Removal of Chloramphenicol from Aqueous Solution Using Low-Cost Activated Carbon Prepared from Typha orientalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Activated Carbon
2.3. Characterization of Activated Carbon
2.4. Adsorption Experiments
2.5. Desorption Experiments
2.6. Adsorption of CAP in Realistic Water Environment
3. Results and Discussion
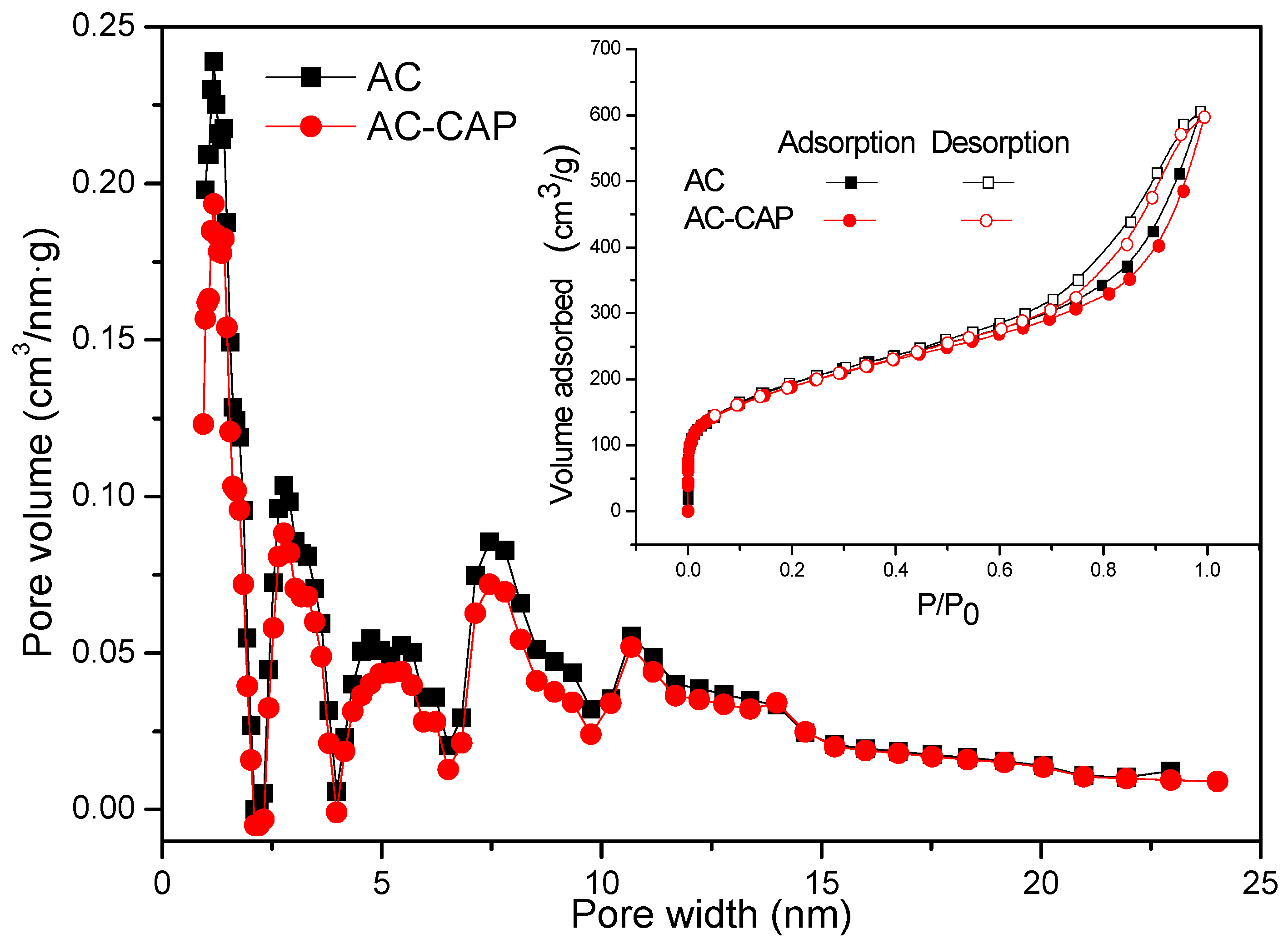
3.1. Physical and Chemical Properties of AC
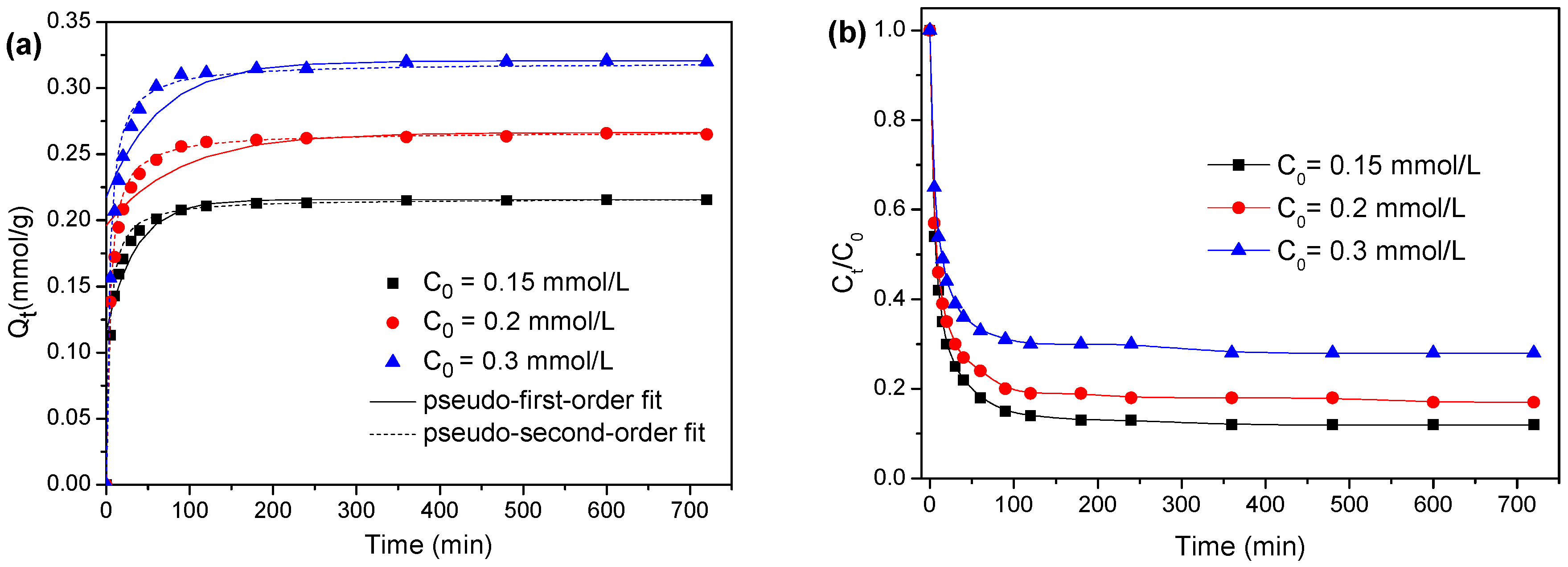
3.2. Adsorption Kinetics
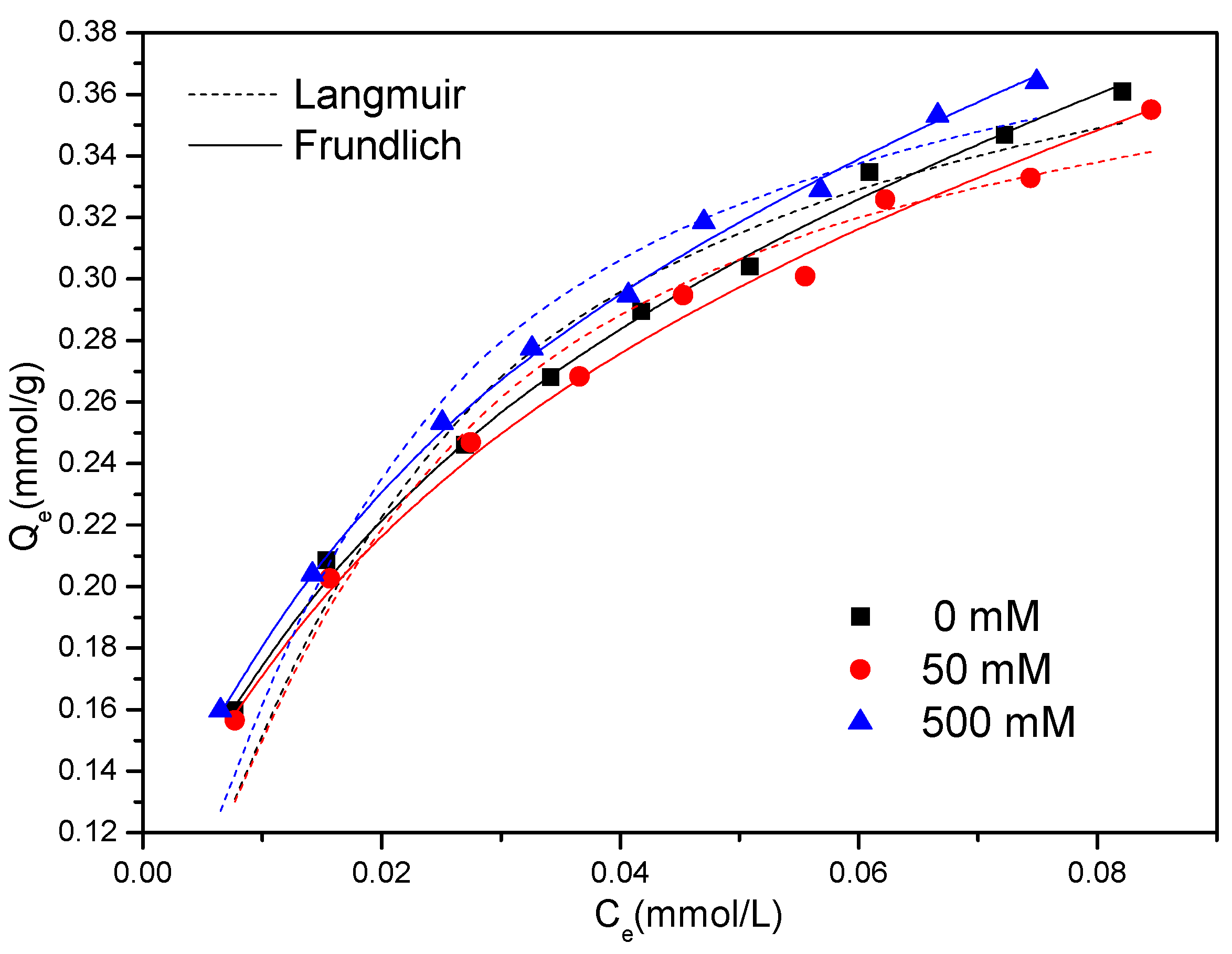
3.3. Adsorption Isotherms
3.4. Effect of the Initial pH
3.5. Desorption Experiment
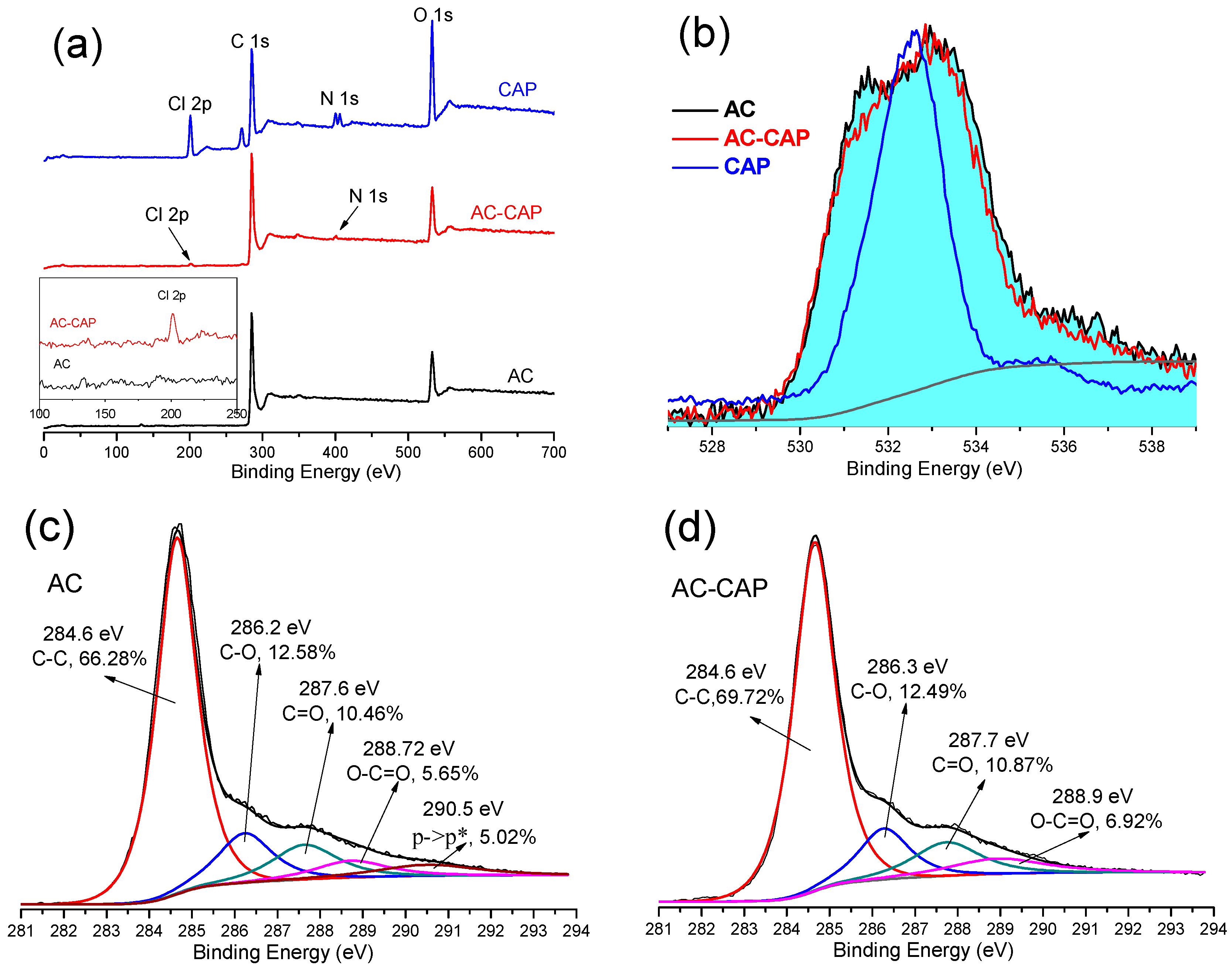
3.6. Mechanism for CAP Adsorption on the AC
3.7. Comparison with Other Adsorbents
3.8. Adsorption of CAP in Realistic Water Environment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hanekamp, J.C.; Bast, A. Antibiotics exposure and health risks: Chloramphenicol. Environ. Toxicol. Pharmacol. 2015, 39, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Hamidi-Asl, E.; Dardenne, F.; Blust, R.; De Wael, K. An improved electrochemical aptasensor for chloramphenicol detection based on aptamer incorporated gelatine. Sensors 2015, 15, 7605–7618. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A. Antibiotic side effects. Med. Clin. N. Am. 2001, 85, 149–185. [Google Scholar] [CrossRef]
- Doody, M.M.; Linet, M.S.; Glass, A.G.; Curtis, R.E.; Pottern, L.M.; Rush, B.B.; Boice, J.D., Jr.; Fraumeni, J.F., Jr.; Friedman, G.D. Risks of non-hodgkin’s lymphoma, multiple myeloma, and leukemia associated with common medications. Epidemiology 1996, 7, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Krasinski, K.; Perkin, R.; Rutledge, J.C. Gray baby syndrome revisited. Clin. Pediatr. 