Hydrochemical Changes and Influencing Factors in the Dongkemadi Region, Tanggula Range, China
Abstract
1. Introduction
2. Materials and Methods
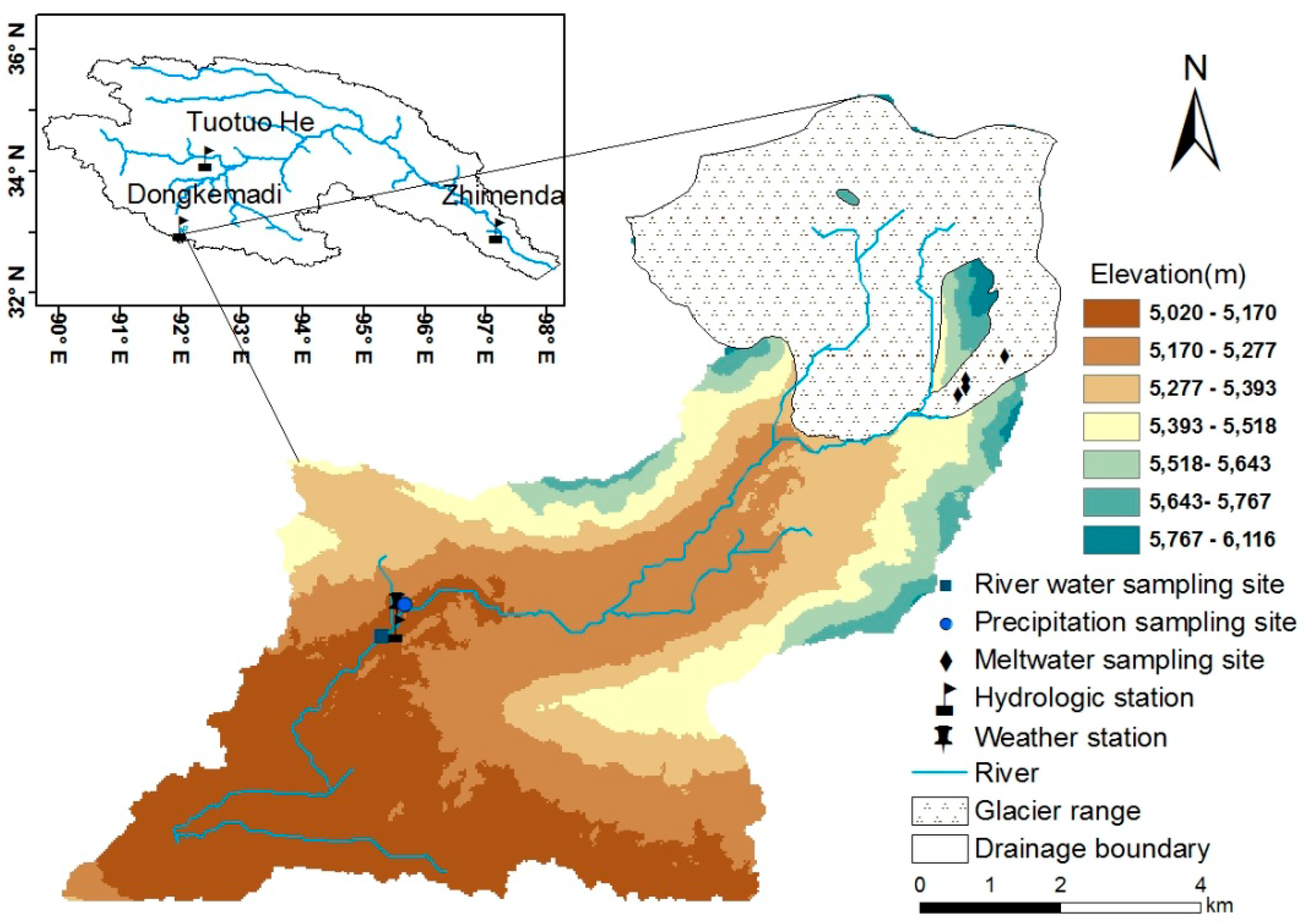
2.1. Overview of the Drainage Basin
2.2. Sampling and Analysis
3. Results
3.1. Characteristics of Hydrochemical Compositions in Different Water Sources
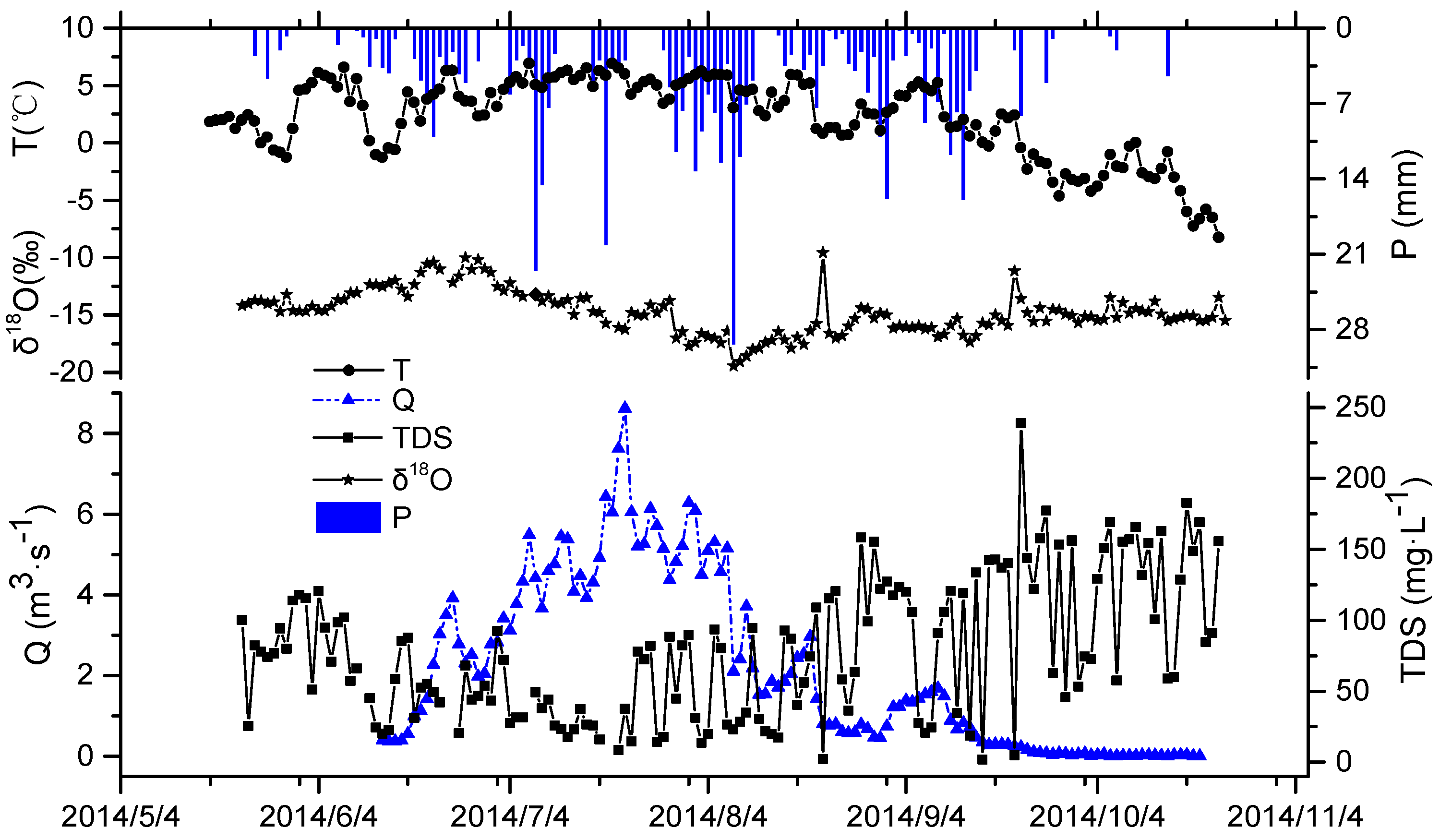
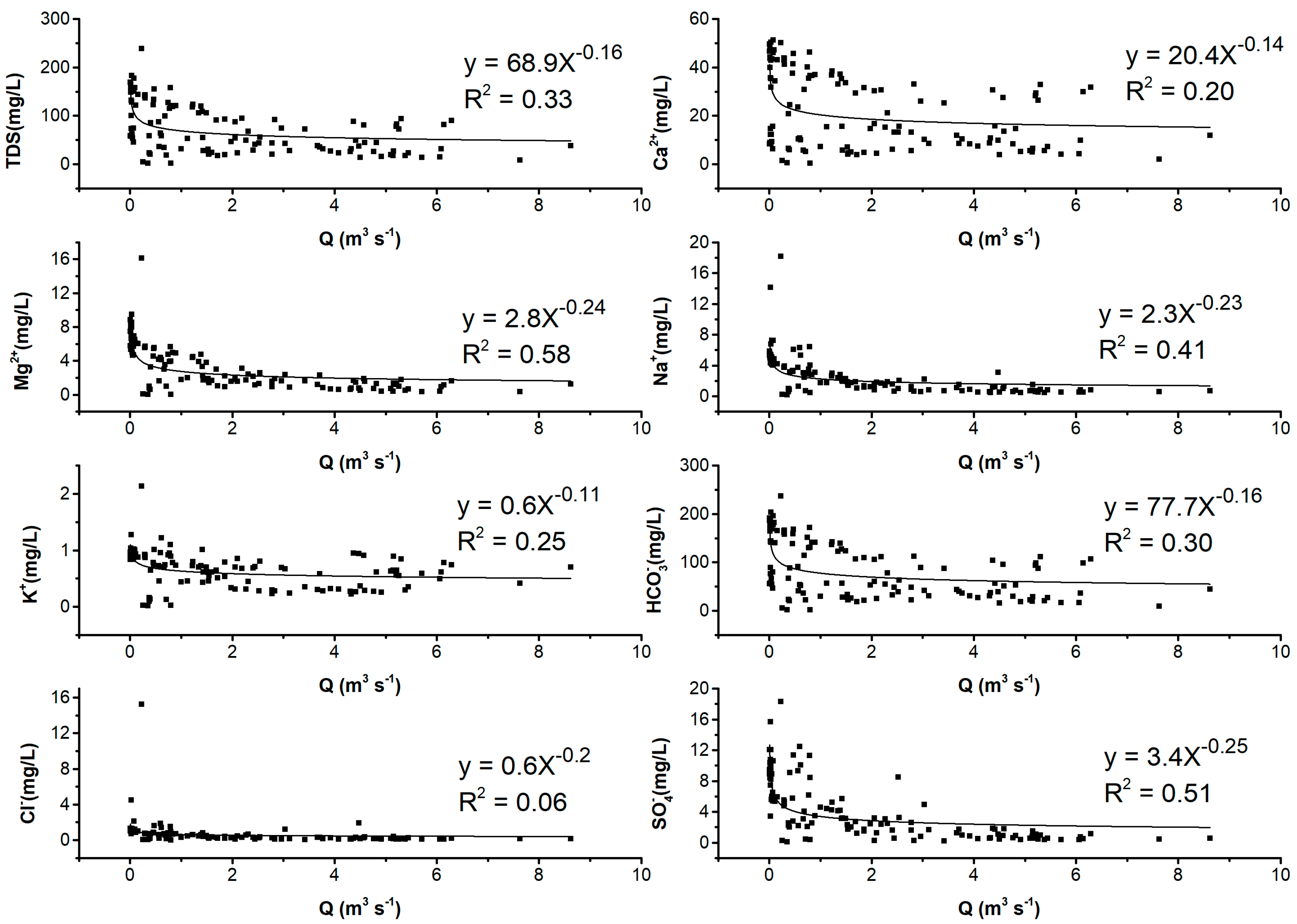
3.2. Relationship between Hydrochemical Compositions and the Runoff in the River
4. Discussion
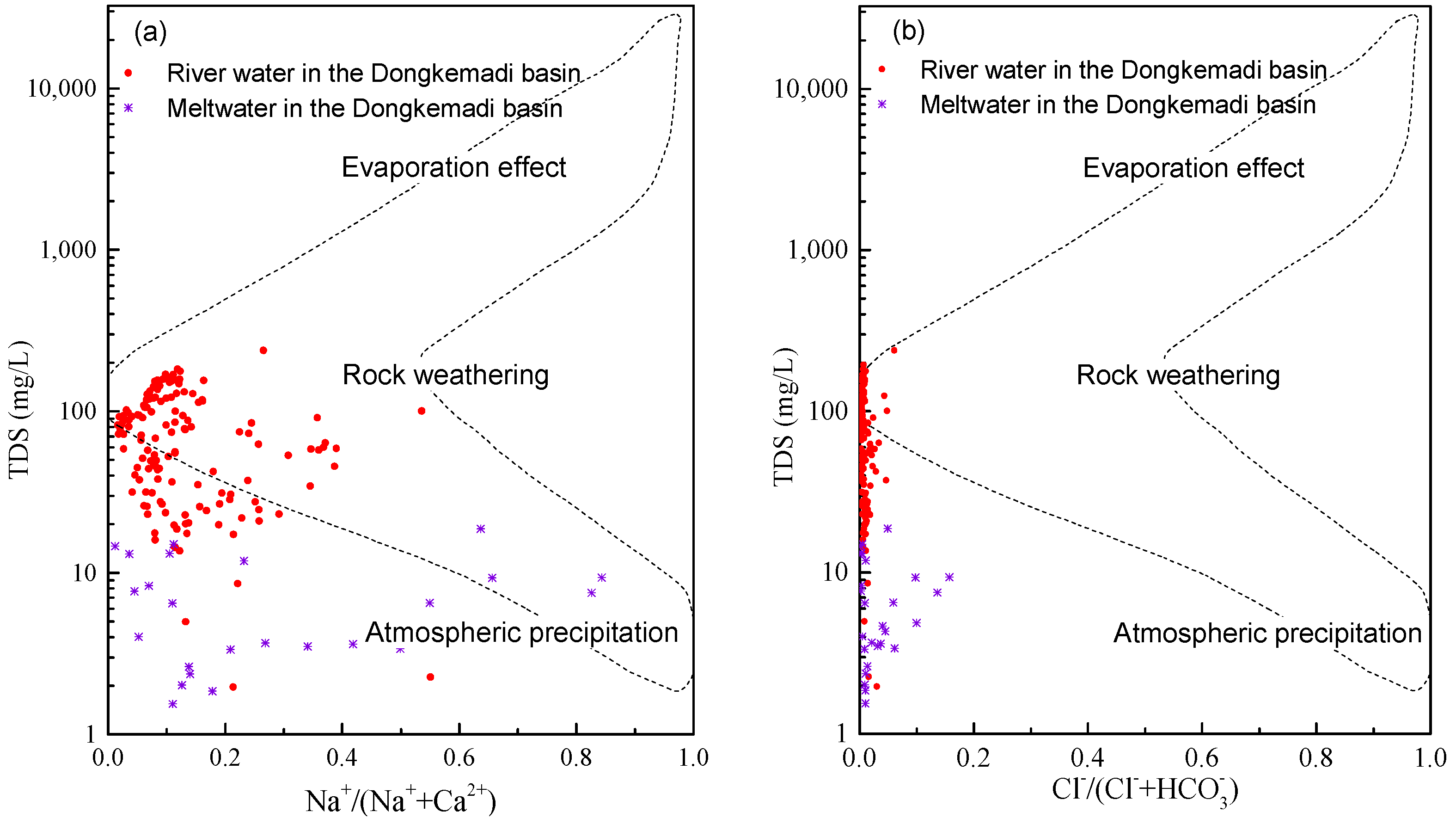
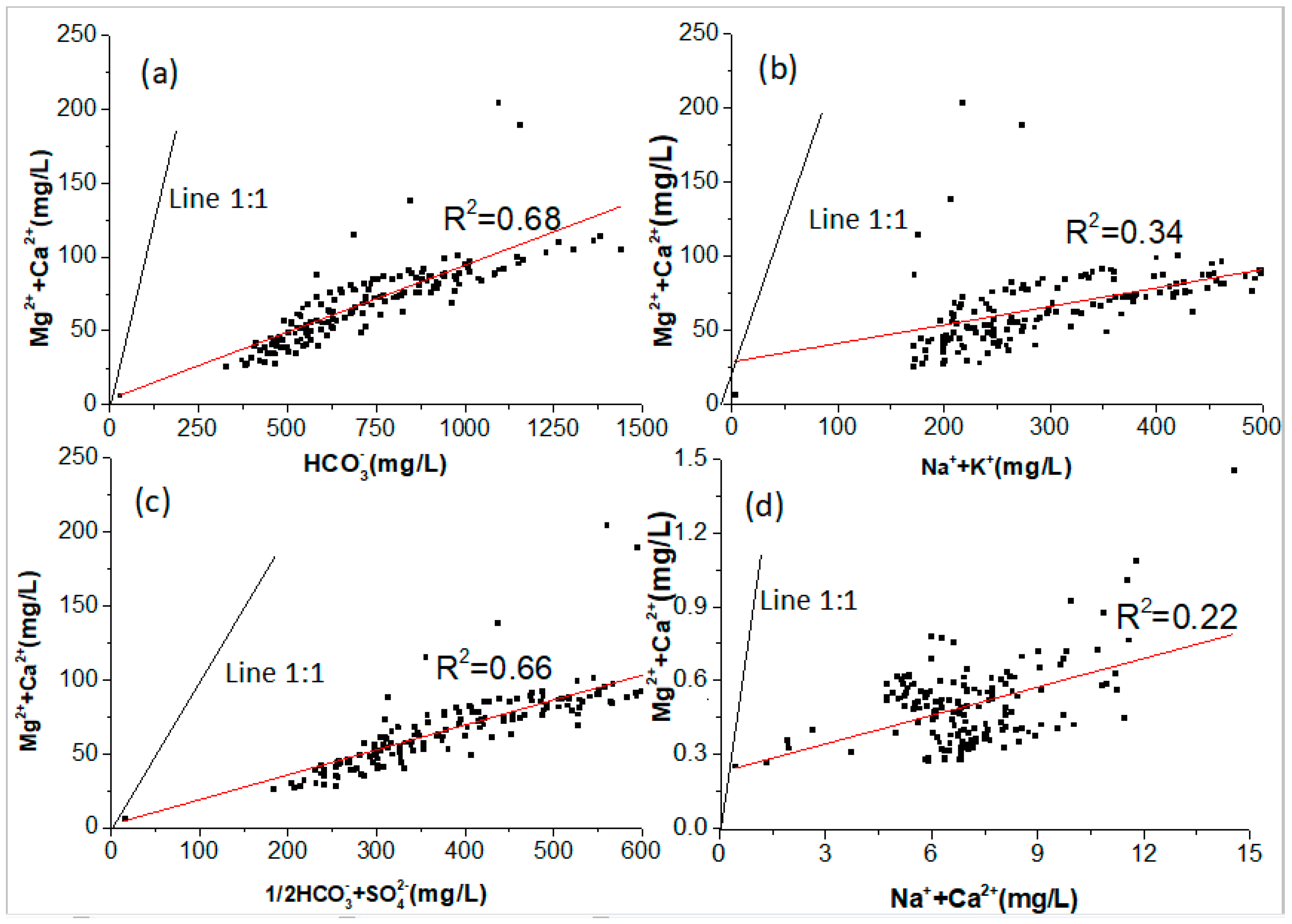
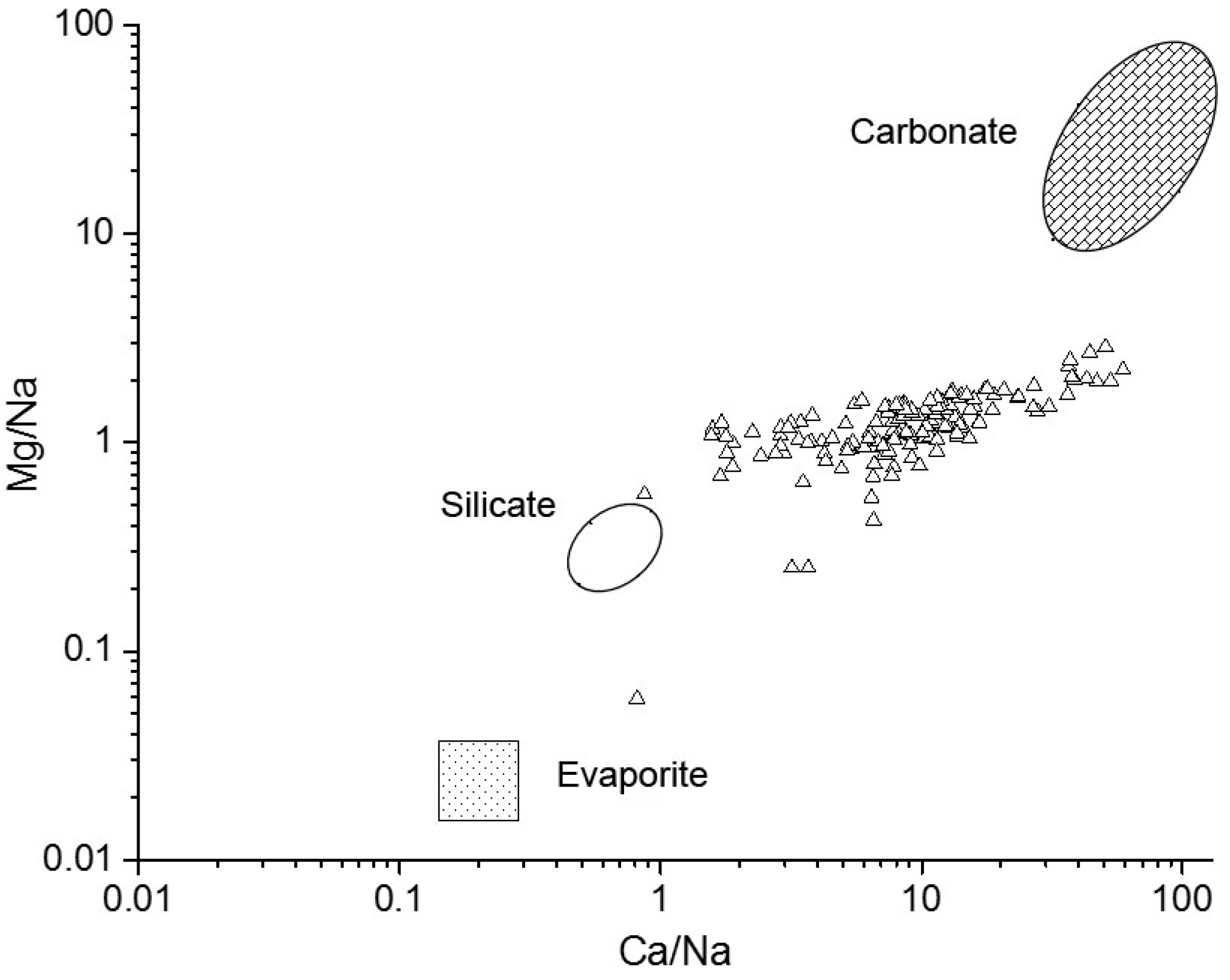
4.1. Sources of Major Ions in River Water and Its Controlling Factors
4.2. Contributions of Different Sources to Dissolved Composition in River Water
4.2.1. Contribution of Precipitation to the Hydrochemistry in the Dongkemadi River
4.2.2. Contributions of the Dissolution of Minerals to River Hydrochemistry
4.3. Rate of Rock Weathering and CO2 Consumption Fluxes in The Drainage Basin
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Summery for Policymaker. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 3–29. [Google Scholar]
- Ren, G.Y.; Chu, Z.Y.; Zhou, Y.Q. Recent progresses in studies of regional temperature changes in China. Clim. Environ. Res. 2005, 10, 701–716. [Google Scholar]
