Sorption of Arsenic from Desalination Concentrate onto Drinking Water Treatment Solids: Operating Conditions and Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. RO Concentrate and Analysis
2.2. DWTS and Characterization
2.3. Sorption Experiments
3. Results and Discussion
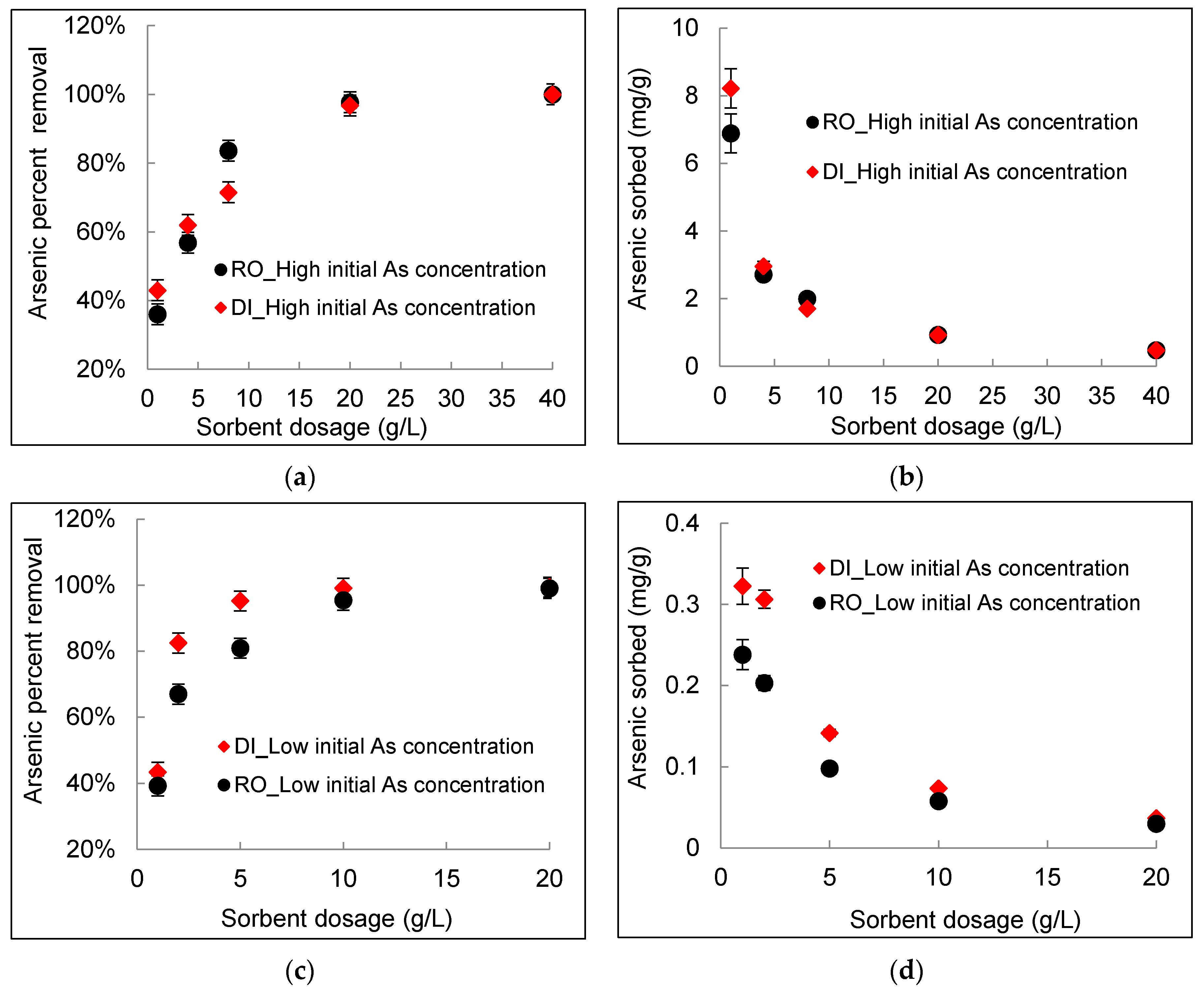
3.1. Effect of DWTS Dosage
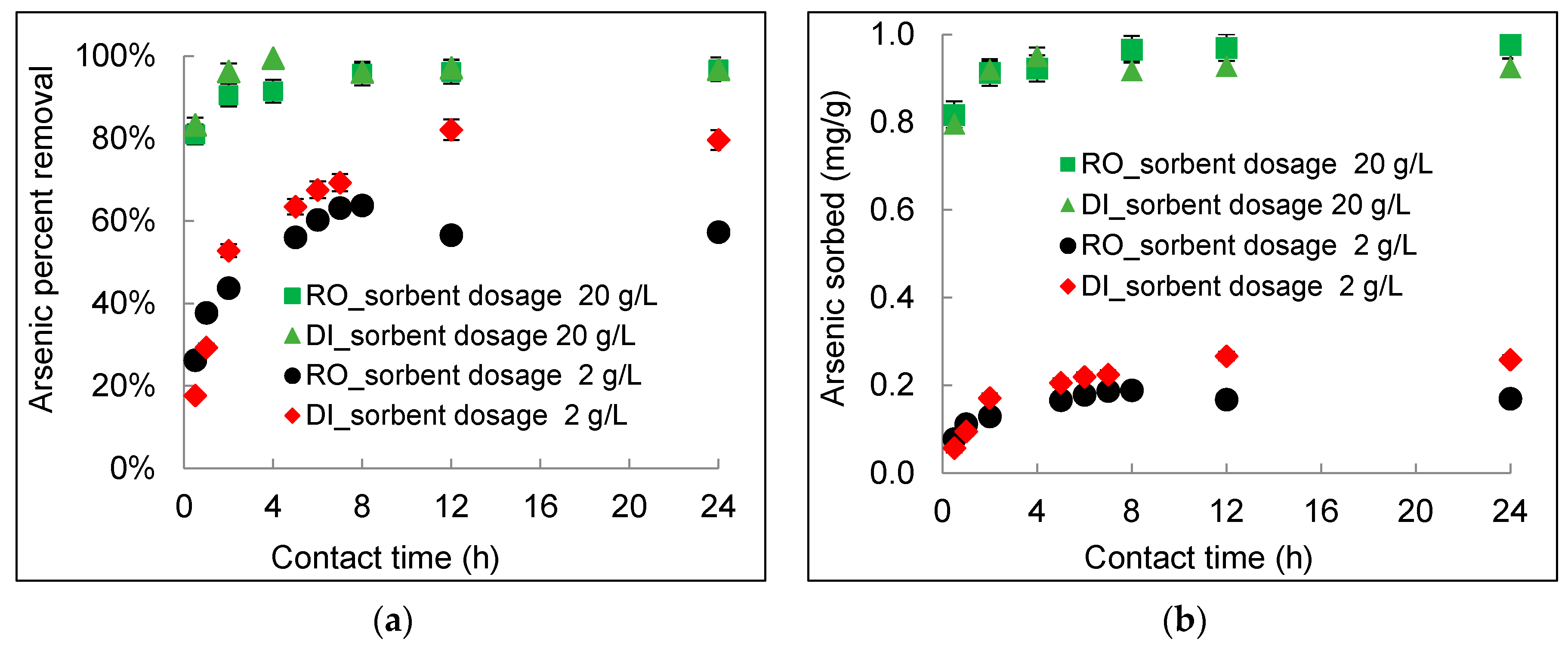
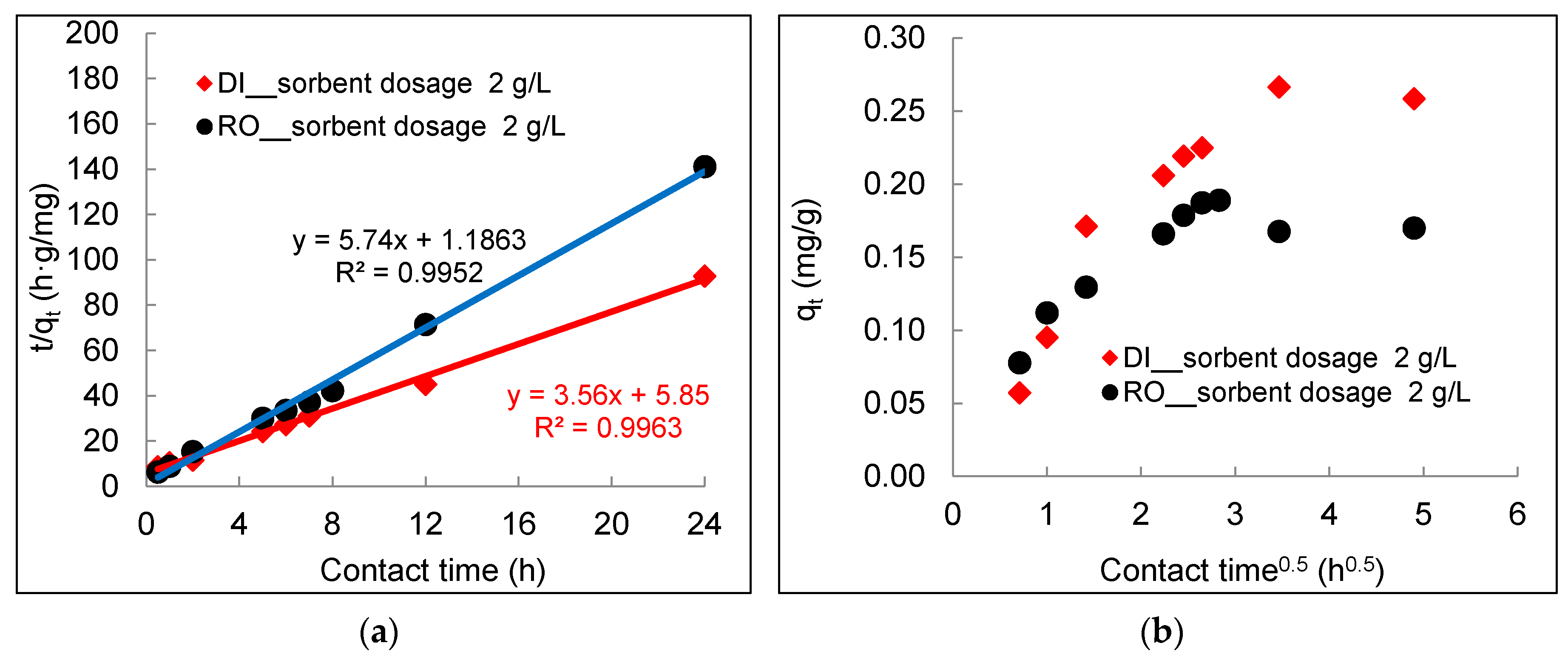
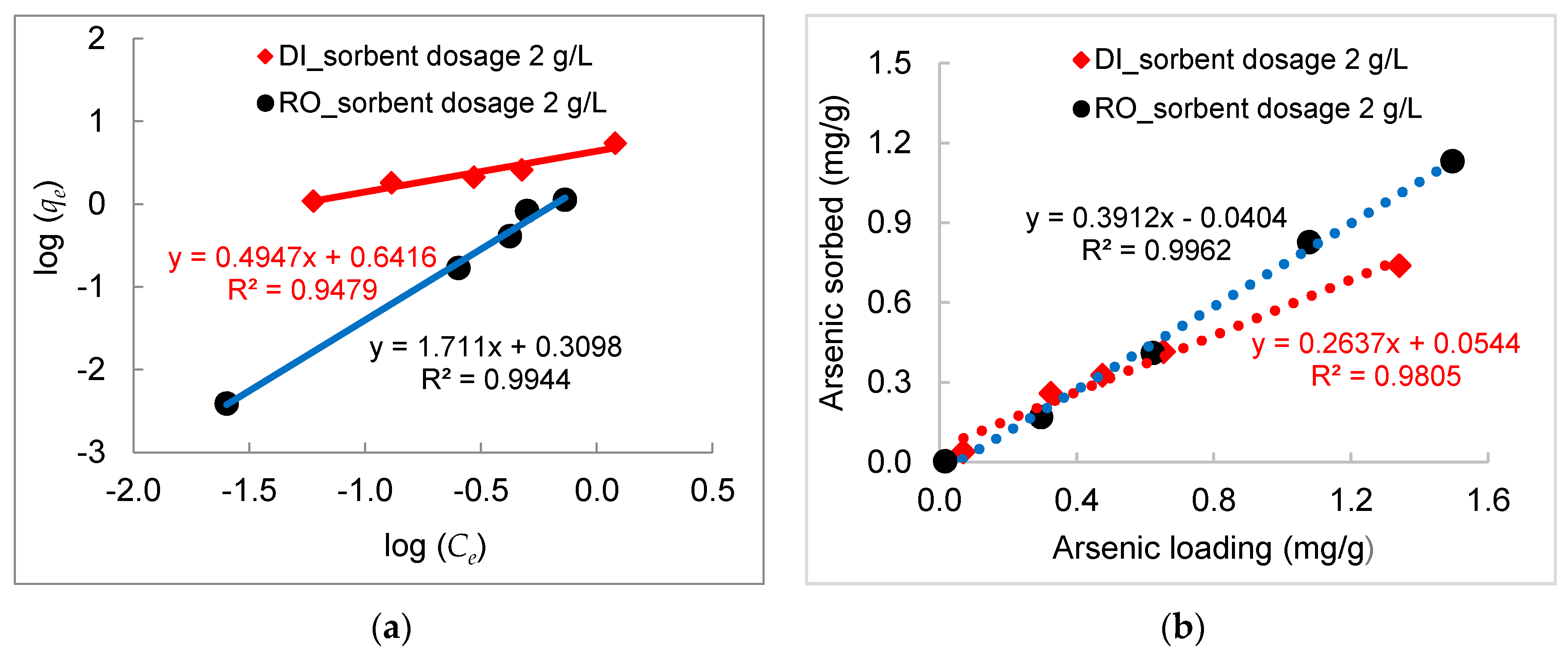
3.2. Kinetics of Arsenic Sorption onto DWTS
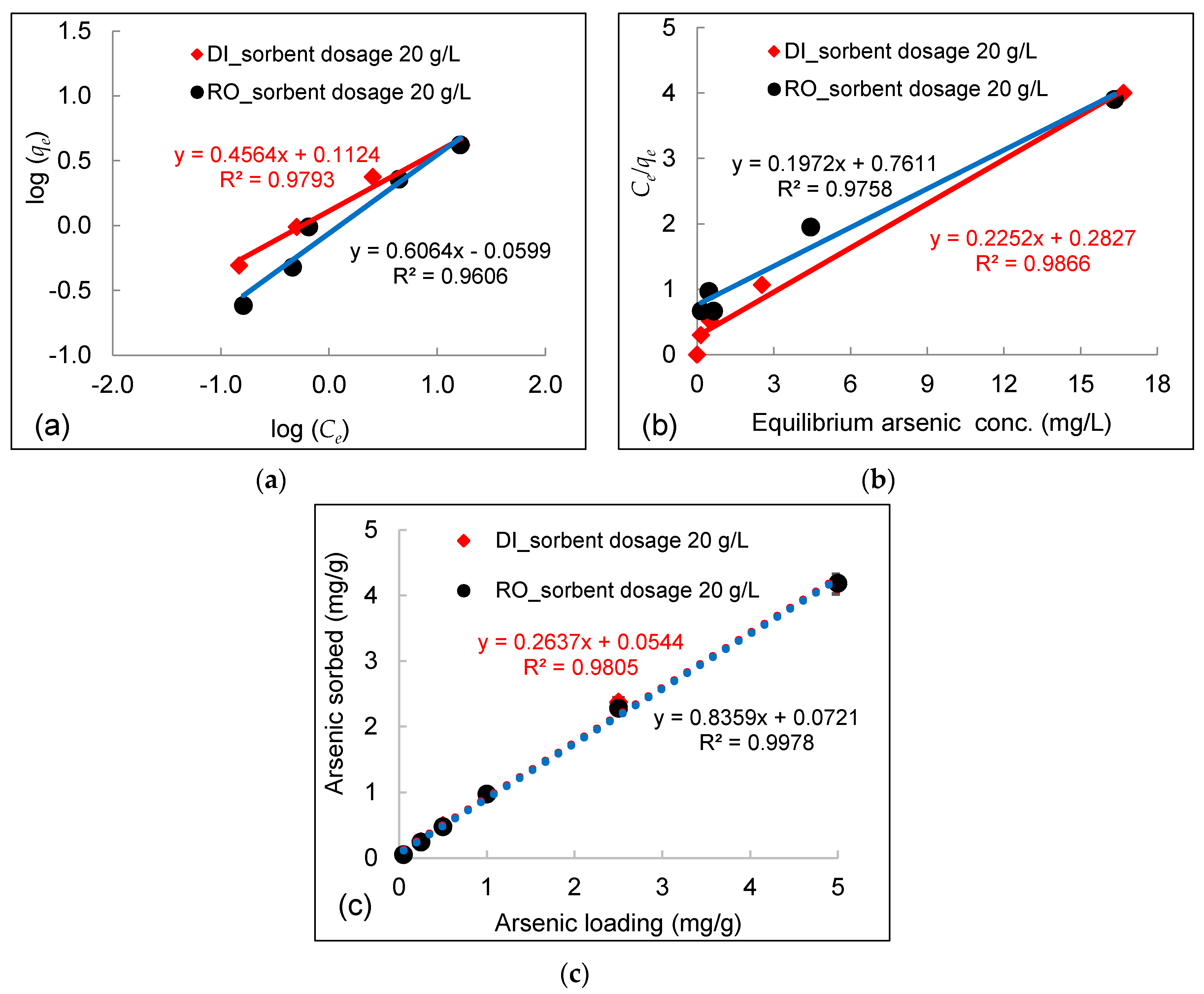
