Floating Photocatalysts for Passive Solar Degradation of Naphthenic Acids in Oil Sands Process-Affected Water
Abstract
:1. Introduction
2. Experimental
2.1. Materials
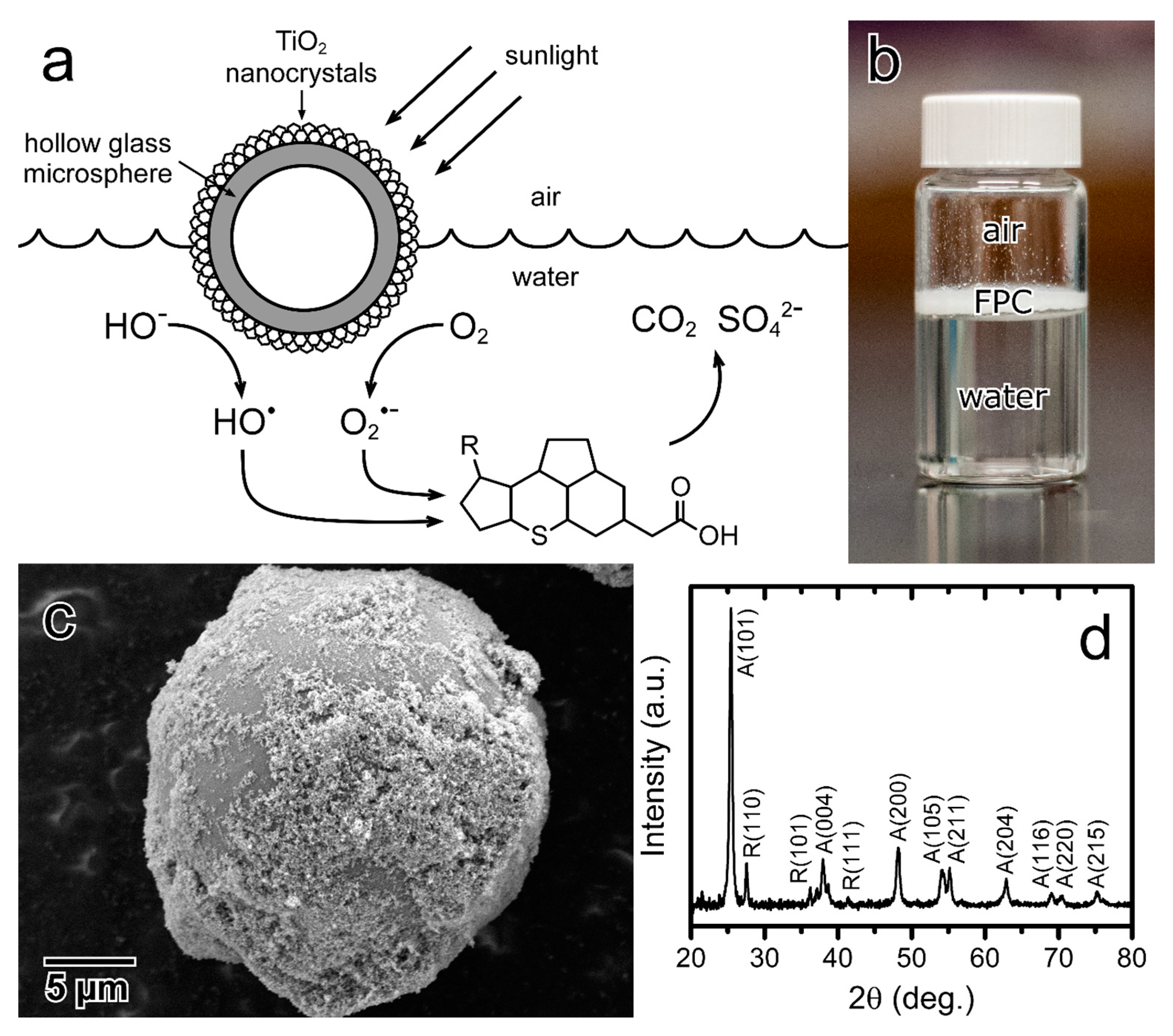
2.2. Floating Photocatalyst Synthesis and Characterization
2.3. Photocatalysis Experiments
2.4. Analysis
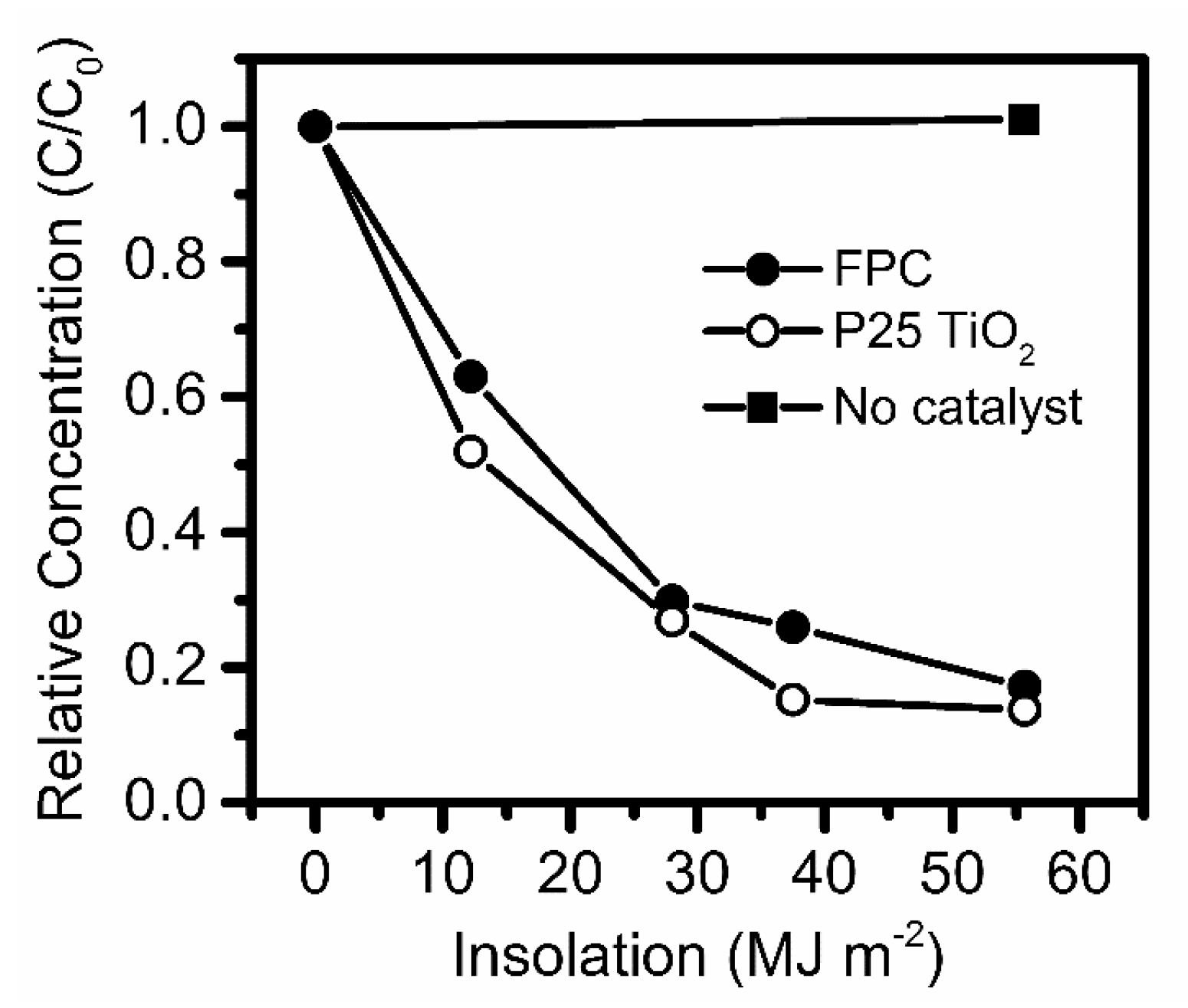
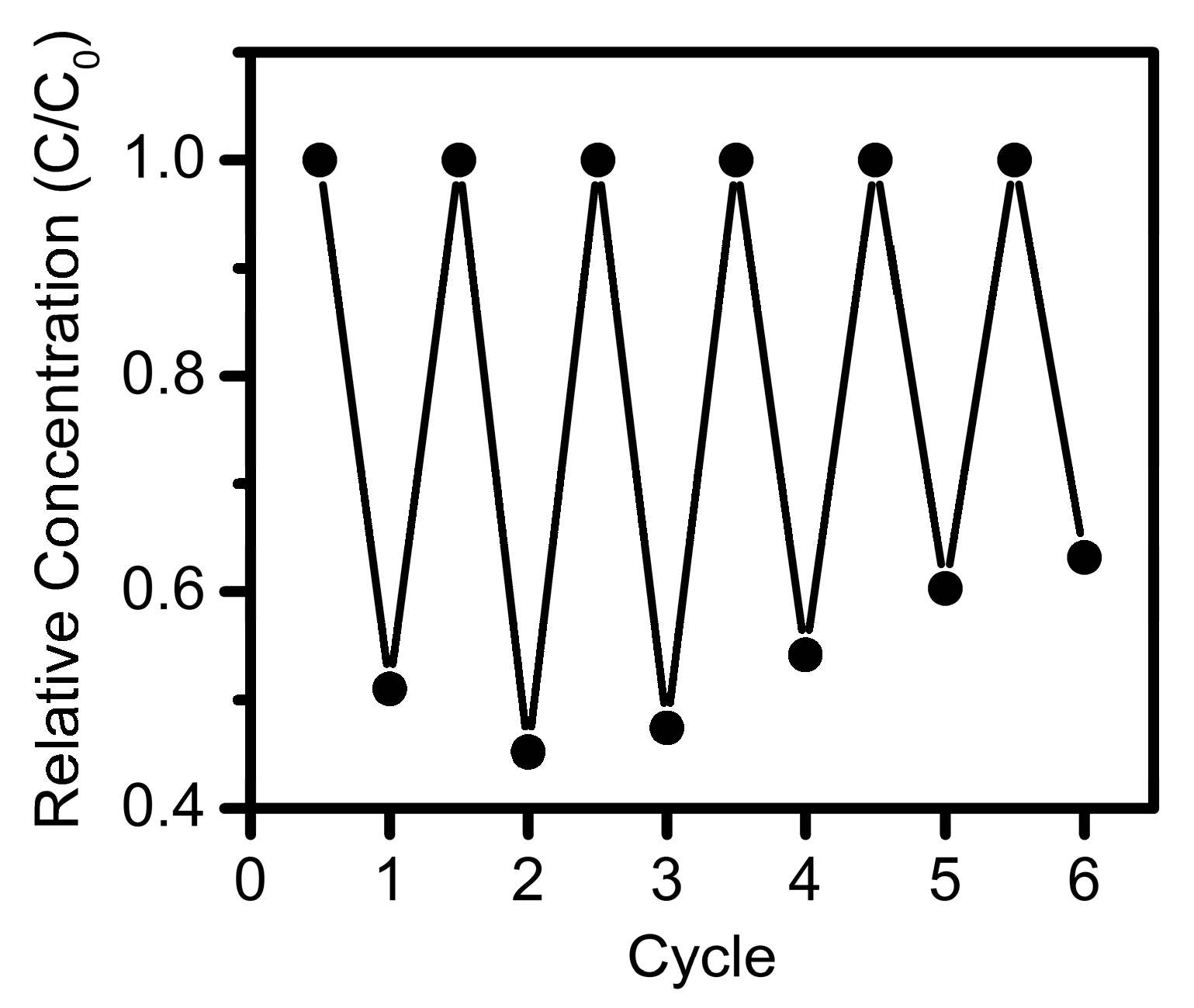
3. Results and Discussion
4. Conclusions
- Due to their buoyancy, FPCs naturally concentrate at the air-water interface where sunlight intensity and dissolved oxygen concentrations are highest (i.e., the optimal treatment zone);
- Substantially less photocatalyst material is needed to achieve the same treatment rate as slurry deployment, since TiO2 comprises only a minority of the buoyant composite mass, while in photocatalytic slurries, the vast majority of particles suspended below the illuminated air-water interface do not participate in the treatment process;
- NAs are naturally enriched at the air-water interface due to their surfactant properties, and are replenished to the surface from deeper OSPW in tailings ponds by methane bubbling, and through natural mixing provided by wind and waves;
