The Contribution of Different Restored Habitats to Fish Diversity and Population Development in a Highly Modified River: A Case Study from the River Günz
Abstract
1. Introduction
2. Materials and Methods
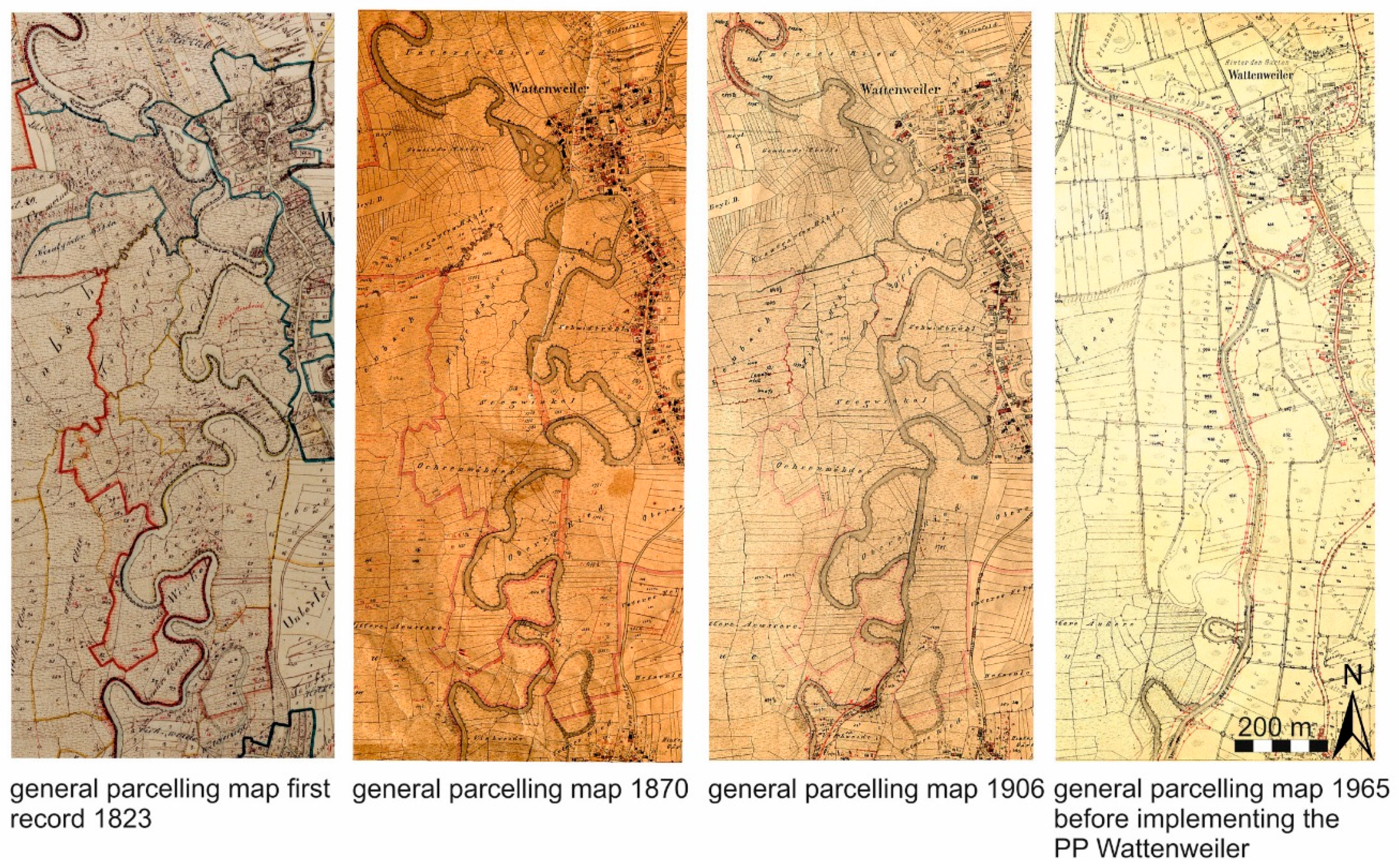
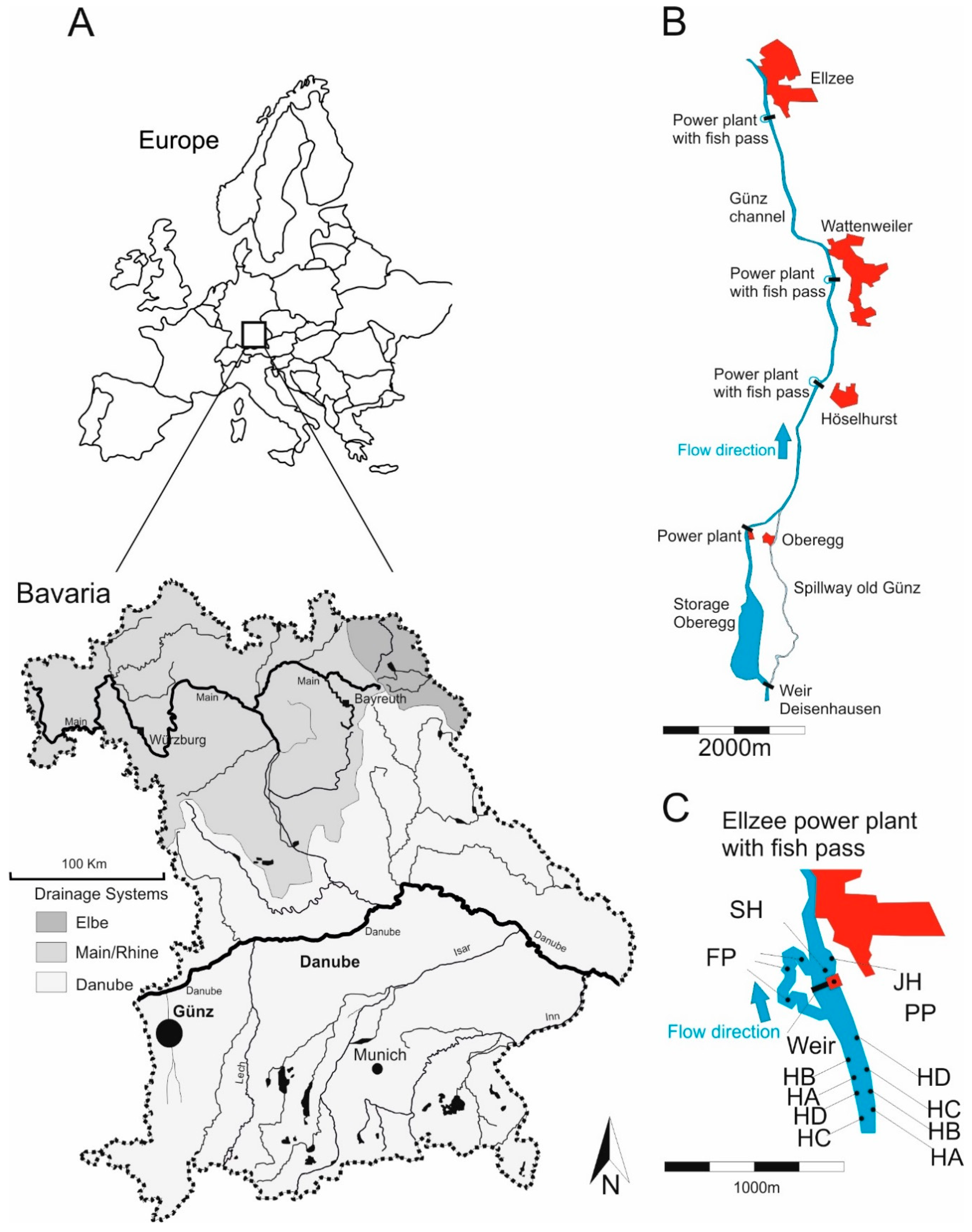
2.1. Study Area
2.2. Study Design
2.3. Restoration Actions in the Compared Habitats
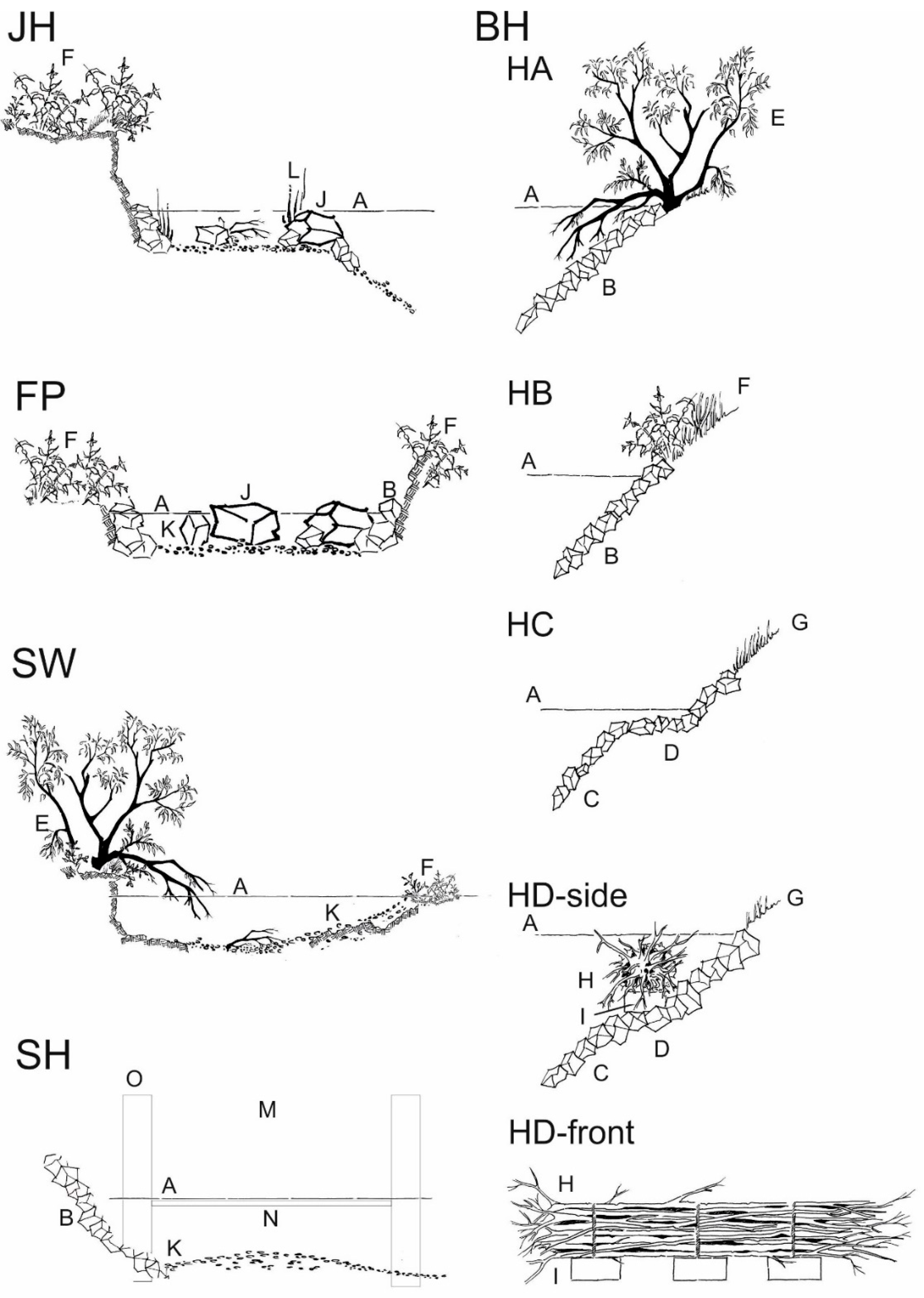
2.3.1. Bank Habitats (BH)
2.3.2. Structured Shallow Water Zones (JH)
2.3.3. Fast Flowing Instream Habitat with Gravel (SH)
2.3.4. Nature Like Fish Passes (FP)
2.3.5. Former River Course below the Spillway (SW)
2.4. Measurement of Physico-Chemical Habitat Characteristics
2.5. Fish Sampling
2.6. Statistical Analysis
3. Results
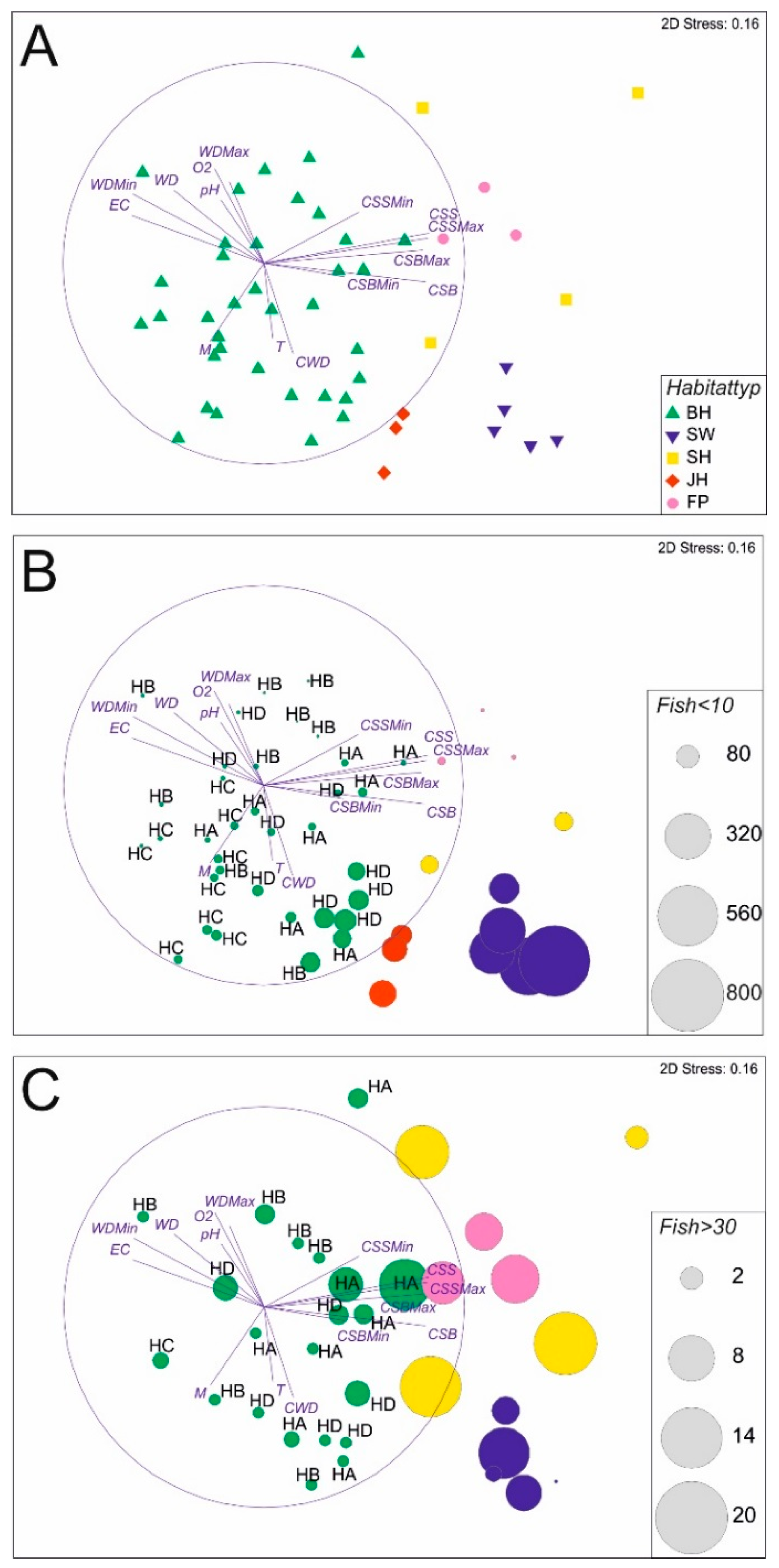
3.1. Abiotic Habitat Characteristics
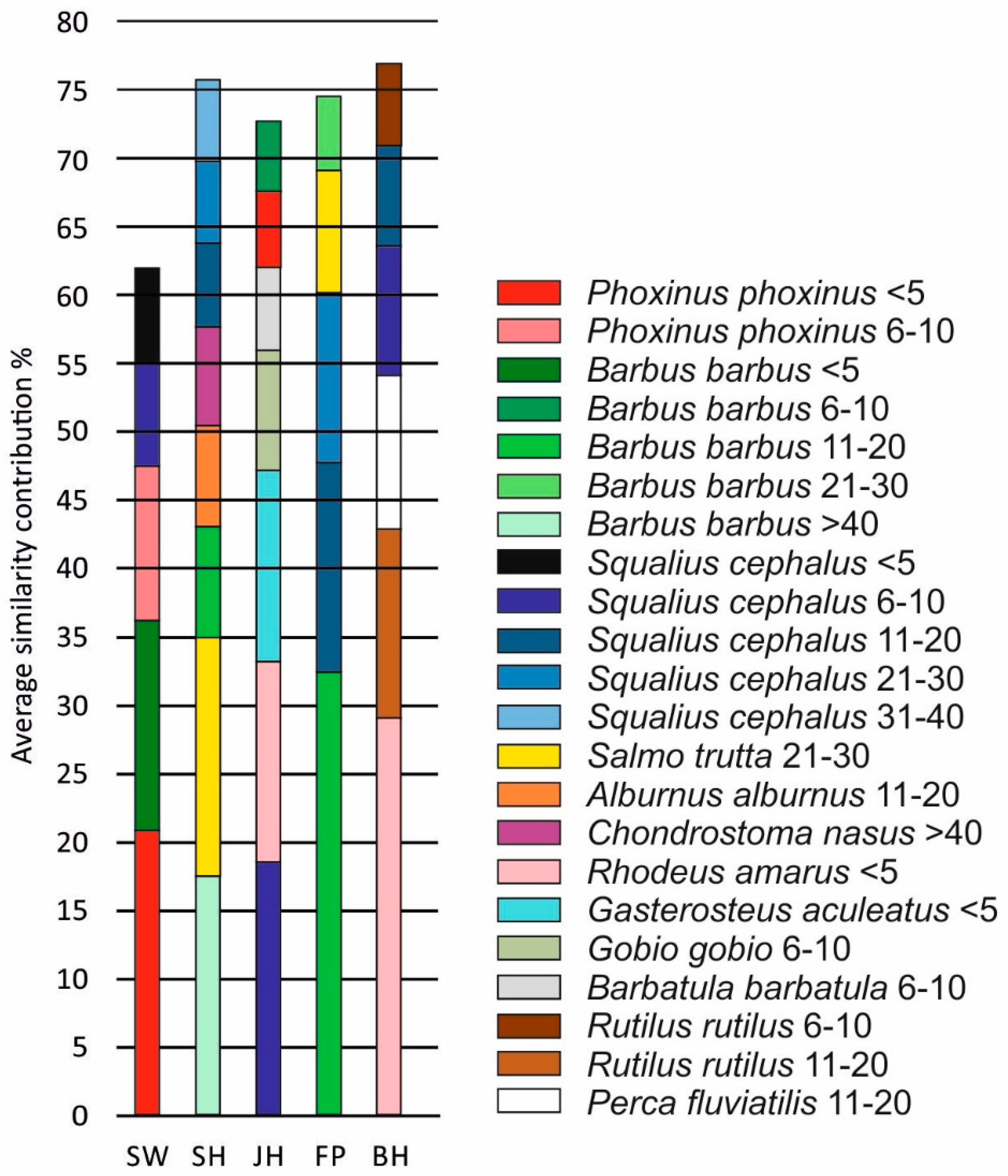
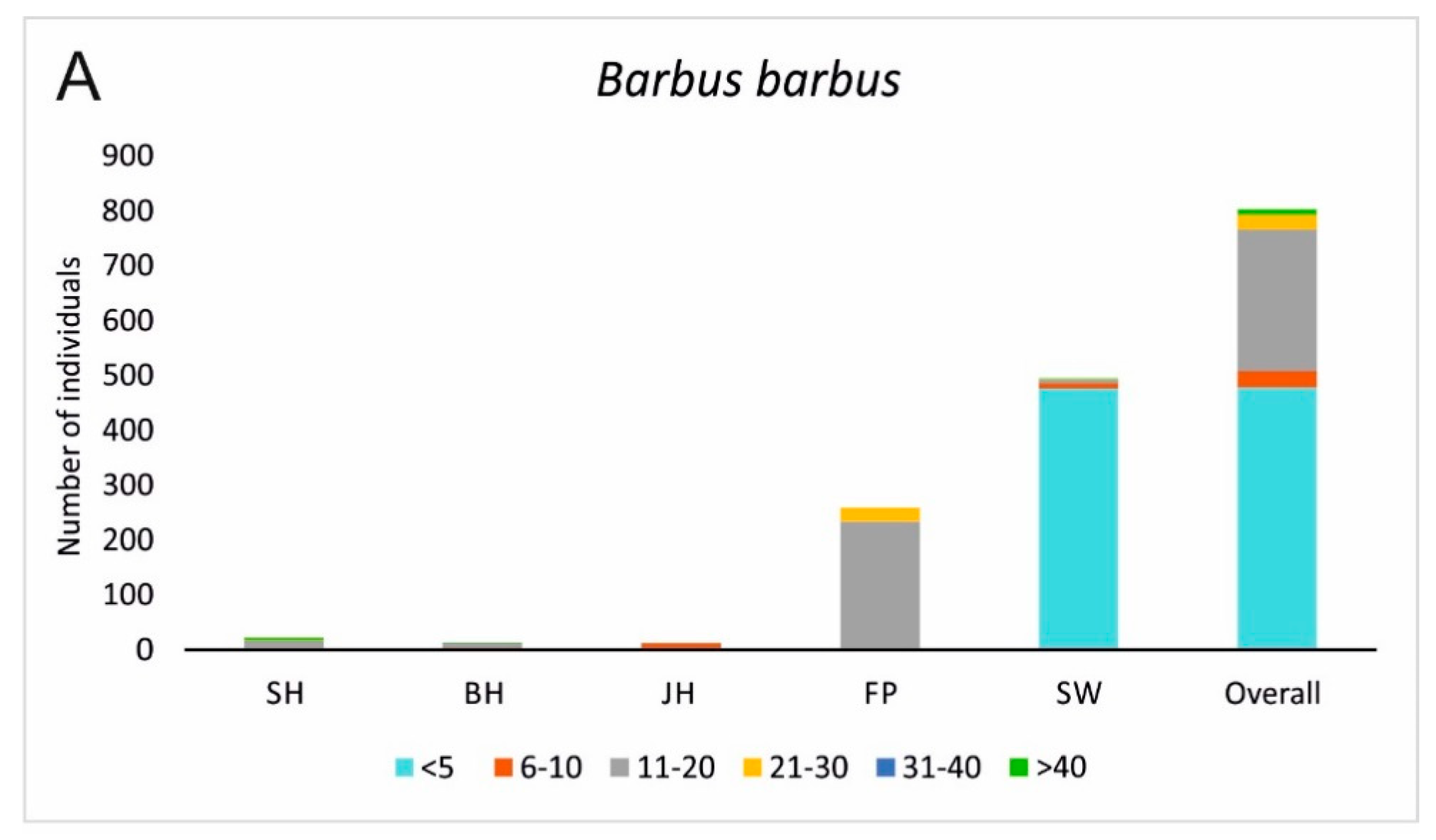
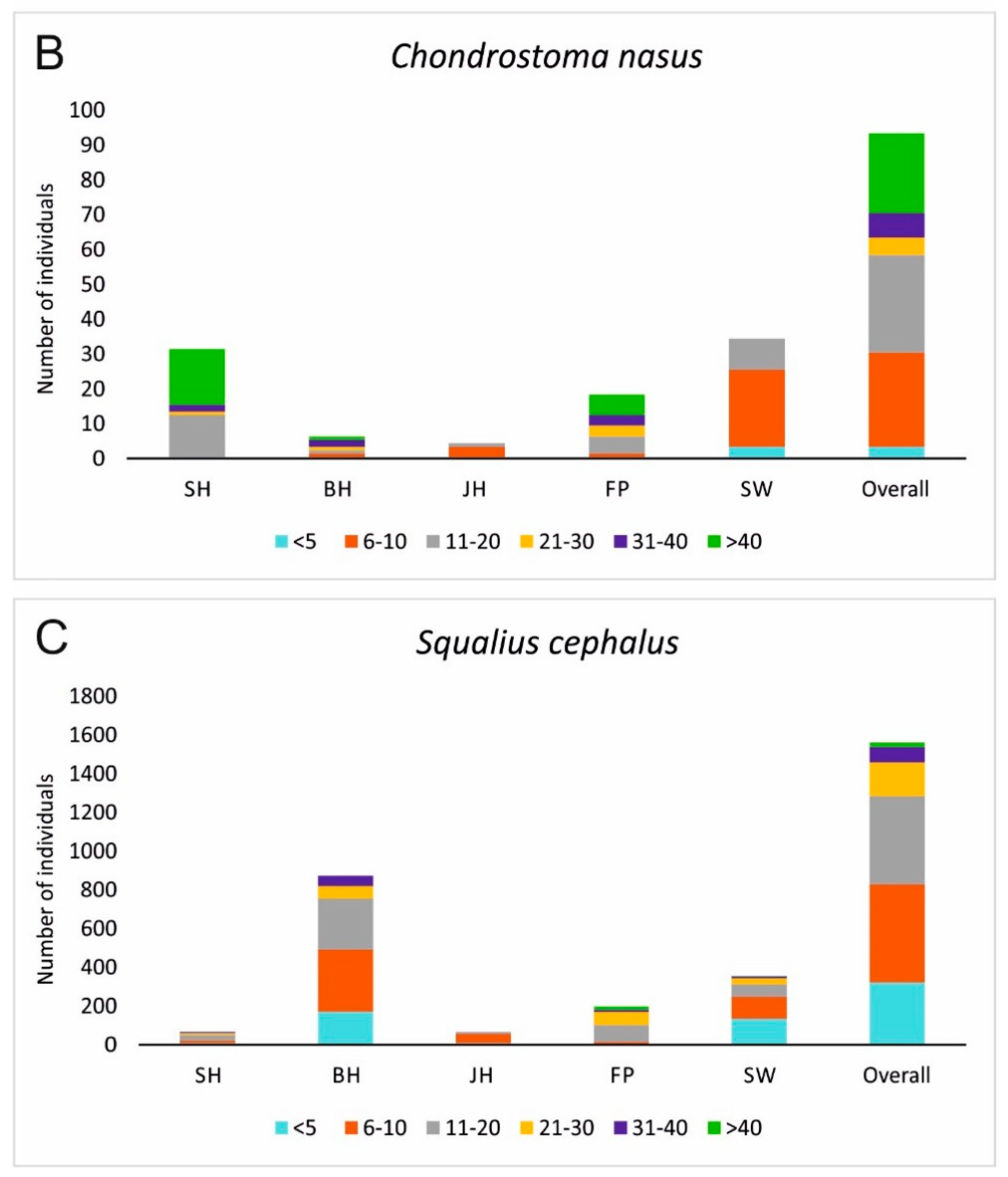
3.2. Fish Habitat Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EU Commission. Towards Sustainable Water Management in the European Union. First Stage in the implementation of the Water Framework Directive 2000⁄60⁄EC; Commission staff Working Document. Accompanying Document to the Communication Forum from the Commission to the European Parliament and the Council COM 2007; EU Commission: Brussels, Belgium, 2007; p. 128. [Google Scholar]
- Marchetti, M.P.; Moyle, P. Effects of flow regime on fish assemblages in a regulated California stream. Ecol. Appl. 2001, 11, 530–539. [Google Scholar] [CrossRef]
- Pander, J.; Geist, J. Seasonal and spatial bank habitat use by fish in highly altered rivers–a comparison of four different restoration measures. Ecol. Freshw. Fish 2010, 19, 127–138. [Google Scholar] [CrossRef]