- Dou, R.Y. The climate change and adaptation strategies for sustainable development in the Three-River headwaters region in Qinghai province in recent half century. Ecol. Econ. (Chinese version) 2016, 32, 165–171. [Google Scholar]
- Shen, Y.P.; Liu, S.Y.; Ding, Y.J. Glacier mass balance change in Tailanhe River Watersheds on the south slope of the Tianshan Mountains and its impact on water resources. J. Glaciol. Geocryol. 2003, 25, 124–129. [Google Scholar]
- Li, Z.X.; He, Y.Q.; Jia, W.X. Response of “glaciers-runoff” system in a typical temperate-glacier, Hailuogou Glacier in Gongga Mountain of China to global change. Sci. Geogr. Sin. 2008, 28, 229–234. [Google Scholar]
- Brown, G.H. Glacier meltwater hydrochemistry. Appl. Geochem. 2002, 17, 855–883. [Google Scholar] [CrossRef]
- Liu, S.Y.; Ding, Y.J.; Li, J. Glaciers in response to recent climate warming in western China. Quat. Sci. 2006, 26, 762–771. [Google Scholar]
- Pu, J.C.; Yao, T.D.; Yang, M.X. Rapid decrease of mass balance observed in the Xiao (Lesser) Dongkemadi Glacier, in the central Tibetan Plateau. Hydrol. Process. 2008, 22, 2953–2958. [Google Scholar] [CrossRef]
- Ke, L.; Ding, X.; Li, W.; Qiu, B. Remote sensing of glacier change in the central Qinghai-Tibet Plateau and the relationship with changing climate. Remote Sens. 2017, 9, 114. [Google Scholar] [CrossRef]
- Singh, A.K.; Hasnain, S.I. Aspects of weathering and solute acquisition processes controlling chemistry of sub-Alpine proglacial streams of Garhwal Himalaya, India. Hydrol. Process. 2002, 16, 835–849. [Google Scholar] [CrossRef]
