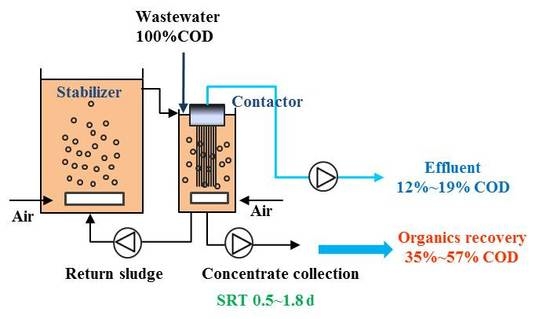
High-Rate Contact Stabilization Process-Coupled Membrane Bioreactor for Maximal Recovery of Organics from Municipal Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Operation
2.2. COD Mass Balance and Oxygen Uptake Rate
2.3. Biochemical Methane Potential
2.4. Analytical Methods
2.5. Membrane Permeability Analysis
3. Results and Discussion
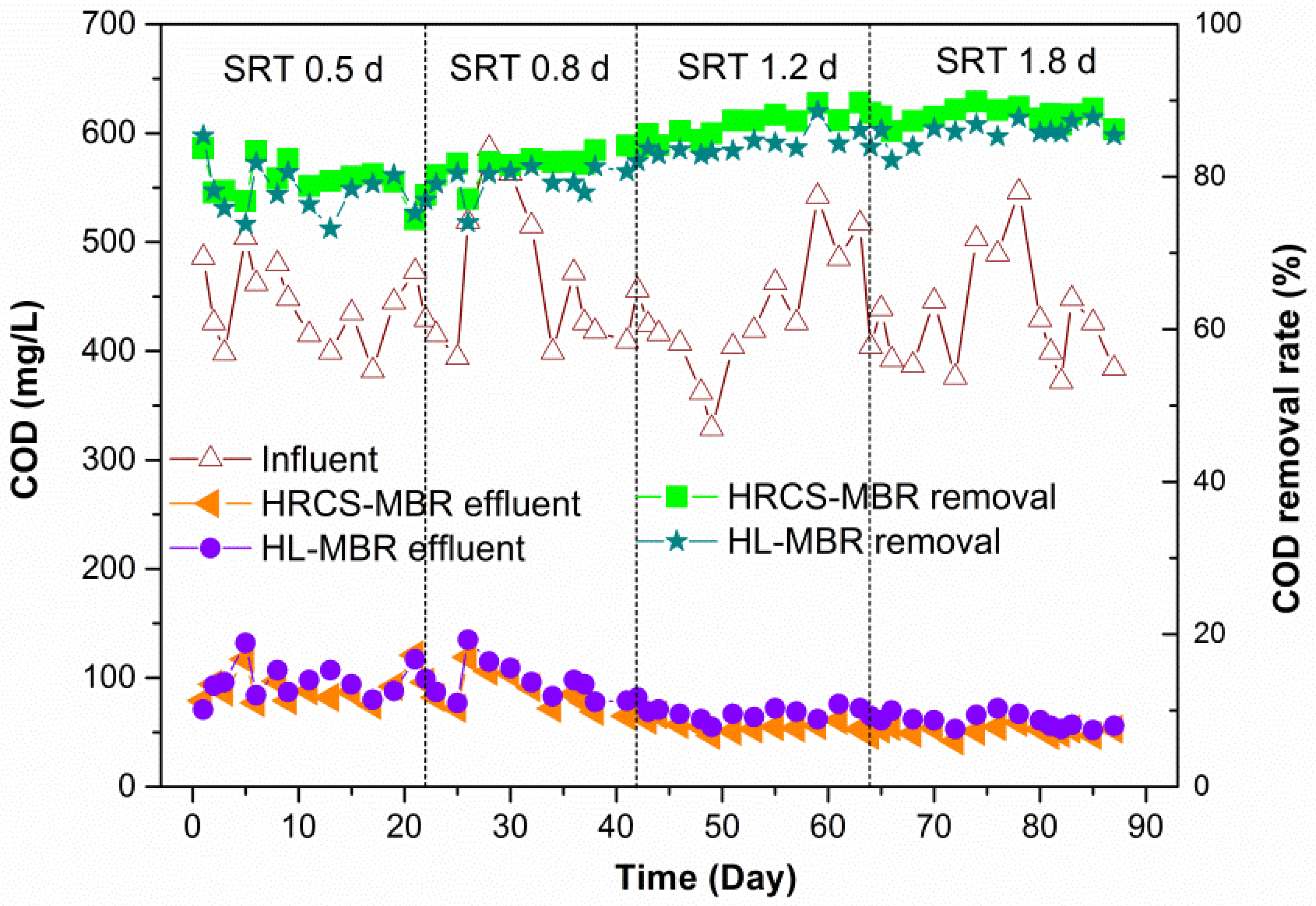
3.1. Permeate Quality
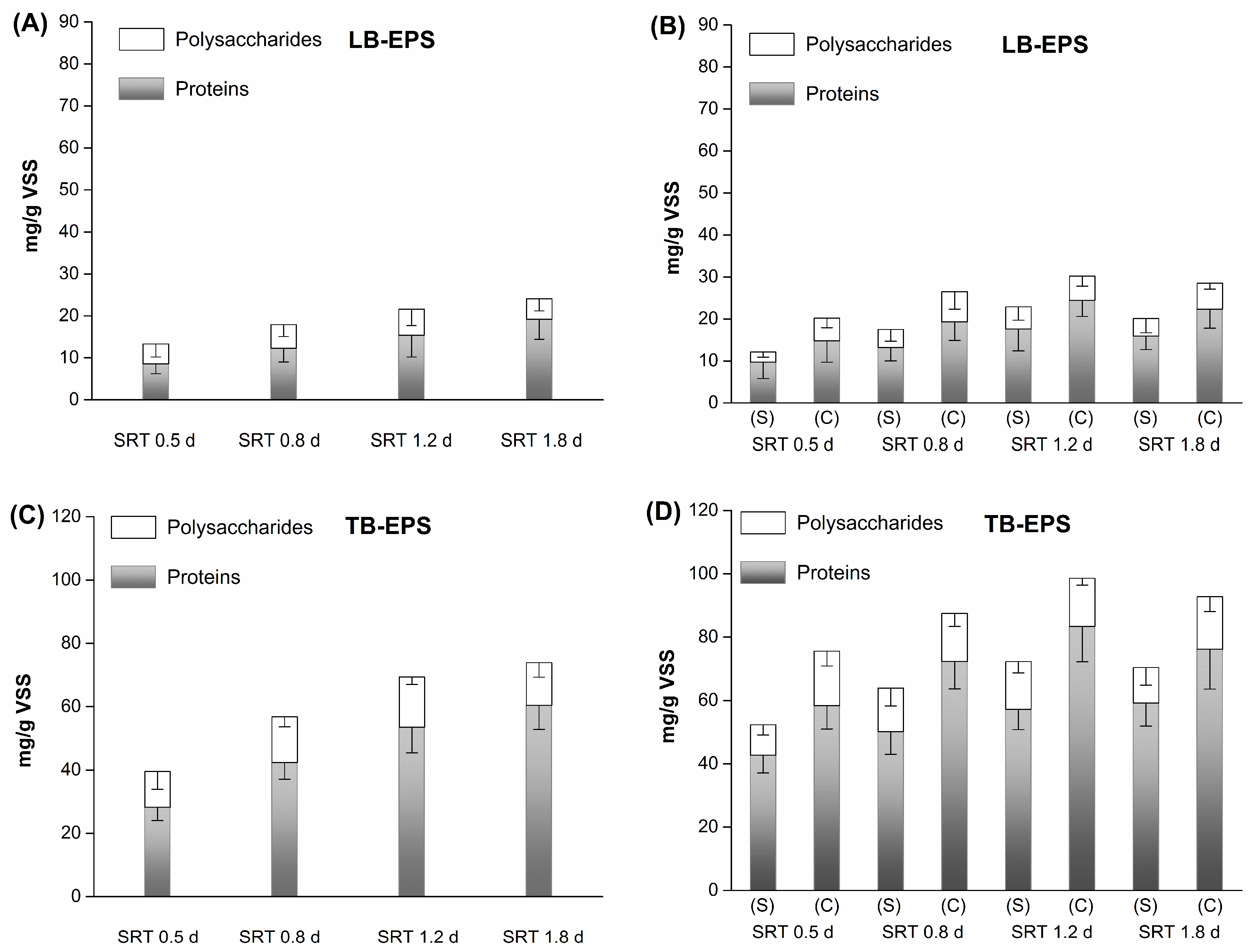
3.2. Bioflocculation Performance and EPS Composition
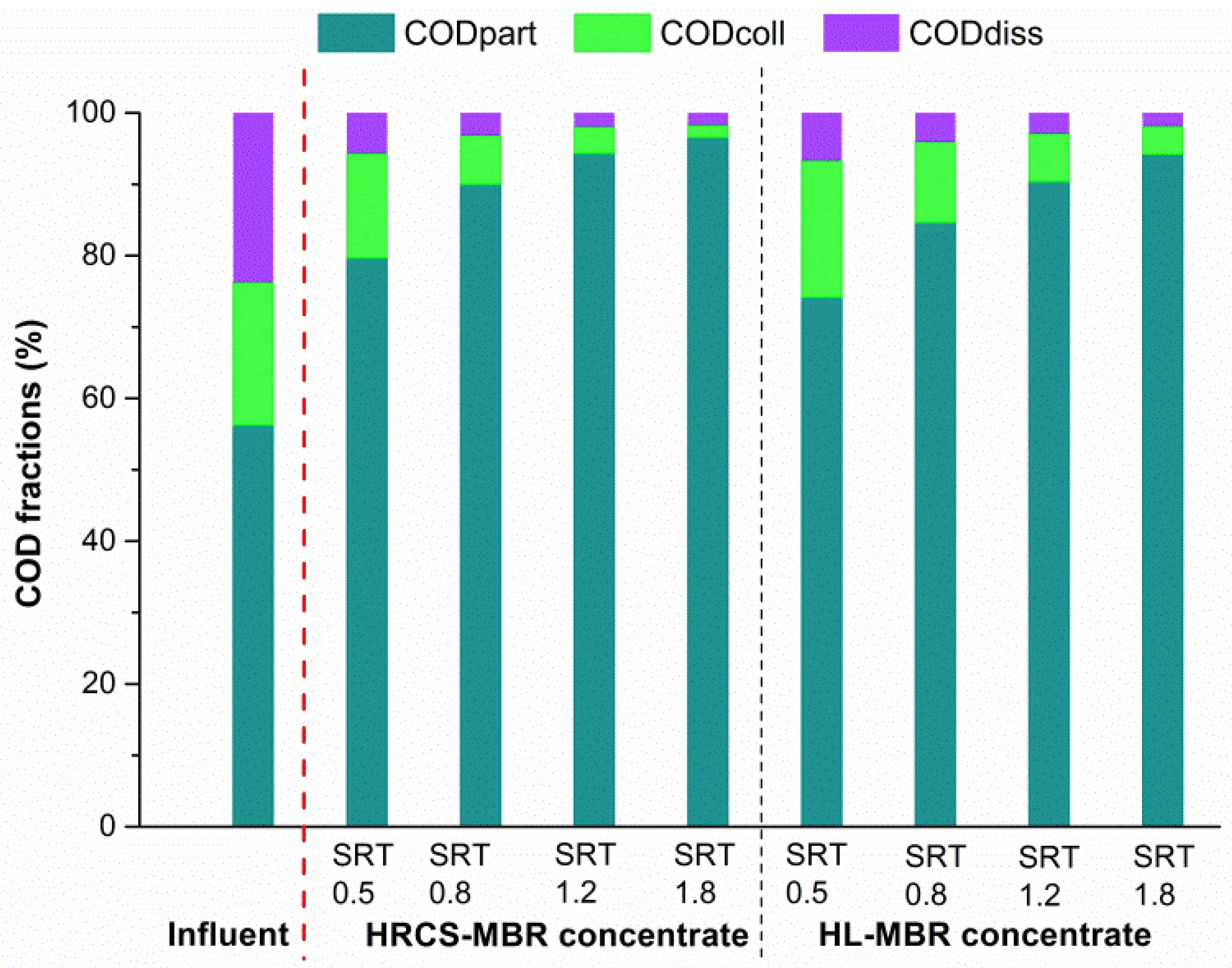
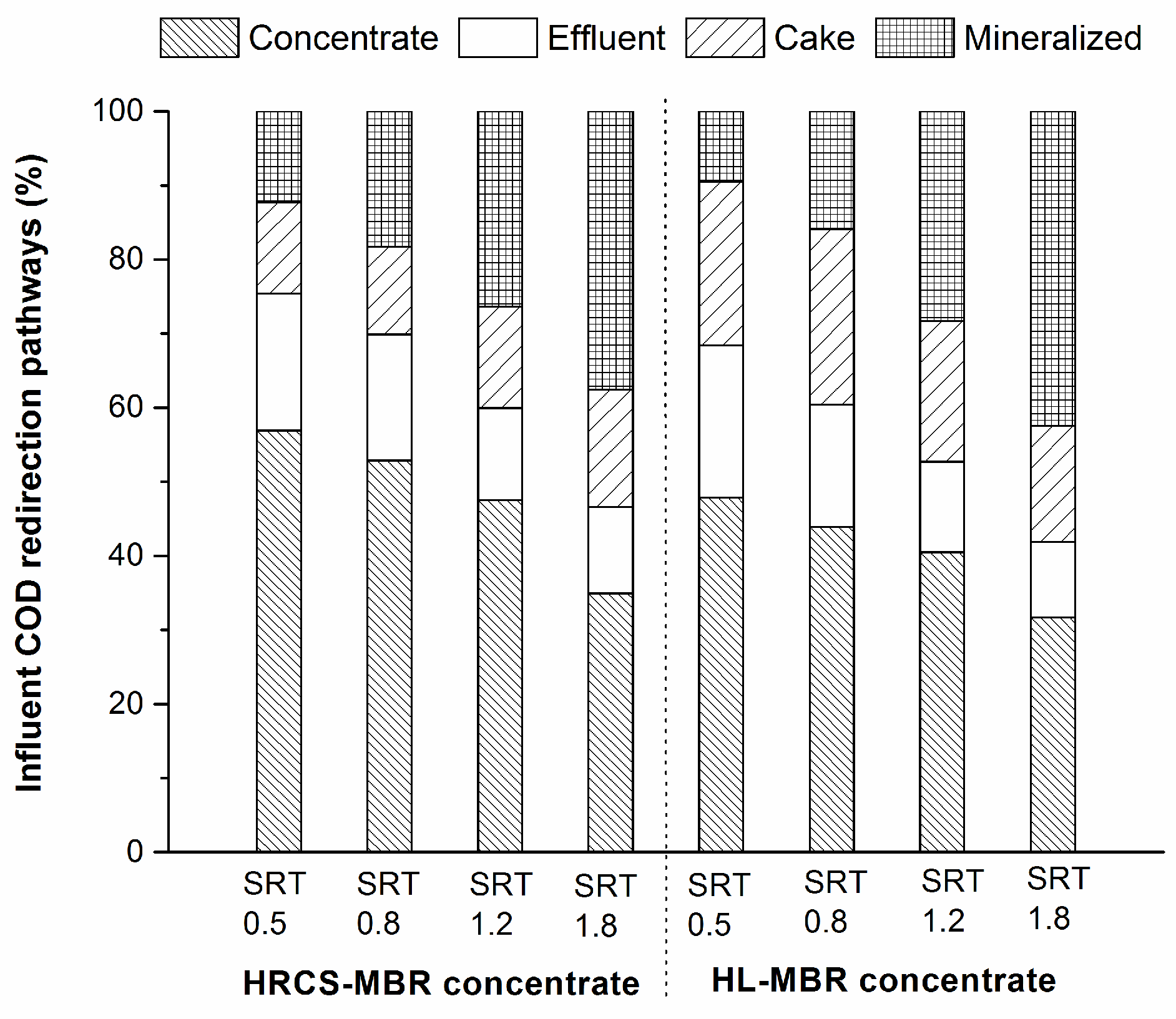
3.3. COD Mass-Balance and Energy Recovery
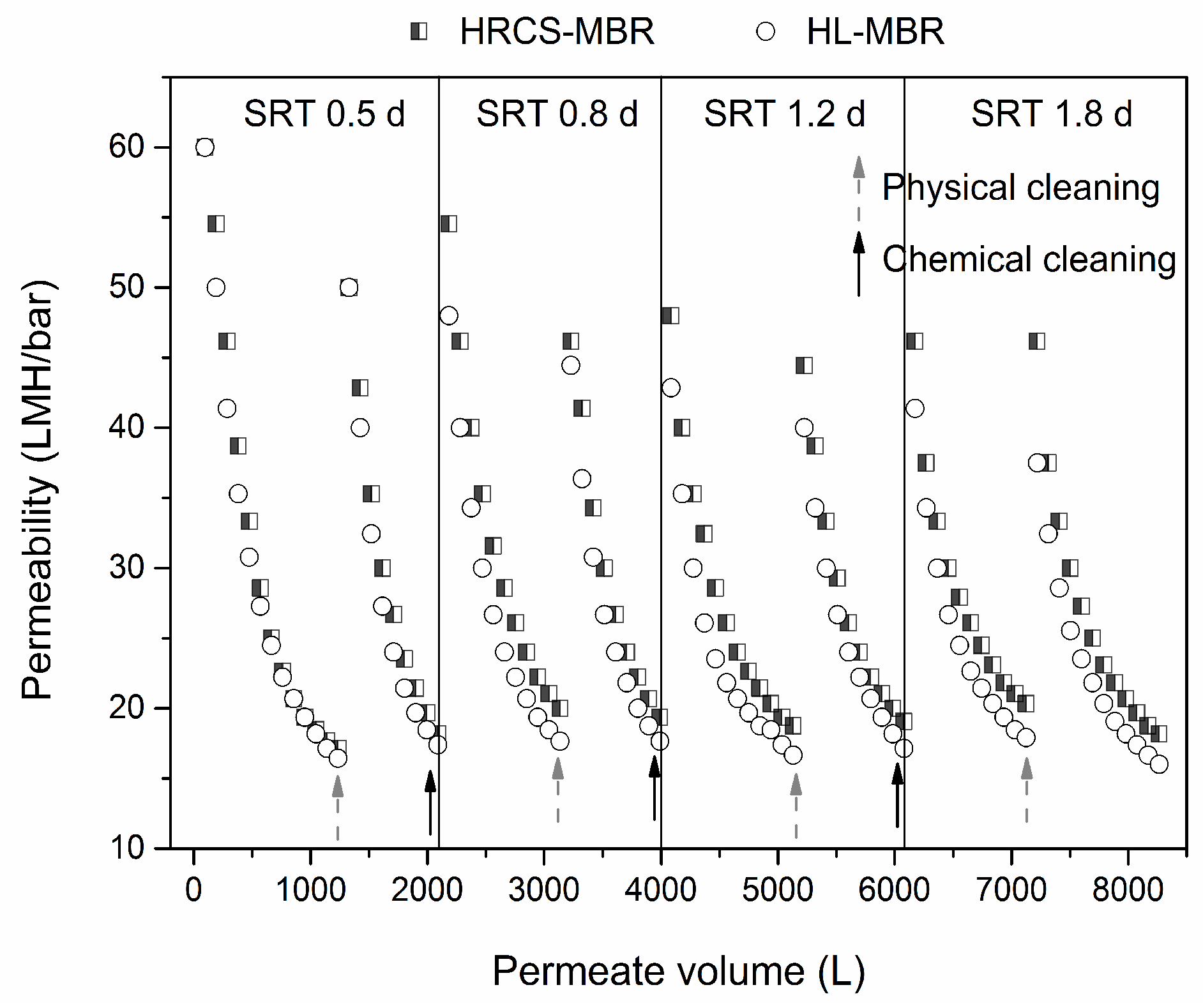
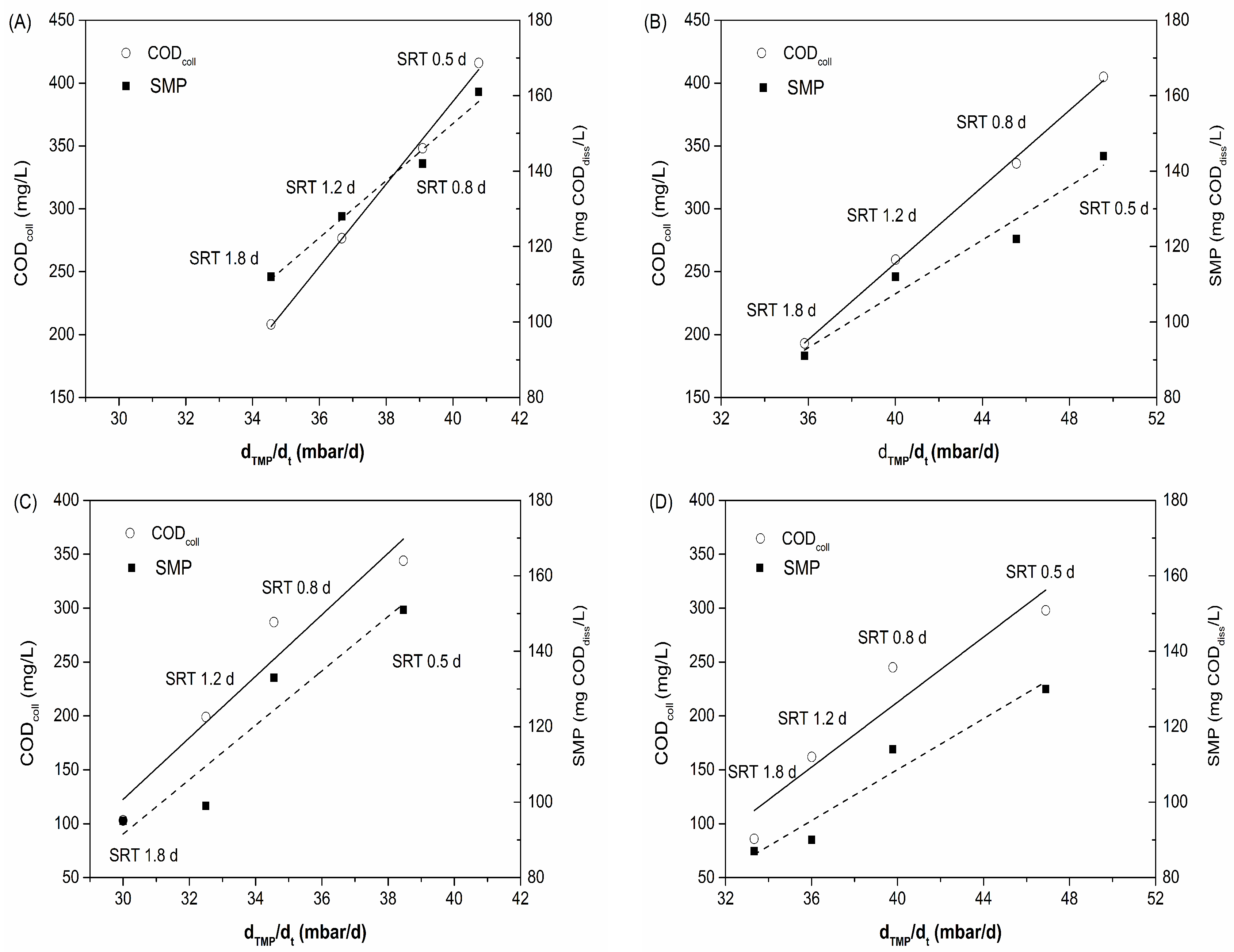
3.4. Membrane Fouling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shizas, I.; Bagley, D.M. Experimental Determination of Energy Content of Unknown Organics in Municipal Wastewater Streams. J. Energy Eng. 2004, 130, 45–53. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Curtis, T.P.; Dolfing, J. Determination of the internal chemical energy of wastewater. Environ. Sci. Technol. 2011, 45, 827–832. [Google Scholar] [CrossRef] [PubMed]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer—Can this be achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Buswell, A.M.; Long, H.L. Microbiology and Theory of Activated Sludge. Am. Water Works Assoc. 1923, 10, 309–321. [Google Scholar]
- Metcalf & Eddy Inc. Wastewater Engineering: Treatment and Reuse/Metcalf and Eddy; McGraw Hill Higher Education: New York, NY, USA, 2002. [Google Scholar]
