Treatment of Palm Oil Mill Effluent Using Membrane Bioreactor: Novel Processes and Their Major Drawbacks
Abstract
1. Introduction
2. Physicochemical Properties and Biodegradability Index of POME
3. Membrane Bioreactor (MBR) Application for POME Treatment
3.1. Membrane in MBR System
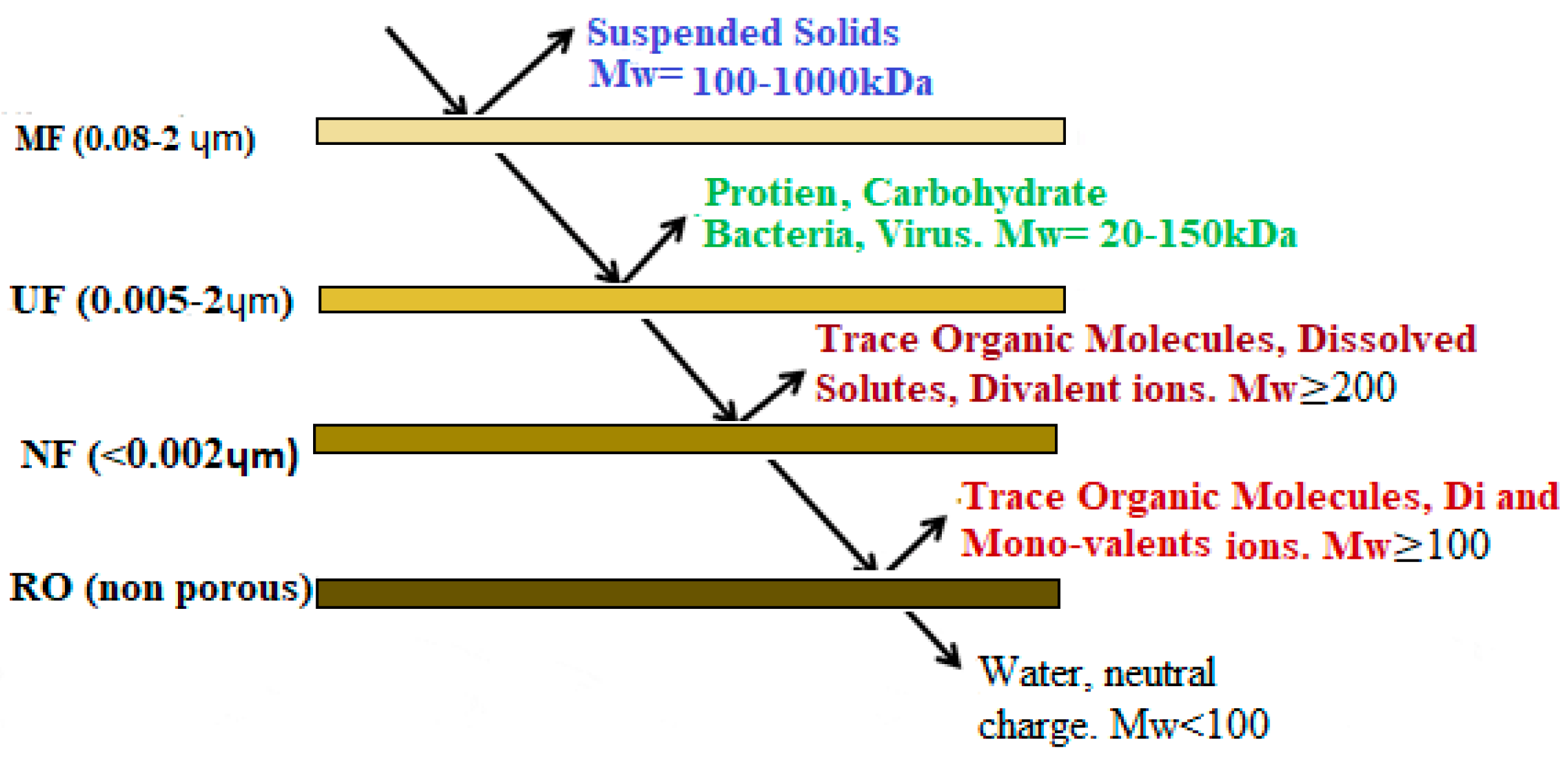
3.1.1. Types of Membrane
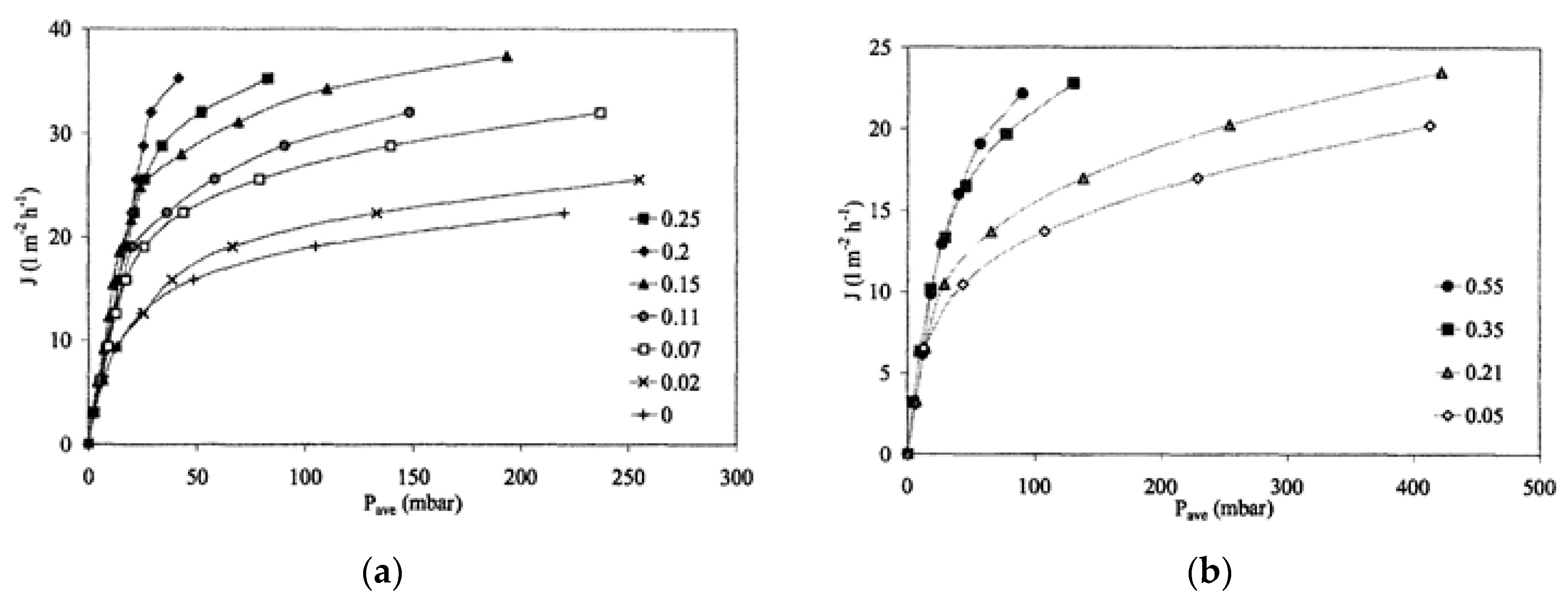
3.1.2. Membrane Performance Indices
3.2. MBR Configurations
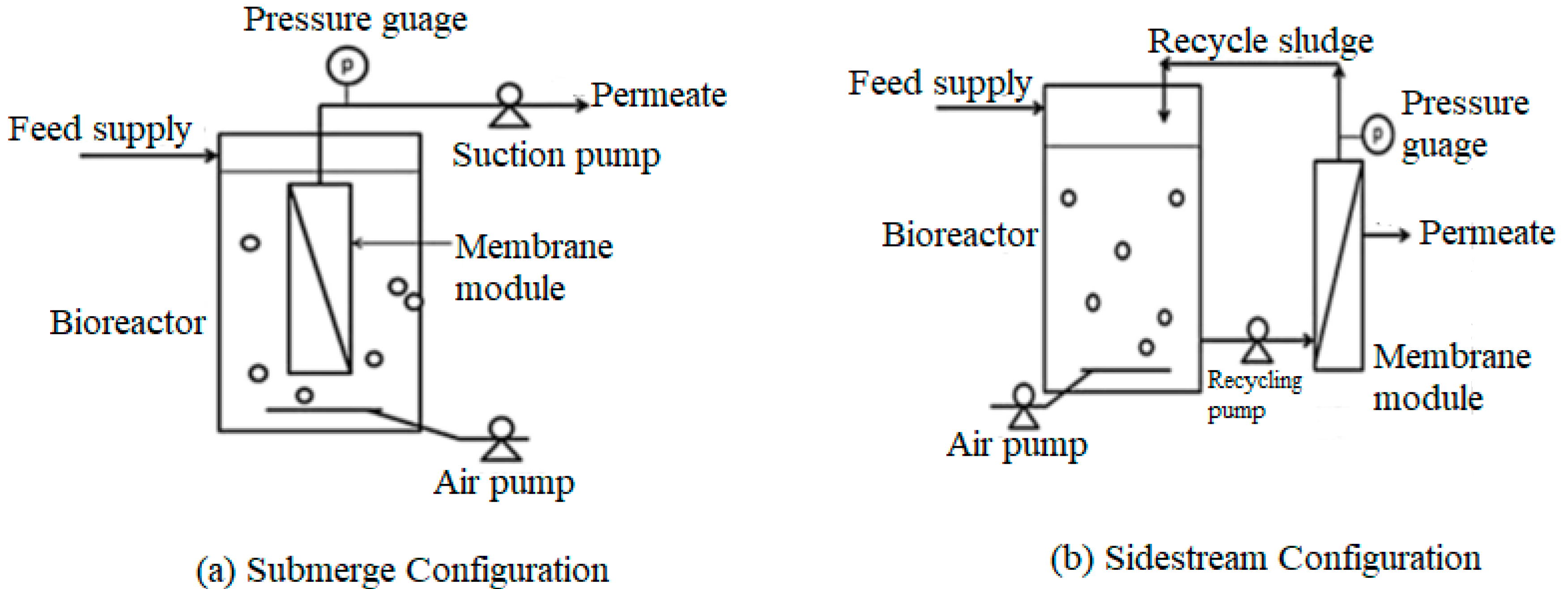
3.2.1. Submerged Configuration (sub-MBR)
3.2.2. Side-stream Configuration (ss-MBR)
3.2.3. Effect of MBR Configuration
3.3. Treatments Process Using MBR
3.3.1 Aerobic Processes in MBR (AerMBR)
3.3.2. Anaerobic Processes in MBR (AnMBR)
3.3.3. Hybrid (Integrated) Processes in MBR (HybMBR)
3.3.4. Sonication Processes in MBR (SonMBR)
3.3.5. A Thermophilic and Mesophilic Condition in MBR (TheMBR)
4. MBR Major Drawbacks
4.1. Types of Foulants as Related to POME Treatment Using MBR
4.1.1. Organic Foulants Generated during POME Treatment
4.1.2. Microbial Foulants as Related to POME Treatment
4.1.3. Inorganic Foulants Generated during POME Treatment
4.1.4. Particulate Foulants as Related to POME Treatment
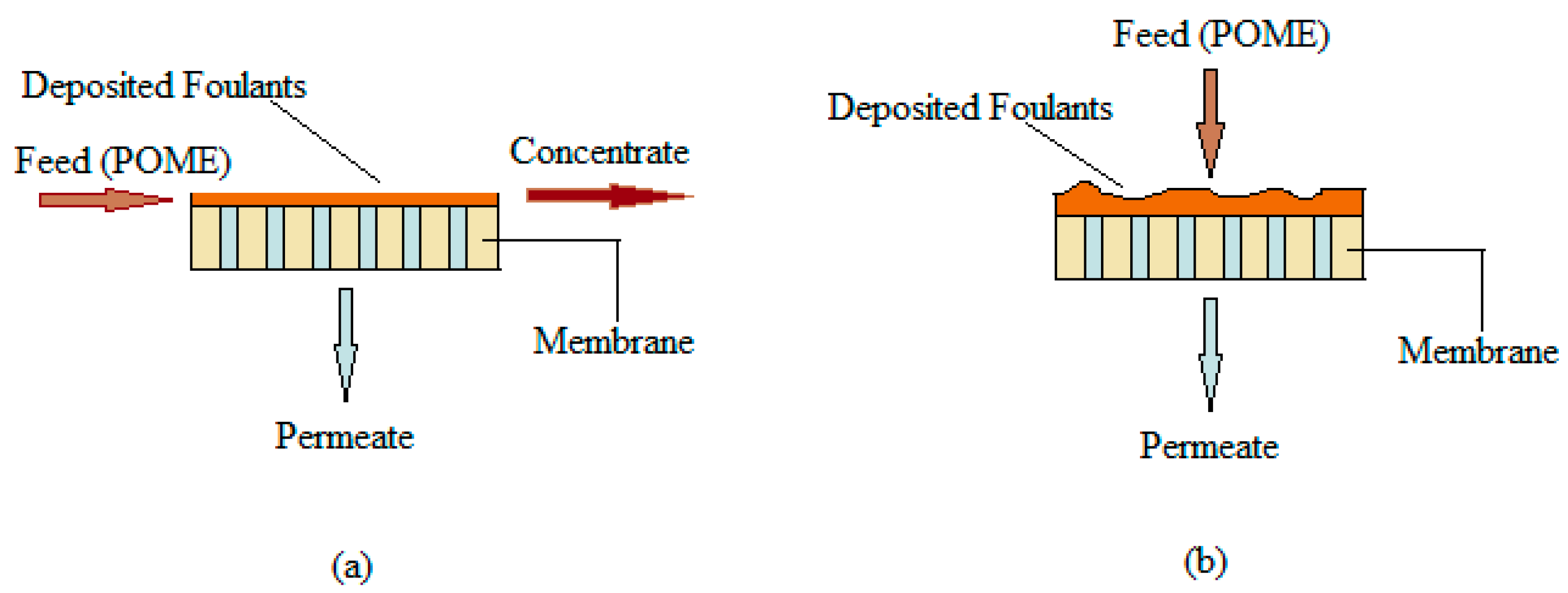
4.2. The Mechanism in Membrane Fouling During POME Treatment
4.2.1. Build-up (Conditioning) Fouling Stage
4.2.2. Steady Fouling Stage
4.2.3. An Abrupt Increase in TMP Stage
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loh, S.K.; Nasrin, A.B.; Mohamad Azri, S.; Nurul Adela, B.; Muzzammil, N.; Daryl Jay, T.; Stasha Eleanor, R.A.; Lim, W.S.; Choo, Y.M.; Kaltschmitt, M. First Report on Malaysia’s experiences and development in biogas capture and utilization from palm oil mill effluent under the Economic Transformation Programme: Current and future perspectives. Renew. Sustain. Energy Rev. 2017, 74, 1257–1274. [Google Scholar] [CrossRef]
- Rupani, P.F.; Rajeev, P.S.; Irahim, M.H.; Esa, N. Review of Current Palm Oil Mill Effluent (POME) Treatment Methods: Vermicomposting as a Sustainable Practice. World Appl. Sci. J. 2010, 11, 70–81. [Google Scholar] [CrossRef]
- Wu, T.Y.; Mohammad, A.W.; Jahim, J.M.; Anuar, N. Pollution control technologies for the treatment of palm oil mill effluent (POME) through end-of-pipe processes. J. Environ. Manag. 2010, 91, 1467–1490. [Google Scholar] [CrossRef] [PubMed]
- Poh, P.E.; Chong, M.F. Development of anaerobic digestion methods for palm oil mill effluent (POME) treatment. Bioresour. Technol. 2009, 100, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boonrod, B.; Prapainainar, C.; Narataruksa, P.; Kantama, A.; Saibautrong, W.; Sudsakorn, K.; Mungcharoen, T.; Prapainainar, P. Evaluating the environmental impacts of bio-hydrogenated diesel production from palm oil and fatty acid methyl ester through life cycle assessment. J. Clean. Prod. 2017, 142, 1210–1221. [Google Scholar] [CrossRef]
- Choong, Y.Y.; Chou, K.W.; Norli, I. Strategies for improving biogas production of palm oil mill effluent (POME) anaerobic digestion: A critical review. Renew. Sustain. Energy Rev. 2018, 82, 2993–3006. [Google Scholar] [CrossRef]
- Ayu, E.D.; Halim, L.; Mellyanawaty, M.; Sudibyo, H.; Budhijanto, W. The effect of natural zeolite as microbial immobilization media in anaerobic digestion at various concentrations of palm oil mill effluent (POME). AIP Conf. Proc. 2017, 1840. [Google Scholar] [CrossRef]
- Okwute, O.L.; Isu, N.R. Impact Analysis of Palm Oil Mill Effluent on The Aerobic Bacterial Density and Ammonium Oxidizers in A Dumpsite in Anyigba, Kogi State. Afr. J. Biotechnol. 2007, 6, 116–119. [Google Scholar]
- Dadrasnia, A.; Usman, M.M.; Lim, K.T.; Velappan, R.D.; Vejan, P.; Mahmud, A.F.; Ismail, S. Microbial Aspects in Wastewater Treatment—A Technical Review. Environ. Pollut. Prot. 2017, 2, 75–84. [Google Scholar]
- Abu Bakar, S.N.H.; Abu Hasan, H.; Mohammad, A.W.; Sheikh Abdullah, S.R.; Haan, T.Y.; Ngteni, R.; Yusof, K.M.M. A review of moving-bed biofilm reactor technology for palm oil mill effluent treatment. J. Clean. Prod. 2018, 171, 1532–1545. [Google Scholar] [CrossRef]
- Ho, K.C.; Teow, Y.H.; Ang, W.L.; Mohammad, A.W. Novel GO/OMWCNTs mixed-matrix membrane with enhanced antifouling property for palm oil mill effluent treatment. Sep. Purif. Technol. 2017, 177, 337–349. [Google Scholar] [CrossRef]
- Azmi, N.S.; Yunos, K.F.M. Wastewater Treatment of Palm Oil Mill Effluent (POME) by Ultrafiltration Membrane Separation Technique Coupled with Adsorption Treatment as Pre-treatment. Agric. Agric. Sci. Procedia 2014, 2, 257–264. [Google Scholar] [CrossRef]
- Daelman, M.R.J.; van Voorthuizen, E.M.; van Dongen, U.G.J.M.; Volcke, E.I.P.; van Loosdrecht, M.C.M. Methane emission during municipal wastewater treatment. Water Res. 2012, 46, 3657–3670. [Google Scholar] [CrossRef] [PubMed]
- Din, A.K. Malaysian Oil Palm Industry Performance 2016 and Prospects for 2017; MPOB: Bandar Baru Bangi, Malaysia, 2017. [Google Scholar]
- Zhang, Y.; Yan, L.; Qiao, X.; Chi, L.; Niu, X.; Mei, Z.; Zhang, Z. Integration of biological method and membrane technology in treating palm oil mill effluent. J. Environ. Sci. 2008, 20, 558–564. [Google Scholar] [CrossRef]
- Taha, M.R.; Ibrahim, A.H. COD removal from anaerobically treated palm oil mill effluent (AT-POME) via aerated heterogeneous Fenton process: Optimization study. J. Water Process Eng. 2014, 1, 8–16. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Ismail, S.; Bhatia, S. Membrane treatment for palm oil mill effluent: Effect of transmembrane pressure and crossflow velocity. Desalination 2005, 179, 245–255. [Google Scholar] [CrossRef]
- Yuniarto, A.; Noor, Z.Z.; Ujang, Z.; Olsson, G.; Aris, A.; Hadibarata, T. Bio-fouling reducers for improving the performance of an aerobic submerged membrane bioreactor treating palm oil mill effluent. Desalination 2013, 316, 146–153. [Google Scholar] [CrossRef]
- Ali Amat, N.A.; Tan, Y.H.; Lau, W.J.; Lai, G.S.; Ong, C.S.; Mokhtar, N.M.; Sani, N.A.A.; Ismail, A.F.; Goh, P.S.; Chong, K.C.; et al. Tackling colour issue of anaerobically-treated palm oil mill effluent using membrane technology. J. Water Process Eng. 2015, 8, 221–226. [Google Scholar] [CrossRef]
- Wang, J.; Mahmood, Q.; Qiu, J.P.; Li, Y.S.; Chang, Y.S.; Li, X.D. Anaerobic Treatment of Palm Oil Mill Effluent in Pilot-Scale Anaerobic EGSB Reactor. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, M.N.; Goh, P.S.; Lau, W.J.; Tan, Y.H.; Ng, B.C.; Ismail, A.F. Hydrophilic hollow fiber PVDF ultrafiltration membrane incorporated with titanate nanotubes for decolourization of aerobically-treated palm oil mill effluent. Chem. Eng. J. 2017, 316, 101–110. [Google Scholar] [CrossRef]
- Ye, Y.; Saikaly, P.E.; Logan, B.E. Simultaneous nitrogen and organics removal using membrane aeration and effluent ultrafiltration in an anaerobic fluidized membrane bioreactor. Bioresour. Technol. 2017, 244, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Judd, S.; Judd, C. The MBR Book: Principles and Applications of Membrane Bioreactors in Water and Wastewater Treatment; Elsevier: New York, NY, USA, 2008; ISBN 9781856174817. [Google Scholar]
- Ahmad, Z.; Ridzuan, M.B.; Daud, Z. Membrane Bioreactor for Palm Oil Mill Effluent and Resource Recovery. In Proceedings of the International Conference on Sustainable Development for Water and Waste Water Treatment, Yogyakarta, Indonesia, 14–15 December 2009; pp. 1–8. [Google Scholar]
- Ghani, M.S.H.; Haan, T.Y.; Lun, A.W.; Mohammad, A.W.; Ngteni, R.; Yusof, K.M.M. Fouling assessment of tertiary palm oil mill effluent (POME) membrane treatment for water reclamation. J. Water Reuse Desalin. 2017, 8, 412–423. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Yin, X.; Tian, L. Membrane fouling in a submerged membrane bioreactor (MBR) under sub-critical flux operation: Membrane foulant and gel layer characterization. J. Membr. Sci. 2008, 325, 238–244. [Google Scholar] [CrossRef]
- Judd, S.J. The status of industrial and municipal effluent treatment with membrane bioreactor technology. Chem. Eng. J. 2015. [Google Scholar] [CrossRef]
- Krzeminski, P.; Leverette, L.; Malamis, S.; Katsou, E. Membrane bioreactors—A review on recent developments in energy reduction, fouling control, novel configurations, LCA and market prospects. J. Membr. Sci. 2017, 527, 207–227. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ahmad, D.; Ezani Bin Abdul Aziz, M. Aerobic treatment of palm oil mill effluent. J. Environ. Manag. 2007, 82, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Rana, S.; Singh, L.; Wahid, Z.; Liu, H. A Recent Overview of Palm Oil Mill Effluent Management via Bioreactor Configurations. Curr. Pollut. Rep. 2017, 3, 254–267. [Google Scholar] [CrossRef]
- Coutte, F.; Lecouturier, D.; Dimitrov, K.; Guez, J.S.; Delvigne, F.; Dhulster, P.; Jacques, P. Microbial lipopeptide production and purification bioprocesses, current progress and future challenges. Biotechnol. J. 2017, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ohimain, E.I.; Izah, S.C. A review of biogas production from palm oil mill effluents using different configurations of bioreactors. Renew. Sustain. Energy Rev. 2017, 70, 242–253. [Google Scholar] [CrossRef]
- Bello, M.M.; Abdul Raman, A.A. Trend and current practices of palm oil mill effluent polishing: Application of advanced oxidation processes and their future perspectives. J. Environ. Manag. 2017, 198, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.R.; Chong, M.F. Treatment and decolorization of biologically treated Palm Oil Mill Effluent (POME) using banana peel as novel biosorbent. J. Environ. Manag. 2014, 132, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Ohimain, E.I.; Izah, S.C.; Jenakumo, N. Physicochemical and microbial screening of palm oil mill effluent for amylase production by mill effluents for amylase production. Greener J. Bol. Sci. 2013, 3, 307–318. [Google Scholar]
- Wang, J.; Mahmood, Q.; Qiu, J.P.; Li, Y.S.; Chang, Y.S.; Chi, L.N.; Li, X.D. Zero discharge performance of an industrial pilot-scale plant treating palm oil mill effluent. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.J.; Chong, M.F.; Law, C.L.; Hassell, D.G. A review on anaerobic-aerobic treatment of industrial and municipal wastewater. Chem. Eng. J. 2009, 155, 1–18. [Google Scholar] [CrossRef]
- Shon, H.K.; Vigneswaran, S.; Snyder, S.A. Effluent organic matter (EfOM) in wastewater: Constituents, effects, and treatment. Crit. Rev. Environ. Sci. Technol. 2006, 36, 327–374. [Google Scholar] [CrossRef]
- Mutamim, N.S.A.; Noor, Z.Z.; Hassan, M.A.A.; Yuniarto, A.; Olsson, G. Membrane bioreactor: Applications and limitations in treating high strength industrial wastewater. Chem. Eng. J. 2013, 225, 109–119. [Google Scholar] [CrossRef]
- Nwuche, C.O.; Aoyagi, H.; Ogbonna, J.C. Treatment of Palm Oil Mill Effluent by a Microbial Consortium Developed from Compost Soils. Int. Sch. Res. Not. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Samudro, G.; Mangkoedihardjo, S. Review on Bod, Cod and Bod/Cod Ratio: A Triangle Zone for Toxic, Biodegradable and Stable Levels. Int. Acad. Res. 2010, 2, 235–239. [Google Scholar]
- Mutamim, N.S.A.; Noor, Z.Z.; Hassan, M.A.A.; Olsson, G. Application of membrane bioreactor technology in treating high strength industrial wastewater: A performance review. Desalination 2012, 305, 1–11. [Google Scholar] [CrossRef]