- FPCs can be easily contained and collected by skimming from the surface, compared with the significant technical challenges of collecting colloidal nanoparticles from a slurry.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Morandi, G.D.; Wiseman, S.B.; Pereira, A.; Mankidy, R.; Gault, I.G.M.; Martin, J.W.; Giesy, J.P. Effects-Directed Analysis of Dissolved Organic Compounds in Oil Sands Process-Affected Water. Environ. Sci. Technol. 2015, 49, 12395–12404. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Ramsay, B.A.; Wang, J.; Ramsay, J. Toxicity and composition profiles of solid phase extracts of oil sands process-affected water. Sci. Total Environ. 2015, 538, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Scott, A.C.; Fedorak, P.M.; Bataineh, M.; Martin, J.W. Influence of Molecular Structure on the Biodegradability of Naphthenic Acids. Environ. Sci. Technol. 2008, 42, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; MacKinnon, M.D.; Martin, J.W. Estimating the in situ biodegradation of naphthenic acids in oil sands process waters by HPLC/HRMS. Chemosphere 2009, 76, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Marentette, J.R.; Frank, R.A.; Bartlett, A.J.; Gillis, P.L.; Hewitt, L.M.; Peru, K.M.; Headley, J.V.; Brunswick, P.; Shang, D.; Parrott, J.L. Toxicity of naphthenic acid fraction components extracted from fresh and aged oil sands process-affected waters, and commercial naphthenic acid mixtures, to fathead minnow (Pimephales promelas) embryos. Aquat. Toxicol. 2015, 164, 108–117. [Google Scholar] [CrossRef] [PubMed]