- Zhang, J.; He, X.B.; Ye, B.S. Rencent Variation of mass balance of the Xiao Dongkemadi Glacier in the Tanggula Range and its influencing factors. J. Glaciol. Geocryol. 2013, 35, 263–271. [Google Scholar]
- Zhang, Y.S.; Yao, T.D.; Pu, J.C. The features of hydrological processing in the Dongkemadi River basin, Tanggula Pass, Tibetan Plateau. J. Glaciol. Geocryol. 1997, 19, 214–222. [Google Scholar]
- Liu, J.F.; Yang, J.P.; Chen, R.S. The simulation of snowmelt runoff model in the Dongkemadi River Basin, headwater of the Yangtze River. Acta Geogr. Sin. 2006, 61, 1149–1159. [Google Scholar]
- Qiao, C.J.; He, X.B.; Ye, B.S. Study of the degree-day factors for snow and ice on the Dongkemadi Glacier, Tanggula Range. J. Glaciol. Geocryol. 2010, 32, 257–264. [Google Scholar]
- Yang, J.P.; Ding, Y.J.; Chen, R.S. Comprehensive Research of Ecological Environment Change in The Yangtze-Yellow Rivers Source Regions; China Meteorological Press: Beijing, China, 2006; pp. 102–123. [Google Scholar]
- Chen, J.S.; Tao, S.; Deng, B.S. Water Environmental Chemistry; Higher Education Press: Beijing, China, 1987; pp. 45–66. [Google Scholar]
- Hou, S.G. Chemical characteristics of precipitation at the headwaters of the Urumqi River in the Tianshan Mountains. J. Glaciol. Geocryol. 2001, 23, 80–84. [Google Scholar]