- Pander, J.; Geist, J. Can fish habitat restoration for rheophilic species in highly modified rivers be sustainable in the long run? Ecol. Eng. 2016, 88, 28–38. [Google Scholar] [CrossRef]
- European Parliament. Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for the Community action in the field of water policy. Off. J. Eur. Union 2000, 327, 1–73. [Google Scholar]
- Pander, J.; Geist, J. Ecological indicators for measuring stream restoration success. Ecol. Indic. 2013, 30, 106–118. [Google Scholar] [CrossRef]
- Boedeltje, G.; Smolders, A.J.P.; Roelofs, J.G.M.; Van Groenendael, J.M. Constructed shallow zones along navigation canals: Vegetation establishment and change in relation to environmental characteristics. Aquat. Conserv. Mar. Freshw. Ecosyst. 2001, 11, 453–471. [Google Scholar] [CrossRef]
- Pulg, U.; Barlaup, B.T.; Sternecker, K.; Trepl, L.; Unfer, G. Restoration of spawning habitats of brown trout (Salmo trutta) in a regulated chalk stream. River Res. Appl. 2013, 29, 172–182. [Google Scholar] [CrossRef]
- Tockner, K.; Schiemer, F.; Ward, J.V. Conservation by restoration: The management concept for a river-floodplain system on the Danube River in Austria. Aquat. Conserv. Mar. Freshw. Ecosyst. 1998, 8, 71–86. [Google Scholar] [CrossRef]
- Pusey, B.J.; Arthington, A.H. Importance of the riparian zone to the conservation and management of freshwater fish: A review. Mar. Freshw. Res. 2003, 54, 1–16. [Google Scholar] [CrossRef]
- Keckeis, H.; Winkler, G.; Flore, L.; Reckendorfer, W.; Schiemer, F. Spatial and seasonal characteristics of 0+ fish nursery habitats of nase, Chondrostoma nasus in the river Danube, Austria. Folia Zool. 1997, 46, 133–150. [Google Scholar]