- Chan, Y.J.; Chong, M.F.; Law, C.L. Bioresource Technology Start-up, steady state performance and kinetic evaluation of a thermophilic integrated anaerobic–aerobic bioreactor (IAAB). Bioresour. Technol. 2012, 125, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Bala, J.D.; Lalung, J.; Ismail, N. Biodegradation of palm oil mill effluent (POME) by bacterial. Int. J. Sci. Res. Publ. 2014, 4, 2250–3153. [Google Scholar]
- Chin, M.J.; Poh, P.E.; Tey, B.T.; Chan, E.S.; Chin, K.L. Biogas from palm oil mill effluent (POME): Opportunities and challenges from Malaysia’s perspective. Renew. Sustain. Energy Rev. 2013, 26, 717–726. [Google Scholar] [CrossRef]
- Tabassum, S.; Zhang, Y.; Zhang, Z. An Integrated Method for Palm Oil Mill Effluent (POME) Treatment for Achieving Zero Liquid Discharge—A Pilot Study; Elsevier Ltd.: New York, NY, USA, 2015; Volume 95, ISBN 1522119574. [Google Scholar]
- Zinatizadeh, A.A.L.; Mohamed, A.R.; Najafpour, G.D.; Hasnain Isa, M.; Nasrollahzadeh, H. Kinetic evaluation of palm oil mill effluent digestion in a high rate up-flow anaerobic sludge fixed film bioreactor. Process Biochem. 2006, 41, 1038–1046. [Google Scholar] [CrossRef]
- Comte, I.; Colin, F.; Whalen, J.K.; Grünberger, O.; Caliman, J.P. Agricultural Practices in Oil Palm Plantations and Their Impact on Hydrological Changes, Nutrient Fluxes and Water Quality in Indonesia. A Review; Elsevier Ltd.: New York, NY, USA, 2012; Volume 116, ISBN 9780123942777. [Google Scholar]
- Guo, W.; Ngo, H.H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.M.; Nourouzi, M.M.; Abdullah, L.C.; Choong, T.S.Y.; Koay, Y.S.; Keshani, S. POME is treated for removal of color from biologically treated POME in fixed bed column: Applying wavelet neural network (WNN). J. Hazard. Mater. 2013, 262, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Khemkhao, M.; Techkarnjanaruk, S.; Phalakornkule, C. Simultaneous treatment of raw palm oil mill effluent and biodegradation of palm fiber in a high-rate CSTR. Bioresour. Technol. 2015, 177, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ghufran, R.; Wahid, Z.A. Bioenergy from anaerobic degradation of lipids in palm oil mill effluent. Rev. Environ. Sci. Biotechnol. 2011, 10, 353–376. [Google Scholar] [CrossRef]
- Alhaji, M.H.; Sanaullah, K.; Lim, S.F.; Khan, A.; Hipolito, C.N.; Abdullah, M.O.; Bhawani, S.A.; Jamil, T. Photocatalytic treatment technology for palm oil mill effluent (POME)—A review. Process Saf. Environ. Prot. 2016, 102, 673–686. [Google Scholar] [CrossRef]
- Chan, Y.J.; Chong, M.F.; Law, C.L. An integrated anaerobic-aerobic bioreactor (IAAB) for the treatment of palm oil mill effluent (POME): Start-up and steady state performance. Process Biochem. 2012, 47, 485–495. [Google Scholar] [CrossRef]
- Damayanti, A.; Ujang, Z.; Salim, M.R. The influenced of PAC, zeolite, and Moringa oleifera as biofouling reducer (BFR) on hybrid membrane bioreactor of palm oil mill effluent (POME). Bioresour. Technol. 2011, 102, 4341–4346. [Google Scholar] [CrossRef] [PubMed]
- Melin, T.; Jefferson, B.; Bixio, D.; Thoeye, C.; De Wilde, W.; De Koning, J.; van der Graaf, J.; Wintgens, T. Membrane bioreactor technology for wastewater treatment and reuse. Desalination 2006, 187, 271–282. [Google Scholar] [CrossRef]
- Ahmad, Z.; Ujang, Z.; Olsson, G. Biomass Effect on Membrane Fouling Using a Hybrid Membrane Bioreactor for Palm Oil Mill Effluent. In Proceedings of the 1st IWA Malaysia Young Water Professionals Conference (IWAYP2010), Kuala, Lumpur, 1–4 March 2010. [Google Scholar]
- Kraume, M.; Drews, A. Membrane Bioreactors in Waste Water Treatment—Status and Trends. Chem. Eng. Technol. 2010, 33, 1251–1259. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Machdar, I.; Gani, A.; Daimon, H. The Combination of Air Flotation and a Membrane Bioreactor for the Treatment of Palm Oil Mill Effluent. Int. J. Technol. 2016, 7, 767. [Google Scholar] [CrossRef]
- Park, H.; Chang, I.; Lee, K. Principles of Membrane Bioreactors for Wastewater Treatment Waste Activated Sludge; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781466590380. [Google Scholar]
- Mamimin, C.; Chaikitkaew, S.; Niyasom, C.; Kongjan, P.; O.-Thong, S. Effect of Operating Parameters on Process Stability of Continuous Biohydrogen Production from Palm Oil Mill Effluent under Thermophilic Condition. Energy Procedia 2015, 79, 815–821. [Google Scholar] [CrossRef]
- Radjenović, J.; Matošić, M.; Mijatović, I.; Petrović, M.; Barceló, D. Membrane Bioreactor (MBR) as an Advanced Wastewater Treatment Technology. In Emerging Contaminants from Industrial and Municipal Waste; The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2008; Volume 5, pp. 37–101. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.S.; Chae, S.R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Verrecht, B.; Judd, S.; Guglielmi, G.; Brepols, C.; Mulder, J.W. An aeration energy model for an immersed membrane bioreactor. Water Res. 2008, 42, 4761–4770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, Z.; Mai, S.; Yang, C.; Wang, X.; An, Y.; Zhou, Z. Research and applications of membrane bioreactors in China: Progress and prospect. Sep. Purif. Technol. 2008, 62, 249–263. [Google Scholar] [CrossRef]
- Fazal, S.; Zhang, B.; Zhong, Z.; Gao, L.; Chen, X. Industrial Wastewater Treatment by Using MBR (Membrane Bioreactor) Review Study. J. Environ. Prot. 2015, 06, 584–598. [Google Scholar] [CrossRef]
- Kizilet, A.; Veral, M.A.; Amar, C.; Isik, O.; Bahramian, M. The Use of Membrane Processes to Promote Sustainable Environmental Protection Practices; DergiPark: Çankaya/Ankara, Turkey, 2017. [Google Scholar]
- Meng, F.; Chae, S.R.; Drews, A.; Kraume, M.; Shin, H.S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Li, Y.; Hu, C. Influence of Filtration Aids on Continuous Filtration in Membrane Bioreactors. Ind. Eng. Chem. Res. 2014, 53, 7202–7208. [Google Scholar] [CrossRef]
- Wan, C.F.; Yang, T.; Lipscomb, G.G.; Stookey, D.J.; Chung, T.S. Design and fabrication of hollow fiber membrane modules. J. Membr. Sci. 2017, 538, 96–107. [Google Scholar] [CrossRef]
- Li, D.; Wang, R.; Chung, T.S. Fabrication of lab-scale hollow fiber membrane modules with high packing density. Sep. Purif. Technol. 2004, 40, 15–30. [Google Scholar] [CrossRef]
- Yang, W.; Cicek, N.; Ilg, J. State-of-the-art of membrane bioreactors: Worldwide research and commercial applications in North America. J. Membr. Sci. 2006, 270, 201–211. [Google Scholar] [CrossRef]
- Wang, Y.; Ong, K.W.; Brannock, M.W.D.; Leslie, G.L. Evaluation of membrane bioreactor performance via residence time distribution: Effects of membrane configuration and mixing. Water Sci. Technol. 2008, 57, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Shakibabarough, A.; Valinejadshoubi, M.; Valinejadshoubi, M. Useable and Precautionary Aspects of Using Nanotechnology and Nano- materials in the Construction Industry. Int. J. Sci. 2014, 3, 841–848. [Google Scholar]
- Drews, A. Membrane fouling in membrane bioreactors—Characterisation, contradictions, cause and cures. J. Membr. Sci. 2010, 363, 1–28. [Google Scholar] [CrossRef]
- Mamimin, C.; Prasertsan, P.; Kongjan, P.; O.-Thong, S. Effects of volatile fatty acids in biohydrogen effluent on biohythane production from palm oil mill effluent under thermophilic condition. Electron. J. Biotechnol. 2017, 29, 78–85. [Google Scholar] [CrossRef]
- Mondal, S. Polymeric membranes for produced water treatment: An overview of fouling behavior and its control. Rev. Chem. Eng. 2016, 32, 611–628. [Google Scholar] [CrossRef]
- Vasanth, D.; Pugazhenthi, G.; Uppaluri, R. Fabrication and properties of low cost ceramic microfiltration membranes for separation of oil and bacteria from its solution. J. Membr. Sci. 2011, 379, 154–163. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, S.; Luan, J.; Mu, Y.; Du, Y.; Wang, G. Fabrication of ultrafiltration membranes with enhanced antifouling capability and stable mechanical properties via the strategies of blending and crosslinking. J. Membr. Sci. 2017, 539, 116–127. [Google Scholar] [CrossRef]
- Fane, A.G.; Wang, R.; Hu, M.X. Synthetic membranes for water purification: Status and future. Angew. Chem. Int. Ed. 2015, 54, 3368–3386. [Google Scholar] [CrossRef] [PubMed]
- Vieira Salla, A.C.; Margarites, A.C.; Seibel, F.I.; Holz, L.C.; Brião, V.B.; Bertolin, T.E.; Colla, L.M.; Costa, J.A.V. Increase in the carbohydrate content of the microalgae Spirulina in culture by nutrient starvation and the addition of residues of whey protein concentrate. Bioresour. Technol. 2016, 209, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Arabi, S.; Nakhla, G. Impact of protein/carbohydrate ratio in the feed wastewater on the membrane fouling in membrane bioreactors. J. Membr. Sci. 2008, 324, 142–150. [Google Scholar] [CrossRef]
- Van Reis, R.; Zydney, A. Bioprocess membrane technology. J. Membr. Sci. 2007, 297, 16–50. [Google Scholar] [CrossRef]
- Çakmakce, M.; Kayaalp, N.; Koyuncu, I. Desalination of produced water from oil production fields by membrane processes. Desalination 2008, 222, 176–186. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Onsekizoglu, P.; Ng, L.Y.; Mohammad, W.A.W. Principle, Advances, Limitations and Future Prospects in Food Industry. J. Membr. Sci. 2015, 344, 226–254. [Google Scholar] [CrossRef]
- Sorayani Bafqi, M.S.; Bagherzadeh, R.; Latifi, M. Fabrication of composite PVDF-ZnO nanofiber mats by electrospinning for energy scavenging application with enhanced efficiency. J. Polym. Res. 2015, 22, 130. [Google Scholar] [CrossRef]
- Yan, Z.Q.; Zeng, L.M.; Li, Q.; Liu, T.Y.; Matsuyama, H.; Wang, X.L. Selective separation of chloride and sulfate by nanofiltration for high saline wastewater recycling. Sep. Purif. Technol. 2016, 166, 135–141. [Google Scholar] [CrossRef]
- Silva, V.; Geraldes, V.; Brites Alves, A.M.; Palacio, L.; Prádanos, P.; Hernández, A. Multi-ionic nanofiltration of highly concentrated salt mixtures in the seawater range. Desalination 2011, 277, 29–39. [Google Scholar] [CrossRef]
- Shanmuganathan, S.; Vigneswaran, S.; Nguyen, T.V.; Loganathan, P.; Kandasamy, J. Use of nanofiltration and reverse osmosis in reclaiming micro-filtered biologically treated sewage effluent for irrigation. Desalination 2015, 364, 119–125. [Google Scholar] [CrossRef]
- Bunani, S.; Yörükoğlu, E.; Yüksel, Ü.; Kabay, N.; Yüksel, M.; Sert, G. Application of reverse osmosis for reuse of secondary treated urban wastewater in agricultural irrigation. Desalination 2015, 364, 68–74. [Google Scholar] [CrossRef]
- Nicolini, J.V.; Borges, C.P.; Ferraz, H.C. Selective rejection of ions and correlation with surface properties of nanofiltration membranes. Sep. Purif. Technol. 2016, 171, 238–247. [Google Scholar] [CrossRef]
- Miller, D.J.; Kasemset, S.; Wang, L.; Paul, D.R.; Freeman, B.D. Constant flux crossflow filtration evaluation of surface-modified fouling-resistant membranes. J. Membr. Sci. 2014, 452, 171–183. [Google Scholar] [CrossRef]
- Tummons, E.N.; Tarabara, V.V.; Chew, J.W.; Fane, A.G. Behavior of oil droplets at the membrane surface during crossflow microfiltration of oil-water emulsions. J. Membr. Sci. 2016, 500, 211–224. [Google Scholar] [CrossRef]
- Winans, J.D.; Smith, K.J.P.; Gaborski, T.R.; Roussie, J.A.; McGrath, J.L. Membrane capacity and fouling mechanisms for ultrathin nanomembranes in dead-end filtration. J. Membr. Sci. 2016, 499, 282–289. [Google Scholar] [CrossRef]