- Zhang, L.T.; Chen, J.S. The relationship between the composition of the major ion of river of China and regional natural factors. Sci. Geogr. Sin. 2000, 20, 236–240. [Google Scholar]
- Gao, T.G.; Kang, S.C.; Zhang, Q.G. Major ionic Ffatures and their sources in the Nam Co Basin over the Tibetan Plateau. Environ. Sci. 2008, 29, 3009–3016. [Google Scholar]
- Qin, J.H.; Huh, Y.; Edmond, J.M.; Du, G.; Ran, J. Chemical and physical weathering in the Min Jiang, a headwater tributary of the Yangtze River. Chem. Geol. 2006, 227, 53–69. [Google Scholar] [CrossRef]
- Liu, F.J.; Conklin, M.H.; Shaw, G.D. Insights into hydrologic and hydrochemical processes based on concentration-discharge and end-member mixing analyses in the mid-Merced River Basin, Sierra Nevada, California. Water. Resour. Res. 2017, 53, 832–850. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Xu, H.; Hou, Z.H.; An, Z.S. Major ion chemistry of waters in Lake Qinghai catchments, NE Qinghai-Tibet plateau, China. Quat. Int. 2010, 212, 35–43. [Google Scholar] [CrossRef]
- Xia, X.H.; Zhang, L.T.; Chen, J.S. The effect of lithology and climate on major ion chemistry of the Yangtze River system. Acta Sci. Nat. Univ. Pekin. 2000, 36, 246–252. [Google Scholar]
- Zhu, B.Q.; Yang, X.P. The ion chemistry of surface and ground waters in the Taklimakan Desert of Tarim Basin, western China. Chin. Sci. Bull. 2007, 52, 2123–2129. [Google Scholar] [CrossRef]
