Abstract
Peatlands play an essential role in the global carbon (C) and nitrogen (N) cycling. In order to ascertain the draining effects on recent accumulation rates of C (RERCA) and N (RERNA) in the Zoige peatland in the eastern Qinghai-Tibet Plateau, the core samples of peat growth, C and N accumulation for both natural and drained peatlands were measured using 210Pb and 137Cs dating methods. As a result, RERCA and RERNA showed an increasing trend from the bottom to the surface of the peatland, which was in accordance with the peat accumulation rates. However, the average RERCA in permanently flooded and seasonally flooded peatlands were 1.5–2.5 times that of drainage peatlands, and the average of RERNA were 1.2–1.7 times. Our findings indicate that the Zoige peatland is still in the stage of peat development with a large carbon sequestration capacity, and drainage from human activities leads to the decreasing of RERCA and RERNA, which will contribute to the selection of the effective ways to slow down the anthropogenic effects on the degradation of the Zoige peatland.
1. Introduction
Peatlands have accumulated thick horizons of organic matter (peat) and recode information about past vegetation dynamics, climate, and microbial process [1,2]. They provide important raw material, which is the foundation of the hydrological reconstructions that provide an important insight into climatic changes during the Holocene [3,4]. Besides, peatlands cover only 3% or so of the global land area, but hold about 30% of the global terrestrial organic carbon (C) pool [5]. Due to primary production exceeding decomposition and other losses, peatlands accumulate C and their C stock continues to increase. Peatlands store C at a disproportionately greater rate than upland ecosystems [6,7]. Therefore, understanding the rate of C accumulation in peatlands is crucial to the global C cycle [7]. C and nitrogen (N) cycling are tightly linked [8]. Peatlands also play an essential role in N cycling [5].
Mire ecosystems have suffered degradation mainly due to the drainage resulted from human activities, which hindered C and N accumulation, disturbed the biogeochemical processes, and disordered ecosystem functions of peatlands [9,10]. In addition, vegetation [11], hydrology, microbial activity [12], and climate also have a significant impact on the development of peatlands. Therefore, research concerning peatlands is of great significance to their conservation and development.
The Zoige alpine peatland in eastern Qinghai-Tibet Plateau in China, covering an area of 4600 km2 with elevations of 3400–3900 m above sea level, is the largest plateau wetland in the world [13,14]. It stores 6.3 × 108 t soil C, and it plays an important role in regulating the climate [15,16]. However, a large area of Zoige Plateau peatland was drained for grazing in the 1970s. The ground water levels is reducing, which exposed peat C to the atmosphere, accelerated the decomposition of organic matter, and influenced C sedimentation and N accumulation [14,17,18]. The microtopography of those peatlands has been altered by drainage. Consequently, the peatlands here have beene severely degraded, drawing widespread attention of eclologists to the studies on peatlands in the Zoige for ecosystem restoration. Lots of previous studies in the Zoige focused on CH4 fluxes [14], soil C stocks [19,20], and measures of ecosystem restoration [21]. However, the peat deposition, C and N accumulation of natural and drained peatlands in the Zoige are not well documented yet. It remains unclear how water table level affects peat deposition and C and N accumulation in this area.
In this study, first, we made the hypothesis that water table level had an impact on C and N accumulation. Then, three flooded peat plots (one permanently flooded and two seasonally flooded) under natural condition and two drained peat plots under human interference were selected. Finally, we aim to provide a scientific support for the selection of effective ways to slow down the anthropogenic effects on the degradation of the Zoige peatland.
2. Materials and Methods
2.1. Site Description
The study area (33°55′ N, 102°46′ E; 3430 m above sea level) was located in the Zoige Wetland Ecosystem Research Station on the eastern Qinghai-Tibet Plateau of China (Figure 1). The area is characterized by cool temperatures and a continental monsoon. The mean annual precipitation is about 656.8 mm, with 86% between April and October, and the mean annual temperature is about 0.7–1.1 °C. The minimum and maximum mean monthly temperature was −10.6 °C in January and 10.8 °C in July [13,14].
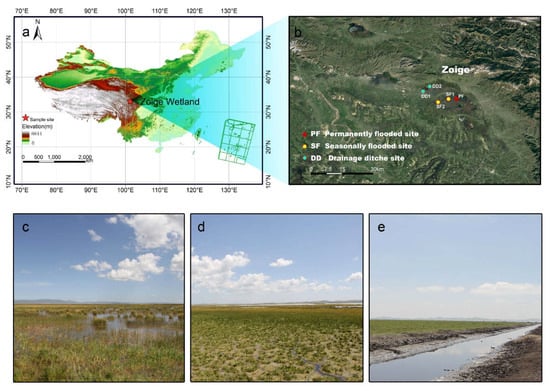
Figure 1.
The location of (a) the study area, (b) sampling sites in the Zoige alpine peatland, (c) permanently flooded peatland, (d) seasonally flooded peatland, and (e) drained peatland.