- Jurajda, P. Comparative nursery habitat use by 0+ fish in a modified lowland river. Regul. Rivers Res. Manag. 1999, 15, 113–124. [Google Scholar] [CrossRef]
- Schiemer, F.; Keckeis, H.; Kamler, E. The early life history stages of riverine fish: Ecophysiological and environmental bottelnecks. Comp. Biochem. Physiol. Part A 2003, 133, 439–449. [Google Scholar] [CrossRef]
- Hauer, C.; Unfer, G.; Schmutz, S.; Habersack, H. Morphodynamic effects on the habitat of juvenile cyprinids (Chondrostoma nasus) in a restored Austrian lowland river. Environ. Manag. 2008, 42, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Melcher, A.H.; Schmutz, S. The importance of structural features for spawning habitat of nase Chondrostoma nasus (L.) and barbel Barbus barbus (L.) in a pre-Alpine river. River Syst. 2010, 19, 33–42. [Google Scholar] [CrossRef]
- Britton, J.R.; Pegg, J. Ecology of European barbel Barbus barbus: Implications for river, fishery and conservation management. Rev. Fish. Sci. 2011, 19, 321–330. [Google Scholar] [CrossRef]
- Jungwirth, M.; Muhar, S.; Schmutz, S. The effects of recreated instream and ecotone structures on the fish fauna of an epipotamal river. Hydrobiologia 1995, 303, 195–206. [Google Scholar] [CrossRef]
- Pander, J.; Mueller, M.; Knott, J.; Egg, L.; Geist, J. Is it Worth the Money? The Functionality of Engineered Shallow Stream Banks as Habitat for Juvenile Fishes in Heavily Modified Water Bodies. River Res. Appl. 2017, 33, 63–72. [Google Scholar] [CrossRef]