- Karis, T.; Silvester, E.; Rees, G. Chemical regulation of alpine headwater streams during a storm event (Bogong High Plains, Victoria, Australia). J. Hydrol. 2016, 542, 317–329. [Google Scholar] [CrossRef]
- Xiao, J.; Jin, Z.D.; Ding, H. Geochemistry and solute sources of surface waters of the Tarim River Basin in the extreme arid region, NW Tibetan Plateau. J. Asian Earth Sci. 2012, 54, 162–173. [Google Scholar] [CrossRef]
- Yang, Q.; Xiao, H.; Zhao, L. Hydrological and isotopic characterization of river water, groundwater, and groundwater recharge in the Heihe River basin, northwestern China. Hydrol. Process. 2011, 25, 1271–1283. [Google Scholar] [CrossRef]
- Galy, A.; France-Lanord, C. Weathering processes in the Ganges-Brahmaputra basin and the riverine alkalinity budget. Chem. Geol. 1999, 159, 31–60. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupré, B.; Louvat, P. Global silicate weathering and CO2, consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Roy, S.; Gaillardet, J.; Allègre, C.J. Geochemistry of dissolved and suspended loads of the Seine River, France: Anthropogenic impact, carbonate and silicate weathering. Geochim. Cosmoschim. Acta 1999, 63, 1277–1292. [Google Scholar] [CrossRef]
- Noh, H.; Huh, Y.; Qin, J.H. Chemical weathering in the Three Rivers region of Eastern Tibet. Geochim. Cosmoschim. Acta 2009, 73, 1857–1877. [Google Scholar] [CrossRef]