A natural site and a drained site in the Zoige Plateau wetland were selected in August 2015. The two sites are near Huahu Lake, and their soils have similar characteristics. The natural site contained a permanently flooded peatland with an average water table depth of 0.21 m and a seasonally flooded peatland with an average water table depth of −0.15 m. Due to water supplies from the Huahu lake, the dominant plant species were Carex muliensis and Potamogeton pusillus in the permanently flooded peatland, and were Kobresia tibetica and Carex muliensis in the seasonally flooded peatland. Drained peatland were excavated for grazing in the 1970s [17,18], and the ditches are now 1–1.5 m deep and 3–7 m wide, with an average water table depth of −0.91 m. The dominant species of drained peatland were Kobresia humilis, Potentilla anserina, and Glaux maritime. The ditches had been blocked at 100 m intervals to carry out the wetland restoration projects in 2008, leading to a rise in the water table level. However, the dams were destroyed by a flood during the rainy season in 2010, and then the water table dropped again [14].
2.2. Core Sampling
According to natural conditions and human activities in this peatland, a permanently flooded peatland plot (PF), two seasonally flooded peatland plots (SF1 and SF2), and two drainage ditch peatland plots (DD1 and DD2) in the Zoige Plateau (Figure 1) were selected for sampling. Five peat cores with size of 10 × 10 × H cm3 of each (H is the depth of a core) were taken from each of peatlands using a titanium Wardenaar peat profile sampler (Eijkelkamp, Giesbeek, Netherlands). Peat sub-samples were sectioned on-site at 1-cm intervals with a stainless steel band saw and were then stored in polyethylene plastic bags under refrigerated condition of 4 °C until laboratory analysis was conducted.
2.3. Physicochemical Analysis
Dry bulk density (DBD), organic carbon (OC), and total nitrogen (TN) analyses were performed at the Laboratory of Institute of Wetland Research, Chinese Academy of Forestry. Soils were dried in a convection oven at 65 °C until a constant mass was obtained. DBD (g cm−3) was calculated from the oven dried weight and the known sample volume. Samples containing carbonates were treated with 0.1 mol L−1 HCl to remove carbonates prior to carbon analysis so measured values represent OC. OC was analyzed using a Perkin-Elmer 2400 CHN analyzer (PerkinElmer, Waltham, MA, USA) and TN was analyzed using a SmartChem200 analyzer (WestCo, Frepillon, France). OC and TN were used to estimate the recent rates of carbon accumulation (RERCA) and the recent rates of nitrogen accumulation (RERNA).
2.4. Dating and Estimating RERCA and RERNA
The process of 210Pb with a half-life of 22.3 years and 137Cs with a half-life of 30.2 years dating was performed at the State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences. These analyses were carried out by direct gamma assay using an ORTEC high-purity Ge series well-type coaxial low-background detector (ORTEC Instruments Ltd., Berwyn, USA). Counting times of 210Pb and 137Cs were typically in the range 50,000–86,000 s, giving a measurement precision of between ca. ±5% and ±10% at the 95% level of confidence, respectively. Core chronologies were calculated using the constant rate of supply model (CRS) [22]. Peat accumulation rate was calculated based on 210Pb-inferred chronology and the 137Cs time marker. The RERCA and RERNA were calculated for each peat profile using the known depth, DBD, OC, TN, and peat age [23]. Thus, these values as functions were determined using the following formulae:
where T is the age of layer at depth L (cm), AT and A0 refer to the inventory of unsupported 210Pb at depth L (cm) and the total inventory of unsupported 210Pb in the core section (both are calculated by direct numerical integration), λ is the 210Pb decay constant (0.0311 yr−1), SL (cm yr−1) represent peat accumulation rate at depth L (cm), DBD (g cm−3) is the dry bulk density, OC (%) and TN (%) are the organic carbon content and the total nitrogen content, and RERCA (g C m−2 yr−1) and RERNA (g N m−2 yr−1) denote the recent rate of C and N accumulation.
2.5. Data Analysis
SPSS 22.0 was used for the calculation of average, SD, and multiple comparisons. One-way ANOVA was adopted to analyze the differences among the peat samples under natural conditions and human interference. Significant differences were calculated on the basis of the T-test, considering a significance level of p < 0.05. The dynamic changes of the specific activity of 210Pb and 137Cs, DBD, OC, TN, and peat accumulation rate, RERCA and RERNA with the depths were illustrated by Sigma-Plot 12.5.
3. Results
3.1. Peat Properties
It was obvious that the dry bulk densities (DBD) of five peat sites increased with the increase of depth (Figure 2a), averaging 0.43–0.62 g cm−3 (Table 1). DBD was significantly higher in DD1 than the others, and that in PF was the lowest (p < 0.05). Across all peatland samples, the content of organic carbon (OC) and total nitrogen (TN) presented a decreasing tendency (Figure 2b,c). The average of OC ranged from 25.91% to 56.10%, with the maximum value in PF (56.10%) and the minimum value in DD1 (25.91%). Similarly, in terms of the average of TN, PF showed the highest value of 2.25% and DD1 (1.41%) was the lowest with the same case as OC, averaging 1.41–2.25%. It can be seen from Figure 2d that C:N of PF increased slightly, while other peat samples did not show the obvious increasing or decreasing tendency. Across all of the samples, C:N of PF were significantly higher than that of others (p < 0.05). On the whole, C:N of five peatlands was above or equal to 20 with an average of 17.64 (SF2)–25.86 (PF) (Table 1).