3.3. Effect of Initial Arsenic Concentration
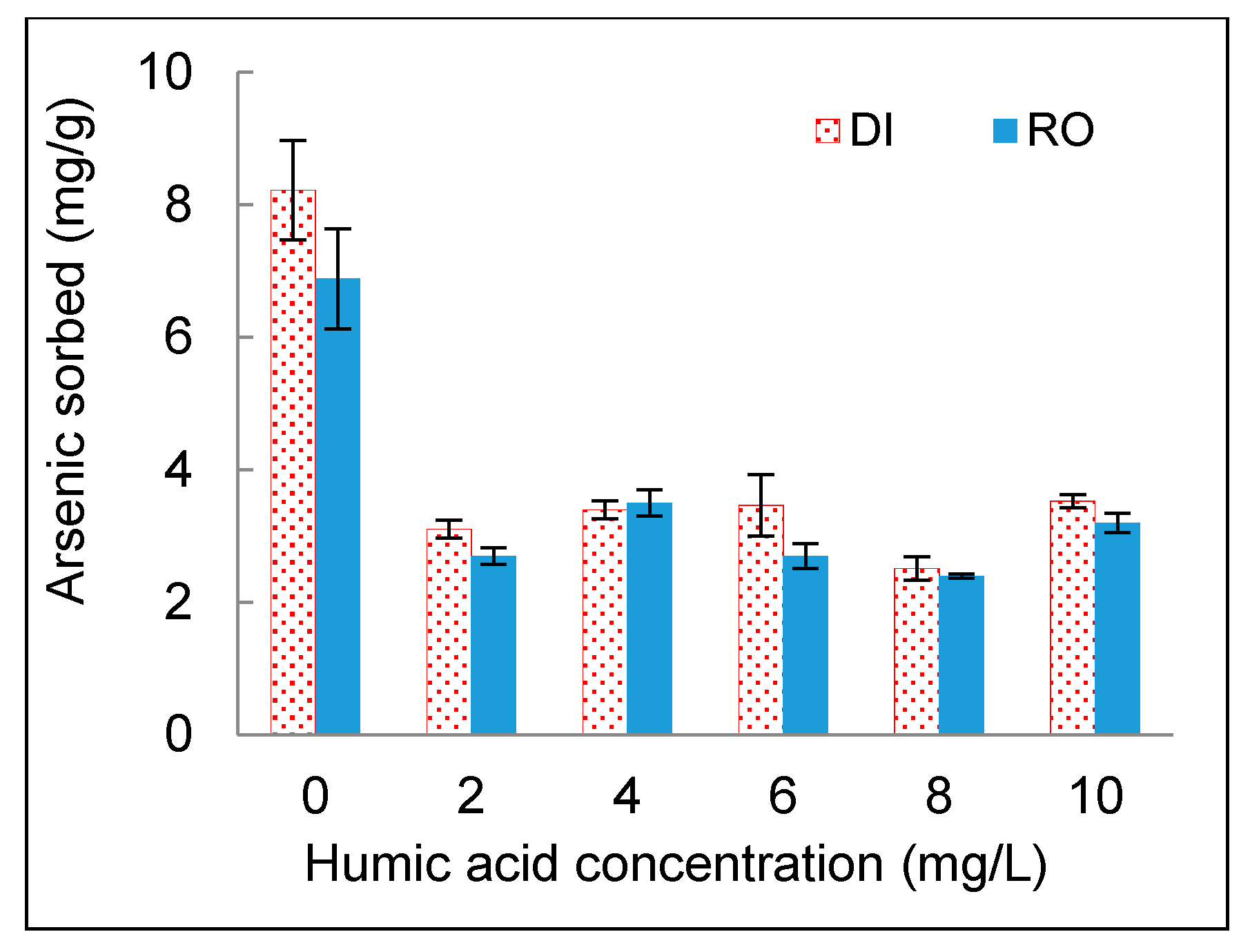
3.4. Effect of NOM
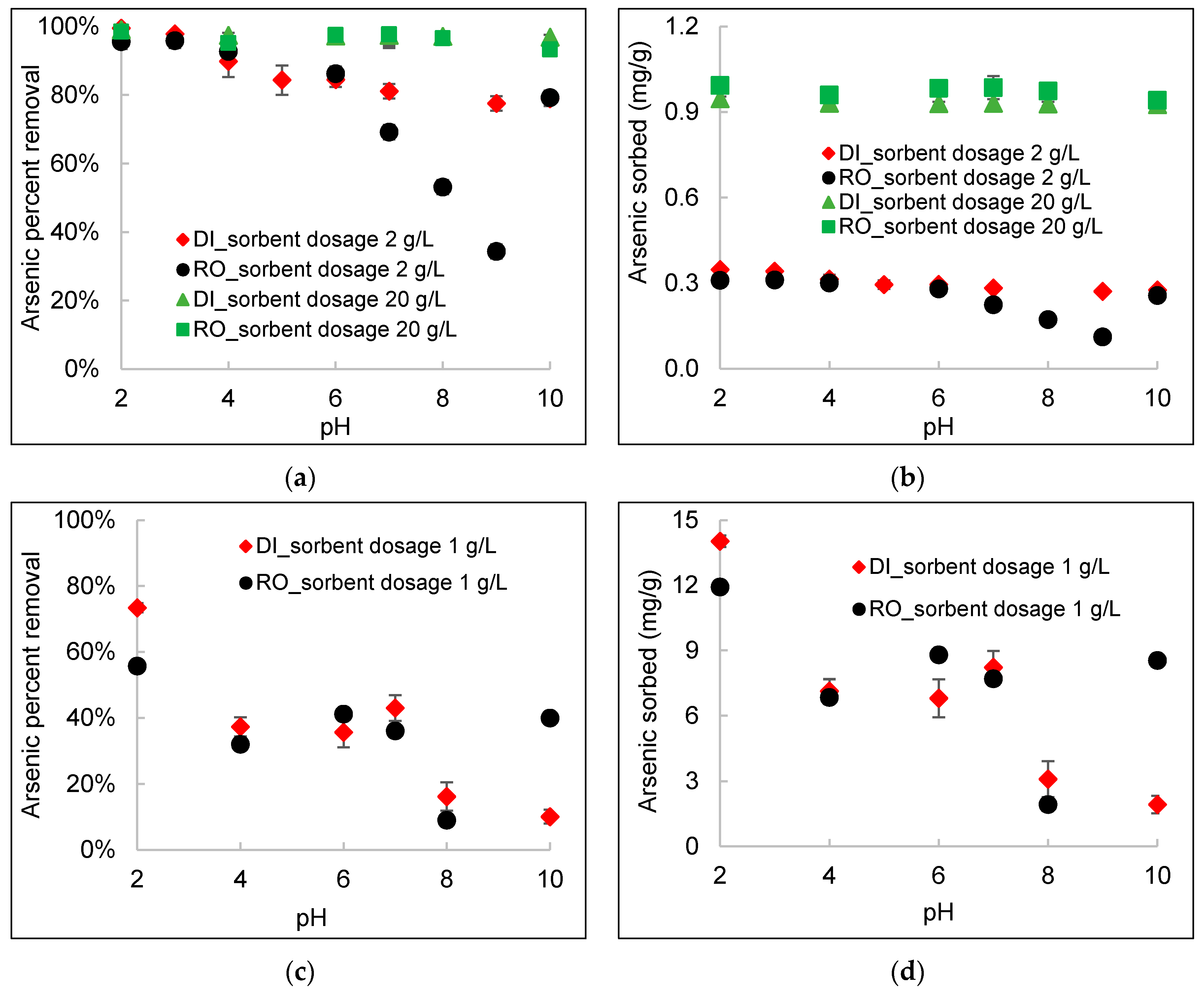
3.5. Effect of pH
4. Conclusions
- The percent removal of arsenic increased significantly with an increasing DWTS dosage from ~40% removal at a 1 g/L sorbent dosage to greater than 95% removal at a sorbent dosage of 20 g/L. However, the sorption capacity decreased with an increasing DWTS dosage. For example, for a high arsenic concentration of 20 mg/L, the amount of arsenic sorbed decreased from 