- Chen, R.; Nie, Y.; Hu, Y.; Miao, R.; Utashiro, T.; Li, Q.; Xu, M. Fouling behaviour of soluble microbial products and extracellular polymeric substances in a submerged anaerobic membrane bioreactor treating low- strength wastewater at room temperature. J. Membr. Sci. 2017, 531, 1–9. [Google Scholar] [CrossRef]
- Bacchin, P. Membranes: A variety of energy landscapes for many transfer opportunities. Membranes (Basel) 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Le Clech, P.; Jefferson, B.; Chang, I.S.; Judd, S.J. Critical flux determination by the flux-step method in a submerged membrane bioreactor. J. Membr. Sci. 2003, 227, 81–93. [Google Scholar] [CrossRef]
- He, Z.; Miller, D.J.; Kasemset, S.; Paul, D.R.; Freeman, B.D. The effect of permeate flux on membrane fouling during microfiltration of oily water. J. Membr. Sci. 2017, 525, 25–34. [Google Scholar] [CrossRef]
- Dutta, K.; Das, S.; Kumar, P.; Kundu, P.P. Polymer electrolyte membrane with high selectivity ratio for direct methanol fuel cells: A preliminary study based on blends of partially sulfonated polymers polyaniline and PVdF-co-HFP. Appl. Energy 2014, 118, 183–191. [Google Scholar] [CrossRef]
- Sert, G.; Bunani, S.; Yörükoğlu, E.; Kabay, N.; Egemen, Ö.; Arda, M.; Yüksel, M. Performances of some NF and RO membranes for desalination of MBR treated wastewater. J. Water Process Eng. 2017, 16, 193–198. [Google Scholar] [CrossRef]
- White, N.; Misovich, M.; Alemayehu, E.; Yaroshchuk, A.; Bruening, M.L. Highly selective separations of multivalent and monovalent cations in electrodialysis through Nafion membranes coated with polyelectrolyte multilayers. Polymer 2016, 103, 478–485. [Google Scholar] [CrossRef]
- Ge, L.; Wu, B.; Yu, D.; Mondal, A.N.; Hou, L.; Afsar, N.U.; Li, Q.; Xu, T.; Miao, J.; Xu, T. Monovalent cation perm-selective membranes (MCPMs): New developments and perspectives. Chin. J. Chem. Eng. 2017, 25, 1606–1615. [Google Scholar] [CrossRef]
- Vaughn, J.T.; Koros, W.J. Analysis of feed stream acid gas concentration effects on the transport properties and separation performance of polymeric membranes for natural gas sweetening: A comparison between a glassy and rubbery polymer. J. Membr. Sci. 2014, 465, 107–116. [Google Scholar] [CrossRef]
- Martin-Garcia, I.; Monsalvo, V.; Pidou, M.; Le-Clech, P.; Judd, S.J.; McAdam, E.J.; Jefferson, B. Impact of membrane configuration on fouling in anaerobic membrane bioreactors. J. Membr. Sci. 2011, 382, 41–49. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Deowan, S.A.; Bouhadjar, S.I.; Hoinkis, J. Membrane bioreactors for water treatment. Adv. Membr. Technol. Water Treat. Mater. Process. Appl. 2015, 155–184. [Google Scholar] [CrossRef]
- Ferrentino, R.; Langone, M.; Merzari, F.; Tramonte, L.; Andreottola, G. A review of anaerobic side-stream reactor for excess sludge reduction: Configurations, mechanisms, and efficiency. Crit. Rev. Environ. Sci. Technol. 2016, 46, 382–405. [Google Scholar] [CrossRef]
- Le-Clech, P.; Jefferson, B.; Judd, S.J. Impact of aeration, solids concentration and membrane characteristics on the hydraulic performance of a membrane bioreactor. J. Membr. Sci. 2003, 218, 117–129. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, H.; Ge, L.; Chen, Z.; Dang, Y.; Sun, D. Comparison of the performance of waste leachate treatment in submerged and recirculated membrane bioreactors. Int. Biodeterior. Biodegrad. 2015, 102, 73–80. [Google Scholar] [CrossRef]
- Morrow, C.P.; McGaughey, A.L.; Hiibel, S.R.; Childress, A.E. Submerged or sidestream? The influence of module configuration on fouling and salinity in osmotic membrane bioreactors. J. Membr. Sci. 2017. [Google Scholar] [CrossRef]
- Aslam, M.; Charfi, A.; Lesage, G.; Heran, M. Membrane Bioreactors for Wastewater Treatment: A review of mechanical cleaning by scouring agents to control membrane fouling. Chem. Eng. J. 2016. [Google Scholar] [CrossRef]
- Thomas, H.; Judd, S.; Murrer, J. Fouling characteristics of membrane filtration in membrane bioreactors. Membr. Technol. 2000, 2000, 10–13. [Google Scholar] [CrossRef]
- Cicek, N.; Winnen, H.; Suidan, M.T.; Wrenn, B.E.; Urbain, V.; Manem, J. Effectiveness of the membrane bioreactor in the biodegradation of high molecular weight compounds. Water Res. 1998, 32, 1553–1563. [Google Scholar] [CrossRef]
- Wisniewski, C. Membrane bioreactor for water reuse. Desalination 2007, 203, 15–19. [Google Scholar] [CrossRef]
- Zsirai, T.; Buzatu, P.; Aerts, P.; Judd, S. Efficacy of relaxation, backflushing, chemical cleaning and clogging removal for an immersed hollow fibre membrane bioreactor. Water Res. 2012, 46, 4499–4507. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, L.; Gómez, M.; Dolina, J.; Černín, A. Anaerobic membrane bioreactors—A mini review with emphasis on industrial wastewater treatment: Applications, limitations and perspectives. Desalin. Water Treat. 2016, 57, 19062–19076. [Google Scholar] [CrossRef]
- Andrade, L.H.; Mendes, F.D.S.; Espindola, J.C.; Amaral, M.C.S. Reuse of dairy wastewater treated by membrane bioreactor and nanofiltration: Technical and economic feasibility. Braz. J. Chem. Eng. 2015, 32, 735–747. [Google Scholar] [CrossRef]
- Gander, M.; Jefferson, B.; Judd, S. Aerobic MBRs for domestic wastewater treatment: A review with cost considerations. Sep. Purif. Technol. 2000, 18, 119–130. [Google Scholar] [CrossRef]
- Jefferson, B.; Laine, A.L.; Judd, S.J.; Stephenson, T. Membrane bioreactors and their role in wastewater reuse. Water Sci. Technol. 2000, 41, 197–204. [Google Scholar] [CrossRef]
- Le-Clech, P. Membrane bioreactors and their uses in wastewater treatments. Appl. Microbiol. Biotechnol. 2010, 88, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, C.; Yang, B.S.; Muttamara, S.; Maythanukhraw, R. Application of air backflushing technique in membrane bioreactor. Water Sci. Technol. 1997, 36, 259–266. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, G.; Deng, B. Graphene oxide (GO) enhanced polyamide (PA) thin-film nanocomposite (TFN) membrane for water purification. Desalination 2016, 379, 93–101. [Google Scholar] [CrossRef]
- Xiao, K.; Shen, Y.X.; Liang, S.; Liang, P.; Wang, X.M.; Huang, X. A systematic analysis of fouling evolution and irreversibility behaviors of MBR supernatant hydrophilic/hydrophobic fractions during microfiltration. J. Membr. Sci. 2014, 467, 206–216. [Google Scholar] [CrossRef]
- Abdurahman, N.H.; Rosli, Y.M.; Azhari, N.H.; Ahmad, A.L.A.; Ghufran, R.; Wahid, Z.A. Hydrogen production by biological processes: a survey of literature. Int. J. Hydrogen Energy 2010, 27, 13–28. [Google Scholar] [CrossRef]
- Chang, I.S.; Le Clech, P.; Jefferson, B.; Judd, S. Membrane Fouling in Membrane Bioreactors for Wastewater Treatment. J. Environ. Eng. 2002, 128, 1018–1029. [Google Scholar]
- Yusuf, Z.; Wahab, N.A.; Sahlan, S. Fouling control strategy for submerged membrane bioreactor filtration processes using aeration airflow, backwash, and relaxation: A review. Desalin. Water Treat. 2015, 57, 17683–17695. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, M.; Wang, F.; Meng, F.; Liao, B.Q.; Hong, H.; Chen, J.; Gao, W. A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: Characteristics, roles in membrane fouling and control strategies. J. Membr. Sci. 2014, 460, 110–125. [Google Scholar] [CrossRef]
- Meng, F.; Yang, F.; Shi, B.; Zhang, H. A comprehensive study on membrane fouling in submerged membrane bioreactors operated under different aeration intensities. Sep. Purif. Technol. 2008, 59, 91–100. [Google Scholar] [CrossRef]
- Ahmad, A.; Buang, A.; Bhat, A.H. Renewable and sustainable bioenergy production from microalgal co-cultivation with palm oil mill effluent (POME): A review. Renew. Sustain. Energy Rev. 2016, 65, 214–234. [Google Scholar] [CrossRef]
- Nurliyana, M.Y.; H’ng, P.S.; Rasmina, H.; Kalsom, M.S.U.; Chin, K.L.; Lee, S.H.; Lum, W.C.; Khoo, G.D. Effect of C/N ratio in methane productivity and biodegradability during facultative co-digestion of palm oil mill effluent and empty fruit bunch. Ind. Crops Prod. 2015, 76, 409–415. [Google Scholar] [CrossRef]
- Shahata, A.; Mohammedadel, A.; Professor, A.; Akimoto, T. Improvement of Membrane Bioreactor Operations for Color and Oil Removal from Wastewater. Ph.D. Thesis, School of Bioscience and Biotechnology, Tokyo University of Technology, Tokyo, Japan, March 2016; pp. 1–102. [Google Scholar]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. 2015, 144, 73–95. [Google Scholar] [CrossRef]
- 0-Thong, S.; Suksong, W.; Promnuan, K. ScienceDirect Two-stage thermophilic fermentation and mesophilic methanogenic process for biohythane production from palm oil mill effluent with methanogenic effluent recirculation for pH control. Int. J. Hydrogen Energy 2016, 41, 21702–21712. [Google Scholar] [CrossRef]
- Krishnan, S.; Singh, L.; Sakinah, M.; Thakur, S.; Wahid, Z.A.; Alkasrawi, M. Process enhancement of hydrogen and methane production from palm oil mill effluent using two-stage thermophilic and mesophilic fermentation. Int. J. Hydrogen Energy 2016, 41, 12888–12898. [Google Scholar] [CrossRef]
- Krishnan, S.; Singh, L.; Sakinah, M.; Thakur, S.; Wahid, Z.A.; Ghrayeb, O.A. Role of organic loading rate in bioenergy generation from palm oil mill effluent in a two-stage up-flow anaerobic sludge blanket continuous-stirred tank reactor. J. Clean. Prod. 2017, 142, 3044–3049. [Google Scholar] [CrossRef]
- Gobi, K.; Vadivelu, V.M. By-products of palm oil mill effluent treatment plant—A step towards sustainability. Renew. Sustain. Energy Rev. 2013, 28, 788–803. [Google Scholar] [CrossRef]
- Liew, W.L.; Kassim, M.A.; Muda, K.; Loh, S.K.; Affam, A.C. Conventional methods and emerging wastewater polishing technologies for palm oil mill effluent treatment: A review. J. Environ. Manag. 2014, 149, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Neoh, C.H.; Yung, P.Y.; Noor, Z.Z.; Razak, M.H.; Aris, A.; Md Din, M.F.; Ibrahim, Z. Correlation between microbial community structure and performances of membrane bioreactor for treatment of palm oil mill effluent. Chem. Eng. J. 2017, 308, 656–663. [Google Scholar] [CrossRef]
- Garritano, N.; Gonc, C.; Maria, D. ScienceDirect Efficient biohydrogen production via dark fermentation from hydrolized palm oil mill effluent by non-commercial enzyme preparation. Int. J. Hydrogen Energy 2017, 42, 29166–29174. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.W.; Kim, Y.M.; Park, C.; Park, K.Y. Performance and fouling in pre-denitrification membrane bioreactors treating high-strength wastewater from food waste disposers. Water (Switzerland) 2017, 9, 512. [Google Scholar] [CrossRef]
- Huang, L.; Lee, D.J. Membrane bioreactor: A mini review on recent R&D works. Bioresour. Technol. 2015, 194, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.K.; Pradhan, N.C.; Adhikari, B. Synthesis and characterization of porous polyurethaneurea membranes for pervaporative separation of 4-nitrophenol from aqueous solution. Bull. Mater. Sci. 2006, 29, 225–231. [Google Scholar] [CrossRef]
- Basile, A.; Cassano, A.; Rastogi, N.K. Advances in Membrane Technologies for Water Treatment: Materials, Processes and Applications; Woodhead Publishing: Cambridge, UK, 2015; ISBN 9781782421269. [Google Scholar]
- Cervantes, F.J.; Pavlostathis, S.G.; Van Haandel, A.C. Advanced Biological Treatment Processes for Industrial Wastewaters: Principles and Applications; IWA Publishing: London, UK, 2006. [Google Scholar]