1982, 21, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Neu, H.C. The crisis in antibiotic resistance. Science 1992, 257, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission decision 2003/181/EC of 13 March 2003. Off. J. Eur. Commun. 2003, 71, 17–18. [Google Scholar]
- Zhou, C.; Zhang, X.; Huang, X.; Guo, X.; Cai, Q.; Zhu, S. Rapid detection of chloramphenicol residues in aquatic products using colloidal gold immunochromatographic assay. Sensors 2014, 14, 21872–21888. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, G.; Liu, C.-Q.; Li, L.; Xiang, M. The occurrence of chloramphenicol and tetracyclines in municipal sewage and the Nanming river, Guiyang city, China. J. Environ. Monit. 2009, 11, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Gu, Z.; Zhang, Z.; Zhang, J.; Hermanowicz, S.W. Removal of chloramphenicol from aqueous solution by nanoscale zero-valent iron particles. Chem. Eng. J. 2014, 257, 98–104. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef] [PubMed]
- Czech, B.; Rubinowska, K. TiO2-assisted photocatalytic degradation of diclofenac, metoprolol, estrone and chloramphenicol as endocrine disruptors in water. Adsorption 2013, 19, 619–630. [Google Scholar] [CrossRef]
- Csay, T.; Rácz, G.; Takács, E.; Wojnárovits, L. Radiation induced degradation of pharmaceutical residues in water: Chloramphenicol. Radiat. Phys. Chem. 2012, 81, 1489–1494. [Google Scholar] [CrossRef]
- Wang, J.; Sun, W.; Xu, C.; Liu, W. Ozone degradation of chloramphenicol: Efficacy, products and toxicity. Int. J. Environ. Technol. Manag. 2012, 15, 180–192. [Google Scholar] [CrossRef]