6.8 mg/g in RO concentrate and 8.2 mg/g in deionized water at 1 g/L sorbent dosage to 0.92 mg/g at sorbent dosage of 20 g/L for both water matrices. For a lower arsenic concentration of 0.7 mg/L, the amount of arsenic sorbed decreased from 0.32 mg/g in deionized water and 0.24 mg/g in RO concentrate at 1 g/L sorbent dosage to 0.03 mg/g at a sorbent dosage of 20 g/L for both deionized water and RO concentrate.
- At a high DWTS dosage of 20 g/L, no pH-dependent behavior was observed within the pH range of 2–10 because of the abundant sorption sites in DWTS. For a low DWTS dosage of 1–2 g/L, the sorption of arsenic increased substantially as the pH decreased, except at pH 10, in RO concentrate. Precipitation of calcium arsenate at a higher pH (>10) increased arsenic sorption onto DWTS in the presence of a high concentration of calcium in RO concentrate.
- The arsenic sorption kinetics was best described by the pseudo-second-order kinetic model. The intra-particle diffusion kinetics model depicted by plotting qt (mg/g) versus t0.5 (h0.5) showed three distinct sorption steps including boundary diffusion, intra-particle diffusion, and the equilibrium of arsenic to the DWTS.
- Experimental data fitted well to the Freundlich equation, demonstrating a multilayer adsorption process between arsenic in water and DWTS due to surface precipitation of calcium arsenate and the formation of ternary complexes between arsenic and NOM bounded by polyvalent cations (e.g., Fe, Al) in DWTS. The maximum sorption capacities calculated from the Langmuir isotherm in RO concentrate and deionized water were 169 mg/g and 172 mg/g, respectively.