- Jimenez, J.A.; La Motta, E.J.; Parker, D.S. Kinetics of removal of particulate chemical oxygen demand in the activated-sludge process. Water Environ. Res. 2005, 77, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Miller, M.; Bott, C.; Murthy, S.; De Clippeleir, H.; Wett, B. High-rate activated sludge system for carbon management—Evaluation of crucial process mechanisms and design parameters. Water Res. 2015, 87, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Akanyeti, I.; Temmink, H.; Remy, M.; Zwijnenburg, A. Feasibility of bioflocculation in a high-loaded membrane bioreactor for improved energy recovery from sewage. Water Sci. Technol. 2010, 61, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, W.; Van de Caveye, P.; Diamantis, V. Maximum use of resources present in domestic “used water”. Bioresour. Technol. 2009, 100, 5537–5545. [Google Scholar] [CrossRef] [PubMed]
- Cornel, P.; Meda, A.; Bieker, S. Wastewater as a Source of Energy, Nutrients, and Service Water. In Treatise Water Science; Elsevier: Amsterdam, The Netherlands, 2011; pp. 337–375. [Google Scholar]
- Hernandez Leal, L.; Temmink, H.; Zeeman, G.; Buisman, C.J. Bioflocculation of grey water for improved energy recovery within decentralized sanitation concepts. Bioresour. Technol. 2010, 101, 9065–9070. [Google Scholar] [CrossRef] [PubMed]
- Faust, L.; Temmink, H.; Zwijnenburg, A.; Kemperman, A.J.; Rijnaarts, H.H. High loaded MBRs for organic matter recovery from sewage: Effect of solids retention time on bioflocculation and on the role of extracellular polymers. Water Res. 2014, 56, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, S.C.; Irvine, R.L. Growth and control of filamentous microbes in activated sludge: An integrated hypothesis. Water Res. 1985, 19, 471–479. [Google Scholar] [CrossRef]