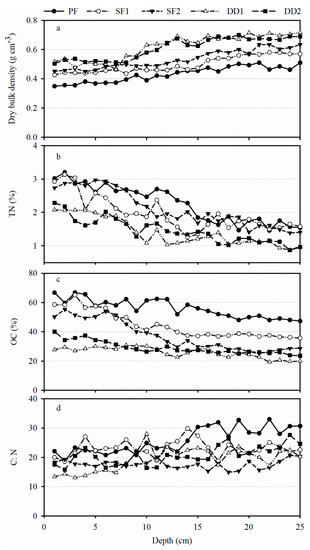
Figure 2.
(a) Dry bulk density (g cm−3), (b) total nitrogen (TN) (%), (c) organic carbon (OC) (%), and (d) C:N in the top 25 cm averaged in all samples. Note: PF represents a permanently flooded peatland; SF1 and SF2 represent two seasonally flooded peatlands; and, DD1 and DD2 represent two drainage peatlands. The same as below.

Table 1.
Peat bulk density, organic carbon, and total nitrogen (mean ± SD) in the five peat sites from the Zoige alpine peatland.
3.2. Age Dating
The specific radioactivity of 210Pb and 137Cs in five peat samples was shown in Figure 3. The specific radioactivity of 210Pb in PF, SF1, SF2, DD1, and DD2 reached the decay balance at a depth of 19 cm, 19 cm, 17 cm, 13 cm, and 13 cm, respectively. This research adopted the upper profile data to calculate the age of sedimentation and sediment flux of each layer. PF, SF1, SF2, DD1, and DD2 at 19 cm, 19 cm, 17 cm, 13 cm, and 13 cm were dating back to the year 1835, 1833, 1847, 1839, and 1837, respectively (Figure 4). The vertical profile distribution of 137Cs activity was shown in Figure 3. The peak of 137Cs in PF, SF1, SF2, DD1 and DD2 were at 4 cm, 5 cm, 4 cm, 4 cm, and 3 cm, respectively, and the corresponding year were in 1988, 1982, 1990, 1984, and 1993 by 210Pb dating (Figure 4). The sedimentation peak that resulted from the global nuclear test was about in 1963, with a large deviation. Therefore, we must consider the reliability that the 137Cs dating applied to the chronosequence in the peat profile.
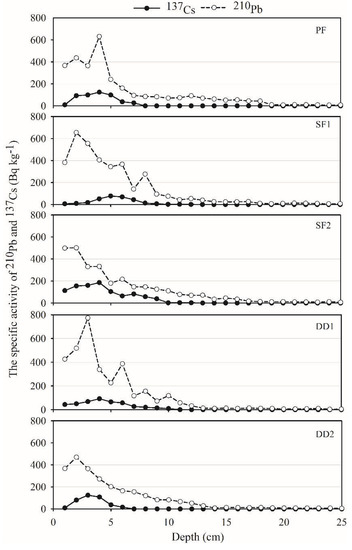
Figure 3.
The specific activity of 210Pb and 137Cs (Bq kg−1).
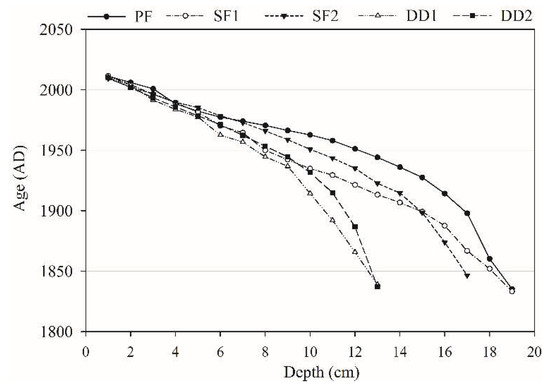
Figure 4.
Soil depth-age relation.
3.3. Peat Accumulation
According to the dating results, the average rate of peat growth of PF, SF1, SF2, DD1, and DD2 were 0.17 (0.09) cm yr−1 since 1835, 0.13 (0.06) cm yr−1 since 1833, 0.13 (0.05) cm yr−1 since 1847, 0.10 (0.06) cm yr−1 since 1839, 0.11 (0.05) cm yr−1 since 1837, respectively (Figure 5a). On the basis of overall trend, the peat deposition rate increased from the bottom to the surface, especially in recent years, the deposition rate of PF, SF1, and SF2 increased more obviously, while the DD1 and DD2 rate increased in a smaller range (Figure 5a).
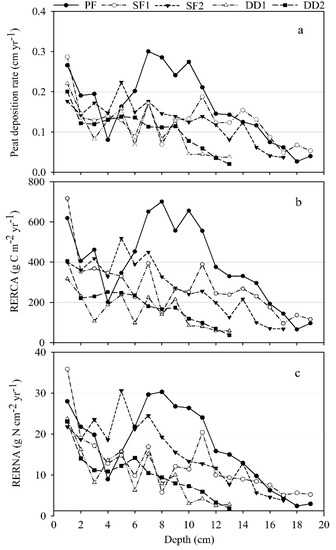
Figure 5.
