Simultaneous Treatment of Agro-Industrial and Industrial Wastewaters: Case Studies of Cr(VI)/Second Cheese Whey and Cr(VI)/Winery Effluents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater Characterization
2.2. Indigenous Microorganisms/Enrichment and Culture Conditions
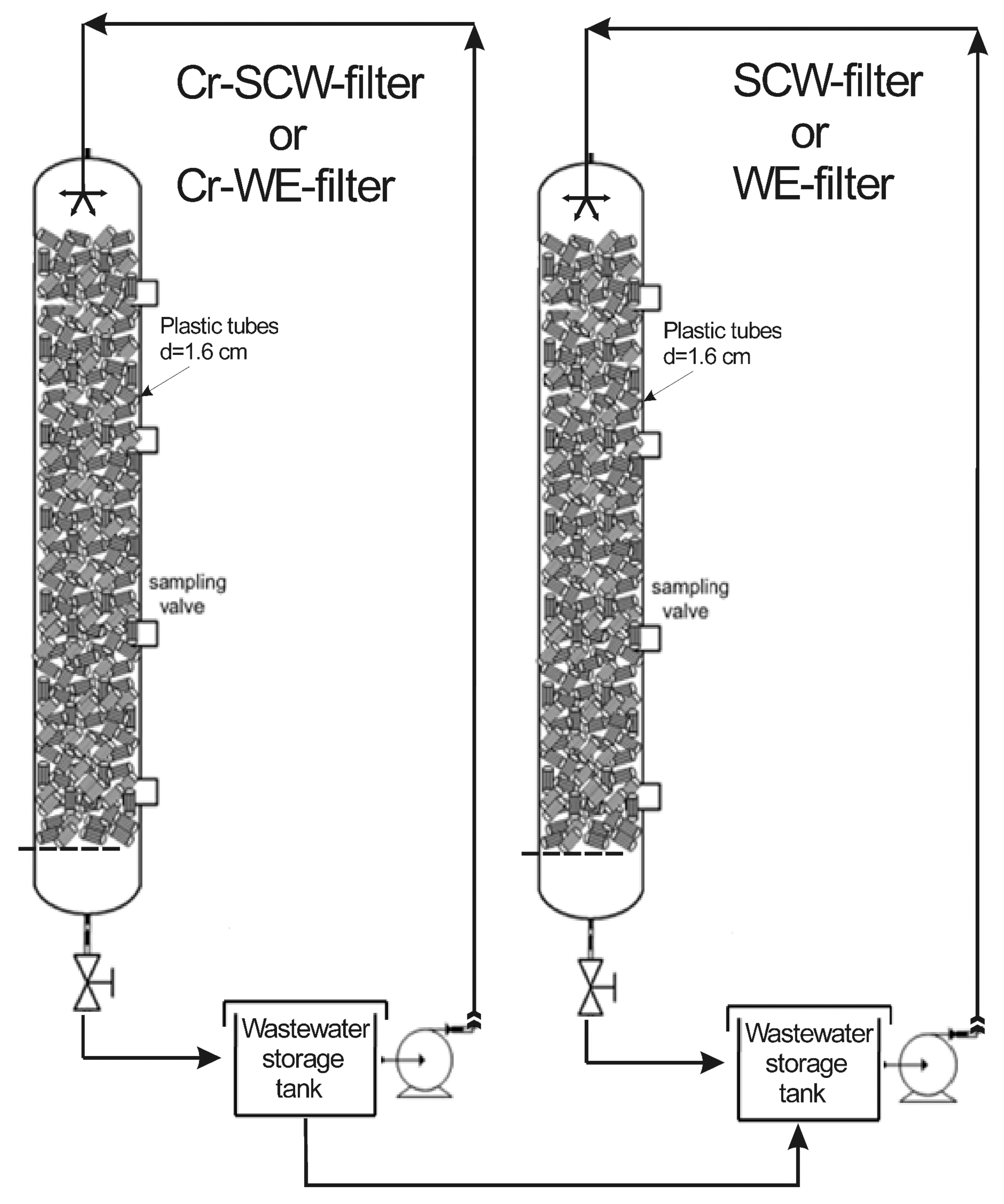
2.3. Packed Bed Reactors (Pilot-Scale Filters)
2.4. Sample Collection and Analyses
2.5. Statistical Analysis
3. Results and Discussion
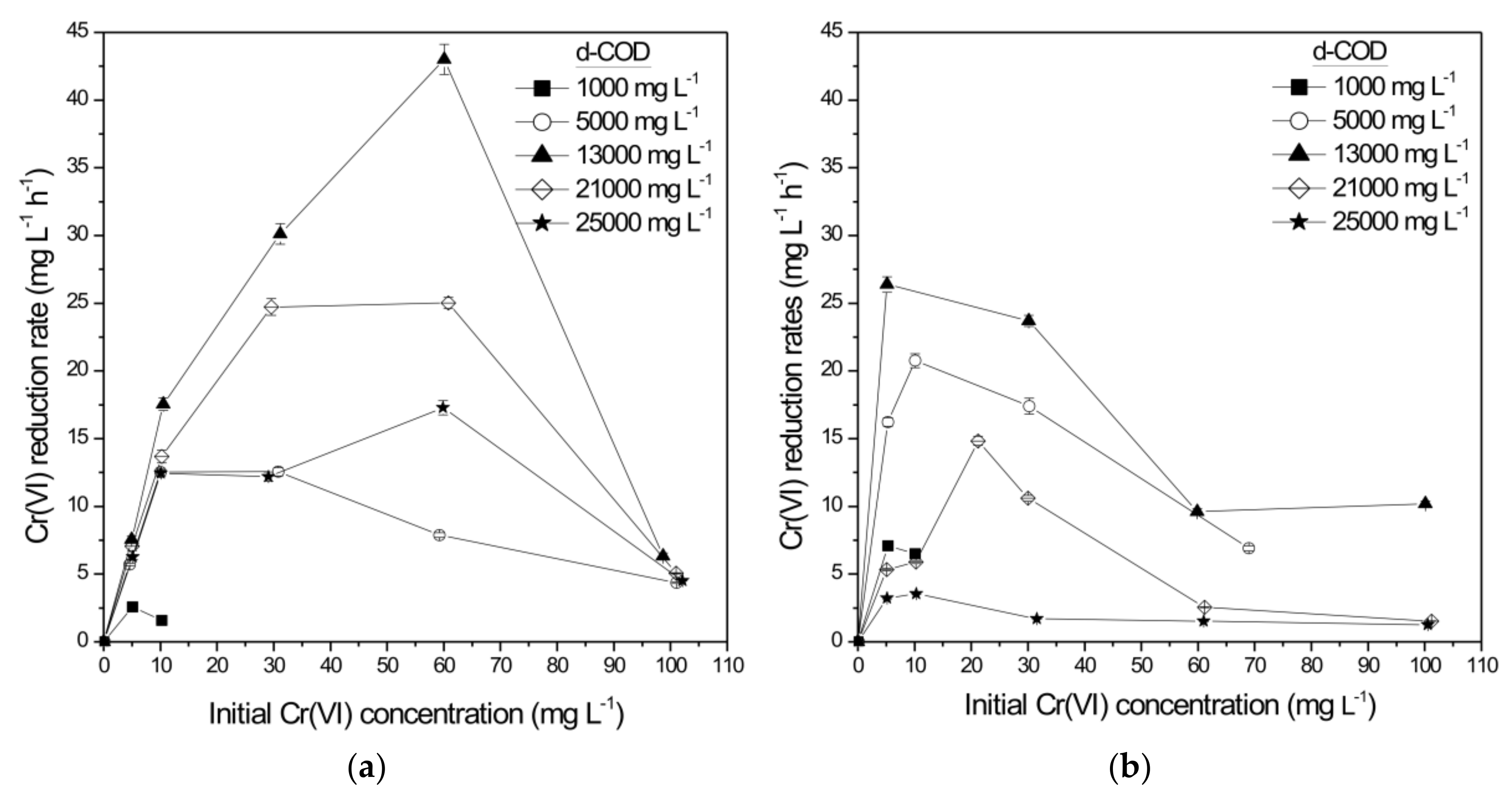
3.1. Packed Bed Reactor Experiments in Cr-SCW-Filter and Cr-WE-Filter
3.1.1. Sequencing Batch Mode Operation
3.1.2. Sequencing Batch Mode Operation with Recirculation
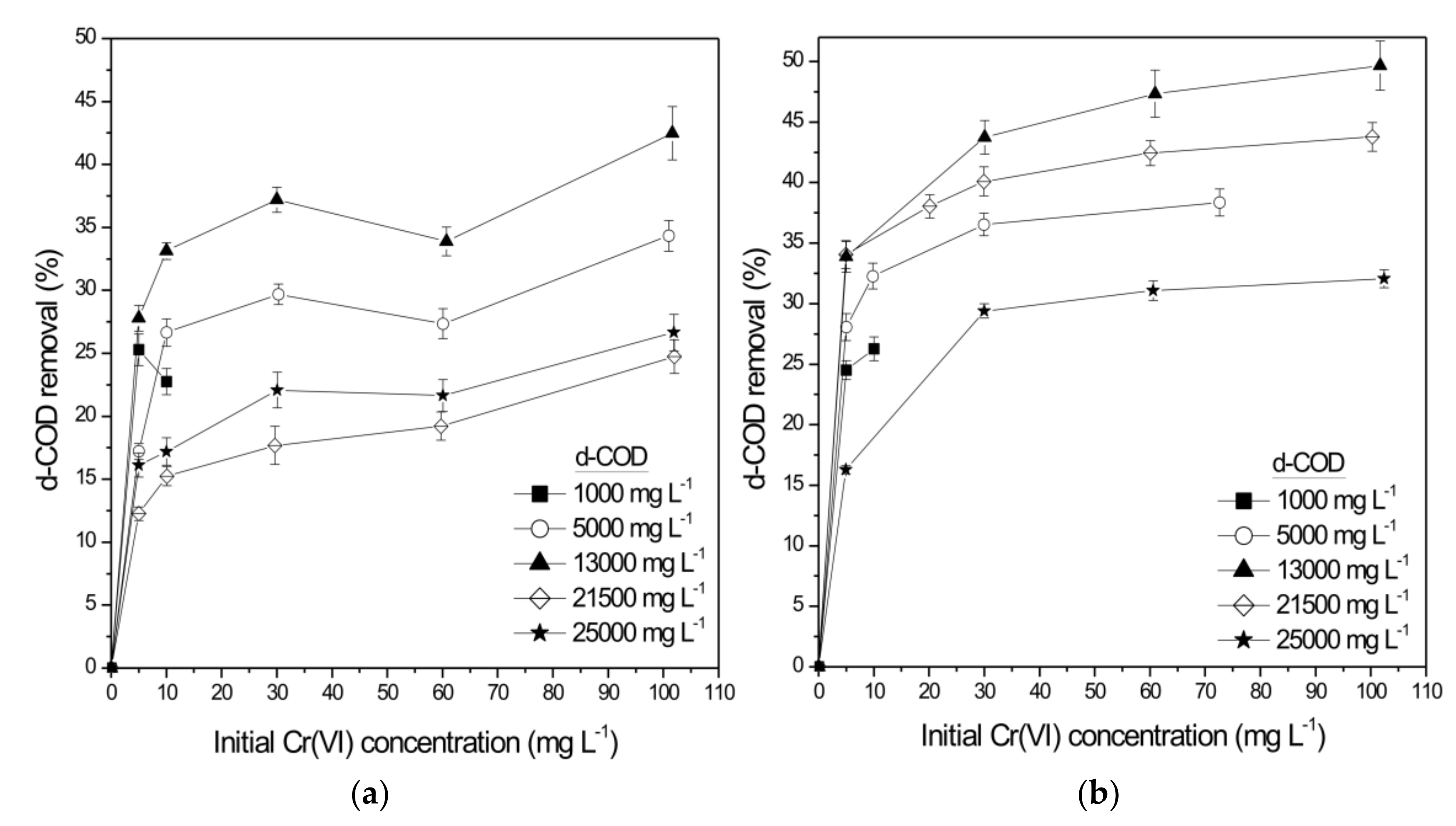
3.2. Packed Bed Reactor Experiments in SCW-Filter and WE-Filter
4. Conclusions
- High percentage biological Cr(VI) reduction can be achieved in an attached growth reactor (99.2–100%) by using an indigenous mixed population and SCW or WE (both very low-cost carbon sources) as the sole electron donor.
- Complete Cr(VI) reduction can be achieved in attached growth reactors operated in batch operation with recirculation of 0.5 L min−1 for all initial Cr(VI) (5–110 mg L−1) and d-COD (1000–25,000 mg L−1) concentrations tested for both agro-industrial effluents (SCW or WE). The reduction rates that are accomplished (35.99 and 43.0 mg L−1 h−1 for SCW and WE, respectively) are the highest reported in the literature to date. With higher recirculation rates (1.0 L min−1) the Cr-SCW-filter or Cr-WE-filter were unable to achieve complete Cr(VI) reduction for initial Cr(VI) concentrations above 30 or 10 mg L−1, respectively, while for 2.0 L min−1, detachment of biofilm led to inadequate operation of the filter. Continuous operating mode with or without recirculation resulted in very low Cr(VI) bioreduction rates.
- Winery effluents presented slightly higher Cr(VI) reduction rates for initial d-COD concentrations up to 13,000 mg L−1. The same was not observed for higher initial concentrations probably due to the presence of higher quantities of phenolic compounds that are associated with microbial toxicity.