- Frissell, C.A.; Liss, W.J.; Warren, C.E.; Hurley, M.D. A Hierarchical Framework for Stream Habitat Classification: Viewing Streams in a Watershed Context. Environ. Manag. 1986, 10, 199–214. [Google Scholar] [CrossRef]
- FAO/DVWK. FishPasses—Design, Dimensions and Monitoring; Food and Agriculture Organization of the United Nations, Deutscher Verband für Wasserwirtschaft und Kulturbau e.V.: Rome, Italy, 2002; p. 118. [Google Scholar]
- Pander, J.; Mueller, M.; Geist, J. Ecological functions of fish bypass channels in streams: Migration corridor and habitat for rheophilic species. River Res. Appl. 2013, 29, 441–450. [Google Scholar] [CrossRef]
- Arthington, A.H.; Bunn, S.E.; Poff, N.L.; Naiman, R.J. The challenge of providing environmental flow rules to sustain river ecosystems. Ecol. Appl. 2006, 16, 1311–1318. [Google Scholar] [CrossRef]
- Poff, N.L.; Zimmerman, J.K. Ecological responses to altered flow regimes: A literature review to inform the science and management of environmental flows. Freshw. Biol. 2010, 55, 194–205. [Google Scholar] [CrossRef]
- Beechie, T.J.; Sear, D.A.; Olden, J.D.; Pess, G.R.; Buffington, J.M.; Moir, H.; Roni, P.; Pollock, M.M. Process-based principles for restoring river ecosystems. BioScience 2010, 60, 209–222. [Google Scholar] [CrossRef]
- Mueller, M.; Pander, J.; Geist, J. The ecological value of stream restoration measures: An evaluation on ecosystem and target species scales. Ecol. Eng. 2014, 62, 129–139. [Google Scholar] [CrossRef]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat: Cornol, Switzerland, 2007; p. 646. [Google Scholar]
- Duerregger, A.; Pander, J.; Palt, M.; Mueller, M.; Nagel, C.; Geist, J. The importance of stream interstitial conditions for the early life stage development of the European nase (Chondrostoma nasus L.). Ecol. Freshw. Fish 2018. [Google Scholar] [CrossRef]