- Bharagava, R.N.; Mishra, S. Ecotoxicology and Environmental Safety Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicol. Environ. Saf. 2018, 147, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Tajuddin, H.A.; Abdullah, L.C.; Idris, A.; Choong, T.S.Y. Effluent Quality of Anaerobic Palm Oil Mill Effluent (POME) Wastewater Using Organic Coagulant. Int. J. Sci. Res. 2015, 4, 667–677. [Google Scholar]
- Hariz, H.B.; Takriff, M.S. Palm oil mill effluent treatment and CO 2 sequestration by using microalgae — sustainable strategies for environmental protection. Environ. Sci. Pollut. Res. 2017, 24, 20209–20240. [Google Scholar] [CrossRef] [PubMed]
- Pretel, R.; Robles, A.; Ruano, M.V.; Seco, A.; Ferrer, J. Economic and environmental sustainability of submerged anaerobic MBR-based (AnMBR-based) technology as compared to aerobic-based technologies for moderate-/high-loaded urban wastewater treatment. J. Environ. Manag. 2016, 166, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.H.; Goh, P.S.; Ismail, A.F.; Ng, B.C.; Lai, G.S. Decolourization of aerobically treated palm oil mill effluent (AT-POME) using polyvinylidene fluoride (PVDF) ultrafiltration membrane incorporated with coupled zinc-iron oxide nanoparticles. Chem. Eng. J. 2017, 308, 359–369. [Google Scholar] [CrossRef]
- Subramaniam, M.N.; Goh, P.S.; Lau, W.J.; Ng, B.C.; Ismail, A.F. AT-POME colour removal through photocatalytic submerged filtration using antifouling PVDF-TNT nanocomposite membrane. Sep. Purif. Technol. 2018, 191, 266–275. [Google Scholar] [CrossRef]
- Mohd Azoddein, A.A.; Haris, H.; Mohd Azli, F.A. Treatment of Palm Oil Mill Effluent (Pome) Using Membrane Bioreactor. Malays. J. Anal. Sci. 2015, 19, 463–471. [Google Scholar]
- Sarma, S.J.; Tay, J.H.; Chu, A. Finding Knowledge Gaps in Aerobic Granulation Technology. Trends Biotechnol. 2017, 35, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, J.; Lee, D. Bioresource Technology Aerobic granular processes: Current research trends. Bioresour. Technol. 2016, 210, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Iorhemen, O.T.; Hamza, R.A.; Tay, J.H. Membrane bioreactor (Mbr) technology for wastewater treatment and reclamation: Membrane fouling. Membranes (Basel) 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Park, J.; Han, G. Toxic/Hazardous Substances and Environmental Engineering Control of membrane fouling with the addition of a nanoporous zeolite membrane fouling reducer to the submerged hollow fiber membrane bioreactor. J. Environ. Sci. Health 2016, 51, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Zhou, Z.; Shen, X.; Qiao, W.; Jiang, L.; Pan, W. Effects of dissolved oxygen on performance and microbial community structure in a micro-aerobic hydrolysis sludge in situ reduction process. Water Res. 2016, 90, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.J.; Chong, M.F.; Law, C.L. Performance and kinetic evaluation of an integrated anaerobic–aerobic bioreactor in the treatment of palm oil mill effluent. Environ. Technol. 2017, 38, 1005–1021. [Google Scholar] [CrossRef] [PubMed]
- Alattabi, A.W.; Harris, C.B.; Alkhaddar, M.; Alzeyadi, A.T. Journal of Water Process Engineering An investigation into the e ff ect of MLSS on the effluent quality and sludge settleability in an aerobic-anoxic sequencing batch reactor (AASBR). J. Water Process Eng. 2017. [Google Scholar] [CrossRef]
- Díaz, O.; González, E.; Vera, L.; Macías-hernández, J.J.; Rodríguez-sevilla, J. Fouling analysis and mitigation in a tertiary MBR operated under restricted aeration. J. Membr. Sci. 2017, 525, 368–377. [Google Scholar] [CrossRef]
- Yang, L.; Liu, L.; Wang, Z. Preparation of PVDF/GO[sbnd]SiO2 hybrid microfiltration membrane towards enhanced perm-selectivity and anti-fouling property. J. Taiwan Inst. Chem. Eng. 2017, 78, 500–509. [Google Scholar] [CrossRef]
- De Temmerman, L.; Maere, T.; Temmink, H.; Zwijnenburg, A.; Nopens, I. The effect of fine bubble aeration intensity onmembrane bioreactor sludge characteristics and fouling. Water Res. 2015, 76, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, Z. A review of membrane fouling in MBRs: Characteristics and role of sludge cake formed on membrane surfaces. Sep. Sci. Technol. 2009, 44, 3571–3596. [Google Scholar] [CrossRef]
- Maqbool, T.; Khan, S.J.; Waheed, H.; Lee, C.H.; Hashmi, I.; Iqbal, H. Membrane biofouling retardation and improved sludge characteristics using quorum quenching bacteria in submerged membrane bioreactor. J. Membr. Sci. 2015, 483, 75–83. [Google Scholar] [CrossRef]
- Rodríguez, F.A.; Reboleiro-Rivas, P.; Osorio, F.; Martínez-Toledo, M.V.; Hontoria, E.; Poyatos, J.M. Influence of mixed liquid suspended solids and hydraulic retention time on oxygen transfer efficiency and viscosity in a submerged membrane bioreactor using pure oxygen to supply aerobic conditions. Biochem. Eng. J. 2012, 60, 135–141. [Google Scholar] [CrossRef]
- Farhan, A.; Udaiyappan, M.; Abu, H.; Sobri, M. Journal of Water Process Engineering A review of the potentials, challenges and current status of microalgae biomass applications in industrial wastewater treatment. J. Water Process Eng. 2017, 20, 8–21. [Google Scholar] [CrossRef]
- Hamza, R.A.; Iorhemen, O.T.; Tay, J.H. Journal of Water Process Engineering Advances in biological systems for the treatment of high-strength wastewater. J. Water Process Eng. 2016, 10, 128–142. [Google Scholar] [CrossRef]
- Manai, I.; Miladi, B.; Mselmi, A.; El Hamdi, M. Improvement of activated sludge resistance to shock loading by fungal enzyme addition during textile wastewater treatment. Environ. Technol. 2016, 38, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.J.; Hai, F.I.; Guo, W.; Ngo, H.H.; Price, W.E.; Nghiem, L.D. Science of the Total Environment Factors governing the pre-concentration of wastewater using forward osmosis for subsequent resource recovery. Sci. Total Environ. 2016, 566–567, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Hu, K. Contribution to modeling of treatment and reuse of industrial wastewater. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 2017. [Google Scholar]
- Arévalo, J.; Moreno, B.; Pérez, J.; Gómez, M.A. Applicability of the Sludge Biotic Index (SBI) for MBR activated sludge control. J. Hazard. Mater. 2009, 167, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, F.; Mehrnia, M.R.; Poostchi, A.A. Optimal operating strategies of SFDM formation for MBR application. Sep. Purif. Technol. 2014, 124, 124–133. [Google Scholar] [CrossRef]
- Ho, J.; Smith, S.; Patamasank, J.; Tontcheva, P.; Kim, G.D. Development of Alternative Energy Saving MBR Using Reciprocating Vibration in Place of Membrane Air Scouring. In Proceedings of the Water Environment Federation, WEFTEC 2013: Session 92 through Session 101; Water Environment Federation: Alexandria, VA, USA, 2013; pp. 6679–6688. [Google Scholar]
- Kumar, A.; Jena, H.M. High surface area microporous activated carbons prepared from Fox nut (Euryale ferox) shell by zinc chloride activation. Appl. Surf. Sci. 2015, 356, 753–761. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Alkhatib, M.F.; Mamun, A.A.; Akbar, I. Application of response surface methodology (RSM) for optimization of color removal from POME by granular activated carbon. Int. J. Environ. Sci. Technol. 2015, 12, 1295–1302. [Google Scholar] [CrossRef]
- Sia, Y.Y.; Tan, I.A.W.; Abdullah, M.O. Adsorption of colour, TSS and COD from palm oil mill effluent (POME) using acid-washed coconut shell activated carbon: Kinetic and mechanism studies. MATEC Web Conf. 2017, 87. [Google Scholar] [CrossRef]
- Guo, W.; Vigneswaran, S.; Ngo, H.H.; Xing, W.; Goteti, P. Comparison of the performance of submerged membrane bioreactor (SMBR) and submerged membrane adsorption bioreactor (SMABR). Bioresour. Technol. 2008, 99, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Wahi, R.; Chuah Abdullah, L.; Nourouzi Mobarekeh, M.; Ngaini, Z.; Choong Shean Yaw, T. Utilization of esterified sago bark fibre waste for removal of oil from palm oil mill effluent. J. Environ. Chem. Eng. 2017, 5, 170–177. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2015. [Google Scholar] [CrossRef]
- Kaman, S.; Tan, I.; Lim, L. Palm oil mill effluent treatment using coconut shell–based activated carbon: Adsorption equilibrium and isotherm. MATEC Web 2017, 87, 3009. [Google Scholar] [CrossRef]
- Pellegrin, M.; Greiner, A.D.; Aguinaldo, J.; Diamond, J.; Gluck, S.; Burbano, M.S.; Arabi, S.; Wert, J.; Mccandless, R.; Padhye, L.P. Membrane Processes. Water Environ. Res. 2012, 84, 1114–1216. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.S.; Lee, S.; Kim, S.H.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Shahkaramipour, N.; Tran, T.N.; Ramanan, S.; Lin, H. Membranes with surface-enhanced antifouling properties for water purification. Membranes (Basel) 2017, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Figoli, A.; Cassano, A.; Basile, A. Membrane Technologies for Biorefining; Elsevier: New York, NY, USA, 2016; ISBN 9780857095213. [Google Scholar]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Budiman, P.M.; Wu, T.Y.; Ramanan, R.N.; Xiao, J.; Hay, W. Treatment and Reuse of E ffl uents from Palm Oil, Pulp, and Paper Mills as a Combined Substrate by Using Purple Nonsulfur Bacteria. Ind. Eng. Chem. Res. 2014, 53, 14921–14931. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Dereli, R.K.; Ersahin, M.E.; Ozgun, H.; Ozturk, I.; Jeison, D.; van der Zee, F.; van Lier, J.B. Potentials of anaerobic membrane bioreactors to overcome treatment limitations induced by industrial wastewaters. Bioresour. Technol. 2012, 122, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Evren, M.; Tao, Y.; Ozgun, H.; Gimenez, J.B.; Spanjers, H.; Lier, B. Van Impact of anaerobic dynamic membrane bioreactor con fi guration on treatment and fi lterability performance. J. Membr. Sci. 2017, 526, 387–394. [Google Scholar] [CrossRef]
- Mamimin, C.; Jehlee, A.; Saelor, S.; Prasertsan, P.; O.-Thong, S. Thermophilic hydrogen production from co-fermentation of palm oil mill effluent and decanter cake by Thermoanaerobacterium thermosaccharolyticum PSU-2. Int. J. Hydrogen Energy 2016, 41, 21692–21701. [Google Scholar] [CrossRef]
- Ravindra, P. Advances in Bioprocess Technology; Springer: Berlin, Germany, 2015; ISBN 9783319179155. [Google Scholar]
- Tan, D.T.; Chin, S.K.; Poh, P.E.; Lee, Y.H. Preservation of thermophilic mixed culture for anaerobic palm oil mill effluent treatment by convective drying methods. Int. J. Environ. Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Abdurahman, N.H.; Rosli, Y.M.; Azhari, N.H. Development of a membrane anaerobic system (MAS) for palm oil mill effluent (POME) treatment. Desalination 2011, 266, 208–212. [Google Scholar] [CrossRef]
- Abdurahman, N.H.; Azhari, N.H. An integrated UMAS for POME treatment. J. Water Reuse Desalin. 2016. [Google Scholar] [CrossRef]
- Ahmad, A.; Ghufran, R. Evaluation of the bio-kinetics of cement kiln dust in an upflow anaerobic sludge blanket reactor for treatment of palm oil mill effluent as a function of hydraulic retention time. Sep. Purif. Technol. 2014, 133, 129–137. [Google Scholar] [CrossRef]