- Meybeck, M. Atmospheric inputs and river transport of dissolved substances. In Dissolved loads of Rivers and Surface Water Quantity/Quality Relationships, Proceedings of A Symposium Held During The XVIIIth General Assembly of the International Union of Geodesy and Geophysics at Hamburg, Germany, 15–27 August 1983; IAHS (International Association of Hydrological Sciences): London, UK, 1983. [Google Scholar]
- Tian, L.; Yao, T.; MacClune, K.; White, J.W.C.; Schilla, A.; Vaughn, B.; Vachon, R.; Ichiyanagi, K. Stable isotopic variations in west China: A consideration of moisture sources. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Pu, T.; He, Y.Q.; Zhu, G.F. Hydrochemical characteristics of Three Rivers around Yulong Mountain in rainy season. Sci. Geogr. Sin. 2011, 31, 734–740. [Google Scholar]
- Wang, Y.S.; Chen, X.X.; Zhang, M.N. Hydrochemistry and chemical weathering processes of Malian River basin. Earth Environ. 2018, 46, 15–22. [Google Scholar]
- Wu, W.H.; Yang, J.D.; Xu, S.J. Geochemistry of the headwaters of the Yangtze River, Tongtian He and Jinsha Jiang: Silicate weathering and CO2 consumption. Appl. Geochem. 2008, 23, 3712–3727. [Google Scholar] [CrossRef]
- Fan, B.L.; Zhao, Z.Q.; Tao, F.X. Characteristics of carbonate, evaporite and silicate weathering in Huanghe River basin: A comparison among the upstream, midstream and downstream. J. Asian Earth Sci. 2014, 96, 17–26. [Google Scholar] [CrossRef]