- DWA 509. Merkblatt Deutsche Vereinigung für Wasserwirtschaft und Abfall e.V., Fischaufstiegsanlagen und Fischpassierbare Bauwerke—Gestaltung, Bemessung, Qualitätssicherung; Eigenverlag: Hennef, Germany, 2014; p. 284. [Google Scholar]
- Schneider, S.; Melzer, A. The trophic index of macrophytes (TIM)—A new tool for indicating the trophic state of running waters. Int. Rev. Hydrobiol. 2003, 88, 49–67. [Google Scholar] [CrossRef]
- Gurnell, A.; Gregory, K.J.; Petts, G.E. The role of coarse woody debris in forest aquatic habitats: Implications for management. Aquat. Conserv. Mar. Freshw. Ecosyst. 1995, 5, 143–166. [Google Scholar] [CrossRef]
- Gurnell, A.; Tockner, K.; Edwards, P.; Petts, G.E. Effects of deposited wood on biocomplexity of river corridors. Front. Ecol. Environ. 2005, 3, 377–382. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde; Springer-Verlag: Wien, Austria, 1964; p. 632. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press Urbana: Champaign, IL, USA, 1949; p. 117. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Zauner, G.; Eberstaller, J. Klassifizierungsschema der österreichischen Flußfischfauna in Bezug auf deren Lebensraumansprüche. Österreichs Fischerei 1999, 52, 198–205. [Google Scholar]
- Dußling, U.; Bischoff, A.; Haberbosch, R.; Hoffmann, A.; Klinger, H.; Wolter, C.; Wysujack, K.; Berg, R. Der Fischregionsindex (FRI)—Ein Instrument zur Fließgewässerbewertung gemäß EG-Wasserrahmenrichtlinie. Wasserwirtschaft 2005, 95, 19–24. [Google Scholar]