- Zhang, Q.; Singh, S.; Stuckey, D.C. Bioresource Technology Fouling reduction using adsorbents/flocculants in a submerged anaerobic membrane bioreactor. Bioresour. Technol. 2017, 239, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, W.; Tang, B.; Bin, L.; Ding, J.; Zheng, Y.; Zhang, Z. Membrane fouling mechanism of biofilm-membrane bioreactor (BF-MBR): Pore blocking model and membrane cleaning. Bioresour. Technol. 2017, 250, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Annop, S.; Sridang, P.; Puetpaiboon, U.; Grasmick, A. Influence of relaxation frequency on membrane fouling control in submerged anaerobic membrane bioreactor (SAnMBR). Desalin. Water Treat. 2014, 52, 4102–4110. [Google Scholar] [CrossRef]
- Said, M.; Wahab Mohammad, A.; Tusirin, M.; Nor, M. Investigation of Three Pre-treatment Methods Prior to Nanofiltration Membrane for Palm Oil Mill Effluent Treatment. Sains Malays. 2015, 44, 421–427. [Google Scholar] [CrossRef]
- Dimitriou, E.; Boutikos, P.; Sh, E.; Koziel, S. Theoretical performance prediction of a reverse osmosis desalination membrane element under variable operating conditions. Desalination 2017, 419, 70–78. [Google Scholar] [CrossRef]
- Annop, S.; Sridang, P.; Puetpaiboon, U.; Grasmick, A. Effect of solids retention time on membrane fouling intensity in two-stage submerged anaerobic membrane bioreactors treating palm oil mill effluent. Environ. Technol. 2014, 35, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Neoh, C.H.; Noor, Z.Z.; Mutamim, N.S.A.; Lim, C.K. Green technology in wastewater treatment technologies: Integration of membrane bioreactor with various wastewater treatment systems. Chem. Eng. J. 2016, 283, 582–594. [Google Scholar] [CrossRef]
- Ziegler, A.S.; McIlroy, S.J.; Larsen, P.; Albertsen, M.; Hansen, A.A.; Heinen, N.; Nielsen, P.H. Dynamics of the fouling layer microbial community in a membrane bioreactor. PLoS ONE 2016, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Damayanti, A.; Ujang, Z.; Salim, M.R.; Olsson, G. The effect of mixed liquor suspended solids (MLSS) on biofouling in a hybrid membrane bioreactor for the treatment of high concentration organic wastewater. Water Sci. Technol. 2011, 63, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Ujang, Z.; Abdul Latiff, A.A.; Ahmat Nor, N.I. Effect on Membrane Fouling and Cake Resistance in a Hybrid Membrane Bioreactor for Palm Oil Mill Effluent Treatment. Int. Conf. Environ. 2008, 2008, 1–12. [Google Scholar]
- Ma, J.; Wang, Z.; Yang, Y.; Mei, X.; Wu, Z. Correlating microbial community structure and composition with aeration intensity in submerged membrane bioreactors by 454 high-throughput pyrosequencing. Water Res. 2013, 47, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xiao, K.; Mo, Y.; Liang, P.; Shen, Y.; Zhu, N.; Huang, X. Seasonal characteristics of supernatant organics and its effect on membrane fouling in a full-scale membrane bioreactor. J. Membr. Sci. 2014, 453, 168–174. [Google Scholar] [CrossRef]
- Khan, S.J.; Visvanathan, C.; Jegatheesan, V. Bioresource Technology Influence of biofilm carriers on membrane fouling propensity in moving biofilm membrane bioreactor. Bioresour. Technol. 2012, 113, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yu, S.; Shi, W.; Heijman, S.G.J.; Rietveld, L.C. Bioresource Technology Effect of different temperatures on performance and membrane fouling in high concentration PAC–MBR system treating micro-polluted surface water. Bioresour. Technol. 2013, 141, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gao, X.; Xu, H.; Cai, Y.; Cui, J. Chemosphere influence of extracellular polymeric substances (EPS) treated by combined ultrasound pretreatment and chemical re-flocculation on water treatment sludge settling performance. Chemosphere 2017, 170, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Tee, P.F.; Abdullah, M.O.; Tan, I.A.W.; Amin, M.A.M.; Nolasco-Hipolito, C.; Bujang, K. Effects of temperature on wastewater treatment in an affordable microbial fuel cell-adsorption hybrid system. J. Environ. Chem. Eng. 2017, 5, 178–188. [Google Scholar] [CrossRef]
- Ma, Z.; Wen, X.; Zhao, F.; Xia, Y.; Huang, X.; Waite, D.; Guan, J. Effect of temperature variation on membrane fouling and microbial community structure in membrane bioreactor. Bioresour. Technol. 2013, 133, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.J.; Leung, K.T.; Qin, W.S.; Liao, B.Q. Bioresource Technology Effects of temperature and temperature shock on the performance and microbial community structure of a submerged anaerobic membrane bioreactor. Bioresour. Technol. 2011, 102, 8733–8740. [Google Scholar] [CrossRef] [PubMed]
- Cologgi, D.; Kasiri, S.; Hofstetter, S.; Donoso-bravo, A.; Ulrich, A. Anaerobic Processes. Water Environ. Res. 213, 85, 1176–1231. [Google Scholar] [CrossRef]
- Shafie, N.F.A.; Mansor, U.Q.A.; Yahya, A.; Som, A.M.; Nour, A.H.; Hassan, Z.; Yunus, R.M. Performance of ultrasonic-assisted membrane anaerobic system (UMAS) for membrane fouling control in palm oil mill effluent (POME) treatment. Adv. Sci. Lett. 2017, 23, 3903–3906. [Google Scholar] [CrossRef]
- Saifuddin, N.; Dinara, S.; Unit, C.; Nasional, U.T. Pretreatment of Palm Oil Mill Effluent (POME) Using Magnetic Chitosan. J. Chem. 2011, 8 (Suppl. S1), S67–S78. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.; Appels, L.; Dewil, R.A.F. Ultrasonic Treatment of Waste Sludge: A Review on Mechanisms and Applications. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1220–1288. [Google Scholar] [CrossRef]
- Carrère, H.; Dumas, C.; Battimelli, A.; Batstone, D.J.; Delgenès, J.P.; Steyer, J.P.; Ferrer, I. Pretreatment methods to improve sludge anaerobic degradability: A review. 2010, 183, 1–15. [Google Scholar] [CrossRef]
- Abdurahman, N.H.; Azhari, N.H.; Rosli, Y.M. Ultrasonic Membrane Anaerobic System (UMAS) for Palm Oil Mill Effluent (POME) Treatment. Int. Perspect. Water Qual. Manag. Pollut. Control 2013, 107–121. [Google Scholar] [CrossRef]
- Leaño, E.P.; Anceno, A.J.; Babel, S. Ultrasonic pretreatment of palm oil mill effluent: Impact on biohydrogen production, bioelectricity generation, and underlying microbial communities. Int. J. Hydrogen Energy 2012, 37, 12241–12249. [Google Scholar] [CrossRef]
- Taha, M.R.; Ibrahim, A.H. Characterization of nano zero-valent iron (nZVI) and its application in sono-Fenton process to remove COD in palm oil mill effluent. J. Environ. Chem. Eng. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Manickam, S.; Abidin, Z.; Parthasarathy, S. Role of H2O2 in the Fluctuating Patterns of COD (Chemical Oxygen Demand) during the treatment of Palm Oil Mill Effluent (POME) Using Pilot Scale Triple Frequency Ultrasound Cavitation Reactor. Ultrason. Sonochem. 2014, 21, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, S.; Mohammed, R.R.; Fong, C.M.; Gomes, R.L.; Manickam, S. A novel hybrid approach of activated carbon and ultrasound cavitation for the intensification of palm oil mill effluent (POME) polishing. J. Clean. Prod. 2016, 112, 1218–1226. [Google Scholar] [CrossRef]
- Xu, M.; Wen, X.; Yu, Z.; Li, Y.; Huang, X. Bioresource Technology A hybrid anaerobic membrane bioreactor coupled with online ultrasonic equipment for digestion of waste activated sludge. Bioresour. Technol. 2011, 102, 5617–5625. [Google Scholar] [CrossRef] [PubMed]
- Abeynayaka, A.; Visvanathan, C. Performance comparison of mesophilic and thermophilic aerobic sidestream membrane bioreactors treating high strength wastewater. Bioresour. Technol. 2011, 102, 5345–5352. [Google Scholar] [CrossRef] [PubMed]
- Stuckey, D.C. Bioresource Technology Recent developments in anaerobic membrane reactors. Bioresour. Technol. 2012, 122, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, C.; Abeynayaka, A. Developments and future potentials of anaerobic membrane bioreactors (AnMBRs). Membr. Water Treat. 2012, 3, 1–23. [Google Scholar] [CrossRef]
- Christensen, M.L.; Keiding, K.; Halkj, P. ScienceDirect Dewatering in biological wastewater treatment: A review. Water Res. 2015, 82, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Massara, T.M.; Tarik, O.; Onur, K.; Senba, S. A Mini Review of the Techno-environmental Sustainability of Biological Processes for the Treatment of High Organic Content Industrial Wastewater Streams. Waste Biomass Valoriz. 2017, 8, 1665–1678. [Google Scholar] [CrossRef]
- Lin, H.J.; Xie, K.; Mahendran, B.; Bagley, D.M.; Leung, K.T.; Liss, S.N.; Liao, B.Q. Sludge properties and their effects on membrane fouling in submerged anaerobic membrane bioreactors (SAnMBRs). Water Res. 2009, 43, 3827–3837. [Google Scholar] [CrossRef] [PubMed]
- Arifin, H.; Choong, T.S.Y.; Rong, C.K.; Ahmadun, F.A.R.; Abdullah, L.C. Forward Osmosis: Temperature effects by using pome as feed solution. ASEAN J. Chem. Eng. 2015, 15, 31–40. [Google Scholar]
- Martinez-Sosa, D.; Helmreich, B.; Horn, H. Anaerobic submerged membrane bioreactor (AnSMBR) treating low-strength wastewater under psychrophilic temperature conditions. Process Biochem. 2012, 47, 792–798. [Google Scholar] [CrossRef]
- Choorit, W.; Wisarnwan, P. Effect of temperature on the anaerobic digestion of palm oil mill effluent. Electron. J. Biotechnol. 2007, 10, 376–385. [Google Scholar] [CrossRef]
- Shao, L.; Wang, Z.X.; Zhang, Y.L.; Jiang, Z.X.; Liu, Y.Y. A facile strategy to enhance PVDF ultrafiltration membrane performance via self-polymerized polydopamine followed by hydrolysis of ammonium fluotitanate. J. Membr. Sci. 2014, 461, 10–21. [Google Scholar] [CrossRef]
- Najib, M.Z.M.; Salmiati; Ujang, Z.; Salim, M.R.; Ibrahim, Z. Developed microbial granules containing photosynthetic pigments for carbon dioxide reduction in palm oil mill effluent. In International Biodeterioration and Biodegradation; Elsevier Ltd.: New York, NY, USA, 2017; Volume 116, pp. 163–170. [Google Scholar]
- Ao, L.; Liu, W.; Zhao, L.; Wang, X. Membrane fouling in ultrafiltration of natural water after pretreatment to different extents. J. Environ. Sci. 2016, 43, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Jalani, N.F.; Aziz, A.A.; Wahab, N.A.; Hasamudin, W.; Hassan, W.; Zainal, N.H. Application of Palm Kernel Shell Activated Carbon for the Removal of Pollutant and Color in Palm Oil Mill Effluent Treatment. J. Earth Environ. Health Sci. 2016, 2, 15–20. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1–15. [Google Scholar] [CrossRef]
- Di Martino, P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018, 4, 274–288. [Google Scholar] [CrossRef]
- Seabra, A.; Durán, N. Nanotoxicology of Metal Oxide Nanoparticles. Metals (Basel) 2015, 5, 934–975. [Google Scholar] [CrossRef]
- Isma, M.I.A.; Idris, A.; Omar, R.; Razreena, A.R.P. Effects of SRT and HRT on Treatment Performance of MBR and Membrane Fouling. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2014, 8, 488–492. [Google Scholar]
- Iorhemen, O.T.; Hamza, R.A.; Tay, J.H. Membrane fouling control in membrane bioreactors (MBRs) using granular materials. Bioresour. Technol. 2017, 240, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.A.; Wong, L.Y.; Chai, H.Y.; Bashir, M.J.K.; Ho, C.-D.; Nisar, H.; Lo, P.K. Investigation on the performance of hybrid anaerobic membrane bioreactors for fouling control and biogas production in palm oil mill effluent treatment. Water Sci. Technol. 2017, 76, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Mai, D.T.; Kunacheva, C.; Stuckey, D.C. Post-treatment of anaerobic membrane bioreactor (AnMBR) effluent using activated carbon. Bioresour. Technol. 2018, 266, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Kennedy, M.D.; van der Meer, W.G.J.; Vanrolleghem, P.A.; Schippers, J.C. The role of blocking and cake filtration in MBR fouling. Desalination 2003, 157, 335–343. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Ismail, S.; Bhatia, S. Water recycling from palm oil mill effluent (POME) using membrane technology. Desalination 2003, 157, 87–95. [Google Scholar] [CrossRef]