- The presence of NOM in an aqueous phase or a solid phase had significant effects on arsenic sorption onto DWTS. NOM in the aqueous phase hindered the sorption of arsenic to DWTS. The high organic matter content in DWTS enhanced the arsenic sorption from the aqueous phase to the solid phase.
- This study demonstrated that DWTS is effective in removing arsenic from desalination concentrate. The experimental data indicated that the DWTS used in this study has a promising potential for arsenic removal as a low-cost alternative sorbent.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mukherjee, A.; Sengupta, M.K.; Hossain, M.A.; Ahamed, S.; Das, B.; Nayak, B.; Lodh, D.; Rahman, M.M.; Chakraborti, D. Arsenic contamination in groundwater: A global perspective with emphasis on the Asian scenario. J. Health Popul. Nutr. 2006, 24, 142–163. [Google Scholar] [PubMed]
- Wang, S.; Mulligan, C.N. Occurrence of arsenic contamination in Canada: Sources, behavior and distribution. Sci. Total Environ. 2006, 366, 701–721. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Cullen, W.R.; Reimer, K.J. Arsenic speciation in the environment. Chem. Rev. 1989, 89, 713–764. [Google Scholar] [CrossRef]
- Smedley, P.; Kinniburgh, D. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Anawar, H.; Akai, J.; Mostofa, K.M.; Safiullah, S.; Tareq, S.M. Arsenic poisoning in groundwater: Health risk and geochemical sources in Bangladesh. Environ. Int. 2002, 27, 597–604. [Google Scholar] [CrossRef]
- Choong, T.S.; Chuah, T.G.; Robiah, Y.; Koay, F.L.G.; Azni, I. Arsenic toxicity, health hazards and removal techniques from water: An overview. Desalination 2007, 217, 139–166. [Google Scholar] [CrossRef]
- Viraraghavan, T.; Subramanian, K.; Aruldoss, J. Arsenic in drinking water—Problems and solutions. Water Sci. Technol. 1999, 40, 69–76. [Google Scholar]
- Ning, R.Y. Arsenic removal by reverse osmosis. Desalination 2002, 143, 237–241. [Google Scholar] [CrossRef]
- Ali, I.; Khan, T.A.; Asim, M. Removal of arsenic from water by electrocoagulation and electrodialysis techniques. Sep. Purif. Rev. 2011, 40, 25–42. [Google Scholar] [CrossRef]
- Kartinen, E.O.; Martin, C.J. An overview of arsenic removal processes. Desalination 1995, 103, 79–88. [Google Scholar] [CrossRef]
- Güell, R.; Fontàs, C.; Salvadó, V.; Anticó, E. Modelling of liquid–liquid extraction and liquid membrane separation of arsenic species in environmental matrices. Sep. Purif. Rev. 2010, 72, 319–325. [Google Scholar] [CrossRef]
- Maji, S.K.; Pal, A.; Pal, T. Arsenic removal from real-life groundwater by adsorption on laterite soil. J. Hazard. Mater. 2008, 151, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Gregor, J. Arsenic removal during conventional aluminium-based drinking-water treatment. Water Res. 2001, 35, 1659–1664. [Google Scholar] [CrossRef]
- Habuda-Stanić, M.; Nujić, M.; Romić, Ž.; Lončarić, A.; Ravančić, M.E.; Kralj, E. Arsenic preoxidation and its removal from groundwater using iron coagulants. Desalin. Water Treat. 2015, 56, 2105–2113. [Google Scholar] [CrossRef]
- Abejón, A.; Garea, A.; Irabien, A. Arsenic removal from drinking water by reverse osmosis: Minimization of costs and energy consumption. Sep. Purif. Technol. 2015, 144, 46–53. [Google Scholar] [CrossRef]
- Seidel, A.; Waypa, J.J.; Elimelech, M. Role of charge (Donnan) exclusion in removal of arsenic from water by a negatively charged porous nanofiltration membrane. Environ. Eng. Sci. 2001, 18, 105–113. [Google Scholar] [CrossRef]
- Truesdall, J.; Mickley, M.; Hamilton, R. Survey of membrane drinking water plant disposal methods. Desalination 1995, 102, 93–105. [Google Scholar] [CrossRef]
- Xu, P.; Cath, T.Y.; Robertson, A.P.; Reinhard, M.; Leckie, J.O.; Drewes, J.E. Critical review of desalination concentrate management, treatment and beneficial use. Environ. Eng. Sci. 2013, 30, 502–514. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Xu, P. Comparative study on pharmaceuticals adsorption in reclaimed water desalination concentrate using biochar: Impact of salts and organic matter. Sci. Total Environ. 2017, 601, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, X.; Papelis, C.; Xu, P. Innovative use of drinking water treatment solids for heavy metals removal from desalination concentrate: Synergistic effect of salts and natural organic matter. Chem. Eng. Res. Des. 2017, 120, 231–239. [Google Scholar] [CrossRef]
- Xu, P.; Capito, M.; Cath, T.Y. Selective removal of arsenic and monovalent ions from brackish water reverse osmosis concentrate. J. Hazard. Mater. 2013, 260, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.A.; Sheng, T.; Hu, Y.; Xu, J.; Xu, X. Arsenic removal from natural water using low cost granulated adsorbents: A review. CLEAN Soil Air Water 2015, 43, 13–26. [Google Scholar] [CrossRef]