- COSIA Challenge #0014: Passive Organics Treatment Technology. Available online: http://www.cosia.ca/initiatives/water/water-challenge-statements (accessed on 29 October 2016).
- Leshuk, T.; Wong, T.; Linley, S.; Peru, K.M.; Headley, J.V.; Gu, F. Solar photocatalytic degradation of naphthenic acids in oil sands process-affected water. Chemosphere 2016, 144, 1854–1861. [Google Scholar] [CrossRef] [PubMed]
- Headley, J.V.; Du, J.-L.; Peru, K.M.; McMartin, D.W. Electrospray ionization mass spectrometry of the photodegradation of naphthenic acids mixtures irradiated with titanium dioxide. J. Environ. Sci. Health Part A 2009, 44, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Leshuk, T.; de Oliveira Livera, D.; Peru, K.M.; Headley, J.V.; Vijayaraghavan, S.; Wong, T.; Gu, F. Photocatalytic degradation kinetics of naphthenic acids in oil sands process-affected water: Multifactorial determination of significant factors. Chemosphere 2016, 165, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.C.; Zubot, W.; MacKinnon, M.D.; Smith, D.W.; Fedorak, P.M. Ozonation of oil sands process water removes naphthenic acids and toxicity. Chemosphere 2008, 71, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Li, C.; Belosevic, M.; Bolton, J.R.; El-Din, M.G. Application of a Solar UV/Chlorine Advanced Oxidation Process to Oil Sands Process-Affected Water Remediation. Environ. Sci. Technol. 2014, 48, 9692–9701. [Google Scholar] [CrossRef] [PubMed]