- Loh, S.K.; Ngatiman, M.; Lim, W.S.; Choo, Y.M. A Zero Discharge Treatment System Of Palm Oil Mill Effluent. Malays. Palm Oil Board Inf. Ser. 2014, 657, 6–9. [Google Scholar]
- Huan, L.; Yiying, J.; Mahar, R.B.; Zhiyu, W.; Yongfeng, N. Effects of ultrasonic disintegration on sludge microbial activity and dewaterability. J. Hazard. Mater. 2009, 161, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Mirbagheri, S.A.; Bagheri, M.; Boudaghpour, S.; Ehteshami, M.; Bagheri, Z. Performance evaluation and modeling of a submerged membrane bioreactor treating combined municipal and industrial wastewater using radial basis function artificial neural networks. J. Environ. Heal. Sci. Eng. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Yamato, N.; Yamamura, H.; Watanabe, Y. Membrane fouling in pilot-scale membrane bioreactors (MBRs) treating municipal wastewater. Environ. Sci. Technol. 2005, 39, 6293–6299. [Google Scholar] [CrossRef] [PubMed]
- Mitri, S.; Clarke, E.; Foster, K.R. Resource limitation drives spatial organization in microbial groups. ISME J. 2016, 10, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Said, M.; Abu Hasan, H.; Mohd Nor, M.T.; Mohammad, A.W. Removal of COD, TSS and colour from palm oil mill effluent (POME) using montmorillonite. Desalin. Water Treat. 2016, 57, 10490–10497. [Google Scholar] [CrossRef]
- Soleimaninanadegani, M.; Manshad, S. Enhancement of Biodegradation of Palm Oil Mill Effluents by Local Isolated Microorganisms. Int. Sch. Res. Not. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Vashi, H.; Iorhemen, O.T.; Tay, J.H. Aerobic granulation: A recent development on the biological treatment of pulp and paper wastewater. Environ. Technol. Innov. 2018, 9, 265–274. [Google Scholar] [CrossRef]
- Schulz, M.; Soltani, A.; Zheng, X.; Ernst, M. Effect of inorganic colloidal water constituents on combined low-pressure membrane fouling with natural organic matter (NOM). J. Membr. Sci. 2016, 507, 154–164. [Google Scholar] [CrossRef]
- Mei, X.; Wang, Z.; Zheng, X.; Huang, F.; Ma, J.; Tang, J.; Wu, Z. Soluble microbial products in membrane bioreactors in the presence of ZnO nanoparticles. J. Membr. Sci. 2014, 451, 169–176. [Google Scholar] [CrossRef]
- Peleato, N.M.; Legge, R.L.; Andrews, R.C. Characterization of UF foulants and fouling mechanisms when applying low in-line coagulant pre-treatment. Water Res. 2017, 126, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, P.K.; Madras, G.; Bose, S. PVDF/PBSA membranes with strongly coupled phosphonium derivatives and graphene oxide on the surface towards antibacterial and antifouling activities. J. Membr. Sci. 2018, 548, 203–214. [Google Scholar] [CrossRef]
- Hu, N.; Xiao, T.; Cai, X.; Ding, L.; Fu, Y.; Yang, X. Preparation and characterization of hydrophilically modified PVDF membranes by a novel nonsolvent thermally induced phase separation method. Membranes (Basel) 2016, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Ngo, H.H.; Urase, T.; Gin, K.Y.H. A critical review on characterization strategies of organic matter for wastewater and water treatment processes. Bioresour. Technol. 2015, 193. [Google Scholar] [CrossRef] [PubMed]
- Mancebo, U.; Hettiaratchi, J.P.A. Rapid assessment of methanotrophic capacity of compost-based materials considering the effects of air-filled porosity, water content and dissolved organic carbon. Bioresour. Technol. 2015, 177, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Gkotsis, P.; Banti, D.; Peleka, E.; Zouboulis, A.; Samaras, P. Fouling Issues in Membrane Bioreactors (MBRs) for Wastewater Treatment: Major Mechanisms, Prevention and Control Strategies. Processes 2014, 2, 795–866. [Google Scholar] [CrossRef]
- Ding, Y.; Tian, Y.; Li, Z.; Zuo, W.; Zhang, J. A comprehensive study into fouling properties of extracellular polymeric substance (EPS) extracted from bulk sludge and cake sludge in a mesophilic anaerobic membrane bioreactor. Bioresour. Technol. 2015, 192, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Sioutopoulos, D.C.; Karabelas, A.J. The effect of permeation flux on the specific resistance of polysaccharide fouling layers developing during dead-end ultrafiltration. J. Membr. Sci. 2015, 473, 292–301. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Ding, S.; Hou, L.A. Dynamics of biofouling development on the conditioned membrane and its relationship with membrane performance. J. Membr. Sci. 2016, 514, 264–273. [Google Scholar] [CrossRef]
- Meng, L.; Xi, J.; Yeung, M. Degradation of extracellular polymeric substances (EPS) extracted from activated sludge by low-concentration ozonation. Chemosphere 2016, 147, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.; Idrus, S.; Che Man, H.; Nik Daud, N. Wastewater Treatment and Biogas Recovery Using Anaerobic Membrane Bioreactors (AnMBRs): Strategies and Achievements. Energies 2018, 11, 1675. [Google Scholar] [CrossRef]
- Neoh, C.H.; Lam, C.Y.; Lim, C.K.; Yahya, A.; Ibrahim, Z. Decolorization of palm oil mill effluent using growing cultures of Curvularia clavata. Environ. Sci. Pollut. Res. 2014, 21, 4397–4408. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.P.; Kong, H.F.; Bashir, M.J.K.; Lo, P.K.; Ho, C.D.; Ng, C.A. Treatment of palm oil mill effluent using combination system of microbial fuel cell and anaerobic membrane bioreactor. Bioresour. Technol. 2017, 245, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, M.; Elimelech, M. Biofouling of reverse osmosis membranes: Role of biofilm-enhanced osmotic pressure. J. Membr. Sci. 2007, 295, 11–20. [Google Scholar] [CrossRef]
- Al Ashhab, A.; Sweity, A.; Bayramoglu, B.; Herzberg, M.; Gillor, O. Biofouling of reverse osmosis membranes: effects of cleaning on biofilm microbial communities, membrane performance, and adherence of extracellular polymeric substances. Biofouling 2017, 33, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Derlon, N.; Grütter, A.; Brandenberger, F.; Sutter, A.; Kuhlicke, U.; Neu, T.R.; Morgenroth, E. The composition and compression of biofilms developed on ultrafiltration membranes determine hydraulic biofilm resistance. Water Res. 2016, 102, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Bucs, S.; Farhat, N.; Kruithof, J.C.; Picioreanu, C.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Review on strategies for biofouling mitigation in spiral wound membrane systems. Desalination 2018, 434, 189–197. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwak, S.; Sohn, B.; Park, T.H. Design of TiO2 nanoparticle self-assembled aromatic polyamide thin- lm-composite (TFC) membrane as an approach to solve biofouling problem. J. Membr. Sci. 2003, 211, 157–165. [Google Scholar] [CrossRef]
- Ayyavoo, J.; Nguyen, T.P.N.; Jun, B.M.; Kim, I.C.; Kwon, Y.N. Protection of polymeric membranes with antifouling surfacing via surface modifications. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 506, 190–201. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Zhang, Y.; Tian, M.; He, T.; Liu, J.; Chen, V. Polymeric antimicrobial membranes enabled by nanomaterials for water treatment. J. Membr. Sci. 2018, 550, 173–197. [Google Scholar] [CrossRef]
- Giwa, A.; Dindi, A.; Kujawa, J. Membrane bioreactors and electrochemical processes for treatment of wastewaters containing heavy metal ions, organics, micropollutants and dyes: Recent developments. J. Hazard. Mater. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ding, L.; Luo, J.; Jaffrin, M.Y.; Tang, B. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review. Chem. Eng. J. 2016, 302, 446–458. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Jiang, L.; Jin, J. Recent progress in developing advanced membranes for emulsified oil/water separation. NPG Asia Mater. 2014, 6, e101. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Ghanbari, M.; Lau, W.J.; Rahbari-Sisakht, M.; Rana, D.; Matsuura, T.; Kruczek, B.; Ismail, A.F. Surface modification of thin film composite membrane by nanoporous titanate nanoparticles for improving combined organic and inorganic antifouling properties. Mater. Sci. Eng. C 2017, 75, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Wang, Z.; Liu, D.Y.; Xiao, K.; Guan, J.; Xie, Y.F.; Wang, X.M.; Waite, T.D. Role of adsorption in combined membrane fouling by biopolymers coexisting with inorganic particles. Chemosphere 2018, 191, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, M.; Bastani, D.; Musavi, S.A. Enhancement of surface properties and performance of reverse osmosis membranes after surface modification: A review. Desalination 2017, 420, 330–383. [Google Scholar] [CrossRef]
- Luo, W.; Hai, F.I.; Price, W.E.; Nghiem, L.D. Water extraction from mixed liquor of an aerobic bioreactor by forward osmosis: Membrane fouling and biomass characteristics assessment. Sep. Purif. Technol. 2015, 145, 56–62. [Google Scholar] [CrossRef]
- Fan, C.; Nguyen, V.; Zeng, Y.; Phadungbut, P.; Horikawa, T.; Do, D.D.; Nicholson, D. Novel approach to the characterization of the pore structure and surface chemistry of porous carbon with Ar, N2, H2O and CH3OH adsorption. Microporous Mesoporous Mater. 2015, 209, 79–89. [Google Scholar] [CrossRef]
- Thommes, M.; Cychosz, K.A. Physical adsorption characterization of nanoporous materials: Progress and challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Li, Z.Y.; Yangali-Quintanilla, V.; Valladares-Linares, R.; Li, Q.; Zhan, T.; Amy, G. Flux patterns and membrane fouling propensity during desalination of seawater by forward osmosis. Water Res. 2012, 46, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Field, E.L.; Howe, K.J.; Thomson, B.M. Effect of Solids Retention Time in Membrane Bioreactors on Reverse Osmosis Membrane Fouling. Ph.D. Thesis, Department of Civil Engineering, University of New Mexico, Albuquerque, NM, USA, 2010; p. 139. [Google Scholar]
- Zhu, X.; Treu, L.; Kougias, P.G.; Campanaro, S.; Angelidaki, I. Converting mesophilic upflow sludge blanket (UASB) reactors to thermophilic by applying axenic methanogenic culture bioaugmentation. Chem. Eng. J. 2018, 332, 508–516. [Google Scholar] [CrossRef]
- Deng, L.; Guo, W.; Ngo, H.H.; Zhang, H.; Wang, J.; Li, J.; Xia, S.; Wu, Y. Biofouling and control approaches in membrane bioreactors. Bioresour. Technol. 2016, 221, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Singh, B.; Upadhyay, S.; Pandey, S. Solution for Sustainable Development for Developing Countries: Waste Water Treatment by Use of Membranes—A Review. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 1212–1228. [Google Scholar] [CrossRef]
- Burman, I.; Sinha, A. A Review on Membrane Fouling in Membrane Bioreactors: Control and Mitigation; Springer: New York, NY, USA, 2018; ISBN 978-981-10-7332-8. [Google Scholar]
- Banti, D.C.; Samaras, P.; Tsioptsias, C.; Zouboulis, A.; Mitrakas, M. Mechanism of SMP aggregation within the pores of hydrophilic and hydrophobic MBR membranes and aggregates detachment. Sep. Purif. Technol. 2018, 202, 119–129. [Google Scholar] [CrossRef]