(a) Peat accumulation rate (cm yr−1), (b) RERCA (g C cm−2 yr−1) and (c) RERNA (g N cm−2 yr−1) with depths.
3.4. Recent Rates of C and N Accumulation
The change and mean level of recent rates of C accumulation (RERCA) and N accumulation (RERNA) are calculated according to the peat deposition rate under different water table levels. The average RERCA of PF, SF1, SF2, DD1 and DD2 were392 (197) g C m−2 yr−1, 279 (140) g C m−2 yr−1, 279 (137) g C m−2 yr−1, 157 (83) g C m−2 yr−1, and 187 (96) g C m−2 yr−1, respectively. That of PF was significantly higher than DD1 and DD2 (p < 0.05) (Figure 5b). The average RERNA of above samples were 17.0 (9.3) g N m−2 yr−1, 12.4 (7.4) g N m−2 yr−1, 15.7 (7.6) g N m−2 yr−1, 9.9 (6.5) g N m−2 yr−1, and 10.1 (5.4) g N m−2 yr−1, respectively (Figure 5c). From the overall variation trend, RERCA and RERNA in peatlands were in accordance with the peat accumulation rate, which showed an increasing trend from the bottom to the surface. In recent decades, both of them increased obviously in PF, SF1, SF2, however, DD1 and DD2 increased slowly.
4. Discussion
4.1. OC and TN in the Zoige Peatland
The variation of DBD was opposite to the content of OC and TN in all of the samples. Flooded peatlands (permanently or seasonally) had lower DBD, but higher OC and TN, whereas drainage ditches in the drained peatlands showed lower OC and TN, but higher DBD. Drainage could lead to the formation of aerobic environment, which accelerated the deposition rate [12]. As a result, OC and TN content were lower in drained peatlands. In this study, the average of OC and TN were similar to the free-floating peatlands in Central Italy (OC%: 35–50%; TN%: 0.6–2.9%) [24] and peatlands of Isla Grande de Chiloé-Chile (TN%: 0.74–2.28%) [7]. Regarded as an important indicator of decomposition of organic matter, C:N of flooded peatlands in this study were similar to the Czech Republic, midwestern and southeastern U.S. (equal to 20) [25]. It thus appeared that the water table level impacted bulk density and the content of carbon and nitrogen.
4.2. Radioisotope Chronology and Peat Deposition
Some scholars have pointed out that it was difficult to use 137Cs for the sediments with a deposition rate <1 cm yr−1 [26]. At the same time, the reliability of 137Cs dating relied on its stability in the sediments. In the peat swamp rich in organic matter, 137Cs was easily affected by pore water and plant uptake, and then migrated [27,28]. In this peat profile, the estimated peat deposition rate was less than l cm yr−1, and the peat profile belonged to the seasonally peat swamp, where soil was rich in organic matter. Therefore, we did not adopt 137Cs dating in this study.
From Figure 5a, it can be seen that the peat accumulation rate among peat samples varied obviously due to the effect of water table level. The peat deposition rate of PF, SF1, SF2 were 59%, 21%, 24% higher than DD1, respectively, and that of PF, SF1, SF2 were 56%, 19%, 22% higher than DD2. As a result, peat accumulation of permanently flooded and seasonally flooded area were higher than that of the drainage. Therefore, ditch drainage would lead to the decrease of peat accumulation capacity and a loss of C through aerobic decomposition and change the properties of the peatlands in this area. Because of large-scale drainage of mires in Estonia between the 1950s and the 1980s, the total area of the country’s open transitional mires decreased from 76,200 ha in the 1950s [29] to 35,000 ha, according to the recent mire inventory [30].
The values of peat accumulation rates of relevant studies worldwide are exhibited in Table 2. The range of peat accumulation rate in this study was similar to the values of 0.22 cm yr−1 in five Sphagnum-dominated peatlands of US that was found by Wieder and co-workers with the 210Pb dating method [31]. The average rate of our results was lower than that of other studies in Table 2, such as 2.52 cm yr−1 in Southwest Florida [32], 1.11 cm yr−1 in Yancheng, Jiangsu province, China [33] and 0.43 cm yr−1 in the paramo ecoregion of northeastern Ecuador [34]. Therefore, the Zoige Plateau peatland has a potential capacity to sequester peat.

Table 2.
Comparison with relevant studies worldwide in peat accumulation rate, RERCA and RERNA.