- Xie, F. Research on irradiation-induced degradation products and mechanism of chloramphenicol in animal derived food [MSc thesis]. China BJ Chin. Acad. Agric. Sci. 2008, 16, 34. [Google Scholar]
- Zhang, Y.; Shao, Y.; Gao, N.; Gao, Y.; Chu, W.; Li, S.; Wang, Y.; Xu, S. Kinetics and by-products formation of chloramphenicol (CAP) using chlorination and photocatalytic oxidation. Chem. Eng. J. 2018, 333, 85–91. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Wu, H.; Guo, Z.; Cheng, C.; Zhang, C. Effect on physical and chemical characteristics of activated carbon on adsorption of trimethoprim: Mechanisms study. RSC Adv. 2015, 5, 85187–85195. [Google Scholar] [CrossRef]
- Zhu, Z.; Xie, J.; Zhang, M.; Zhou, Q.; Liu, F. Insight into the adsorption of PPCPs by porous adsorbents: Effect of the properties of adsorbents and adsorbates. Environ. Pollut. 2016, 214, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; He, J.; Xie, A.; Gao, L.; Pan, J.; Chen, X.; Zhou, Z.; Wei, X.; Yan, Y. Novel pitaya-inspired well-defined core–shell nanospheres with ultrathin surface imprinted nanofilm from magnetic mesoporous nanosilica for highly efficient chloramphenicol removal. Chem. Eng. J. 2016, 284, 812–822. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, X.; Cao, Z.; Zhan, Y.; Shi, X.; Yang, Y.; Zhou, J.; Xu, J. Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes. J. Hazard. Mater. 2016, 310, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Shi, Y.; Xu, W.; Potter, N.; Li, Z.; Zhu, J. Modification of clays and zeolites by ionic liquids for the uptake of chloramphenicol from water. Chem. Eng. J. 2017, 313, 336–344. [Google Scholar] [CrossRef]