- Feng, F.; Li, Z.Q.; Zhang, M.J. Hydrochemical characteristics and controls of runoff at the headwaters of the Urumqi River, eastern Tianshan Mountain. Resour. sci. 2011, 33, 2238–2247. [Google Scholar]
- Anderson, S.P.; Drever, J.I.; Humphrey, N.F. Chemical weathering in glacial environments. Geology 1997, 25, 399–402. [Google Scholar] [CrossRef]
| Water Sample | Eigen-Value | Ca2+ | Mg2+ | Na+ | K+ | SO42− | NO3− | Cl− | HCO3− | TDS | Hydrochemical Characteristics |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Runoff | max mid-value min | 51.36 21.33 0.37 | 16.14 2.34 0.03 | 18.16 1.86 0.15 | 2.13 0.70 nd | 18.32 3.07 0.06 | 260.08 0.42 0.02 | 15.27 0.44 0.03 | 237.11 81.30 1.93 | 238.76 73.61 1.97 | HCO3−-Ca2+ |
| Precipitation | max mid-value min | 43.34 0.80 0.17 | 8.38 0.04 0.01 | 27.24 0.53 0.03 | 1.95 0.10 0.01 | 37.60 0.37 0.01 | 5.72 0.34 0.02 | 1.13 0.07 0.01 | 168.15 4.55 0.69 | 143 7.54 2.16 | HCO3−-SO42−-Ca2+ |
| Meltwater | max mid-value min | 5.42 0.48 0.21 | 0.54 0.07 0.01 | 2.14 0.16 0.05 | 4.36 0.07 0.01 | 0.31 0.05 0.01 | nd 0.08 0.33 | 1.06 0.03 0.01 | 17.76 2.65 1.06 | 18.74 4.87 1.54 | HCO3−-Ca2+ |
| Drainage Basin | Runoff (108 m3 a−1) | Drainage Area (103 km2) | SWR (t km−2 a−1) | CWR (t km−2 a−1) | TDStotal (t km−2 a−1) | ΦCO2−sil (105 mol km−2 a−1) | ΦCO2−carb (105 mol km−2 a−1) | Source |