4.3. RERCA and RERNA in the Zoige Peatland
The carbon and nitrogen accumulation rate in peatlands was influenced by many factors, such as climatic hydrological conditions, geographical location, peatland development, peatland type, and surface disturbance. Relevant studies on RERCA and RERNA around the world were presented in Table 2 to ensure the reliability of the results in this study. Our results were similar to the range of RERCA with a value of 124–293 g C m−2 yr−1 via 210Pb dating method in the Changbai Mountains [23], 224 g C m−2 yr−1 via 14C method in the paramo ecoregion of northeastern Ecuador [34], 129–204 g C m−2 yr−1 via 137Cs and 210Pb method in northeast China [36], 173–328 g C m−2 yr−1 in estuarine emergent peatland, and 141–372 g C m−2 yr−1 in palustrine forested peatland in northern Gulf of Mexico via 137Cs method [37], and 306 g C m−2 yr−1 via 137Cs and 210Pb method in slow flow rainforest swamp in northeastern Costa Rica, as reported [38]. However, the values of RERCA of several studies in Table 2 were lower than our results. The average of RERCA (259 g C m−2 yr−1) in this study was 7.4 times of that in Isla Grande de Chiloé [7], 2.6 times of that in mangrove swamps in southwest Florida [32], 3.3 times of that estuarine forested peatland in northern Gulf of Mexico [37], and 3.07 times of that in depressional isolated rainforest and seasonal riverine wetland and 6.1 times of that in seasonal riverine floodplain in northeastern Costa Rica [38]. As for RERNA, the average value (13.0 g N m−2 yr−1) in this study was higher than that of studies that are shown in Table 2, with 14.8 times of RERNA value in Isla Grande de Chiloé [7], 5.7 times of that in eastern Canada [39], and 4.6 times of that Yancheng, Jiangsu province, China [33]. According to the results compared with other studies, it can be found that the Zoige Plateau peatland is still in the stage of development with a larger carbon sequestration capacity, and its vital function on the climate regulation shouldn’t be overlooked.
4.4. Water Table Level and Drainage Impacts
From the results of peat accumulation, RERCA and RERNA changes in five peat samples, it can be seen that all of them varied obviously due to the effect of water table level. The average RERCA of PF, SF1, SF2 were 2.5 times, 1.8 times, 1.8 times of DD1, and 2.1 times, 1.5 times, and 1.5 times of DD2. According to the RERCA in peatlands, carbon reserves in nearly 150 years of PF, SF1, SF2, DD1, and DD2 were, respectively, obtained: 58.74 (29.52) kg C m−2, 41.85 (21.08) kg C m−2, 41.79 (20.54) kg C m−2, 23.57 (12.50) kg C m−2, 28.07 (14.37) kg C m−2. RERNA of PF, SF1, SF2 were 1.7 times, 1.3 times, 1.6 times of DD1 and 1.7 times, 1.2 times, and 1.5 times of DD2. On the basis of RERNA in peatlands, nitrogen reserves in nearly 150 years of PF, SF1, SF2, DD1, and DD2 were respectively obtained: 2.55 (1.40) kg N m−2, 1.86 (1.11) kg N m−2, 2.36 (1.14) kg N m−2, 1.49 (0.98) kg N m−2, 1.52 (0.81) kg N m−2. As a consequence, compared with drainage, high water table level has a positive effect on RERCA and RERNA.
Water table, along with site differences such as peat bulk density, organic matter content, moisture content and site identity [3,21], can influence physicochemical peat properties and is positively associated with peat growth rate, explaining 44% of the variation. Some studies have shown that wetland carbon accumulation mainly relied on the balance between high plant growth and low decomposition of organic matter, which was mainly dependent upon water level [41,42]. On one hand, a clear difference was found in the dominant species among the core samples due to the water table level in this study, which would have contributed to variation in the rates of peat (carbon) accumulation among the sites [11]. A high water level will slow the decay rate under anoxic conditions due to weak respiration pulses and hold high rates of RERCA and RERNA [36]. Abundant rains play a vital role in keeping biomass productive in the ecosystem, but the lack of standing water for a long time likely to lead organic matter oxidizing and plant debris decomposing very quickly [38]. With the exception of climatic effect, human activity can influence vegetation change and water table level via ditch drainage and other mechanisms, and these factors, in turn, alter moisture availability and community composition, the primary drivers of peat production and decay rates [41]. What is more, vegetation can provide continual inputs of organic material for peatland growth and carbon accumulation, and it was reported to be indicative of wetland degradation [20,43]. On the other hand, high water table levels can lead to the formation of anaerobic environment, which decreases the peat microbial activities and biodiversity [12]. Consequently, the decomposition rate of litter and organic matters are greatly reduced. On the contrary, drainage could likely lead to turn the peatland environment from anaerobic to aerobic, and then increased the aerobic microbial activities, which made the residue decomposition accelerate [21]. At the depth of 6–12 cm, higher rates were observed in peat accumulation, RERCA and RERNA in peatlands under natural condition. The reason was that the growing season was prolonged by climate warming, which might contribute to peat C accumulation by promoting net primary production [44,45]. However, due to ditching drainage for grazing in the 1970s, water table depth began to decline, and the microenvironment of this area changed. The quantity and composition of vegetation have been greatly influenced [17,18]. As a result, the inputs of plant growth largely decreased. Bagchi and Ritchie pointed out that indirect impacts on vegetation composition are equally important in influencing soil C sequestration in grazing ecosystems [46].
It can be seen that drainage has an adverse impact on peat, carbon, and nitrogen accumulation. In this study, drainage peatlands hold a higher bulk density, but smaller peat accumulation rate, RERCA and RERNA due to the lower water table level. In other words, the microtopography of drainage peatlands has been altered, leading to a serious degradation of these peatlands. Bernal and Mitsch reported wetland soils shrink as they dry and swell as they become moist [38]. Stivrins et al. pointed out that drainage explains a substantial part (20%) of the variation in peat growth rate [3]. Anderson reported that the slowdown of peat deposition was attributed to the drier conditions [47]. Peat drainage resulted from anthropogenic disturbances did not directly damage or trigger significant changes in species composition on the peat, release peat carbon to the atmosphere [35], and crucially impact C accumulation in peatlands [48], and consequently affect the C balance of the Tăul Muced bog [5]. In addition, drainage caused the mean CH4 emission fluxes in the three drained plots to be 99.3% significantly lower than in the four natural plots, presumably because the water table level was lower [14]. Therefore, ditch drainage would lead to the decrease of carbon and nitrogen sequestration capacity, and it changed the stability of the organic carbon storage in the peatlands in this area.