- Liao, P.; Zhan, Z.; Dai, J.; Wu, X.; Zhang, W.; Wang, K.; Yuan, S. Adsorption of tetracycline and chloramphenicol in aqueous solutions by bamboo charcoal: A batch and fixed-bed column study. Chem. Eng. J. 2013, 228, 496–505. [Google Scholar] [CrossRef]
- Mohd Din, A.T.; Ahmad, M.A.; Hameed, B.H. Ordered mesoporous carbons originated from non-edible polyethylene glycol 400 (PEG-400) for chloramphenicol antibiotic recovery from liquid phase. Chem. Eng. J. 2015, 260, 730–739. [Google Scholar] [CrossRef]
- Muller, J.; Huaux, F.; Moreau, N.; Misson, P.; Heilier, J.-F.; Delos, M.; Arras, M.; Fonseca, A.; Nagy, J.B.; Lison, D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl. Pharm. 2005, 207, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, B.; Yuan, S.; Wu, X.; Chen, J.; Wang, L. Adsorptive removal of chloramphenicol from wastewater by NaOH modified bamboo charcoal. Bioresour. Technol. 2010, 101, 7661–7664. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, W.; Zhang, J.; Zhang, C.; Ren, L.; Li, Y. Removal of cephalexin from aqueous solutions by original and Cu(II)/Fe(III) impregnated activated carbons developed from lotus stalks kinetics and equilibrium studies. J. Hazard. Mater. 2011, 185, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhang, J.; Zhang, C.; Li, C.; Zhang, B.; Hu, W.; Xu, J.; Zhao, R. Preparation of activated carbon from cattail and its application for dyes removal. J. Environ. Sci. 2010, 22, 91–97. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Q.; Zhang, C.; Xu, J.; Zhai, B.; Zhang, B. Adsorption of neutral red onto Mn-impregnated activated carbons prepared from Typha orientalis. Bioresour. Technol. 2008, 99, 8974–8980. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Adams, C. Potentiometric determination of acid dissociation constants (pKa) for human and veterinary antibiotics. Water Res. 2004, 38, 2874–2890. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Boehm, H.P. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Noh, J.S.; Schwarz, J.A. Estimation of the point of zero charge of simple oxides by mass titration. J. Colloid Interface Sci. 1989, 130, 157–164. [Google Scholar] [CrossRef]
- Eaton, A.; Clesceri, L.; Greenberg, A. Standard Methods for Examination of Water and Wastewater; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 1998. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark Alexander, V.; Olivier James, P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing Kenneth, S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar]
- Gabyshev, V.A.; Gabysheva, O.I. Adsorption of tetracycline and sulfamethoxazole on crop residue-derived ashes: Implication for the relative importance of black carbon to soil sorption. Environ. Sci. Technol. 2011, 45, 5580–5586. [Google Scholar]
- Kasaoka, S.; Sakata, Y.; Tanaka, E.; Naitoh, R. Design of molecular-sieve carbon. Studies on the adsorption of various dyes in the liquid phase. Int. Chem. Eng. 1989, 29, 734–742. [Google Scholar]
- Xue, C.; Qi, P.; Li, M.; Liu, Y. Characterization and sorptivity of the plesiomonas shigelloides strain and its potential use to remove Cd2+ from wastewater. Water 2016, 8, 241. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Qin, L.; Zhou, Z.; Dai, J.; Ma, P.; Zhao, H.; He, J.; Xie, A.; Li, C.; Yan, Y. Novel N-doped hierarchically porous carbons derived from sustainable shrimp shell for high-performance removal of sulfamethazine and chloramphenicol. J. Taiwan Inst. Chem. E 2016, 62, 228–238. [Google Scholar] [CrossRef]