- Initial d-COD concentration was found to effect Cr(VI) reduction rate. The feed SCW or WE concentration of 1000 mg d-COD L−1 was limiting and caused microbial growth limitation by carbon during the process.
- Due to the high residual d-COD concentration of the treated wastewater, a post-treatment stage was required. The use of mixed indigenous microorganisms originating from SCW or WE provides high degradation rates (total d-COD removal above 97% and 90.5% for SCW and WE, respectively) and durability under various operating conditions. In cases where final d-COD concentrations of the second biofilters are still above the maximum permitted limit of 125 mg L−1, a suitable post-treatment step (e.g., a constructed wetland) should be applied to improve the quality of the final outflow.
Author Contributions
Conflicts of Interest
References
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K.S.M. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef]
- Gröhlich, A.; Langer, M.; Mitrakas, M.; Zouboulis, A.; Katsoyiannis, I.; Ernst, M. Effect of Organic Matter on Cr(VI) Removal from Groundwaters by Fe(II) Reductive Precipitation for Groundwater Treatment. Water 2017, 9, 389. [Google Scholar] [CrossRef]
- Costa, M. Potential hazards of hexavalent chromate in our drinking water. Toxicol. Appl. Pharmacol. 2003, 188, 1–5. [Google Scholar] [CrossRef]
- Stylianou, S.; Simeonidis, K.; Mitrakas, M.; Zouboulis, A.; Ernst, M.; Katsoyiannis, I.A. Reductive precipitation and removal of Cr(VI) from groundwaters by pipe flocculation-microfiltration. Environ. Sci. Pollut. Res. 2017, 1–7, in press. [Google Scholar] [CrossRef] [PubMed]
- Hossini, H.; Makhdoumi, P.; Mohammadi-Moghadam, F.; Ghaffari, H.R.; Mirzaei, N.; Ahmadpour, M. A review of toxicological, environmental and health effects of chromium from aqueous medium; available removal techniques. Acta Medica Mediterr. 2016, 32, 1463–1469. [Google Scholar]
- DeFlora, S.; Bagnasco, M.; Serra, D.; Zanacchi, P. Genotoxicity of chromium compounds: A review. Mutat. Res. 1990, 238, 99–172. [Google Scholar] [CrossRef]
- Anonymous. Directive on the Quality of Water Intended for Human Consumption. In EC-Official Journal of the European Communities; 12 December 1998, L330/32; Commission of the European Communities: Brussels, Belgium.