5. Conclusions
It was found that peat accumulation rate and RERCA and RERNA of permanently flooded peatlands and seasonally flooded peatlands under natural condition were higher than that of drainage peatlands under human interferences due to different water table level. From the results of comparison with relevant published studies, it can be seen that the Zoige peatland is still in the stage of peat development with a large carbon sequestration capacity, and its vital function on the climate regulation shouldn’t be overlooked. However, ditch drainage that resulted from human activities often seriously decreased the water table level and made further influence on the carbon accumulation capacity even the climate in the Zoige peatland. Therefore, effective approaches should be put forward to regulate and manage human activities, and to maintain the balance in carbon accumulation, hydrological condition, vegetation composition, productivity, and biodiversity of this peatland ecosystem. Some researches about alterations of temperature and precipitation affecting the hydrological condition of peatlands needed to be conducted. Because of the complexity of peatland ecosystem and the diversity of human activities, continuous researches on the carbon and nitrogen accumulation rate in peatlands are needed to ensure the sustainable carbon sequestration capacity.
Author Contributions
Chunyi Li and Lijuan Cui conceived and designed the experiments; Chunyi Li performed the experiments; Huanhuan Guo and Yilan Huang analyzed the data; Wei Li contributed materials and analysis tools; Chunyi Li and Lijuan Cui wrote the paper; Chunyi Li and Huanhuan Guo revised the manuscript.
Funding
This research was supported by the Fundamental Research Funds for the Central Non-Profit Research Institution of Chinese Academy of Forestry (CAFYBB2014QA030) and (CAFINT2015C12).
Acknowledgments
The authors thank Xiaodong Zhang, Xiaoming Kang, Xu Pan and Yifei Wang from Sichuan Zoige Wetland Ecosystem Research Station for their assistance with the field work. They also thank Professor Tianshan Zha from Beijing Forestry University for polishing the English text of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hribljan, J.A.; Cooper, D.J.; Sueltenfuss, J.; Wolf, E.C.; Heckman, K.A.; Lilleskov, E.A.; Chimner, R.A. Carbon storage and long-term rate of accumulation in high-altitude Andean peatlands of Bolivia. Mires Peat 2015, 15, 1–14. [Google Scholar]
- Hobbie, E.A.; Chen, J.; Hanson, P.J.; Iversen, C.M.; McFarlane, K.J.; Thorp, N.R.; Hofmockel, K.S. Long-term Carbon and Nitrogen Dynamics at SPRUCE Revealed through Stable Isotopes in Peat Profiles. Biogeosciences 2017, 14, 2481–2494. [Google Scholar] [CrossRef]
- Stivrins, N.; Ozola, I.; Gałka, M.; Kuske, E.; Alliksaar, T.; Andersen, T.J.; Lamentowicz, M.; Wulf, S.; Reitalu, T. Drivers of peat accumulation rate in a raised bog: Impact of drainage, climate, and local vegetation composition. Mires Peat 2017, 19, 1–19. [Google Scholar] [CrossRef]
- Turner, T.E.; Swindles, G.T.; Roucoux, K.H. Late Holocene ecohydrological and carbon dynamics of a UK raised bog: Impact of human activity and climate change. Quat. Sci. Rev. 2014, 84, 65–85. [Google Scholar] [CrossRef]
- Panait, A.; Diaconu, A.; Galka, M.; Grindean, R.; Hutchinson, S.M.; Hickler, T.; Lamentowicz, M.; Mulch, A.; Tanţău, I.; Werner, C.; et al. Hydrological conditions and carbon accumulation rates reconstructed from a mountain raised bog in the Carpathians: A multi-proxy approach. CATENA 2017, 152, 57–68. [Google Scholar] [CrossRef]
- Ott, C.A.; Chimner, R.A. Long-term peat accumulation in temperate forested peatlands (Thuja occidentalis swamps) in the Great Lakes region of North America. Mires Peat 2016, 18, 1–9. [Google Scholar] [CrossRef]
- León, C.A.; Oliván, G. Recent rates of carbon and nitrogen accumulation in peatlands of Isla Grande de Chiloé-Chile. Rev. Chil. Hist. Nat. 2014, 87, 26. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Cole, J.J.; Finzi, A.C.; Holland, E.A. Introduction to coupled biogeochemical cycles. Front. Ecol. Environ. 2011, 9, 5–8. [Google Scholar] [CrossRef]