- Huang, J.C.; Li, L. An innovative approach to maximize primary treatment performance. Water Sci. Technol. 2000, 42, 209–222. [Google Scholar] [CrossRef]
- Meerburg, F.A.; Boon, N.; Van Winckel, T.; Vercamer, J.A.; Nopens, I.; Vlaeminck, S.E. Toward energy-neutral wastewater treatment: A high-rate contact stabilization process to maximally recover sewage organics. Bioresour. Technol. 2015, 179, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Mosquera, M.; Thomas, W.; Jimenez, J.A.; Bott, C.; Wett, B.; Al-Omari, A.; Murthy, S.; Riffat, R.; De Clippeleir, H. Impact of aerobic famine and feast condition on extracellular polymeric substance production in high-rate contact stabilization systems. Chem. Eng. J. 2017, 328, 74–86. [Google Scholar] [CrossRef]
- Ng, H.Y.; Hermanowicz, S.W. Membrane bioreactor operation at short solids retention times: Performance and biomass characteristics. Water Res. 2005, 39, 981–992. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 20th ed.; United Book Press: Gwynn Oak, MD, USA, 1998. [Google Scholar]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.W.; Forster, C.F.; Evison, L. A comparative study of the nature of biopolymers extracted from anaerobic and activated sludges. Water Res. 1990, 24, 743–750. [Google Scholar] [CrossRef]