- EPA (United States Environmental Protection Agency). Parameters of Water Quality, Interpretation and Standards; United States Environmental Protection Agency: Washington, DC, USA, 2001.
- Owlad, M.; Aroua, M.; Daud, W.; Baroutian, S. Removal of Hexavalent Chromium-Contaminated Water and Wastewater: A Review. Water Air Soil Pollut. 2008, 200, 59–77. [Google Scholar] [CrossRef]
- Hawley, E.L.; Deeb, R.; Kavanaugh, M.C.; Jacobs, J.R.G. 8-Treatment Technologies for Chromium(VI); Chemical Rubber Company CRC Press LLC: Boca Raton, FL, USA, 2005; p. 273. [Google Scholar]
- Kaprara, E.; Simeonidis, K.; Zouboulis, A.; Mitrakas, M. Evaluation of current treatment technologies for Cr(VI) removal from water sources at sub-ppb levels. In Proceedings of the 13th International Conference on Environmental Science and Technology, Athens, Greece, 5–7 September 2013. [Google Scholar]
- Najm, I.; Brown, N.P.; Blute, N.; Kader, S. Impact of Water Quality on Cr(VI) treatment efficiency and cost results of WaterRF project 4450. In Proceedings of the ACE 2013–2013 AWWA Annual Conference and Exposition, Denver, CO, USA, 9–13 June 2013. Code 101497. [Google Scholar]
- Joutey, N.T.; Sayel, H.; Bahafid, W.; El Ghachtouli, N. Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev. Environ. Contam. Toxicol. 2015, 233, 45–69. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhu, X.; Song, T.; Zhang, L.; Jia, H.; Wei, P. Effect of acclimatization on hexavalent chromium reduction in a biocathode microbial fuel cell. Bioresour. Technol. 2015, 180, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Molokwane, P.E.; Meli, K.C.; Nkhalambayausi-Chirwa, E.M. Chromium (VI) reduction in activated sludge bacteria exposed to high chromium loading: Brits culture (South Africa). Water Res. 2008, 42, 4538–4548. [Google Scholar] [CrossRef] [PubMed]
- Panousi, E.; Mamais, D.; Noutsopoulos, C.; Mpertoli, K.; Kantzavelou, C.; Nyktari, E.; Kavallari, I.; Nasioka, M.; Kaldis, A. Biological groundwater treatment for hexavalent chromium removal at low chromium concentrations under anoxic conditions. Environ. Technol. (UK) 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Panousi, E.; Mamais, D.; Noutsopoulos, C.; Antoniou, K.; Koutoula, K.; Mastrantoni, S.; Koutsogiannis, C.; Gkioni, A. Biological treatment of groundwater with a high hexavalent chromium content under anaerobic and anoxic conditions. J. Chem. Technol. Biot. 2016, 91, 1681–1687. [Google Scholar] [CrossRef]
- Carlos, F.S.; Giovanella, P.; Bavaresco, J.; Borges, C.S.; Camargo, F.A.O. A Comparison of Microbial Bioaugmentation and Biostimulation for Hexavalent Chromium Removal from Wastewater. Water Air Soil Pollut. 2016, 227, 175. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, A.; Kirrolia, A.; Kumar, R.; Yadav, N.; Bishnoi, N.R.; Lohchab, R.K. Removal of sulphate, COD and Cr(VI) in simulated and real wastewater by sulphate reducing bacteria enrichment in small bioreactor and FTIR study. Bioresour. Technol. 2011, 102, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Tekerlekopoulou, A.; Tsiamis, G.; Dermou, E.; Siozios, S.; Bourtzis, K.; Vayenas, D.V. The effect of carbon source on microbial community structure and Cr(VI) reduction rate. Biotechnol. Bioeng. 2010, 107, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.A.; Apel, W.A.; Petersen, J.N.; Peyton, B.M. Effect of carbon and energy source on bacterial chromate reduction. Bioremediat. J. 2002, 6, 205–215. [Google Scholar] [CrossRef]