- Ma, W.; Dai, J.; Dai, X.; Da, Z.; Yan, Y. Core–shell molecularly imprinted polymers based on magnetic chitosan microspheres for chloramphenicol selective adsorption. Monatshefte Chem. 2015, 146, 465–474. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Higuchi, T.; Marcus, A.D.; Bias, C.D. The kinetics of degradation of chloramphenicol in solution. II. Over-all disappearance rate from buffered solutions. J. Am. Pharm. Assoc. 1954, 43, 129–134. [Google Scholar] [CrossRef]
- Mitchell, S.M.; Ullman, J.L.; Teel, A.L.; Watts, R.J. Hydrolysis of amphenicol and macrolide antibiotics: Chloramphenicol, florfenicol, spiramycin, and tylosin. Chemosphere 2015, 134, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.-R.; Wang, Y.-Y.; Liu, W.-J.; Wang, Y.-K.; Jiang, H. Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar. Chem. Eng. J. 2014, 248, 168–174. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Sui, Z.-J.; Zhu, J.; Li, P.; Chen, D.; Dai, Y.-C.; Yuan, W.-K. Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR. Carbon 2007, 45, 785–796. [Google Scholar] [CrossRef]
- Xiao, F.; Pignatello, J.J. π+–π interactions between (hetero)aromatic amine cations and the graphitic surfaces of pyrogenic carbonaceous materials. Environ. Sci. Technol. 2015, 49, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K.; Sahedur Rahman, M. Chloramphenicol interaction with functionalized biochar in water: Sorptive mechanism, molecular imprinting effect and repeatable application. Sci. Total Environ. 2017, 609, 885–895. [Google Scholar] [CrossRef] [PubMed]
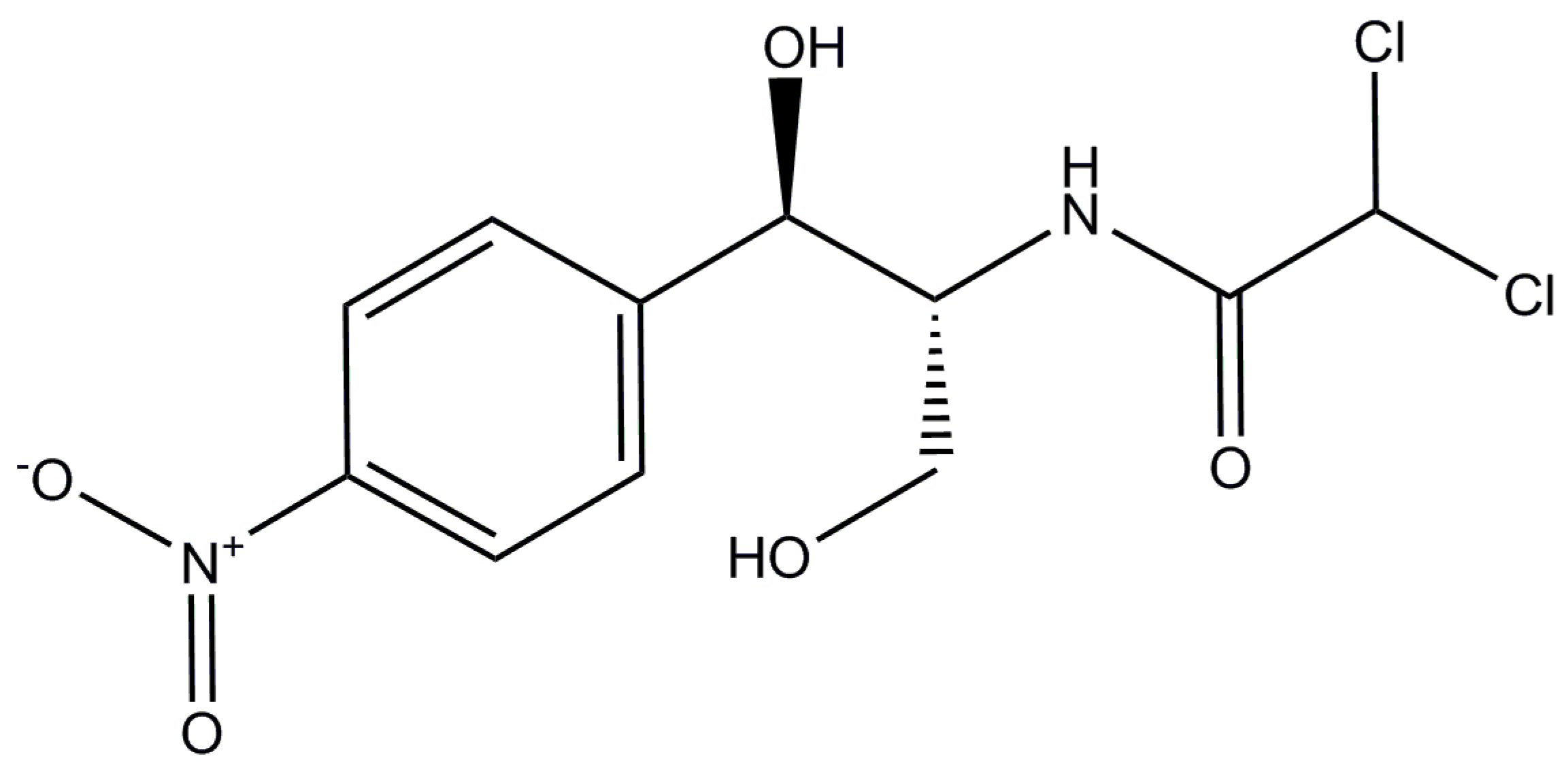
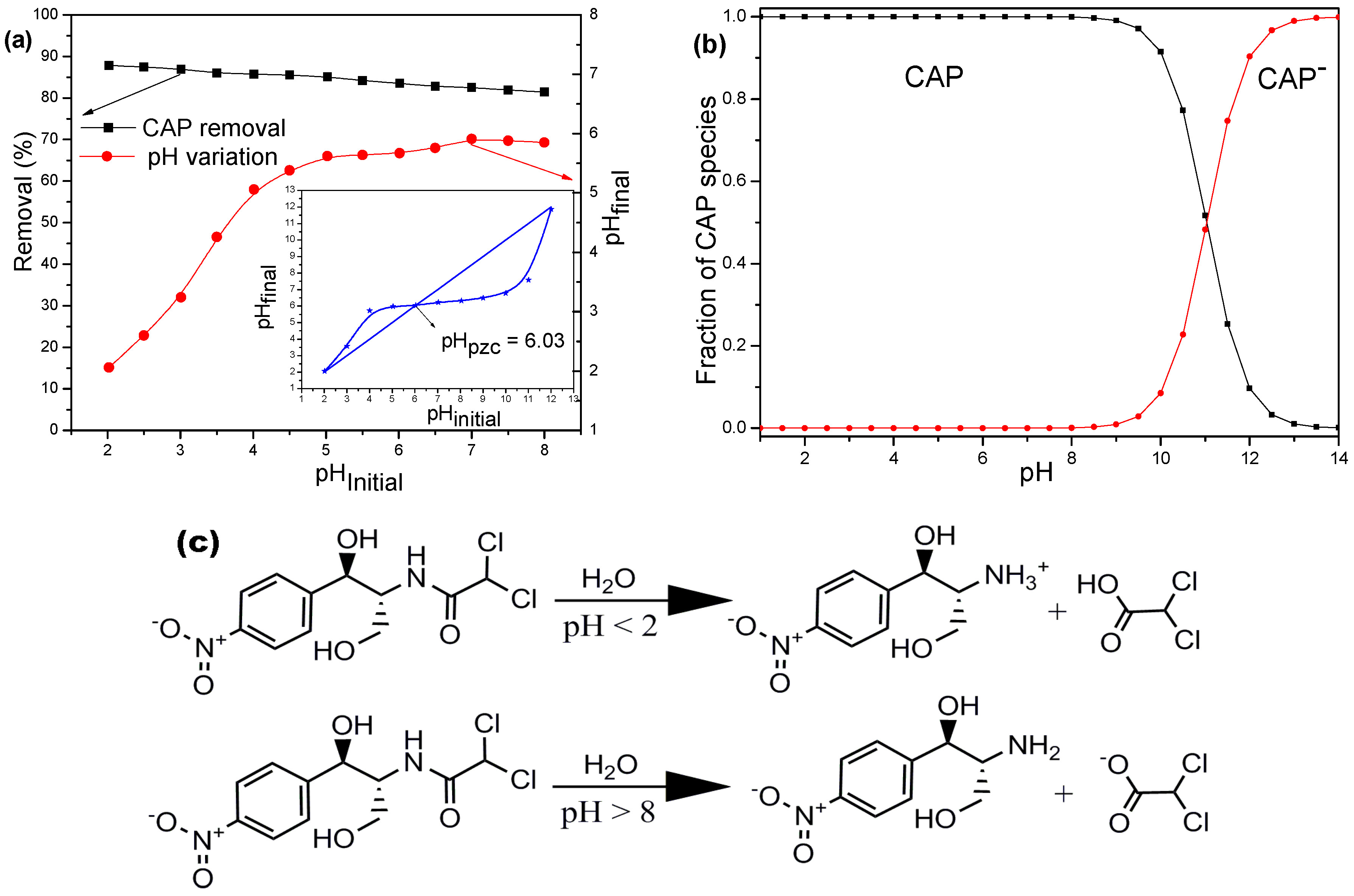