- Hartree, E.F. Determination of protein: A modification of the Lowry method that gives a linear photometric response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, H.; Yang, F.; Zhang, S.; Li, Y.; Zhang, X. Identification of activated sludge properties affecting membrane fouling in submerged membrane bioreactors. Sep. Purif. Technol. 2006, 51, 95–103. [Google Scholar] [CrossRef]
- Ordóñez, R.; Hermosilla, D.; Pío, I.S.; Blanco, Á. Evaluation of MF and UF as pretreatments prior to RO applied to reclaim municipal wastewater for freshwater substitution in a paper mill: A practical experience. Chem. Eng. J. 2011, 166, 88–98. [Google Scholar] [CrossRef]
- Federation, W.E. Membrane Systems for Wastewater Treatment; WEF Press: Alexandria, VA, USA, 2005. [Google Scholar]
- Meerburg, F.A.; Boon, N.; Van Winckel, T.; Pauwels, K.T.; Vlaeminck, S.E. Live Fast, Die Young: Optimizing Retention Times in High-Rate Contact Stabilization for Maximal Recovery of Organics from Wastewater. Environ. Sci. Technol. 2016, 50, 9781–9790. [Google Scholar] [CrossRef] [PubMed]
- Chudoba, J.; Dohanyos, M.; Grau, P. Control of Activated Sludge Filamentous Bulking—IV. Effect of Sludge Regeneration; Iwa Publishing: London, UK, 1982. [Google Scholar]
- Rahman, A.; Meerburg, F.A.; Ravadagundhi, S.; Wett, B.; Jimenez, J.; Bott, C.; Al-Omari, A.; Riffat, R.; Murthy, S.; De Clippeleir, H. Bioflocculation management through high-rate contact-stabilization: A promising technology to recover organic carbon from low-strength wastewater. Water Res. 2016, 104, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Majone, M.; Dircks, K.; Beim, J.J. Aerobic storage under dynamic conditions in activated sludge processes. The state of the art. Water Sci. Technol. 1999, 39, 61–73. [Google Scholar] [CrossRef]