- Robinson, C.T.; Tockner, K.; Ward, J.V. The fauna of dynamic riverine landscapes. Freshw. Biol. 2002, 47, 661–677. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008; p. 212. [Google Scholar]
- Lytle, D.A.; Poff, N.L. Adaptation to natural flow regimes. Trends Ecol. Evol. 2004, 19, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Reinartz, R. Untersuchungen zur Gefährdungssituation der Fischart Nase (Chondrostoma nasus L.) in Bayerischen Gewässern; Technische Universität München, Institut für Tierwissenschaften–Dissertation: München, Germany, 1997; p. 241. [Google Scholar]
- Ebel, G. Untersuchungen zur Stabilisierung von Barbenpopulationen–Dargestellt am Beispiel eines Mitteldeutschen Fließgewässers; Eigenverlag: Halle, Deutschland, 2002; p. 148. [Google Scholar]
- Peňáz, M. Chondrostoma nasus-its reproduction strategy and possible reasons for a widely observed population decline—A review. In Conservation of Endangered Freshwater Fish in Europe; Birkhäuser: Basel, Switzerland, 1996; pp. 279–285. [Google Scholar]
- Dávidová, M.; Blažek, R.; Trichkova, T.; Koutrakis, E.; Gaygusuz, Ö.; Ercan, E.; Ondračková, M. The role of the European bitterling (Rhodeus amarus, Cyprinidae) in parasite accumulation and transmission in riverine ecosystems. Aquat. Ecol. 2011, 45, 377–387. [Google Scholar] [CrossRef]
- Hering, D.; Borja, A.; Carstensen, J.; Carvalho, L.; Elliott, M.; Feld, C.K.; Heiskanen, A.S.; Johnson, R.K.; Moe, J.; Pont, D.; et al. The European Water Framework Directive at the age of 10: A critical review of the achievements with recommendations for the future. Sci. Total Environ. 2010, 408, 4007–4019. [Google Scholar] [CrossRef] [PubMed]