- Valencia, S.; Marín, J.; Restrepo, G. Photocatalytic Degradation of Humic Acids with Titanium Dioxide Embedded into Polyethylene Pellets to Enhance the Postrecovery of Catalyst. Environ. Eng. Sci. 2017. [Google Scholar] [CrossRef]
- Tu, W.; Lin, Y.-P.; Bai, R. Removal of phenol in aqueous solutions by novel buoyant Composite photocatalysts and the kinetics. Sep. Purif. Technol. 2013, 115, 180–189. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, W.; Huo, P.; Luo, Y.; He, M.; Pan, J.; Li, C.; Yan, Y. Performance of a novel TiO2 photocatalyst based on the magnetic floating fly-ash cenospheres for the purpose of treating waste by waste. Chem. Eng. J. 2013, 225, 34–42. [Google Scholar] [CrossRef]
- Damasceno, F.C.; Gruber, L.D.A.; Geller, A.M.; de Campos, M.C.V.; Gomes, A.O.; Guimarães, R.C.L.; Péres, V.F.; Jacques, R.A.; Caramão, E.B. Characterization of naphthenic acids using mass spectroscopy and chromatographic techniques: Study of technical mixtures. Anal. Methods 2014, 6, 807–816. [Google Scholar] [CrossRef]
- Halász, G.; Gyüre, B.; Jánosi, I.M.; Szabó, K.G.; Tél, T. Vortex flow generated by a magnetic stirrer. Am. J. Phys. 2007, 75, 1092–1098. [Google Scholar] [CrossRef]
- Current Readings for UW Weather Station. Available online: http://weather.uwaterloo.ca/ (accessed on 16 December 2014).