- Rynk, R. Bioremediation with cheese whey. BioCycle 2004, 45, 26–28. [Google Scholar]
- Elangovan, R.; Philip, L. Performance evaluation of various bioreactors for the removal of Cr(VI) and organic matter from industrial effluent. Biochem. Eng. J. 2009, 44, 174–186. [Google Scholar] [CrossRef]
- Chen, Z.F.; Zhao, Y.S.; Zhang, J.W.; Bai, J. Mechanism and Kinetics of Hexavalent Chromium Chemical Reduction with Sugarcane Molasses. Water Air Soil Pollut. 2015, 226, 363. [Google Scholar] [CrossRef]
- Michailides, M.K.; Tekerlekopoulou, A.G.; Akratos, C.S.; Coles, S.; Pavlou, S.; Vayenas, D.V. Molasses as an efficient low-cost carbon source for biological Cr(VI) removal. J. Hazard. Mater. 2015, 281, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.A.; Ahmad, W.A.; Zakaria, Z.; Razali, F.; Karim, N.A.; Sum, M.M.; Sidek, M.S.M. Bacterial reduction of Cr(VI) at technical scale - The Malaysian experience. Appl. Biochem. Biotechnol. 2012, 167, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Orozco, A.M.F.; Contreras, E.M.; Zaritzky, N.E. Cr(Vi) reduction capacity of activated sludge as affected by nitrogen and carbon sources, microbial acclimation and cell multiplication. J. Hazard. Mater. 2010, 176, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.M.; Orozco, A.M.F.; Zaritzky, N.E. Factors affecting the biological removal of hexavalent chromium using activated sludges. In Management of Hazardous Residues Containing Cr(VI); Balart Murria, M.J., Ed.; Nova Science Publishers: New York, NY, USA, 2011; pp. 109–134. [Google Scholar]
- Contreras, E.M.; Ferro Orozco, A.M.; Zaritzky, N.E. Biological Cr(VI) removal coupled with biomass growth, biomass decay, and multiple substrate limitation. Water Res. 2011, 45, 3034–3046. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, P.; Aquilanti, G.; Bardelli, F.; Iadecola, A.; Rosellini, I.; Tassi, E.; Pezzarossa, B.; Petruzzelli, G. Spectroscopic evidence of Cr(VI) reduction in a contaminated soil by in situ treatment with whey. Agrochimica 2015, 59, 218–230. [Google Scholar] [CrossRef]
- Němeček, J.; Pokorný, P.; Lacinová, L.; Černík, M.; Masopustová, Z.; Lhotský, O.; Filipová, A.; Cajthaml, T. Combined abiotic and biotic in-situ reduction of hexavalent chromium in groundwater using nZVI and whey: A remedial pilot test. J. Hazard. Mater. 2015, 300, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Němeček, J.; Pokorný, P.; Lhotský, O.; Knytl, V.; Najmanová, P.; Steinová, J.; Černík, M.; Filipová, A.; Filip, J.; Cajthaml, T. Combined nano-biotechnology for in-situ remediation of mixed contamination of groundwater by hexavalent chromium and chlorinated solvents. Sci. Total Environ. 2015, 563–564, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Mamais, D.; Noutsopoulos, C.; Kavallari, I.; Nyktari, E.; Kaldis, A.; Panousi, E.; Nikitopoulos, G.; Antoniou, K.; Nasioka, M. Biological groundwater treatment for chromium removal at low hexavalent chromium concentrations. Chemosphere 2016, 152, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Tatoulis, T.I.; Tekerlekopoulou, A.G.; Akratos, C.S.; Pavlou, S.; Vayenas, D.V. Aerobic biological treatment of second cheese whey in suspended and attached growth reactors. J. Chem. Technol. Biot. 2015, 90, 2040–2049. [Google Scholar] [CrossRef]