- Kim, E.S.; Hwang, G.; Gamal El-Din, M.; Liu, Y. Development of nanosilver and multi-walled carbon nanotubes thin-film nanocomposite membrane for enhanced water treatment. J. Membr. Sci. 2012, 394–395, 37–48. [Google Scholar] [CrossRef]
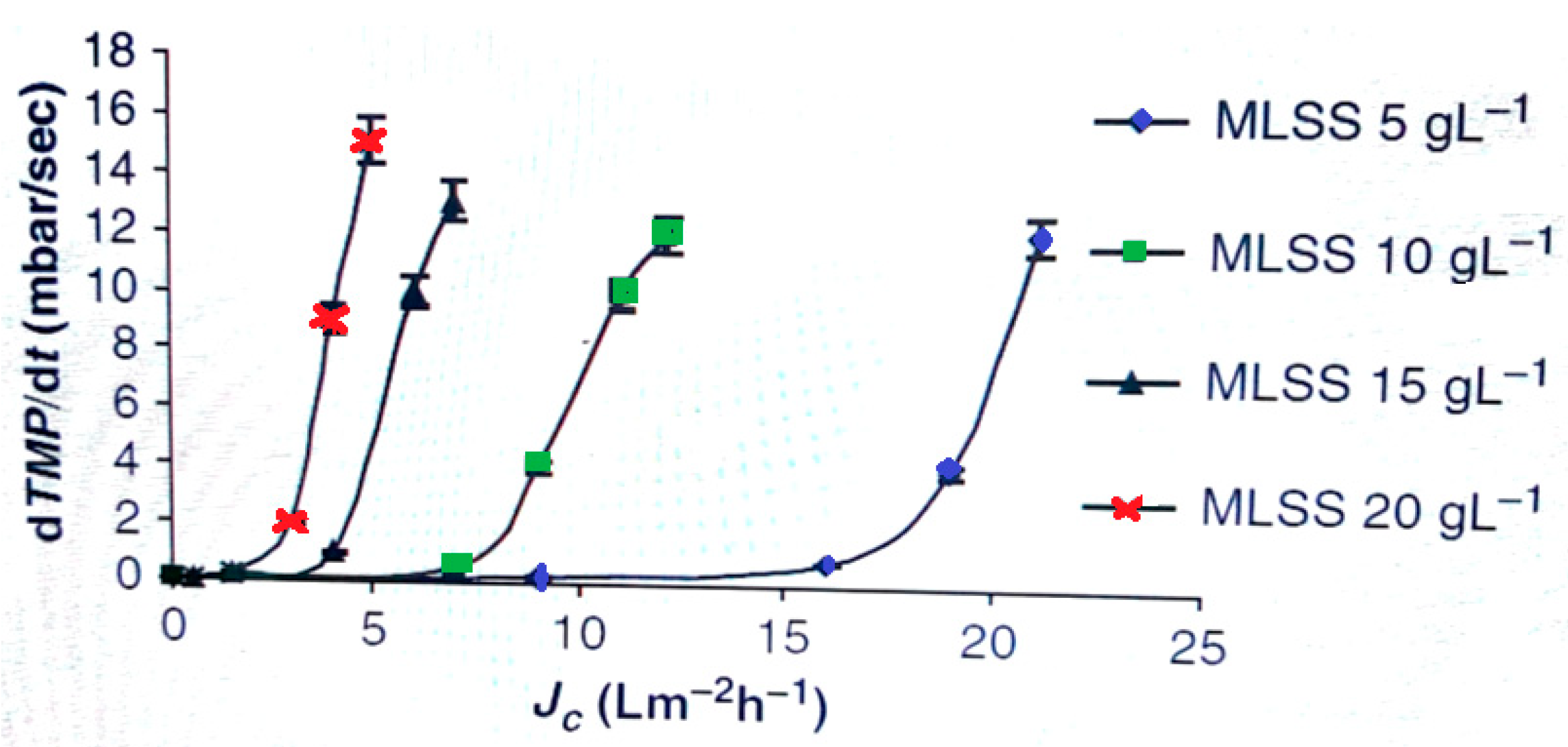
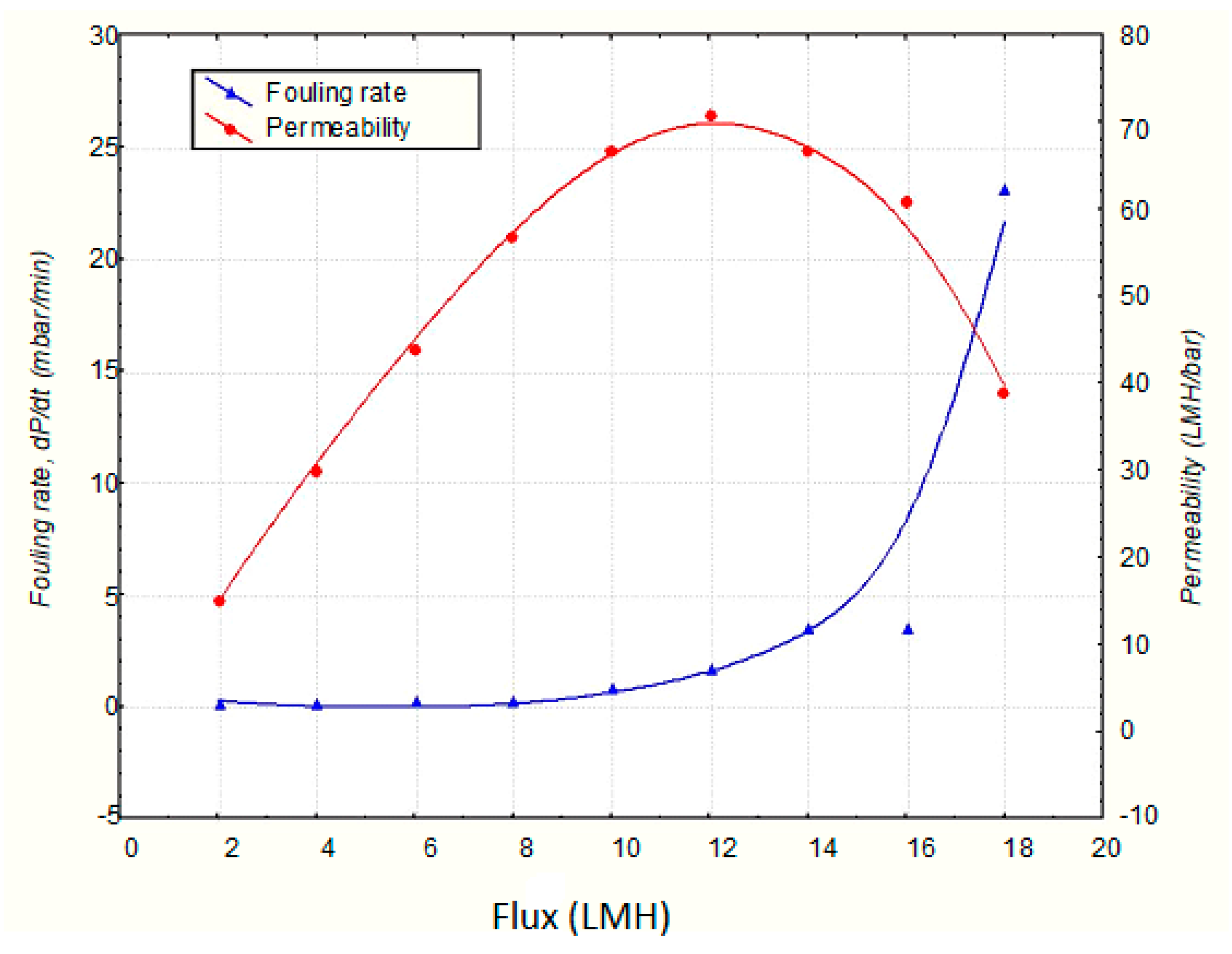

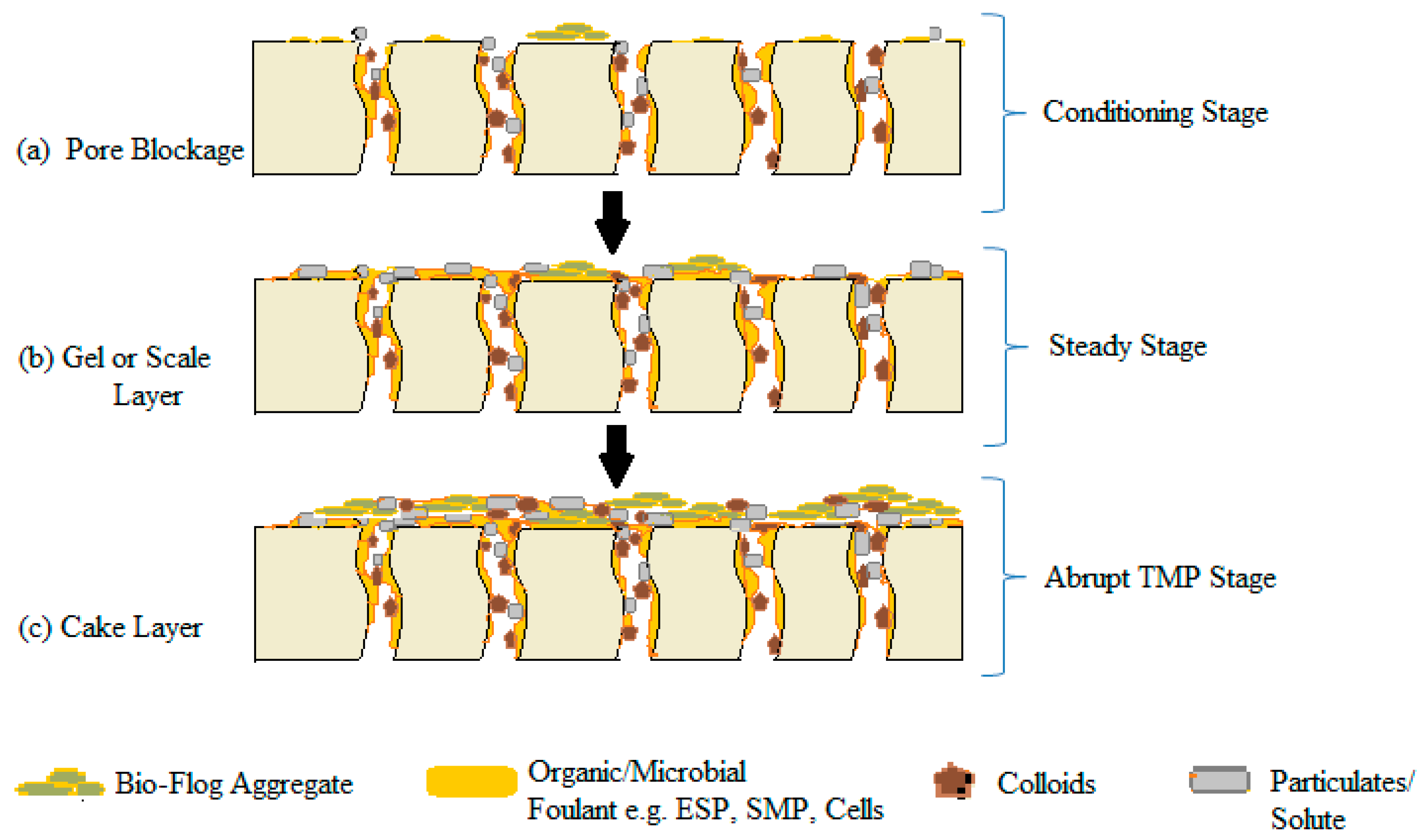
| Parameters | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BOD | COD | BI | pH | T (°C) | TS | TSS | TVS | TN | Oil/Fat | NH3-N | TP | |
| 27,000 | 51,000 | 0.5294 | 4.2 | - | 40,000 | 18,000 | 34,000 | 750 | 6000 | - | - | [51] |
| 30,000 | 50,000 | 0.6000 | 4.5 | - | 16,495 | 59,350 | - | 1820 | - | - | - | [17] |
| 32,500 | 64,500 | 0.5039 | 4.65 | 88 | - | - | - | 41 | 1950 | - | - | [14] |
| 34,950 ± 1450 | 70,500 ± 917 | 0.4957 | 4.72 | - | 51,880 ± 300 | 26,547 ± 3043 | 43,260 ± 140 | 1620 ± 26 | - | - | - | [52] |
| 40,000 | 65,000 | 0.6154 | 4.5 | 55.5 | 45,000 | 20,000 | 26,300 | 890 | 1500 | 90 | 950 | [53] |
| 25,000 | 50,000 | 0.5000 | 4.7 | 85 | 40,500 | 18,000 | 34,000 | 750 | 4000 | - | 180 | [54] |
| 27,000 | 75,000 | 0.3600 | 4.3 | - | 100,000 | 50,000 | 80,000 | - | - | - | - | [46] |
| 30,000 ± 10,391 | 70,000 ± 7612 | 0.4286 | 4.75 | - | - | 28,900 ± 3065 | - | 980 ± 50 | 10,540 ± 1000 | - | 608 ± 81 | [55] |
| 24,500 | 49,100 | 0.4989 | 4.1 | - | - | 18,000 | 2600 | 600 | 5300 | - | - | [48] |
| 45,357 | 73,498 | 0.6171 | 4.5 | - | 56,279 | 32,005.5 | 41,650 | 760 | 6670.5 | 69 | - | [49] |
| - | 4500 | - | 5.6 | - | 4300 | 8200 | 4000 | 500 | - | 200 | - | [56] |
| 25,000 | 50,000 | 0.5000 | 4.7 | 85 | 40,500 | 18,000 | 34,000 | 75,000 | - | 3500 | - | [2] |
| MBR-Configuration | Parameters | Reference | |||||
|---|---|---|---|---|---|---|---|
| TMP bar | Permeate L/m2h | CFV m/s | Air flow m/s | EDP, kW/m3 | EDA, kW/m3 | ||
| sub-MBR | 0.1 | 7.9 | 0.5 | NA | 4 | 4 | [122] |
| sub-MBR | 0.13 | 8 | - | NA | 0.14 | 0.0055 | [124] |
| sub-MBR | 26 | 26 | 0.25 | 0.25 | - | - | [108] |
| sub-MBR | 0.2–0.5 | 20–50 | 0.8696 | 0.3–0.6 | - | [64] | |
| sub-MBR | 30 | 25 | 0.3 | 20 | - | - | [26] |
| ss-MBR | 4 | 50–100 | - | - | - | 4–12 | [124] |
| ss-MBR | 46 | 16 | 0.35 | 0.35 | - | - | [108] |
| ss-MBR | 2 | 175 | 3 | - | 9.9 | 2.8 | [113] |
| ss-MBR | 0.6 | 115 | 3 | - | - | - | [126] |
| ss-MBR | 2 | 10–30 | 1–1.1 | - | - | - | [127] |
| Treatment Configuration/Process | Primary Procedure | Operating Conditions | Major Results and Contaminants Removal | Critical Findings and Effect of Treatment Process on Membrane Fouling | References |
|---|---|---|---|---|---|
| AerMBR (Pure Oxygen) | OTE under pure oxygen treatment at varied MLSS and HRT was investigated | MLSS = 4071 to 11,192 mg/L, HRT = 12–18 h; AF = 141 L/h, DO = 2 mg O2/L | HRT 18 h, varying MLSS from 4300–10,275 mg/L: Alpha aeration-factor decrease from 0.6115 to 0.1223; while at HRT 12 h and MLSS of 4017 to 11,192 mg/L; it decreases from 0.2787 to 0.0221 | MLSS strongly influence OTE; OTE increased by 400% as MLSS decreased with increase in HRT. | [167] |
| AerMBR + Agitation | Effects of aeration and agitation on POME treatment were investigated. | pH = 5–9; three tanks = Ta, Ts and Tas | 61.2 and 58.9% removal efficiency for BOD and COD respectively after 6 d; UF membrane improved the treatment with the overall performance of 99.9% at pH 7.39 | Integrated bioremediation and ultrafiltration membrane improves the total treatment performance to 99.9%. also, the bio pre-treatment reduces fouling propensity | [154] |
| AerMBR + Nano-composite-membrane | The composite UF-PVDF-ZIO was evaluated with aim of deteriorating colour and fouling rate. | ZIO = 0.0, 0.5, 1.0, 1.5 and 2.0 wt %. | Best colour removal and permeability was 80.5% and 50.18 LMH respectively at 0.5 wt % of ZIO dosage | ZIO nanoparticle improves colour removal, permeability; reduce the fouling rate but collapse after 4 cycles. M2.0 retain it throughout | [152] |
| Also, ZIO oxidises to release antimicrobial and hydrophilic radicals. This makes it a good antifouling material | [152,240,241] | ||||
| ZIO reduces fouling | [152] | ||||
| AerMBR + Microbial | The experiment investigates the correlation between the microbial community and MBR performance for POME treatment under alternating conditions. | Aeration period = 40 d, non-aeration period = 10 d; sample collection interval = 25 d, 50 d and 75 d | Aerobic conditions favour microbial as follows: Proteobacteria (19–23%), ODI (11–15%), Chloroflexi (11–13%). While; in the Non-aerobic condition: ODI (20%), proteobacteria (18%) and plantomycetes (16%) were visible | Protein was the main constituent of ESP. Taxonomic profile on the day 75 is similar to day 25. Proteobacteria survives in both conditions but ODI dominates under no aeration condition. | [141,152] |
| ESP constitutes the major component of the accumulated biofilm | [242] | ||||
| AerMBR + TNT nanoparticle PVDF | Composite PVDF-TNT was fabricated with variable TNT amount and evaluated under varied POME concentration with aim of decolourization | TNT load = 0–1 wt %; POME concentrations = 100, 75 and 50% with DF: 1, 2, 3 | At PVDF-TNT 0.5; colour removal = 67.3%. but 5.7% flux reduction was observed after 5 cycles of filtrations | TNT improve the colour removal, also, it is a good antimicrobial material. Hence, capable of mitigating membrane fouling. | [21] |
| However, TNT could be exorbitantly expensive compared to other antimicrobial nanoparticles | [243] | ||||
| AerMBR + Adsorbent (AC + Zeolite) | Performances of 2 different adsorbents were compared with SubMBR evaluated without adsorbent same conditions. | Dosage: AC = 2, 4 g/L; Zeolite = 2 g/L; SRT = 70 d | Total of COD removal with adsorbents ranged from 97.5–98.5% and without was 95.2%, colour reduced to 16–26 Pt-Co while without adsorbent was 80 Pt-Co and improve flux to 42 LMH | Adsorbent improve flux, TMP, and reduce SMP deposition, COD and colour significantly | [18] |
| The improvement in flux indicates that the membrane is less prone to fouling due to the significant reduction in contaminants | [185] | ||||
| AerMBR + AC | The performances of SubMBR with and without adsorbent were compared. | SRT = 20 d; HRT = 3.1 h; MLSS = 1.25 g/L; PAC dosage = 5 g/L; Backwash at F-R = 1 h per 1 min with 30 L/m2h | With PAC, COD removal = 100%, DOC removal = 99% requires 7.5 kPa But without; COD removal = 94% and DOC = 95%. Requires 20 kPa to obtained the maximum flux of 20 L/m2 | PAC lowered the operating TMP, improves organic matter removal and mitigate fouling rate | [180] |
| AerMBR | effect of SRT and HRT on membrane fouling was studied under 10 min-4 min intermittent operation | SRT = 30, 15, 4 d; HRT= 12, 8, 4 h; pH = 7.2 ± 0.1; DO = 2.0 mg/L; flux = 4 L/m2/h; Temp. = 25 to 35 °C | 17 μm cake layer observed after 4 d of SRT and 12 h HRT; COD removal = 93%, TSS = 98%, NH3-N = 80% and PO4 = 30% | SRT influence organic matter removal. But equal removal of TSS at all SRT, higher HRT with shorter SRT induced faster fouling | [244] |
| AerMBR | Fouling behaviour was studied in three separate Submerged MBR: A,B,C under varied aeration intensity as 150, 400 and 800 L/h respectively at constant TMP. | HRT= 10–12 h; MLSS = 6000 mg/L; SRT = 30 d; Aeration:= 150, 400 and 800 L/h; DO= 3.21, 4.76 and 6.5 mg/L; TMP = 3.97 kPa | permeate of A and C, decline after 10 h operation; B reach a steady value after 100 h. Sludge bulking observed at 2.0 mg/L DO | Low DO causes sludge bulking because of the overgrowth of filamentous bacteria. | [131] |
| Fouling rate decrease with an increase in aeration at the initial stage but breaks flocs to smaller sizes after sometimes hence more formation of colloids and higher fouling. | [245] | ||||
| AnMBR | Kinetic coefficient of the POME was studied. | MLSS = 11,760–20,800 mg/L, MLVSS = 8938–17,680 mg/L, OLR = 1–11 kg COD/m3-d, HRT = 600.4 to 6.8 d | GYC and SGR was 0.67 g vss/g COD, and 0.24 d−1 respectively. COD removal efficiency varied from 96.6 to 98.4% while the CH4 yield varied between 0.25 to 0.87 L/g | Biomass concentration has a significant influence on CH4 production. Concentrated biomass yield more CH4 but the membrane is more prone to fouling | [196] |
| AnMBR | Kinetic coefficient was investigated under anaerobic condition and the result was compared with kinetic equation models | MLSS = 8220 to 15,400 mg/L, MLVSS = 6329 to 13,244 mg/L, OLR = 2–13 kg COD/m3-d, HRT = 400.6 to 5.7 d | COD removal efficiency varied between 94.8 to 96.5%; GYC was 0.62 g vss/g COD and SD was 0.21 d−1 | The treatment process deteriorates COD significantly, varying MLSS concentration influences CH4 yield | [222] |
| AnMBR | Effect of intermittent Filtration-Relaxation (F-R) and membrane fouling under anaerobic conditions were investigated | F-R: L1 = 240 s–30 s; L2= 480 s–30 s; L3 = 720 s–30 s and L4 = 960 s–30 s | Protein and carbohydrate dominates and L1 gives the least fouling rate with 50% lower in Hydraulic Resistance | More Frequent F-R reduced prevents fouling by 50%. SMP and EPS contributes to fouling mechanism | [201] |
| AnMBR | Under a specified range of TMP (125–130 mbar), the influence of SRT on membrane fouling under double stage anaerobic conditions was studied. | SRT = 15, 30 and 60 d; Flux = 2.41 L/m2-h; TMP = 125–130 mbar | Foulants deposited for SRT = 15, 30 and 60 d was 34.28–16.81; 64.1–26.07 and 84.19–37.18 mg/g vss respectively | The longer the SRT, the denser the biofilm hence the more the severity of the fouling | [204] |
| AnMBR + | Effect of Baffled Aeration Flotation (BAF) system on AnMBR performance was studied. | Number of BAF = 5; HRT = 3, 4 and 5 d; AFR = 11, 8 and 5 L/min | At optimal condition of 5 d HRT and 11 L/min aeration: BAF system gives 35.5, 86.4, 57.7, 57.3, 59.7 and 52.6% removal of while the overall system gives 97, 93.9, 99.8, 94.5, 96.1 and 99.9% respectively | BAF system improves the performance of the AnMBR in terms of contaminants removal and reduces the fouling rate | [61] |
| HybMBR (+PAC) | Effect of PAC particle sizes: fine = Mf; medium = Mm; coarse = Mc, on POME treatment were studied and compared with treatment without PAC under anaerobic | MLSS = 8050 mg/L; MLVSS = 6850 mg/L; SRT = 30 d; HRT = 6 d PAC dosage: Mb = 0; Mc = 5 g/L; Mm = 5 g/L; Mf = 5 g/L; Temp.= 45 °C; OLR= 7658 ± 408 mg COD/L-d | Without PAC: removal efficiency are COD: 64.90 ± 1.46%; protein: 2407 ± 230 mg/L; polysaccharide: 71 ± 1.827 mg/L. With PAC: Mf present the best performance with 78.53 ± 0.66% COD removal; protein: 1647 ± 175 mg/L; Mc: least COD removal with 72.99 ± 1.47% and protein: 2075 ± 305 mg/L | The Fine PAC (Mf) presents better COD removal of 90.55 ± 0.21 than medium PAC (Mm), particle size influence adsorption performance, biogas yield and reduce fouling rate | [246] |
| This shows that PAC of fine particles sizes present more active sites for COD adsorption | [26] | ||||
| HybMBR | POME was pre-treated using hybrid anaerobic and aerobic process-then polished with membrane techniques. The treatment lasted for a period of a year | Anaerobic HRT = 9.8 d; Aerobic HRT = 48 h; DO = 3.5 mg/L | 55.6% of the waste oil in raw POME was recovered at anaerobic treatment; aerobic treatment degenerate BOD3 level to less than 20 mg/L | Deterioration of COD is more significant during anaerobic treatment, while the aerobic process is more suitable for BOD removal. | [69] |