- Geist, J. Trends and Directions in Water Quality and Habitat Management in the Context of the European Water Framework Directive. Fisheries 2014, 39, 219–220. [Google Scholar] [CrossRef]
- Geist, J. Seven steps towards improving freshwater conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2015, 25, 447–453. [Google Scholar] [CrossRef]
- Bohl, E.; Kleisinger, H.; Leuner, E. Rote Liste gefährdeter Fische (Pisces) und Rundmäuler (Cyclostomata) Bayerns; Bayrisches Landesamt für Umwelt: Augsburg, Germany, 2003; pp. 52–55. [Google Scholar]
- Freyhof, J. Rote Liste der im Süßwasser reproduzierenden Neunaugen und Fische (Cyclostomata & Pisces). Naturschutz und Biologische Vielfalt 2009, 70, 291–316. [Google Scholar]
| Habitat | WD | CSS | CSB | O2 | T | EC | pH | CWD | M |
|---|---|---|---|---|---|---|---|---|---|
| JH | 27 a | 0.19 a | 0.13 a | 6.4 a | 17.4 a | 596 a | 9.4 a | 10 a | 12 a |
| 5–27 | 0.01–0.40 | 0.01–0.29 | 6.2–6.6 | 16.6–18.0 | 589–601 | 9.1–9.6 | 5–15 | 5–15 | |
| BH | 128 b | 0.10 b | 0.06 b | 7.4 b | 17.3 a | 609 b | 8.7 b | 5 a | 5 b |
| 83–175 | 0.01–0.30 | 0.01–0.30 | 7.0–7.7 | 16.6–18.0 | 594–624 | 6.9–9.8 | 0–35 | 0–15 | |
| SH | 116 bc | 0.75 c | 0.39 c | 7.3 c | 17.4 a | 615 c | 8.3 c | 0 b | 0.3 c |
| 83–154 | 0.24–1.10 | 0.11–0.90 | 7.1–7.6 | 16.6–18.0 | 594–624 | 7.8–8.5 | 0 | 0–1 | |
| FP | 55 d | 0.35 ad | 0.24 ad | 9.0 c | 16.1 b | 599 d | 8.2 d | 0.5 c | 1 d |
| 24–102 | 0.01–0.99 | 0.01–1.08 | 7.7–9.9 | 15.6–16.5 | 595–601 | 8.1–8.3 | 0–1 | 1–3 | |
| SW | 48 d | 0.32 d | 0.30 dc | 8.8 c | 16.0 c | 599 d | 7.8 e | 9 ab | 0.6 e |
| 12–105 | 0.03–0.87 | 0.02–1.00 | 8.5–9.8 | 15.5–16.5 | 594–603 | 7.7–8.7 | 3–15 | 0–1 |
| Habitat | S | CPUE (Individuals/300 m2) | M (kg/ha) | MFM (g) | H | J | FRI |