- Sultana, M.-Y.; Tatoulis, T.I.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Effect of operational parameters on the performance of a horizontal subsurface flow constructed wetland treating secondary cheese whey and Cr(VI) wastewater. Int. J. Civ. Struct. Eng. 2015, 2, 286–289. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Symeonidou, M.P.; Matis, K.A. Technologies of winery wastewater treatment: A critical approach. Desalin. Water Treat. 2016, 57, 3372–3386. [Google Scholar] [CrossRef]
- Mosteo, R.; Sarasa, J.; Ormad, M.P.; Ovelleiro, J.L. Sequential solar photo-fenton-biological system for the treatment of winery wastewaters. J. Agric. Food Chem. 2008, 56, 7333–7338. [Google Scholar] [CrossRef] [PubMed]
- Andreottola, G.; Foladori, P.; Ziglio, G. Biological treatment of winery wastewater: An overview. Water Sci. Technol. 2009, 60, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- APHA (American Public Health Association); AWWA (American Water Works Association); WPCF (Water Pollution Control Federation). Standard Methods for the Examination of Water and Wastewater, 17th ed.; American Public Health Association, American Water Works Association and Water Pollution Control Federation: Washington, DC, USA, 1989; ISBN 087553161X. [Google Scholar]
- Waterman, P.G.; Mole, S. Analysis of phenolic plant metabolites. In Methods in Ecology; Lawton, J.H., Likens, G.E., Eds.; Blackwell Scientific: Oxford, UK, 1994; pp. 83–85. ISBN 0632029692. [Google Scholar]
- Nakatsu, C.H.; Carmosini, N.; Baldwin, B.; Beasley, F.; Kourtev, P.; Konopka, A. Soil Microbial Community Responses to Additions of Organic Carbon Substrates and Heavy Metals (Pb and Cr). Appl. Environ. Microbiol. 2005, 71, 7679–7689. [Google Scholar] [CrossRef] [PubMed]
- Welz, P.J.; Holtman, G.; Haldenwang, R.; le Roes-Hill, M. Characterisation of winery wastewater from continuous flow settling basins and waste stabilisation ponds over the course of 1 year: Implications for biological wastewater treatment and land application. Water Sci. Technol. 2016, 74, 2036–2050. [Google Scholar] [CrossRef] [PubMed]
- Ramond, J.B.; Welz, P.J.; Tuffin, M.I.; Burton, S.G.; Cowan, D.A. Assessment of temporal and spatial evolution of bacterial communities in a biological sand filter mesocosm treating winery wastewater. J. Appl. Microbiol. 2013, 115, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Tekerlekopoulou, A.G.; Tsiflikiotou, M.; Akritidou, L.; Viennas, A.; Tsiamis, G.; Pavlou, S.; Bourtzis, K.; Vayenas, D.V. Modelling of biological Cr(VI) removal in draw-fill reactors using microorganisms in suspended and attached growth systems. Water Res. 2013, 47, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.A.; Zakaria, Z.; Surif, S.; Ahmad, W.A. Biological detoxification of Cr(VI) using wood-husk immobilized Acinetobacter haemolyticus. J. Hazard. Mater. 2007, 148, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Dermou, E.; Velissariou, A.; Xenos, D.; Vayenas, D.V. Biological chromium(VI) reduction using a trickling filter. J. Hazard. Mater. 