| Also, the hybrid system is suitable for resource recovery, such as biogas | [140] | ||||
| HybMBR | The HybMBR was acclimatized for 45 days with 4 different samples at a varied MLSS to observe the fouling rate on the membrane. | MLSS = 5–20 g/L; HRT = 11, 7 and 8 h; SRT: 70 days, 120 rpm; flux: 11 L/m2-h | As varied from MLSS from 5 to 20 g/L, the flux reduced down to 400%. However, 97% denitrification and nitrification was obtained at 20 g/L MLSS | MLSS concentration strongly influences fouling. | [207] |
| But, higher MLSS concentration promotes NH4-N and nitrogen substance removal in MBR | [162,207] | ||||
| HybMBR (+adsorbent) | The HybMBR was Evaluated under different adsorbent and varied dosage but same SRT. | Dosage: AC = 4 g, Zeolite = 8 g, Moringa Oliefera = 12 g and SRT = 30 days | Adsorbent: 58, 48 and 42% removal of SMP was observed by AC, Zeolite and MO respectively. | Adsorbents reduce SMP significantly. At optimal operating conditions, AC gives better performance. | [56] |
| At an optimal dosage of AC; 70 and 85% SMP removal efficiency and fouling reduction were archived respectively | The improvement in performance was attributed to the availability of the active sites to adsorb the SMP | [56,247,248] | |||
| HybMBR | TMP and flux was continuously monitored for a complete season under varied temperature to investigate the membrane fouling characteristics | SRT= 20 days, HRT = 17 h, Temp. = 27 to 13 °C | At summer the TMP was 25 kPa with 10 L/m2-h permeability flux while at winter the TMP was 60 kPa at 10 L/m2-h. | This study revealed that temperature influences the organic concentration of the supernatant, filtration resistance, viscosity and humic substance. | [210] |
| Higher temperature disintegrates the flocs aggregates, thereby increasing the concentration of the foulants of smaller sizes. | [141,214] | ||||
| HybMBR | Effect of biomass concentration on POME treatment and membrane fouling with fitted controllable recycling device and agitator at the anoxic and anaerobic condition | MLSS = 4000–8000 mg/L, OLR = 1.77 to 1.87 kg COD/m3, steady inflow = 108 m3/d, flux = 15 L/m2h | The removal efficiency of COD, SS, TN and TP was 94, 98, 83 and 64% respectively | Both Protein and carbohydrate contribute to the fouling. | [58] |
| The hybrid system deteriorates the organic pollutants significantly | [226] | ||||
| HybMBR | Each sections of the hybrid system was subjected to different operating conditions. Also, the MLSS concentration was varied with the aim of investigating the fouling effect | Anaerobic: HRT = 12 h, pH = 5.5 to 6.5, DO = 0-0.1 mg/L Anoxic: HRT = 6 h, pH = 7.2–8.5, DO= 0.3–0.6 mg/L Aerobic: HRT = 4 h, pH=7–7.5, DO= 6–8 mg/L. MLSS = 4–8 g/L | 94, 98, 83 and 64% for COD, SS, TN and TP removal efficiency respectively. At 12 LMH flux, permeability increased to 70 LMH. | Initially, the filtration rate was steady, then, diminished abruptly after the critical flux 16 LMH. | [208] |
| This was due to the fouling which often causes TMP jump. | [65,108] | ||||
| HybMBR | An integrated Biological-composite membrane system was acclimatized at room temperature. The anaerobic and aerobic section takes about 111 and 10 days respectively, for complete acclimation | Influent COD: 4331 to 35,000 mg/L, UF membrane TMP = 200,000 Pa; RO membrane TMP = 1,300,000 Pa Temperature = 25 °C, OLR = 10 kg COD/m3-d | Anaerobic deterioration of COD = 93%, While, 22% of COD was removed in aerobic. UF membrane removes SS and turbidity with a total of 99.288% efficiency the RO membrane removed all the remnants contaminants with 100% efficiency. | Anaerobic performed excellently in terms of COD removal. UF membrane is suitable for removing SS and turbidity. | [15] |
| RO remove all the contaminants including the colour but it requires high TMP. | [249,250] | ||||
| The setup mitigate fouling rate and also improved membrane flux recovery (between 91.7 to 95.3%) | [15] | ||||
| HybMBR | POME passes through the integrated system for treatment and recourse recovery (biogas and bio fertilizer) | Screening operation, biological treatment, reclamation operation, biogas generation, sludge disposal system | About 94% of COD removal was achieved during anaerobic treatment; improvement in the COD removal of 97% was observed after Nano air flotation treatment. | Zero discharge from POME was achieved; SS, residual oil, NH4-N, toxic material and colour were removed in the aeration section. In overall, less fouling was observed using the integrated system | [47] |
| BOD is less than 20 mg/L after the final treatment with 80% consistency | |||||
| SonMBR | Effect of sonication on MBR was studying for different sonication durations: 2 h and 1 h | Acclimatization period = 2 days, pH = 6.8 to 7.8, pressure = 2 bar | COD removal efficiency for 2 h and 1 h sonication was 98.75 and 97.71% respectively; more CH4 yield was obtained at 2 h sonication | Sonication improves organic matter removal, CH4 production rate and mitigating fouling. This implies that the biomass degradation is more at longer sonication duration | [218] |
| SonMBR | The study was conducted to investigate the effect of sonication on the kinetic coefficient of POME prepared in three samples: Sc, Sp and Sr | Sonication time = 3 h, at constant MLSS concentration | Specific Growth Rate in Sc, Sp and Sr was 0.0115, 0.279 and 0.3550 kg VSS/kg COD respectively. 87.5% and 85% COD removal were observed with and without sonication respectively | The higher growth rate was observed in reacted sample Sr under sonication with improved COD removal. This implies that the microbes are more active in the reacted sample under the applied sonication | [218,219] |
| SonMBR anaerobic | Effect of sonication in anaerobic degradation and CH4 production was investigated | MLSS = 13,800 to 22,600 mg/L; MLVSS = 10,400 to 17,350 mg/L; OLR = 1–15 kg COD/m3-d; SRT = 15.8–300 d; HRT = 500.8 to 8.6 days; pH = 2 to 12; pressure = 2–4 bars | COD removal were between 92.8–98.3%; Growth Yield Coefficient = 0.73 g VSS/g COD, SMD rate= 0.28 , CH4 yield= 0.27–0.62 L/g COD-d | Maximum yield of CH4 (0.7 L/g COD/d) was obtained at highest biomass concentration (22,600 mg/L) and highest HRT | [197] |
| While the least yield was obtained at the lowest MLSS concentration. | [188,196] | ||||
| Also, SRT has an insignificant effect on CH4 production rate | [188] | ||||
| SonMBR + Microwave | The effect of combined microwave and sonication process on organic degradation and CH4 production rate was investigated | Temp. = 32–37 °C; TVS = 32–34 g/L; MF = 2450 Hz; IT = 3 to 15 min; UF: = 18 Hz; intensity = 0.2 to 0.5 WmL−1; ST = 1–30 min; HRT = 15–2 d | At optimal microwave and 11 min sonication duration, the SCOD/TCOD and BOD/SCOD ratio increased to 0.77 and 0.95 respectively. Also, about 44 mL of CH4 obtained | SCOD/TCOD ratio and BI increase significantly under sonication and microwave process. The higher CH4 yield obtained under sonication | [219,251] |
| Son MBR | The combined ultrasonic and sono-thermal was applied in an MBR system for pre-treating the POME at a varied temperature and time. | Temperature = 45, 55, 65 and 75 °C. Ultrasonic frequency = 37 Hz, sonication time = 1–6 h | 39.05% increment in degradation was observed when ultrasonic-sono-thermal pretreatment were applied | The ultrasonic and sono-thermal pretreatment promotes degradation and is more rapid at elevated temperature (75 °C) | [222] |
| Also, after the pretreatment, the average particles size 321.94 µm reduced to 79.16 µm | |||||
| SonMBR | Application of ultrasonic pretreatment to accelerate bio-hydrogen production. | Ultrasound dose = 0,91, 143 and 195 J/mL; pH = 7; | Average COD removal = 65% Bio-H2 yield = 0.7 mmol H2/g COD In overall, 38 and 20% increment in bio-H2 yield and COD removal, respectively were obtained. | The ultrasonic improve the overall performance of the reactor significantly. The best performance was obtained at the highest ultrasound dose (195 J/mL) | [223] |
| SonMBR | The application of nano zero valent ions to treat POME was accelerated by applying ultrasound. | pH = 2–4; sonication intensity = 10–50%; sonication time = 3–10 min | At 50% intensity, COD was deteriorated by 80% within 2 h | Significant improvement in COD removal was observed when ultrasound was applied. | [16] |
| TherMBR + MFC | Effect of temperature and microbial in MBR was investigated under intermittent operation and variation in TMP was monitored | Temp. = 8.7–19.7 °C; F-R = 10–2 min; HRT = 4.9 h; SRT = 40 d, TMP = 60 kPa | As temperature increases from 8.7 to 19.7 °C, the MLSS of SMP reduces from 28.1 to 2.2 mg/g. also, Proteobactaria, bacteriodestes, nitrospira, firmicutes and acidobacteria dominate with 41–51.8%, 6.7–22.2%, 8.9–15.1%, 4.32–10% and 2.2–7.0% respectively | Increase in temperature decreases SMP. Lower temperature is more suitable for Proteobacteria, while the higher temperature is capable of activating some bacteria such as Zoogloea from a dormant state. | [224] |
| TherMBR + MFC | The effect of temperature and MFC in an MBR was investigated. R1, R2, and R3 were run under mesophilic while R4 was under thermophilic. | Sample: reactor R1, reactor R2, reactor R3, reactor R4; SRT = 30 d; PAC = 5 g/L dosage; Temp.= 35, 45 and 55 °C | R3 shows the best removal of COD and polysaccharide with 95.6 ± 0.3% and 73.01% respectively, under the mesophilic condition. While R4 under thermophilic present COD removal with 79.23 ± 9.36%. Also, highest MLVSS was obtained in R1 with 38,133±1804 mg/L while least was observed in R3 with 16,467 ± 3239 mg/L | Best performance as obtained at mesophilic conditions. This indicates that mesophilic conditions are more suitable for biodegradation. | [212] |
| Fouling rate is higher at the mesophilic condition. | [31,195,252] | ||||
| TherMBR | The Effect of varied temperature on MBR performance was investigated. The MBR system was incorporated with PAC | PAC dosage = 50 g/L, Temperature = 10–20 °C | At 10 °C, Start-up time = 9 days; Complete nitrification was attained after 10 days. NH3-N removal = 90% at both 10 and 20 °C | Lower temperature (10 °C) cause delay in the start-up of the system, nitrification process. The membrane is less susceptible to fouling at this temperature | [234] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulsalam, M.; Che Man, H.; Isma Idris, A.; Faezah Yunos, K.; Zainal Abidin, Z. Treatment of Palm Oil Mill Effluent Using Membrane Bioreactor: Novel Processes and Their Major Drawbacks. Water 2018, 10, 1165. https://doi.org/10.3390/w10091165
Abdulsalam M, Che Man H, Isma Idris A, Faezah Yunos K, Zainal Abidin Z. Treatment of Palm Oil Mill Effluent Using Membrane Bioreactor: Novel Processes and Their Major Drawbacks. Water. 2018; 10(9):1165. https://doi.org/10.3390/w10091165
Chicago/Turabian StyleAbdulsalam, Mohammed, Hasfalina Che Man, Aida Isma Idris, Khairul Faezah Yunos, and Zurina Zainal Abidin. 2018. "Treatment of Palm Oil Mill Effluent Using Membrane Bioreactor: Novel Processes and Their Major Drawbacks" Water 10, no. 9: 1165. https://doi.org/10.3390/w10091165
APA StyleAbdulsalam, M., Che Man, H., Isma Idris, A., Faezah Yunos, K., & Zainal Abidin, Z. (2018). Treatment of Palm Oil Mill Effluent Using Membrane Bioreactor: Novel Processes and Their Major Drawbacks. Water, 10(9), 1165. https://doi.org/10.3390/w10091165