- Genç-Fuhrman, H.; Tjell, J.C.; McConchie, D. Adsorption of arsenic from water using activated neutralized red mud. Environ. Sci. Technol. 2004, 38, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Raven, K.P.; Jain, A.; Loeppert, R.H. Arsenite and arsenate adsorption on ferrihydrite: Kinetics, equilibrium, and adsorption envelopes. Environ. Sci. Technol. 1998, 32, 344–349. [Google Scholar] [CrossRef]
- Suzuki, T.M.; Bomani, J.O.; Matsunaga, H.; Yokoyama, T. Preparation of porous resin loaded with crystalline hydrous zirconium oxide and its application to the removal of arsenic. React. Funct. Polym. 2000, 43, 165–172. [Google Scholar] [CrossRef]
- Singh, D.; Prasad, G.; Rupainwar, D. Adsorption technique for the treatment of As (V)-rich effluents. Colloids Surf. A Physicochem. Eng. Asp. 1996, 111, 49–56. [Google Scholar] [CrossRef]
- Fendorf, S.; Eick, M.J.; Grossl, P.; Sparks, D.L. Arsenate and chromate retention mechanisms on goethite. 1. Surface structure. Environ. Sci. Technol. 1997, 31, 315–320. [Google Scholar] [CrossRef]
- Makris, K.C.; Sarkar, D.; Parsons, J.G.; Datta, R.; Gardea-Torresdey, J.L. X-ray absorption spectroscopy as a tool investigating arsenic (III) and arsenic (V) sorption by an aluminum-based drinking-water treatment residual. J. Hazard. Mater. 2009, 171, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.; SenGupta, A.K. Selective coagulant recovery from water treatment plant residuals using Donnan membrane process. Environ. Sci. Technol. 2003, 37, 4468–4474. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-F.; Haynes, R.J. Removal of Pb (II), Cr (III) and Cr (VI) from aqueous solutions using alum-derived water treatment sludge. Water Air Soil Pollut. 2011, 215, 631–643. [Google Scholar] [CrossRef]
- Impellitteri, C.A.; Scheckel, K.G. The distribution, solid-phase speciation, and desorption/dissolution of as in waste iron-based drinking water treatment residuals. Chemosphere 2006, 64, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qi, Y.; Pei, Y. Laboratory investigation of phosphorus immobilization in lake sediments using water treatment residuals. Chem. Eng. J. 2012, 209, 379–385. [Google Scholar] [CrossRef]
- Wang, C.; Pei, Y. The removal of hydrogen sulfide in solution by ferric and alum water treatment residuals. Chemosphere 2012, 88, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, X.; Papelis, C.; Cath, T.Y.; Xu, P. Sorption of metals and metalloids from reverse osmosis concentrate on drinking water treatment solids. Sep. Purif. Technol. 2014, 134, 37–45. [Google Scholar] [CrossRef]
- Babatunde, A.; Zhao, Y. Constructive approaches toward water treatment works sludge management: An international review of beneficial reuses. Crit. Rev. Environ. Sci. Technol. 2007, 37, 129–164. [Google Scholar] [CrossRef]
- Makris, K.C.; Sarkar, D.; Datta, R. Evaluating a drinking-water waste by-product as a novel sorbent for arsenic. Chemosphere 2006, 64, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lin, L.; Papelis, C.; Myint, M.; Cath, T.Y.; Xu, P. Use of drinking water treatment solids for arsenate removal from desalination concentrate. J. Colloid Interface Sci. 2015, 445, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Clesceri, L.S.G.; Eaton, A.D.; Rice, E.W.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Garg, U.; Kaur, M.P.; Jawa, G.K.; Sud, D.; Garg, V.K. Removal of cadmium (II) from aqueous solutions by adsorption on agricultural waste biomass. J. Hazard. Mater. 2008, 154, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Sen, T.K.; Gomez, D. Adsorption of zinc (Zn2+) from aqueous solution on natural bentonite. Desalination 2011, 267, 286–294. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Huang, H.; Zeng, G.; Liu, Y.; Wang, X.; Lin, N.; Qi, Y. Adsorption characteristics and behaviors of graphene oxide for Zn (II) removal from aqueous solution. Appl. Surf. Sci. 2013, 279, 432–440. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.; Choi, I.; Rengaraj, S.; Yi, J. Arsenic removal using mesoporous alumina prepared via a templating method. Environ. Sci. Technol. 2004, 38, 924–931. [Google Scholar] [CrossRef] [PubMed]
- El-Moselhy, M.M.; Ates, A.; Çelebi, A. Synthesis and characterization of hybrid iron oxide silicates for selective removal of arsenic oxyanions from contaminated water. J. Colloid Interface Sci. 2017, 488, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yuan, N.; Pei, Y. Effect of pH on metal lability in drinking water treatment residuals. J. Environ. Qual. 2014, 43, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Caporale, A.G.; Punamiya, P.; Pigna, M.; Violante, A.; Sarkar, D. Effect of particle size of drinking-water treatment residuals on the sorption of arsenic in the presence of competing ions. J. Hazard. Mater. 2013, 260, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Thanabalasingam, P.; Pickering, W. Arsenic sorption by humic acids. Environ. Pollut. Ser. B Chem. Phys. 1986, 12, 233–246. [Google Scholar] [CrossRef]