|---|---|---|---|---|---|---|---|
| JH | 19 | 292 | 86 | 8 | 2.48 | 0.84 | 6.12 |
| BH | 21 | 80 | 135 | 55 | 1.82 | 0.67 | 6.48 |
| SW | 21 | 664 | 416 | 15 | 2.12 | 0.70 | 5.75 |
| SH | 18 | 132 | 722 | 165 | 2.09 | 0.77 | 6.02 |
| FP | 17 | 207 | 1043 | 135 | 1.58 | 0.56 | 5.70 |
| Comparisons | R | Significance Level p |
|---|---|---|
| BH-SW | 0.772 | <0.001 |
| BH-SH | 0.687 | <0.001 |
| BH-JH | 0.443 | <0.01 |
| BH-FP | 0.663 | <0.001 |
| SW-SH | 0.619 | <0.01 |
| SW-JH | 1.000 | <0.05 |
| SW-FP | 1.000 | <0.05 |
| SH-JH | 0.481 | >0.05 |
| SH-FP | −0.093 | >0.05 |
| JH-FP | 1.000 | >0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pander, J.; Geist, J. The Contribution of Different Restored Habitats to Fish Diversity and Population Development in a Highly Modified River: A Case Study from the River Günz. Water 2018, 10, 1202. https://doi.org/10.3390/w10091202
Pander J, Geist J. The Contribution of Different Restored Habitats to Fish Diversity and Population Development in a Highly Modified River: A Case Study from the River Günz. Water. 2018; 10(9):1202. https://doi.org/10.3390/w10091202
Chicago/Turabian StylePander, Joachim, and Juergen Geist. 2018. "The Contribution of Different Restored Habitats to Fish Diversity and Population Development in a Highly Modified River: A Case Study from the River Günz" Water 10, no. 9: 1202. https://doi.org/10.3390/w10091202
APA StylePander, J., & Geist, J. (2018). The Contribution of Different Restored Habitats to Fish Diversity and Population Development in a Highly Modified River: A Case Study from the River Günz. Water, 10(9), 1202. https://doi.org/10.3390/w10091202