|---|---|---|---|---|---|---|---|---|
| Dongkemadi | 0.24 | 0.039 | 1.98 | 12.30 | 14.82 | 0.91 | 3.28 | This study |
| Tuotuohe | 13.37 | 15.92 | 1.19 | 1.61 | 2.80 | 0.46 | 1.35 | [37] |
| Jinsha Jiang | 394 | 229 | 1.39 | 16.93 | 18.32 | 0.34 | 1.40 | [37] |
| Upstream Yellow River | 380 | 223 | 2.33 | 27.4 | 29.73 | 0.14 | 3.04 | [38] |
| Glacier No.1 | 0.02 | 0.003 | - | - | 18.1 | - | - | [39] |
| Haut Glacier | - | 0.012 | - | - | 13.7 | - | - | [40] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, T.; Li, Y.; Qin, J.; Li, X.; Yang, Q.; He, X. Hydrochemical Changes and Influencing Factors in the Dongkemadi Region, Tanggula Range, China. Water 2018, 10, 1856. https://doi.org/10.3390/w10121856
Han T, Li Y, Qin J, Li X, Yang Q, He X. Hydrochemical Changes and Influencing Factors in the Dongkemadi Region, Tanggula Range, China. Water. 2018; 10(12):1856. https://doi.org/10.3390/w10121856
Chicago/Turabian StyleHan, Tianding, Yuping Li, Jia Qin, Xiangying Li, Qin Yang, and Xiaobo He. 2018. "Hydrochemical Changes and Influencing Factors in the Dongkemadi Region, Tanggula Range, China" Water 10, no. 12: 1856. https://doi.org/10.3390/w10121856
APA StyleHan, T., Li, Y., Qin, J., Li, X., Yang, Q., & He, X. (2018). Hydrochemical Changes and Influencing Factors in the Dongkemadi Region, Tanggula Range, China. Water, 10(12), 1856. https://doi.org/10.3390/w10121856