- Mikutta, C.; Kretzschmar, R. Spectroscopic evidence for ternary complex formation between arsenate and ferric iron complexes of humic substances. Environ. Sci. Technol. 2011, 45, 9550–9557. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, M.K.; Gagnon, G.A. Adsorption of arsenic from a Nova Scotia groundwater onto water treatment residual solids. Water Res. 2010, 44, 5740–5749. [Google Scholar] [CrossRef] [PubMed]
- Altundoğan, H.S.; Altundoğan, S.; Tümen, F.; Bildik, M. Arsenic removal from aqueous solutions by adsorption on red mud. Waste Manag. 2000, 20, 761–767. [Google Scholar] [CrossRef]
- Ladeira, A.C.; Ciminelli, V.S. Adsorption and desorption of arsenic on an oxisol and its constituents. Water Res. 2004, 38, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhang, G.; Li, H. Efficient removal of arsenic from water using a granular adsorbent: Fe–Mn binary oxide impregnated chitosan bead. Bioresour. Technol. 2015, 193, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, J.; Kappeler, A.; Lindauer, U.; Kistler, D.; Berg, M.; Sigg, L. Arsenite and arsenate binding to dissolved humic acids: Influence of pH, type of humic acid, and aluminum. Environ. Sci. Technol. 2006, 40, 6015–6020. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhao, D.; Yang, S. Impact of solution chemistry conditions on the sorption behavior of Cu (II) on Lin'an montmorillonite. Desalination 2011, 269, 84–91. [Google Scholar] [CrossRef]
- Redman, A.D.; Macalady, D.L.; Ahmann, D. Natural organic matter affects arsenic speciation and sorption onto hematite. Environ. Sci. Technol. 2002, 36, 2889–2896. [Google Scholar] [CrossRef] [PubMed]
- Nagar, R.; Sarkar, D.; Makris, K.C.; Datta, R. Effect of solution chemistry on arsenic sorption by Fe-and Al-based drinking-water treatment residuals. Chemosphere 2010, 78, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Bothe, J.V.; Brown, P.W. Arsenic immobilization by calcium arsenate formation. Environ. Sci. Technol. 1999, 33, 3806–3811. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Haynes, R.J. Sorption of heavy metals by inorganic and organic components of solid wastes: Significance to use of wastes as low-cost adsorbents and immobilizing agents. Crit. Rev. Environ. Sci. Technol. 2010, 40, 909–977. [Google Scholar] [CrossRef]
| Water Type | DWTS Dosage | Arsenic Conc. | Arsenic Loading | Kad | qe (Experiment) | qe (model) * | R2 |
|---|---|---|---|---|---|---|---|
| g/L | mg/L | mg/g | (h−1) | (mg/g) | (mg/g) | ||
| RO concentrate | 2 | 0.65 | 0.30 | 27.8 | 0.170 | 0.174 | 0.995 |
| Deionized water | 2 | 0.60 | 0.32 | 2.17 | 0.260 | 0.281 | 0.996 |
| Water Type | DWTS Dosage | Arsenic conc. | Arsenic Loading | Freundlich | Langmuir | Langmuir qmax |
|---|---|---|---|---|---|---|
| g/L | mg/L | mg/g | R2 | R2 | mg/g | |
| RO concentrate | 20 | 1–100 | 0.05–5 | 0.96 | 0.98 | 5.07 |
| Deionized water | 20 | 1–100 | 0.05–5 | 0.98 | 0.99 | 4.44 |
| RO concentrate | 2 | 0.1–3 | 0.05–1.3 | 0.99 | 0.69 | / |
| Deionized water | 2 | 0.075–3 | 0.04–1.5 | 0.95 | 0.60 | / |
| Adsorbents | Adsorption Capacity (mg/g) | Water Type | As Conc. (µM) | pH | Temp. (°C) | Langmuir R2 | Ref. |
|---|---|---|---|---|---|---|---|
| DWTS | 172 | RO concentrate | 17.7~4004 | 7.0 | 23 | 0.99 | This study |
| Fe-based DWTS | 42.9 | Groundwater (fresh) | 0.58~ | 8.1 | 22 | 0.98 | [50] |
| Al-based DWTS | 47.4 | Synthetic water | 125~6250 | 6.0 | 20 | 0.95 | [47] |
| Red mud | 0.51 | Synthetic water (0.1 M NaCl) | 33.3~400 | 3.2 | 25 | 0.99 | [51] |
| Goethite | 12.4 | Synthetic water | 133~13,348 | 5.5 | 25 | - | [52] |
| Chitosan bead | 39.1 | Synthetic water | 66.7~800 | 7.0 | 25 | 0.91 | [53] |
| Mesoporous alumina | 121 | Synthetic water | 100~20,000 | 5.0 | 25 | 0.98 | [44] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Lin, L.; Papelis, C.; Xu, P. Sorption of Arsenic from Desalination Concentrate onto Drinking Water Treatment Solids: Operating Conditions and Kinetics. Water 2018, 10, 96. https://doi.org/10.3390/w10020096
Xu X, Lin L, Papelis C, Xu P. Sorption of Arsenic from Desalination Concentrate onto Drinking Water Treatment Solids: Operating Conditions and Kinetics. Water. 2018; 10(2):96. https://doi.org/10.3390/w10020096
Chicago/Turabian StyleXu, Xuesong, Lu Lin, Charalambos Papelis, and Pei Xu. 2018. "Sorption of Arsenic from Desalination Concentrate onto Drinking Water Treatment Solids: Operating Conditions and Kinetics" Water 10, no. 2: 96. https://doi.org/10.3390/w10020096
APA StyleXu, X., Lin, L., Papelis, C., & Xu, P. (2018). Sorption of Arsenic from Desalination Concentrate onto Drinking Water Treatment Solids: Operating Conditions and Kinetics. Water, 10(2), 96. https://doi.org/10.3390/w10020096