- Teksoy Başaran, S.; Aysel, M.; Kurt, H.; Ergal, İ.; Kumru, M.; Akarsubaşı, A.; Sözen, S.; Orhon, D. Removal of readily biodegradable substrate in super fast membrane bioreactor. J. Membr. Sci. 2012, 423–424, 477–486. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Orhon, D. The Mechanism and Design of the Contact Stabilization Activated Sludge Process; Advances in Water Pollution Research; University of California: Berkeley, CA, USA, 1973; pp. 353–365. [Google Scholar]
- Tsang, Y.F.; Chua, H.; Sin, S.N.; Tam, C.Y. A novel technology for bulking control in biological wastewater treatment plant for pulp and paper making industry. Biochem. Eng. J. 2006, 32, 127–134. [Google Scholar] [CrossRef]
- Sharghi, E.A.; Bonakdarpour, B. The study of organic removal efficiency and halophilic bacterial mixed liquor characteristics in a membrane bioreactor treating hypersaline produced water at varying organic loading rates. Bioresour. Technol. 2013, 149, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, L.H.; Nielsen, P.H. Quantification of the bond energy of bacteria attached to activated sludge floc surfaces. Water Sci. Technol. 2001, 43, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, J.C.; Liao, B.; Droppo, I.G.; Leppard, G.G.; Liss, S.N. The relationship between the structure of activated sludge flocs and the sorption of hydrophobic pollutants. Water Sci. Technol. 1998, 37, 353–357. [Google Scholar] [CrossRef]
- Liu, H.; Fang, H.H. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [CrossRef]