- Jivraj, M.N.; MacKinnon, M.; Fung, B. Naphthenic Acid Extraction and Quantitative Analysis with FT-IR Spectroscopy; Syncrude Canada Ltd.: Edmonton, Alberta, 1995. [Google Scholar]
- Holowenko, F.M.; MacKinnon, M.D.; Fedorak, P.M. Naphthenic acids and surrogate naphthenic acids in methanogenic microcosms. Water Res. 2001, 35, 2595–2606. [Google Scholar] [CrossRef]
- Grewer, D.M.; Young, R.F.; Whittal, R.M.; Fedorak, P.M. Naphthenic acids and other acid-extractables in water samples from Alberta: What is being measured? Sci. Total Environ. 2010, 408, 5997–6010. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.B.; Wang, C.M.; Luo, Z.; Schwitzgebel, J.; Ekerdt, J.G.; Brock, J.R.; Heller, A. Attachment of TiO2 Powders to Hollow Glass Microbeads: Activity of the TiO2—Coated Beads in the Photoassisted Oxidation of Ethanol to Acetaldehyde. J. Electrochem. Soc. 1991, 138, 3660–3664. [Google Scholar] [CrossRef]
- Espino-Estévez, M.R.; Fernández-Rodríguez, C.; González-Díaz, O.M.; Navío, J.A.; Fernández-Hevia, D.; Doña-Rodríguez, J.M. Enhancement of stability and photoactivity of TiO2 coatings on annular glass reactors to remove emerging pollutants from waters. Chem. Eng. J. 2015, 279, 488–497. [Google Scholar] [CrossRef]
- Lim, L.L.P.; Lynch, R.J.; In, S.-I. Comparison of simple and economical photocatalyst immobilisation procedures. Appl. Catal. A 2009, 365, 214–221. [Google Scholar] [CrossRef]
- Wang, G.; Xu, L.; Zhang, J.; Yin, T.; Han, D. Enhanced Photocatalytic Activity of TiO2 Powders (P25) via Calcination Treatment. Int. J. Photoenergy 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Velásquez, J.; Valencia, S.; Rios, L.; Restrepo, G.; Marín, J. Characterization and photocatalytic evaluation of polypropylene and polyethylene pellets coated with P25 TiO2 using the controlled-temperature embedding method. Chem. Eng. J. 2012, 203, 398–405. [Google Scholar] [CrossRef]
- Fabiyi, M.E.; Skelton, R.L. Photocatalytic mineralisation of methylene blue using buoyant TiO2-coated polystyrene beads. J. Photochem. Photobiol. A 2000, 132, 121–128. [Google Scholar] [CrossRef]
- Magalhães, F.; Moura, F.C.C.; Lago, R.M. TiO2/LDPE composites: A new floating photocatalyst for solar degradation of organic contaminants. Desalination 2011, 276, 266–271. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Chen, Y.; Shi, L.; Zhu, Y. Solid-phase photocatalytic degradation of polyethylene plastic under UV and solar light irradiation. J. Mol. Catal. A 2007, 268, 101–106. [Google Scholar] [CrossRef]
- Shang, J.; Chai, M.; Zhu, Y. Photocatalytic degradation of polystyrene plastic under fluorescent light. Environ. Sci. Technol. 2003, 37, 4494–4499. [Google Scholar] [CrossRef] [PubMed]
- Tennakone, K.; Kottegoda, I.R.M. Photocatalytic mineralization of paraquat dissolved in water by TiO2 supported on polythene and polypropylene films. J. Photochem. Photobiol. A 1996, 93, 79–81. [Google Scholar] [CrossRef]