2005, 126, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.R.; Philip, L. Bioremediation of Cr(VI) in contaminated soils. J. Hazard. Mater. 2005, 121, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Chirwa, N.E.M.; Wang, Y.T. Modeling hexavalent chromium removal in a Bacillus sp. fixed-film bioreactor. Biotechnol. Bioeng. 2004, 87, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Establishment of measures, conditions and procedures for the reuse of treated wastewater and other provisions. Gazette of the Government (GR), 8 March 2011; 2011/354B. [Google Scholar]
- Sultana, M.-Y.; Mourti, C.; Tatoulis, T.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Effect of hydraulic retention time, temperature, and organic load on a horizontal subsurface flow constructed wetland treating cheese whey wastewater. J. Chem. Technol. Biotechnol. 2016, 91, 726–732. [Google Scholar] [CrossRef]
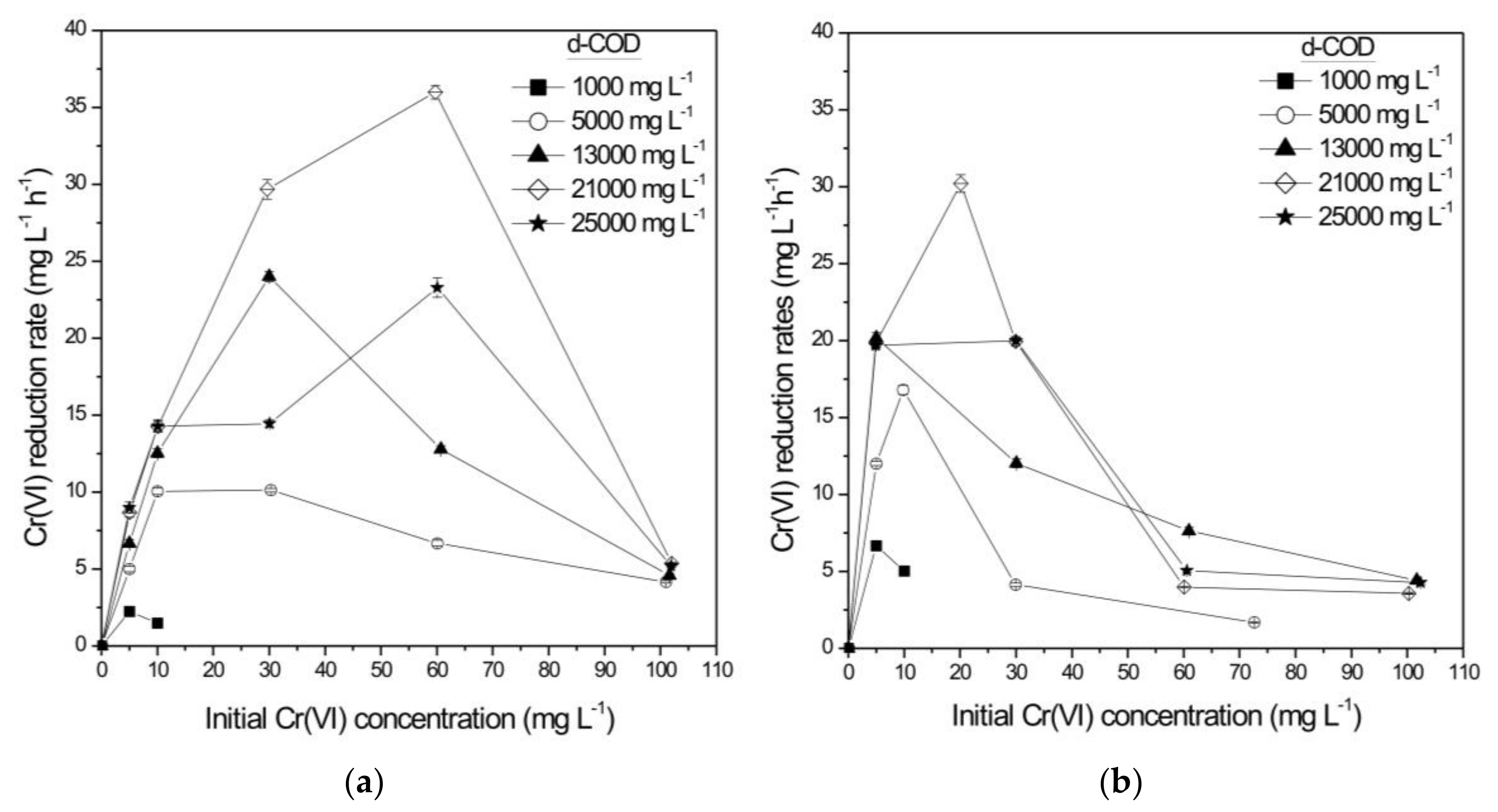
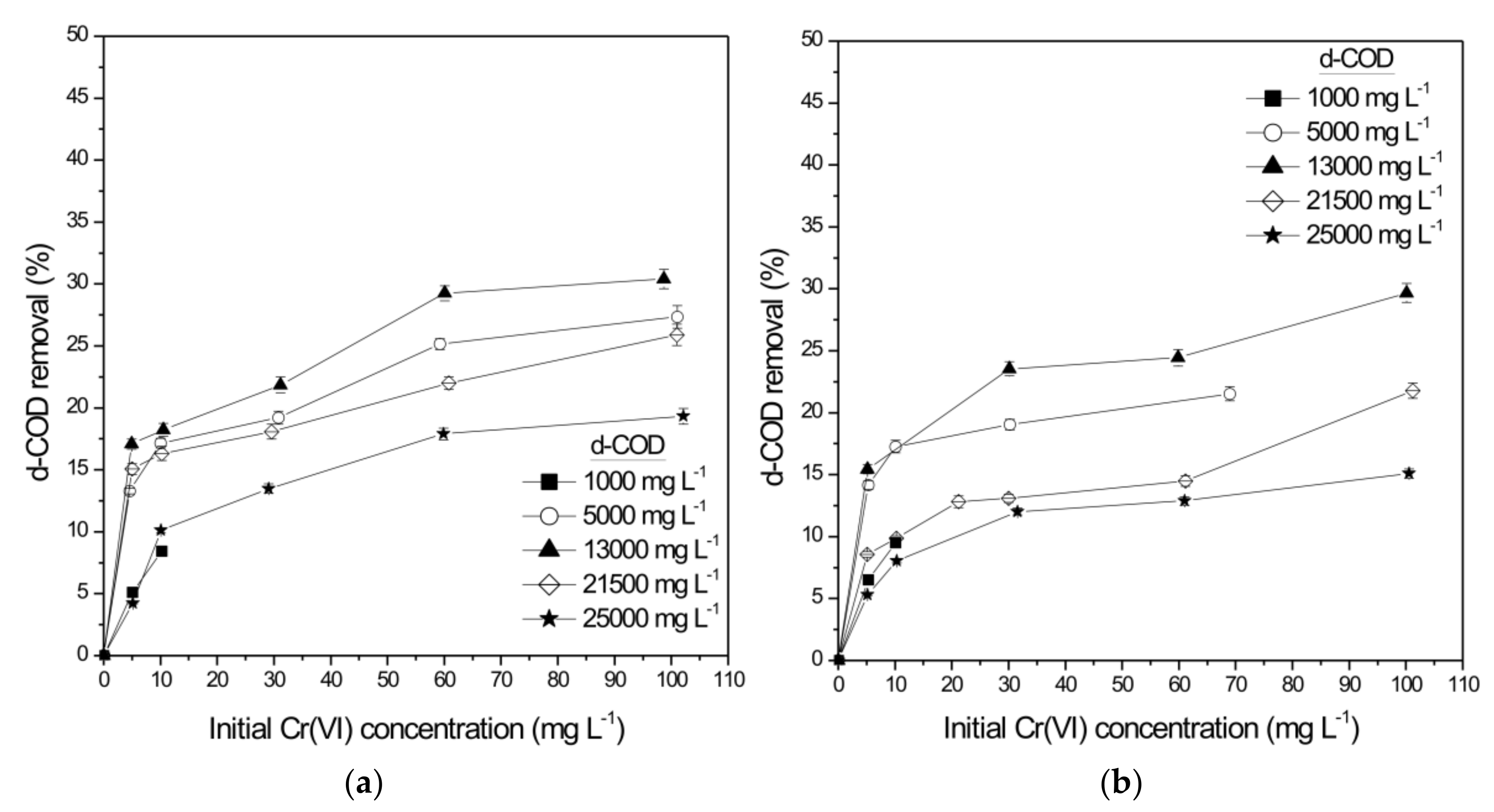
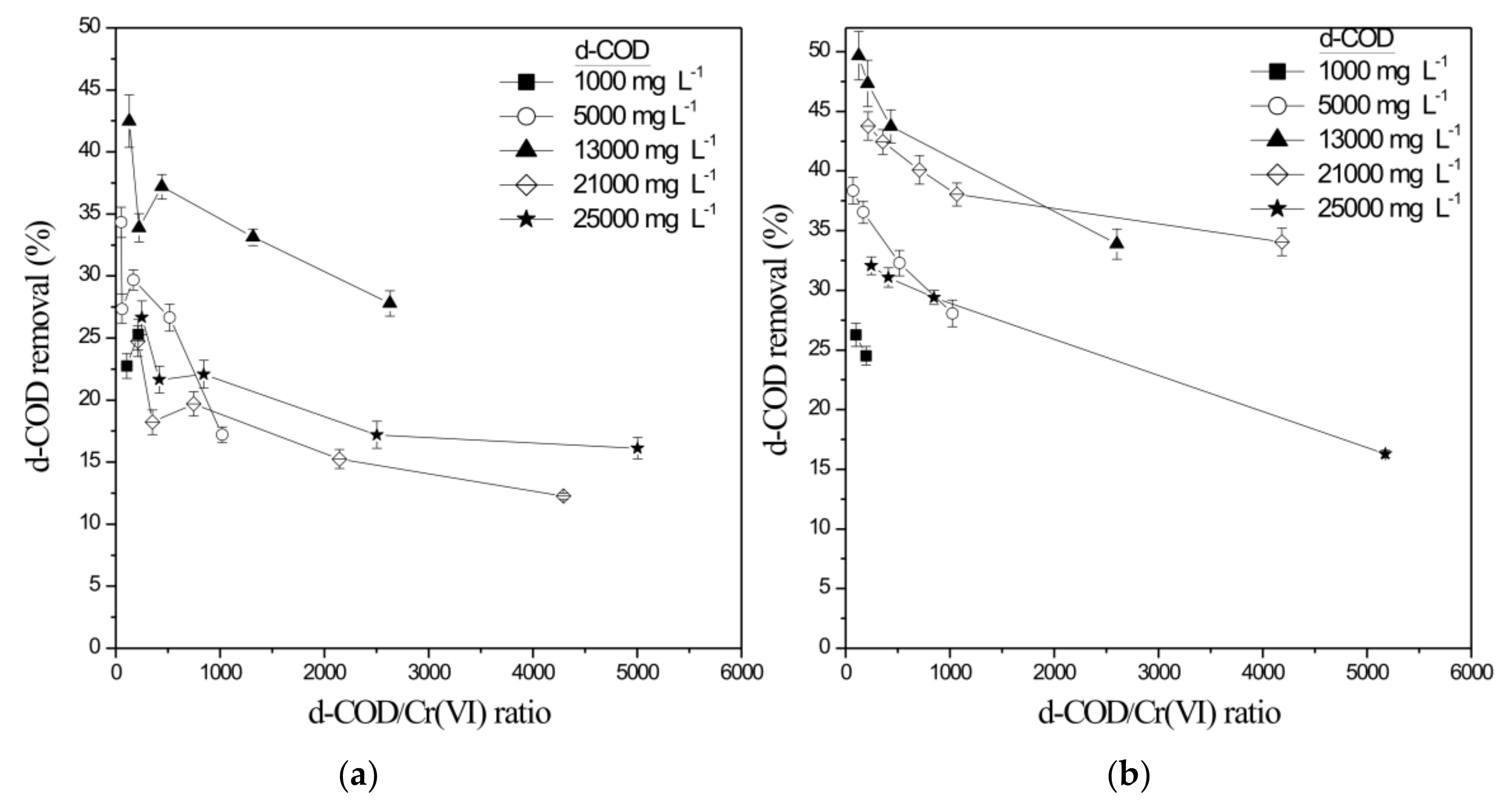
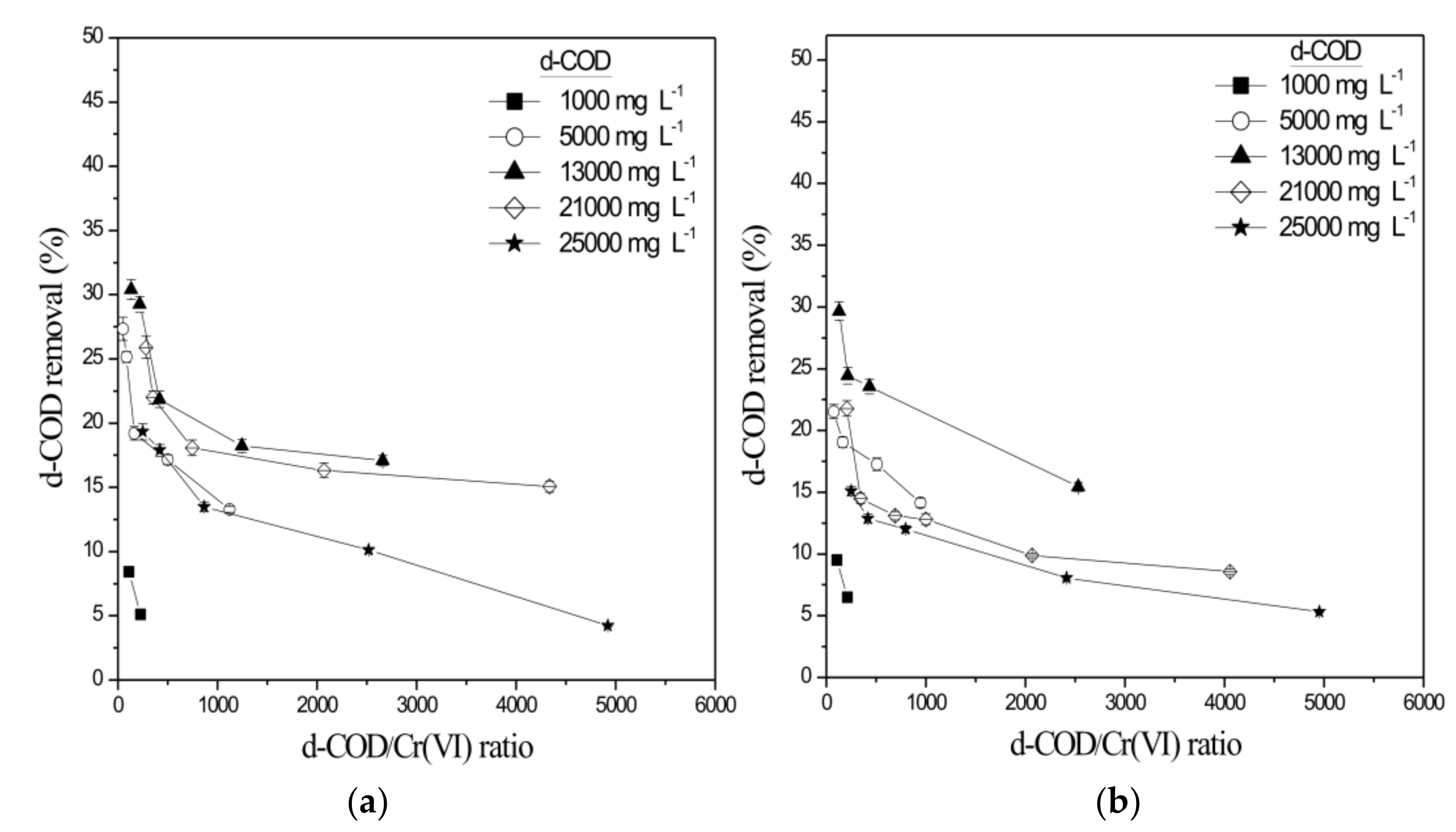
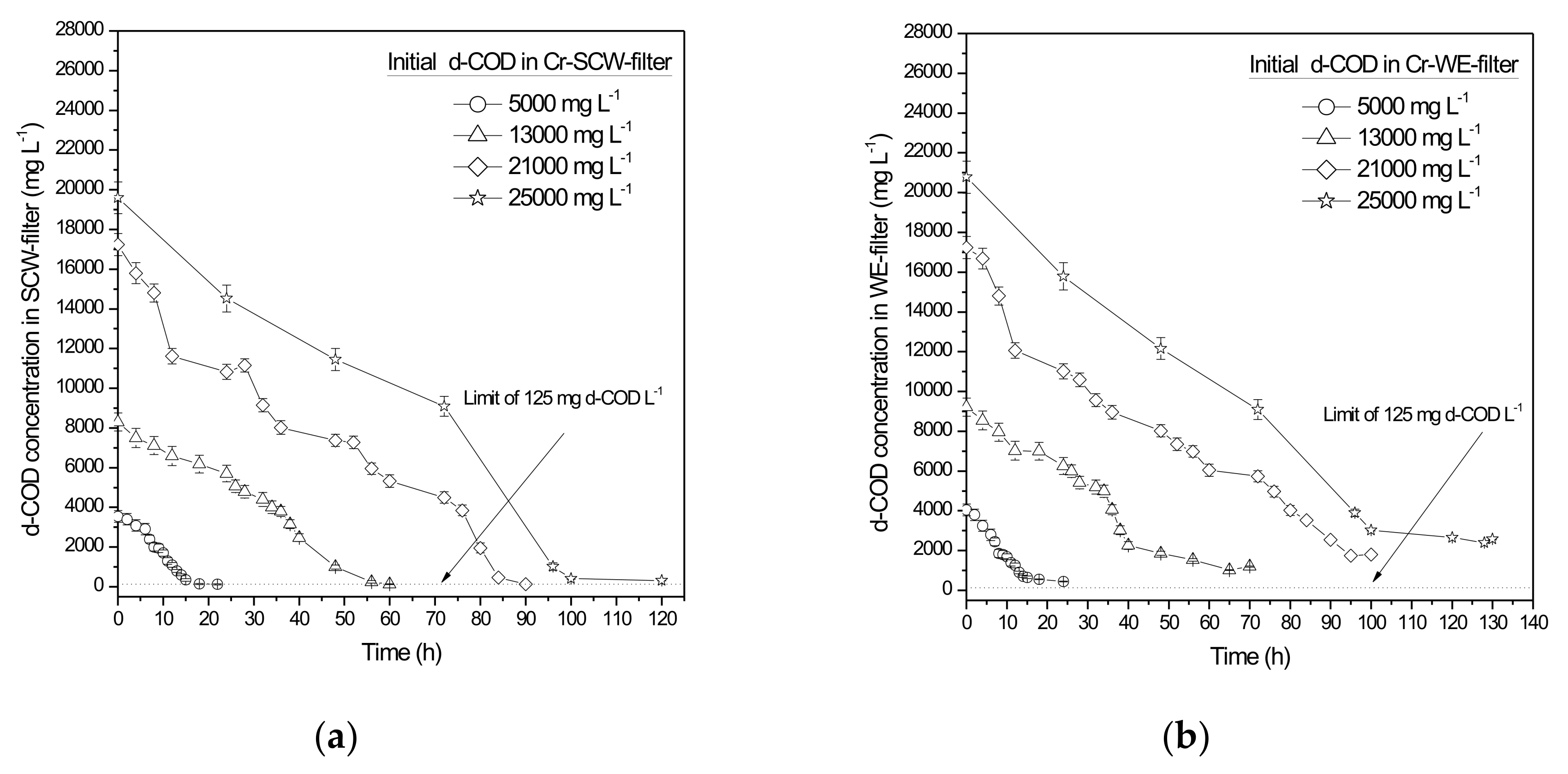
| Initial Cr(VI) (mg L−1) | Initial d-COD (mg L−1) | Cr(VI) Reduction Rate (mg L−1 h−1) | Cr(VI) Reduction Rate (g m−2 d−1) | d-COD Removal (Cr-SCW-Filter) (%) | Total d-COD Removal (SCW-Filter) (%) |
|---|---|---|---|---|---|
| 5.02 ± 0.08 | 1076 ± 22 | 2.23 ± 0.04 | 50.53 ± 0.9 | 25.28 ± 1.25 | - |
| 10.01 ± 0.14 | 1053 ± 33 | 1.48 ± 0.03 | 33.58 ± 0.68 | 22.74 ± 1.04 | - |
| 5.00 ± 0.04 | 5098 ± 87 | 5.00 ± 0.18 | 150.99 ± 5.43 | 17.21 ± 0.64 | - |
| 10.05 ± 0.13 | 5185 ± 36 | 10.05 ± 0.21 | 227.66 ± 4.75 | 26.65 ± 1.09 | - |
| 30.26 ± 0.02 | 5035 ± 121 | 10.12 ± 0.11 | 161.25 ± 1.75 | 29.69 ± 0.81 | 97.57 ± 1.12 |
| 60.10 ± 0.69 | 5018 ± 141 | 6.67 ± 0.19 | 142.13 ± 4.05 | 27.35 ± 1.19 | - |
| 100.94 ± 1.46 | 5010 ± 69 | 4.16 ± 0.08 | 94.27 ± 1.81 | 34.33 ± 1.21 | - |
| 5.01 ± 0.08 | 13155 ± 362 | 6.68 ± 0.24 | 151.19 ± 5.43 | 27.78 ± 1.03 | - |
| 10.03 ± 0.12 | 13200 ± 121 | 12.53 ± 0.29 | 283.59 ± 6.56 | 33.13 ± 0.66 | - |
| 30.02 ± 1.14 | 13295 ± 305 | 24.01 ± 0.34 | 543.81 ± 7.70 | 37.19 ± 0.99 | 99.06 ± 0.56 |
| 60.76 ± 0.18 | 13425 ± 251 | 12.79 ± 0.22 | 289.67 ± 4.98 | 33.89 ± 1.14 | - |
| 101.66 ± 0.29 | 13075 ± 315 | 4.59 ± 0.09 | 104.03 ± 2.03 | 42.49 ± 2.12 | - |
| 5.03 ± 0.04 | 21610 ± 805 | 8.68 ± 0.32 | 196.51 ± 7.24 | 12.26 ± 0.24 | - |
| 10.07 ± 0.03 | 21600 ± 925 | 14.28 ± 0.33 | 323.29 ± 7.47 | 15.25 ± 0.76 | - |
| 29.68 ± 0.11 | 22190 ± 358 | 29.68 ± 0.63 | 672.13 ± 14.27 | 19.69 ± 0.99 | - |
| 59.75 ± 0.29 | 21080 ± 287 | 35.99 ± 0.45 | 815.10 ± 10.19 | 18.22 ± 0.98 | 99.40 ± 2.04 |
| 102.00 ± 2.44 | 21710 ± 1002 | 5.36 ± 0.09 | 120.00 ± 2.02 | 24.74 ± 1.24 | - |
| 5.03 ± 0.01 | 25175 ± 1205 | 8.98 ± 0.35 | 202.57 ± 7.89 | 16.12 ± 0.86 | - |
| 10.06 ± 0.02 | 25200 ± 925 | 14.42 ± 0.40 | 326.46 ± 9.05 | 17.20 ± 1.09 | - |
| 30.05 ± 0.10 | 25413 ± 1102 | 14.45 ± 0.24 | 327.23 ± 5.43 | 22.09 ± 1.12 | - |
| 60.08 ± 0.25 | 25013 ± 852 | 23.29 ± 0.64 | 527.40 ± 14.49 | 21.64 ± 1.08 | 98.70 ± 1.22 |
| 101.87 ± 2.02 | 25425 ± 912 | 5.20 ± 0.07 | 151.29 ± 2.04 | 26.65 ± 1.34 | - |
| Initial Cr(VI) (mg L−1) | Initial d-COD (mg L−1) | Cr(VI) Reduction Rate (mg L−1 h−1) | Cr(VI) Reduction Rate (g m−2 d−1) | d-COD Removal (Cr-WE-Filter) (%) | Total d-COD Removal (%) |