- Pretel, R.; Robles, A.; Ruano, M.V.; Seco, A.; Ferrer, J. The operating cost of an anaerobic membrane bioreactor (AnMBR) treating sulphate-rich urban wastewater. Sep. Purif. Technol. 2014, 126, 30–38. [Google Scholar] [CrossRef]
- Cho, B.D.; Fane, A.G. Fouling phenomena in a MBR: Transmembrane pressure transients and the role of EPS (extracellular polymeric substances). Water Sci. Technol. Water Supply 2003, 3, 261–266. [Google Scholar] [CrossRef]
- Ng, H.Y.; Hermanowicz, S.W. Specific resistance to filtration of biomass from membrane bioreactor reactor and activated sludge: Effects of exocellular polymeric substances and dispersed microorganisms. Water Environ. Res. 2005, 77, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, M.; Wang, F.; Meng, F.; Liao, B.-Q.; Hong, H.; Chen, J.; Gao, W. A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: Characteristics, roles in membrane fouling and control strategies. J. Membr. Sci. 2014, 460, 110–125. [Google Scholar] [CrossRef]
- Gao, W.J.J.; Lin, H.J.; Leung, K.T.; Liao, B.Q. Influence of elevated pH shocks on the performance of a submerged anaerobic membrane bioreactor. Process Biochem. 2010, 45, 1279–1287. [Google Scholar] [CrossRef]
- Laspidou, C.S.; Rittmann, B.E. A unified theory for extracellular polymeric substances, soluble microbial products, and active and inert biomass. Water Res. 2002, 36, 2711–2720. [Google Scholar] [CrossRef]
- Hwang, B.K.; Lee, W.N.; Park, P.K.; Lee, C.H.; Chang, I.S. Effect of membrane fouling reducer on cake structure and membrane permeability in membrane bioreactor. J. Membr. Sci. 2007, 288, 149–156. [Google Scholar] [CrossRef]
 indicates sampling points.