- Faramarzpour, M.; Vossoughi, M.; Borghei, M. Photocatalytic degradation of furfural by titania nanoparticles in a floating-bed photoreactor. Chem. Eng. J. 2009, 146, 79–85. [Google Scholar] [CrossRef]
- Wang, B.; Li, Q.; Wang, W.; Li, Y.; Zhai, J. Preparation and characterization of Fe3+-doped TiO2 on fly ash cenospheres for photocatalytic application. Appl. Surf. Sci. 2011, 257, 3473–3479. [Google Scholar] [CrossRef]
- Hosseini, S.N.; Borghei, S.M.; Vossoughi, M.; Taghavinia, N. Immobilization of TiO2 on perlite granules for photocatalytic degradation of phenol. Appl. Catal. B 2007, 74, 53–62. [Google Scholar] [CrossRef]
- Matthews, R.W. Photooxidative degradation of coloured organics in water using supported catalysts. TiO2 on sand. Water Res. 1991, 25, 1169–1176. [Google Scholar] [CrossRef]
- Pozzo, R.L.; Giombi, J.L.; Baltanás, M.A.; Cassano, A.E. The performance in a fluidized bed reactor of photocatalysts immobilized onto inert supports. Catal. Today 2000, 62, 175–187. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Malato, S.; Fernandez-Ibanez, P.; Maldonado, M.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl. Catal. B 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- Lawrence, G.A.; Ward, P.R.B.; MacKinnon, M.D. Wind-wave-induced suspension of mine tailings in disposal ponds—A case study. Can. J. Civ. Eng. 1991, 18, 1047–1053. [Google Scholar] [CrossRef]
- Lawrence, G.A.; Tedford, E.W.; Pieters, R. Suspended solids in an end pit lake: Potential mixing mechanisms. Can. J. Civ. Eng. 2015, 43, 211–217. [Google Scholar] [CrossRef]
- Dompierre, K.A.; Barbour, S.L. Characterization of physical mass transport through oil sands fluid fine tailings in an end pit lake: A multi-tracer study. J. Contam. Hydrol. 2016, 189, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Holowenko, F.M. Methanogenesis and Fine Tailings Waste from Oil Sands Extraction: A Microcosm-Based Laboratory Examination. Master’s Thesis, University of Alberta, Edmonton, Alberta, 2000. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leshuk, T.; Krishnakumar, H.; De Oliveira Livera, D.; Gu, F. Floating Photocatalysts for Passive Solar Degradation of Naphthenic Acids in Oil Sands Process-Affected Water. Water 2018, 10, 202. https://doi.org/10.3390/w10020202
Leshuk T, Krishnakumar H, De Oliveira Livera D, Gu F. Floating Photocatalysts for Passive Solar Degradation of Naphthenic Acids in Oil Sands Process-Affected Water. Water. 2018; 10(2):202. https://doi.org/10.3390/w10020202
Chicago/Turabian StyleLeshuk, Tim, Harish Krishnakumar, Diogo De Oliveira Livera, and Frank Gu. 2018. "Floating Photocatalysts for Passive Solar Degradation of Naphthenic Acids in Oil Sands Process-Affected Water" Water 10, no. 2: 202. https://doi.org/10.3390/w10020202
APA StyleLeshuk, T., Krishnakumar, H., De Oliveira Livera, D., & Gu, F. (2018). Floating Photocatalysts for Passive Solar Degradation of Naphthenic Acids in Oil Sands Process-Affected Water. Water, 10(2), 202. https://doi.org/10.3390/w10020202



