Bonnet, Ina de Souza Nogueira, Maria Tereza Morais Pereira Souza Lobo, David da Motta Marques, Jérémie Garnier, and Ludgero Cardoso Galli Vieira. 2019. "Unraveling Flooding Dynamics and Nutrients’ Controls upon Phytoplankton Functional Dynamics in Amazonian Floodplain Lakes" Water 11, no. 1: 154. https://doi.org/10.3390/w11010154
APA StyleKraus, C. N., Bonnet, M.-P., de Souza Nogueira, I., Morais Pereira Souza Lobo, M. T., da Motta Marques, D., Garnier, J., & Cardoso Galli Vieira, L. (2019). Unraveling Flooding Dynamics and Nutrients’ Controls upon Phytoplankton Functional Dynamics in Amazonian Floodplain Lakes. Water, 11(1), 154. https://doi.org/10.3390/w11010154