indicates sampling points.
 indicates sampling points.
indicates sampling points.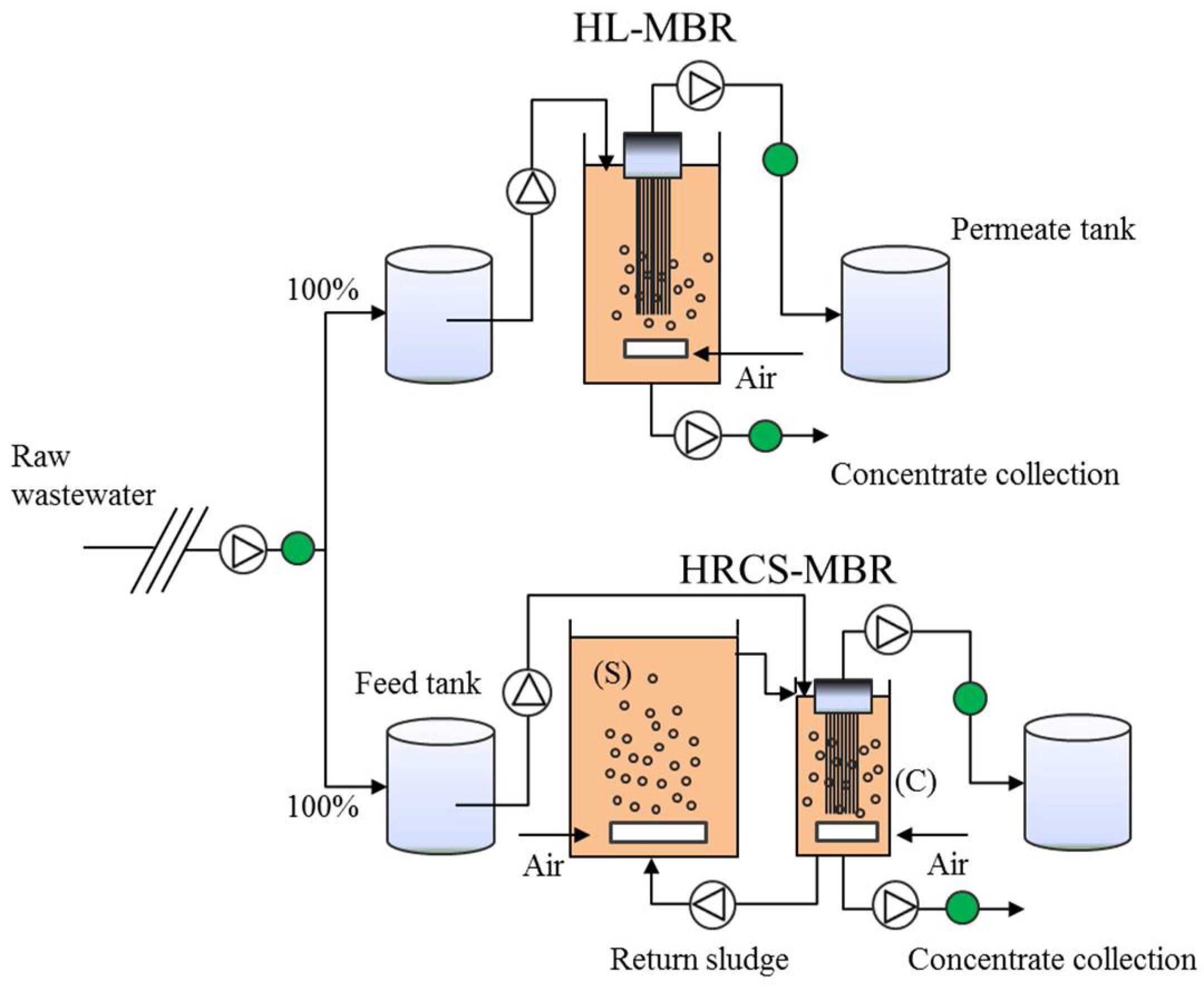
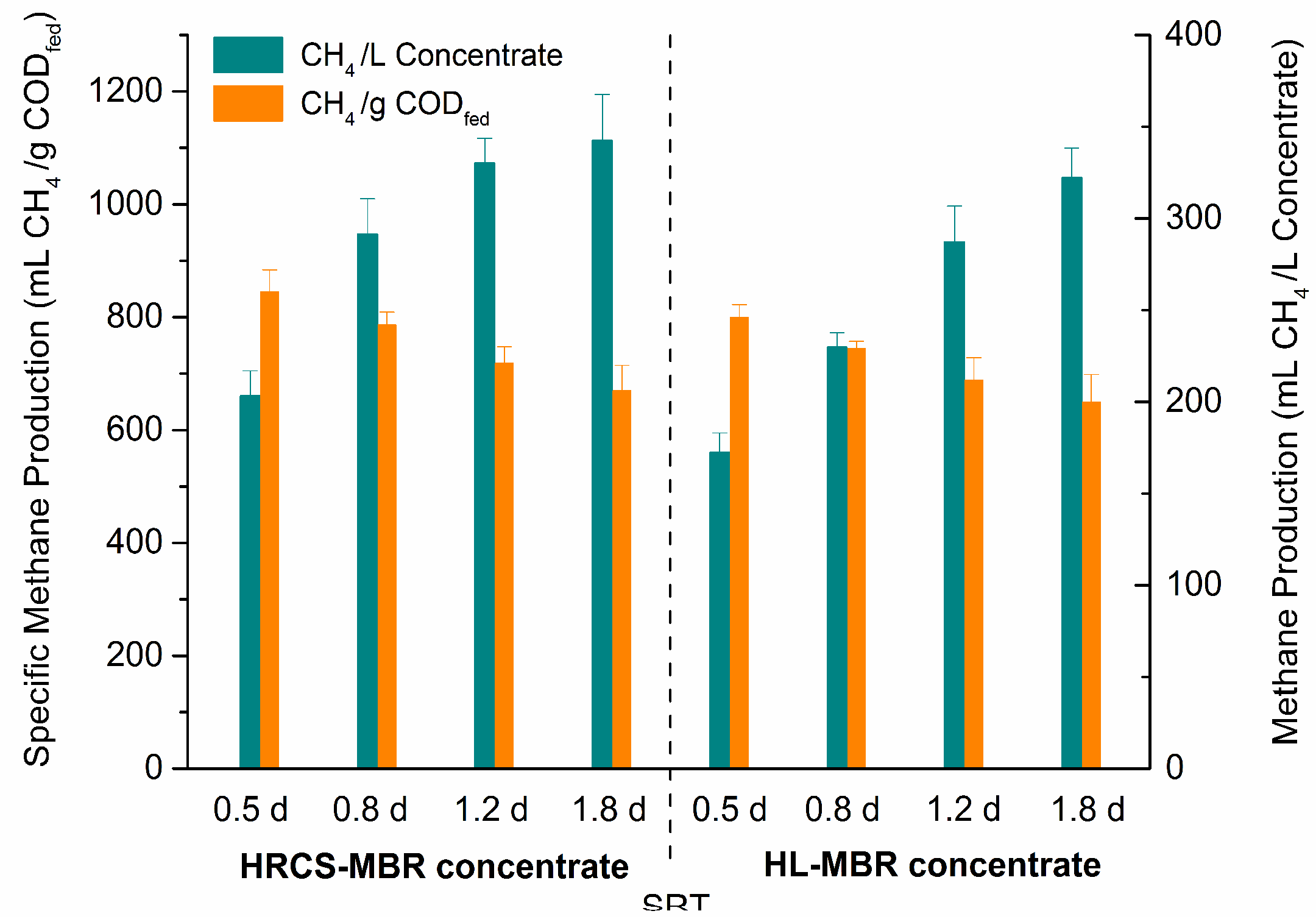
| Characteristic | Unit | Value | COD Fraction (%) |
|---|---|---|---|
| Total COD | mg/L | 441 ± 33 | 100 |
| Particulate COD | mg/L | 244 ± 16 | 55.1 |
| Colloidal COD | mg/L | 90 ± 16 | 20.4 |
| Dissolved COD | mg/L | 108 ± 2 | 24.5 |
| Total suspended solids | mg/L | 187 ± 54 | |
| Volatile suspended solids | mg/L | 164 ± 46 | |
| NH4+-N | mg/L | 41.3 ± 7.2 | |
| Total phosphorus | mg/L | 3.7 ± 1.2 |
| SRT (Day) | Reactor | MLVSS (mg/L) | VSS/SS | |
|---|---|---|---|---|
| HL-MBR | 0.5 | 612 ± 122 | 0.94 | |
| 0.8 | 764 ± 84 | 0.88 | ||
| 1.2 | 1391 ± 163 | 0.83 | ||
| 1.8 | 1622 ± 186 | 0.82 | ||
| HRCS-MBR | 0.5 | C | 652 ± 79 | 0.96 |
| S | 818 ± 126 | 0.92 | ||
| 0.8 | C | 862 ± 110 | 0.93 | |
| S | 1037 ± 84 | 0.88 | ||
| 1.2 | C | 1382 ± 142 | 0.91 | |
| S | 1696 ± 122 | 0.86 | ||
| 1.8 | C | 1526 ± 153 | 0.87 | |
| S | 1985 ± 136 | 0.84 |
| dTMP/dt | ||||
|---|---|---|---|---|
| rp | p | |||
| HL-MBR | Physical cleaning | CODcoll | 0.998 | 0.002 |
| SMP | 0.992 | 0.008 | ||