|---|---|---|---|---|---|
| 5.12 ± 0.04 | 1151 ± 38 | 2.59 ± 0.04 | 58.62 ± 0.9 | 5.10 ± 0.06 | - |
| 10.22 ± 0.11 | 1109 ± 27 | 1.58 ± 0.02 | 35.76 ± 0.45 | 8.44 ± 0.12 | - |
| 4.56 ± 0.03 | 5108 ± 99 | 5.72 ± 0.09 | 151.05 ± 2.38 | 13.28 ± 0.24 | - |
| 10.01 ± 0.14 | 5005 ± 105 | 12.54 ± 0.27 | 284.28 ± 6.12 | 17.15 ± 0.39 | - |
| 30.82 ± 0.74 | 5010 ± 89 | 12.56 ± 0.31 | 279.08 ± 6.89 | 19.22 ± 0.52 | 91.10 ± 2.04 |
| 59.20 ± 0.33 | 5118 ± 152 | 7.89 ± 0.18 | 178.58 ± 4.07 | 25.15 ± 0.43 | - |
| 101.04 ± 1.03 | 5110 ± 122 | 4.36 ± 0.06 | 98.68 ± 1.36 | 27.33 ± 0.91 | - |
| 4.91 ± 0.05 | 13065 ± 208 | 7.55 ± 0.24 | 170.89 ± 5.43 | 17.08 ± 0.41 | - |
| 10.53 ± 0.18 | 13120 ± 305 | 17.55 ± 0.47 | 397.23 ± 10.63 | 18.23 ± 0.51 | - |
| 31.12 ± 0.22 | 13030 ± 287 | 30.12 ± 0.75 | 681.74 ± 16.97 | 21.85 ± 0.63 | - |
| 60.06 ± 0.49 | 13025 ± 299 | 43.01 ± 1.12 | 973.50 ± 25.35 | 29.25 ± 0.61 | 92.01 ± 1.89 |
| 98.7 ± 0.89 | 13295 ± 327 | 6.32 ± 0.25 | 143.05 ± 5.66 | 30.39 ± 0.78 | - |
| 5.03 ± 0.02 | 21800 ± 758 | 7.06 ± 0.31 | 227.70 ± 10.00 | 15.06 ± 0.44 | - |
| 10.27 ± 0.18 | 21250 ± 993 | 13.69 ± 0.47 | 309.86 ± 10.64 | 16.32 ± 0.57 | - |
| 29.55 ± 0.59 | 22000 ± 875 | 24.73 ± 0.61 | 636.20 ± 15.69 | 18.08 ± 0.61 | - |
| 60.77 ± 1.51 | 21380 ± 741 | 25.01 ± 0.41 | 566.08 ± 9.28 | 21.99 ± 0.49 | 91.80 ± 1.04 |
| 101.00 ± 2.01 | 21010 ± 974 | 5.06 ± 0.05 | 114.53 ± 1.13 | 25.89 ± 0.86 | - |
| 5.13 ± 0.03 | 25250 ± 1108 | 6.26 ± 0.19 | 232.22 ± 7.05 | 4.22 ± 0.04 | - |
| 10.10 ± 0.09 | 25450 ± 899 | 12.46 ± 0.22 | 304.66 ± 5.38 | 10.12 ± 0.21 | - |
| 29.05 ± 0.48 | 25150 ± 948 | 12.20 ± 0.34 | 298.77 ± 8.32 | 13.49 ± 0.38 | - |
| 59.88 ± 0.97 | 25300 ± 832 | 17.29 ± 0.52 | 536.61 ± 16.14 | 17.89 ± 0.47 | 90.50 ± 2.84 |
| 102.10 ± 1.98 | 25400 ± 1032 | 4.52 ± 0.05 | 102.23 ± 1.13 | 19.32 ± 0.64 | - |
| Initial Cr(VI) (mg L−1) | Initial d-COD (mg L−1) | Cr(VI) Reduction Rate (mg L−1 h−1) | Cr(VI) Reduction Rate (g m−2 d−1) | d-COD Removal (Cr-SCW-Filter) (%) |
|---|---|---|---|---|
| 5.01 ± 0.03 | 987 ± 10.21 | 6.68 ± 0.29 | 151.19 ± 6.56 | 24.52 ± 0.78 |
| 10.04 ± 0.25 | 996 ± 21.99 | 5.02 ± 0.14 | 113.68 ± 3.17 | 26.27 ± 0.99 |
| 5.01 ± 0.06 | 5123 ± 132 | 12.02 ± 0.14 | 272.12 ± 3.17 | 28.06 ± 1.12 |
| 9.79 ± 0.58 | 5058 ± 99 | 16.79 ± 0.31 | 380.24 ± 7.02 | 32.28 ± 1.07 |
| 29.96 ± 0.22 | 4988 ± 138 | 4.13 ± 0.11 | 93.60 ± 2.49 | 36.54 ± 0.93 |
| 72.59 ± 0.66 | 5029 ± 103 | 1.68 ± 0.03 | 38.03 ± 0.68 | 38.35 ± 1.11 |
| 5.05 ± 0.05 | 13120 ± 322 | 20.18 ± 0.34 | 457.08 ± 7.07 | 33.88 ± 1.27 |
| 30.09 ± 0.04 | 12995 ± 205 | 12.04 ± 0.29 | 272.61 ± 6.57 | 43.75 ± 1.38 |
| 60.94 ± 0.44 | 12800 ± 259 | 7.62 ± 0.25 | 172.51 ± 5.66 | 47.34 ± 1.94 |
| 101.69 ± 1.50 | 12715 ± 223 | 4.42 ± 0.09 | 100.13 ± 2.04 | 49.67 ± 2.02 |
| 4.96 ± 0.14 | 20760 ± 823 | 19.85 ± 0.28 | 449.46 ± 6.34 | 34.06 ± 1.14 |
| 20.09 ± 0.29 | 21400 ± 742 | 30.21 ± 0.58 | 684.26 ± 10.87 | 38.08 ± 0.96 |
| 29.94 ± 0.62 | 21200 ± 906 | 19.96 ± 0.31 | 452.00 ± 7.02 | 40.09 ± 1.21 |
| * 60.09 ± 0.12 | 21420 ± 759 | 3.98 ± 0.03 | 90.08 ± 0.68 | 42.44 ± 1.04 |
| * 100.27 ± 1.68 | 21400 ± 1014 | 3.57 ± 0.06 | 80.80 ± 1.36 | 43.78 ± 1.19 |
| 4.92 ± 0.09 | 25488 ± 1017 | 19.69 ± 0.19 | 445.94 ± 4.30 | 16.28 ± 0.34 |
| 30.00 ± 0.06 | 25475 ± 898 | 20.00 ± 0.24 | 452.88 ± 4.43 | 29.32 ± 0.59 |