| Compound | Chemical Formula | Molecular Weight | log Kow [23] | pKa [30] |
|---|---|---|---|---|
| CAP | C11H12·Cl2O5N2 | 323.13 | 1.14 | 11.03 |
| Sample | SBET a (m2/g) | Sext b (m2/g) | Smic b (m2/g) | Vtot c (cm3/g) | Vmic b (cm3/g) | Vmic/Vtot (%) | Dp d (nm) | Yield (%) |
|---|---|---|---|---|---|---|---|---|
| AC | 794.8 | 543.4 | 251.4 | 1.266 | 0.154 | 12.2 | 6.37 | 40.03 |
| AC-CAP | 672.3 | 503.8 | 168.5 | 1.136 | 0.106 | 9.3 | 5.51 | - |
| Sample | Carboxyl (mmol/g) | Lactone (mmol/g) | Phenolic (mmol/g) | Total Acidic (mmol/g) | Total Base (mmol/g) | Total Groups (mmol/g) | pHpzc |
|---|---|---|---|---|---|---|---|
| AC | 0.695 | 0.596 | 0.787 | 2.078 | 0.995 | 3.073 | 6.03 |
| %/total groups | 22.62 | 19.39 | 25.61 | 67.62 | 32.38 | - | - |
| C0 (mmol/L) | Qe (exp) (mmol/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| Qe (cal) (mmol/g) | k1 (min−1) | R2 | Qe (cal) (mmol/g) | k2 (g/mg·min) | R2 | ||
| 0.15 | 0.215 | 0.0100 | 0.0282 | 0.9122 | 0.216 | 0.0143 | 0.9999 |
| 0.2 | 0.265 | 0.0702 | 0.0112 | 0.7532 | 0.266 | 0.0116 | 0.9999 |
| 0.3 | 0.320 | 0.0935 | 0.0157 | 0.7854 | 0.318 | 0.0097 | 0.9999 |
| Sample | Ionic Strength (mM) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| Qm (mmol/g) | KL (L/mmol) | R2 | KF (mmol1−1/n L1/n/g) | 1/n | R2 | ||
| AC | 0 | 0.424 | 58.2 | 0.9888 | 0.118 | 0.343 | 0.9979 |
| 50 | 0.407 | 61.2 | 0.9903 | 0.117 | 0.336 | 0.9959 | |
| 500 | 0.424 | 65.5 | 0.9893 | 0.123 | 0.432 | 0.9983 | |
| Desorption Treatment | Distilled Water | 0.01 M NaCl | 1 M NaCl | 0.1 M NaOH | 95% Ethanol |
|---|---|---|---|---|---|
| Desorption efficiency | 10.6% | 12.7% | 15.7% | 58.1% | 39.5% |
| Sample | O/C% | N/C% | Cl/C% |