| Chemical cleaning | CODcoll | 0.999 | 0.001 | |
| SMP | 0.980 | 0.020 | ||
| HRCS-MBR | Physical cleaning | CODcoll | 0.969 | 0.031 |
| SMP | 0.953 | 0.047 | ||
| Chemical cleaning | CODcoll | 0.953 | 0.047 | |
| SMP | 0.972 | 0.028 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, W.; Xu, X.; Yang, F. High-Rate Contact Stabilization Process-Coupled Membrane Bioreactor for Maximal Recovery of Organics from Municipal Wastewater. Water 2018, 10, 878. https://doi.org/10.3390/w10070878
Dai W, Xu X, Yang F. High-Rate Contact Stabilization Process-Coupled Membrane Bioreactor for Maximal Recovery of Organics from Municipal Wastewater. Water. 2018; 10(7):878. https://doi.org/10.3390/w10070878
Chicago/Turabian StyleDai, Wenchen, Xiaochen Xu, and Fenglin Yang. 2018. "High-Rate Contact Stabilization Process-Coupled Membrane Bioreactor for Maximal Recovery of Organics from Municipal Wastewater" Water 10, no. 7: 878. https://doi.org/10.3390/w10070878
APA StyleDai, W., Xu, X., & Yang, F. (2018). High-Rate Contact Stabilization Process-Coupled Membrane Bioreactor for Maximal Recovery of Organics from Municipal Wastewater. Water, 10(7), 878. https://doi.org/10.3390/w10070878