- Paal, J.; Jürjendal, I.; Suija, A.; Kull, A. Impact of drainage on vegetation of transitional mires in Estonia. Mires Peat 2016, 18, 1–19. [Google Scholar] [CrossRef]
- Gao, J.Q.; Zhang, X.W.; Lei, G.C.; Wang, G.X. Soil Organic Carbon and its Fractions in Relation to Degradation and Restoration of Wetlands on the Zoigê Plateau, China. Wetlands 2014, 34, 235–241. [Google Scholar] [CrossRef]
- Nakatsubo, T.; Uchida, M.; Sasaki, A.; Kondo, M.; Yoshitake, S.; Kanda, H. Carbon accumulation rate of peatland in the High Arctic, Svalbard: Implications for carbon sequestration. Polar Sci. 2015, 9, 267–275. [Google Scholar] [CrossRef]
- Olson, K.R.; Al-Kaisi, M.M.; Lal, R.; Lowery, B. Experimental Consideration, Treatments, and Methods in Determining Soil Organic Carbon Sequestration Rates. Soil Sci. Soc. Am. J. 2014, 78, 348–360. [Google Scholar] [CrossRef]
- Xiang, S.A.; Guo, R.Q.; Wu, N.; Sun, S.C. Current status and future prospects of Zoige Marsh in eastern Qinghai-Tibet Plateau. Ecol. Eng. 2009, 35, 553–562. [Google Scholar] [CrossRef]
- Zhou, W.C.; Cui, L.J.; Wang, Y.F.; Li, W. Methane emissions from natural and drained peatlands in the Zoigê, eastern Qinghai-Tibet Plateau. J. For. Res. 2017, 28, 539–547. [Google Scholar] [CrossRef]
- Wang, G.; Qian, J.; Cheng, G.; Lai, Y. Soil organic carbon pool of grassland soils on the Qinghai–Tibetan Plateau and its global implication. Sci. Total Environ. 2002, 291, 207–217. [Google Scholar] [CrossRef]
- Luan, J.W.; Cui, L.J.; Xiang, C.H.; Wu, J.H.; Song, H.T.; Ma, Q.F.; Hu, Z.D. Different grazing removal exclosures effects on soil C stocks among alpine ecosystems in east Qinghai–Tibet Plateau. Ecol. Eng. 2014, 64, 262–268. [Google Scholar] [CrossRef]
- Zhang, W.J.; Lu, Q.F.; Song, K.C.; Qin, G.H.; Wang, Y.; Wang, X.; Li, H.X.; Li, J.; Liu, G.D.; Li, H. Remotely sensing the ecological influences of ditches in Zoige peatland, eastern Tibetan Plateau. Int. J. Remote Sens. 2014, 35, 5186–5197. [Google Scholar] [CrossRef]
- Zhao, K.Y.; He, C.Q. Influence of human activities on the mire in Zoige Plateau and countermeasure. Sci. Geogr. Sin. 2000, 20, 444–449. [Google Scholar]
- Luan, J.W.; Cui, L.J.; Xiang, C.H.; Wu, J.H.; Song, H.T.; Ma, Q.F. Soil carbon stocks and quality across intact and degraded alpine wetlands in Zoige, east Qinghai-Tibet Plateau. Wetlands Ecol. Manag. 2014, 22, 427–438. [Google Scholar] [CrossRef]
- Gao, J.; Li, X.L.; Cheung, A.; Yang, Y.W. Degradation of Wetlands on the Qinghai-Tibet Plateau: A Comparison of the Effectiveness of Three Indicators. J. Mt. Sci. 2013, 10, 658–667. [Google Scholar] [CrossRef]
- Cui, L.J.; Kang, X.M.; Li, W.; Hao, Y.B.; Zhang, Y.; Wang, J.Z.; Yan, L.; Zhang, X.D.; Zhang, M.Y.; Zhou, J.; et al. Rewetting Decreases Carbon Emissions from the Zoige Alpine Peatland on the Tibetan Plateau. Sustainability 2017, 9, 948. [Google Scholar] [CrossRef]
- Appleby, P.G.; Oldfield, F. The calculation of 210Pb dates assuming a constant rate of supply of unsupported 210Pb to the sediment. CATENA 1978, 5, 1–8. [Google Scholar] [CrossRef]
- Bao, K.S.; Yu, X.F.; Jia, L.; Wang, G.P. Recent Carbon Accumulation in Changbai Mountain Peatlands, Northeast China. Mt. Res. Dev. 2010, 30, 33–41. [Google Scholar] [CrossRef]
- Zaccone, C.; Lobianco, D.; Shotyk, W.; Ciavatta, C.; Appleby, P.G.; Brugiapaglia, E.; Casella, L.; Miano, T.M.; D’Orazio, V. Highly anomalous accumulation rates of C and N recorded by a relic, free-floating peatland in Central Italy. Sci. Rep.-UK 2017, 7, 43040. [Google Scholar] [CrossRef] [PubMed]
- Craft, C.; Vymazal, J.; Kröpfelová, L. Carbon sequestration and nutrient accumulation in floodplain and depressional wetlands. Ecol. Eng. 2018, 114, 137–145. [Google Scholar] [CrossRef]
- Ritchie, J.C.; McHenry, J.R. Application of radioactive fallout Cesium-137 for measuring soil erosion and sediment accumulation rates and patterns: A review. J. Environ. Qual. 1990, 19, 215–233. [Google Scholar] [CrossRef]
- Fesenko, S.V.; Spiridonov, S.I.; Sanzharova, N.I.; Anisimov, V.S.; Aleksakhin, R.M. Simulation of 137Cs Migration over the Soil-plant System of Peat Soils Contaminated at the Chernobyl Accident. Russ. J. Ecol. 2002, 33, 170–177. [Google Scholar] [CrossRef]