| * 60.61 ± 0.74 | 24760 ± 988 | 5.04 ± 0.08 | 114.07 ± 1.81 | 31.07 ± 0.81 |
| * 102.41 ± 1.79 | 25123 ± 1003 | 4.26 ± 0.11 | 96.42 ± 2.48 | 32.06 ± 0.74 |
| Initial Cr(VI) (mg L−1) | Initial d-COD (mg L−1) | Cr(VI) Reduction Rate (mg L−1 h−1) | Cr(VI) Reduction Rate (g m−2 d−1) | d-COD Removal (Cr-WE-Filter) (%) |
|---|---|---|---|---|
| 5.32 ± 0.03 | 1120 ± 19 | 7.09 ± 0.19 | 160.47 ± 4.3 | 6.50 ± 0.12 |
| 10.12 ± 0.18 | 1080 ± 28 | 6.50 ± 0.11 | 147.12 ± 2.48 | 9.50 ± 0.24 |
| 5.27 ± 0.04 | 4980 ± 95 | 16.23 ± 0.32 | 367.36 ± 7.24 | 14.16 ± 0.37 |
| 10.13 ± 0.11 | 5132 ± 156 | 20.76 ± 0.52 | 469.89 ± 11.77 | 17.26 ± 0.51 |
| 30.25 ± 0.22 | 4990 ± 87 | 17.41 ± 0.59 | 394.06 ± 6.56 | 19.05 ± 0.43 |
| 68.96 ± 0.51 | 5110 ± 190 | 6.90 ± 0.19 | 156.17 ± 4.3 | 21.52 ± 0.57 |
| 5.15 ± 0.02 | 13030 ± 197 | 26.40 ± 0.55 | 597.54 ± 12.45 | 15.44 ± 0.38 |
| 30.15 ± 0.31 | 13100 ± 209 | 23.71 ± 0.41 | 536.66 ± 9.28 | 23.55 ± 0.58 |
| 59.84 ± 0.47 | 12900 ± 274 | 9.62 ± 0.21 | 341.77 ± 7.46 | 24.44 ± 0.67 |
| 100.09 ± 1.21 | 12855 ± 328 | 10.20 ± 0.19 | 230.87 ± 4.3 | 29.67 ± 0.76 |
| 5.06 ± 0.04 | 20520 ± 487 | 5.32 ± 0.09 | 120.41 ± 2.04 | 8.55 ± 0.23 |
| 10.22 ± 0.31 | 21100 ± 854 | 5.89 ± 0.12 | 133.31 ± 2.72 | 9.87 ± 0.19 |
| * 21.19 ± 0.38 | 21150 ± 902 | 14.83 ± 0.34 | 335.66 ± 7.70 | 12.81 ± 0.47 |
| * 30.04 ± 0.29 | 20800 ± 855 | 10.60 ± 0.22 | 239.92 ± 4.98 | 13.09 ± 0.32 |
| * 61.19 ± 0.61 | 20950 ± 932 | 2.56 ± 0.03 | 57.94 ± 0.68 | 14.49 ± 0.42 |
| * 101.17 ± 1.32 | 21100 ± 844 | 1.53 ± 0.02 | 34.63 ± 0.45 | 21.78 ± 0.62 |
| 5.13 ± 0.03 | 25400 ± 1009 | 3.22 ± 0.06 | 72.88 ± 1.35 | 5.32 ± 0.07 |
| 10.32 ± 0.27 | 24900 ± 954 | 3.54 ± 0.05 | 80.13 ± 1.13 | 8.05 ± 0.17 |
| * 31.55 ± 0.37 | 25150 ± 784 | 1.71 ± 0.04 | 15.66 ± 0.09 | 12.02 ± 0.27 |
| * 61.01 ± 0.54 | 25600 ± 1109 | 1.52 ± 0.02 | 34.40 ± 0.45 | 12.88 ± 0.35 |
| * 100.55 ± 0.98 | 24980 ± 1005 | 1.26 ± 0.01 | 28.51 ± 0.22 | 15.09 ± 0.37 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatoulis, T.I.; Michailides, M.K.; Tekerlekopoulou, A.G.; Akratos, C.S.; Pavlou, S.; Vayenas, D.V. Simultaneous Treatment of Agro-Industrial and Industrial Wastewaters: Case Studies of Cr(VI)/Second Cheese Whey and Cr(VI)/Winery Effluents. Water 2018, 10, 382. https://doi.org/10.3390/w10040382
Tatoulis TI, Michailides MK, Tekerlekopoulou AG, Akratos CS, Pavlou S, Vayenas DV. Simultaneous Treatment of Agro-Industrial and Industrial Wastewaters: Case Studies of Cr(VI)/Second Cheese Whey and Cr(VI)/Winery Effluents. Water. 2018; 10(4):382. https://doi.org/10.3390/w10040382
Chicago/Turabian StyleTatoulis, Triantafyllos I., Michail K. Michailides, Athanasia G. Tekerlekopoulou, Christos S. Akratos, Stavros Pavlou, and Dimitrios V. Vayenas. 2018. "Simultaneous Treatment of Agro-Industrial and Industrial Wastewaters: Case Studies of Cr(VI)/Second Cheese Whey and Cr(VI)/Winery Effluents" Water 10, no. 4: 382. https://doi.org/10.3390/w10040382
APA StyleTatoulis, T. I., Michailides, M. K., Tekerlekopoulou, A. G., Akratos, C. S., Pavlou, S., & Vayenas, D. V. (2018). Simultaneous Treatment of Agro-Industrial and Industrial Wastewaters: Case Studies of Cr(VI)/Second Cheese Whey and Cr(VI)/Winery Effluents. Water, 10(4), 382. https://doi.org/10.3390/w10040382