|---|---|---|---|
| CAP | 97.61% | 22.97% | 36.66% |
| AC | 52.69% | 2.23% | - |
| AC-CAP | 56.51% | 5.74% | 1.69% |
| Sorbent | Qmax (mg/g) | Q50 (mg/g) a | Reference |
|---|---|---|---|
| TO-based activated carbon | 137.1 | 69.5 | This work |
| Calgon F400 | 140.3 | 38.4 | This work |
| Bamboo charcoal | 8.1 | - | [23] |
| Commercial BC-NaOH | <3 b | - | [26] |
| fBC-2 | 75.3 | - | [49] |
| MMSNs@MIPs | 41.7 | - | [20] |
| MMIPs | 17 | - | [41] |
| MWCNT-10 | 107.9 | - | [21] |
| ABA-16 (ordered mesoporous carbon) | 209.7 | 40.8 | [24] |
| Water types | pH | TOC (mg/L) | Total Hardness CaCO3 (mg/L) | Q (mmol/g) | Removal Rate (%) |
|---|---|---|---|---|---|
| Distilled water | 6.35 | 0 | 0 | 0.278 | 83.4 |
| Groundwater | 7.78 | 2.3 | 320.8 | 0.273 | 80.9 |
| Treated wastewater | 7.72 | 18.9 | 297.5 | 0.219 | 65.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, J.; Liu, H. Removal of Chloramphenicol from Aqueous Solution Using Low-Cost Activated Carbon Prepared from Typha orientalis. Water 2018, 10, 351. https://doi.org/10.3390/w10040351
Li Y, Zhang J, Liu H. Removal of Chloramphenicol from Aqueous Solution Using Low-Cost Activated Carbon Prepared from Typha orientalis. Water. 2018; 10(4):351. https://doi.org/10.3390/w10040351
Chicago/Turabian StyleLi, Yiran, Jian Zhang, and Hai Liu. 2018. "Removal of Chloramphenicol from Aqueous Solution Using Low-Cost Activated Carbon Prepared from Typha orientalis" Water 10, no. 4: 351. https://doi.org/10.3390/w10040351
APA StyleLi, Y., Zhang, J., & Liu, H. (2018). Removal of Chloramphenicol from Aqueous Solution Using Low-Cost Activated Carbon Prepared from Typha orientalis. Water, 10(4), 351. https://doi.org/10.3390/w10040351