- Rosen, K.; Vinichuk, M.; Johanson, K.J. 137Cs in a raised bog in central Sweden. J. Environ. Radioact. 2009, 100, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Laasimer, L. Eesti NSV taimkate. In Vegetation of the Estonian S.S.R.; Valgus: Tallinn, Estonia, 1965. [Google Scholar]
- Paal, J.; Leibak, E. Estonian Mires: Inventory of Habitats; Regio Ltd.: Tartu, Estonia, 2011. [Google Scholar]
- Wieder, R.K.; Novak, M.; Schell, W.R.; Rhodes, T. Rates of peat accumulation over the past 200 years in five Sphagnum-dominated peatlands in the United States. J. Paleolimnol. 1994, 12, 35–47. [Google Scholar] [CrossRef]
- Marchio, D.A.; Savarese, M.; Bovard, B.; Mitsch, W.J. Carbon Sequestration and Sedimentation in Mangrove Swamps Influenced by Hydrogeomorphic Conditions and Urbanization in Southwest Florida. Forests 2016, 7, 116. [Google Scholar] [CrossRef]
- Bao, K.S.; Quan, G.X.; Liu, F.G. Recent History of Carbon and Nitrogen Accumulation Rates in Yancheng Coastal Wetland, China. Open J. Nat. Sci. 2015, 3, 137–146. [Google Scholar] [CrossRef]
- Hribljan, J.A.; Suarez, E.; Heckman, K.A.; Lilleskov, E.A.; Chimner, R.A. Peatland carbon stocks and accumulation rates in the Ecuadorian paramo. Wetlands Ecol. Manag. 2016, 24, 113–127. [Google Scholar] [CrossRef]
- Kurnianto, S.; Warren, M.; Talbot, J.; Kauffman, B.; Murdiyarso, D.; Frolking, S. Carbon accumulation of tropical peatlands over millennia: A modeling approach. Glob. Chang. Biol. 2015, 21, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.S.; Wang, G.P.; Xing, W.; Shen, J. Accumulation of organic carbon over the past 200 years in alpine peatlands, northeast China. Environ. Earth Sci. 2015, 73, 7489–7503. [Google Scholar] [CrossRef]
- Hansen, V.D.; Nestlerode, J.A. Carbon sequestration in wetland soils of the northern Gulf of Mexico coastal region. Wetlands Ecol. Manag. 2014, 22, 289–303. [Google Scholar] [CrossRef]
- Bernal, B.; Mitsch, W.J. Carbon sequestration in freshwater wetlands in Costa Rica and Botswana. Biogeochemistry 2013, 115, 77–93. [Google Scholar] [CrossRef]
- Turunen, J.; Roulet, N.T.; Moore, T.R. Nitrogen deposition and increased carbon accumulation in ombrotrophic peatlands in eastern Canada. Glob. Biogeochem. Cycles 2004, 18, 1–12. [Google Scholar] [CrossRef]
- Tolonen, K.; Turunen, J. Accumulation rates of carbon in mires in Finland and implications for climate change. Holocene 1996, 6, 171–178. [Google Scholar] [CrossRef]
- Clymo, R.S. The limits to peat bog growth. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1984, 303, 605–654. [Google Scholar] [CrossRef]
- Gorham, E. Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecol. Appl. 1991, 1, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Squeo, F.A.; Warner, B.G.; Aravena, R.; Espinoza, D. Bofedales: High altitude peatlands of the central Andes. Rev. Chil. Hist. Nat. 2006, 79, 245–255. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, H.; Ji, L.; Lei, L.; Wang, C.; Yan, D.; Li, B.; Li, J. Vegetation greenness trend (2000 to 2009) and the climate controls in the Qinghai-Tibetan Plateau. Appl. Remote Sens. 2013, 7, 073572. [Google Scholar] [CrossRef]
- Wang, M.; Yang, G.; Gao, Y.H.; Chen, H.; Wu, N.; Peng, C.H.; Zhu, Q.A.; Zhu, D.; Wu, J.H.; He, Y.X.; et al. Higher recent peat C accumulation than that during the Holocene on the Zoige Plateau. Quat. Sci. Rev. 2015, 114, 116–125. [Google Scholar] [CrossRef]
- Bagchi, S.; Ritchie, M.E. Introduced grazers can restrict potential soil carbon sequestration through impacts on plant community composition. Ecol. Lett. 2010, 13, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E. Carbon Accumulation and C/N Ratios of Peat Bogs in North-West Scotland. Scott. Geogr. J. 2002, 118, 323–341. [Google Scholar] [CrossRef]
- Lindsay, R.; Campus, S.; Lane, W. Peatbogs and Carbon: A Critical Synthesis to Inform Policy Development in Oceanic Peat Bog Conservation and Restoration in the Context of Climate Change; University of East London: London, UK, 2010. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).