Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review
Abstract
1. Introduction
- As mentioned, SO4˙− possesses a high oxidation potential (2.5–3.1 V) comparable or even higher than ˙OH.
- Sulfate radical reacts more selectively and efficiently via electron transfer with organic compounds that contain unsaturated bonds or aromatic π electrons. By contrast, ˙OH is a non-selective radical and may also react with the diverse background constituted by hydrogen abstraction or electrophilic addition [28,29].
- SO4˙− reacts efficiently with organic compounds over a wide pH range of 2–8, reaching higher standard oxidation potential than hydroxyl radical at neutral pH [19].
- The half-life of sulfate radicals is supposed to be 30–40 µs, which enables SO4˙− to have more stable mass transfer and better contact with target compounds than hydroxyl radicals, whose half-life is 20 ns [30].
2. Chemistry of Peroxymonosulfate (PMS) and Persulfate (PS)
3. Activation Methods and Application in Micropollutants Removal
3.1. Radiation Activation
3.2. Thermal Activation
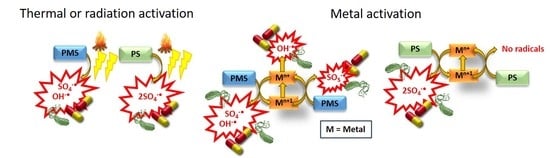
3.3. Metal Catalyst
3.4. Carbon-Based Catalysts
3.5. Hybrid Activation Treatments
4. Sulfate Radicals Applied in Disinfection
5. Coupling with Ultrafiltration Membrane
6. Economic Cost of Sulfate Radical-Based Advanced Oxidation Processes (SR-AOPs)
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WWAP (United Nations World Water Assessment Programme). The United Nations World Water Development Report 2015: Water for a Sustainable World; UNESCO: Paris, France, 2015. [Google Scholar]
- Licciardello, F.; Milani, M.; Consoli, S.; Pappalardo, N.; Barbagallo, S.; Cirelli, G. Wastewater tertiary treatment options to match reuse standards in agriculture. Agric. Water Manag. 2018, 210, 232–242. [Google Scholar] [CrossRef]
- Koshy, N.; Singh, D.N. Fly ash Zeolites for Water Treatment Applications. J. Environ. Chem. Eng. 2016, 4, 1460–1472. [Google Scholar] [CrossRef]
- Gibson, K.E. Viral pathogens in water: Occurrence, public health impact, and available control strategies. Curr. Opin. Virol. 2014, 4, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Quintiliani, C.; Di Cristo, C.; Leoparti, A. Vulnerability assessment to trihalomethane exposure in water distribution. Water 2018, 10, 912. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Alternative Disinfectants and Oxidants Guidance Manual; U.S. Environmental Protection Agency: Washington, DC, USA, 1999.
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Y.; Chu, Y.; Baoyou, G.; Yue, Q.; Yang, Z.; Li, Q. Reduction of organic matter and trihalomethane formation potential in reclaimed water from treated municipal wastewater by coagulation and adsorption. Chem. Eng. J. 2013, 223, 696–703. [Google Scholar] [CrossRef]
- Mohd Zainudin, F.; Abu Hasan, H.; Sheikh Abdullah, S.R. An overview of the technology used to remove trihalomethane (THM), trihalomethane precursors, and trihalomethane formation potential (THMFP) from water and wastewater. J. Ind. Eng. Chem. 2018, 57, 1–14. [Google Scholar] [CrossRef]
- Verma, K.; Gupta, D.; Gupta, A.B. Optimization of ozone disinfection and its effect on trihalomethanes. J. Environ. Chem. Eng. 2016, 4, 3021–3032. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Tufano, T.P.; Dionysiou, D.D. Chemical and microbial decontamination of pool water using activated potassium peroxymonosulfate. Water Res. 2008, 42, 2899–2910. [Google Scholar] [CrossRef]
- Gosselin, F.; Madeira, L.M.; Juhna, T.; Block, J.C. Drinking water and biofilm disinfection by fenton-like reaction. Water Res. 2013, 47, 5631–5638. [Google Scholar] [CrossRef]
- Bianco, A.; Polo-López, M.I.; Fernández-Ibáñez, P.; Brigante, M.; Mailhot, G. Disinfection of water inoculated with enterococcus faecalis using solar/Fe(III)EDDS-H2O2 or S2O82− process. Water Res. 2017, 118, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Ruales-Lonfat, C.; Barona, J.F.; Sienkiewicz, A.; Vélez, J.; Benítez, L.N.; Pulgarín, C. Bacterial inactivation with iron citrate complex: A new source of dissolved iron in solar photo-fenton process at near-neutral and alkaline pH. Appl. Catal. B Environ. 2016, 180, 379–390. [Google Scholar] [CrossRef]
- Díaz-Garduño, B.; Pintado-Herrera, M.G.; Biel-Maeso, M.; Rueda-Márques, J.J.; Lara-Martín, P.A.; Perales, J.A.; Manzano, M.A.; Garrido-Pérez, C.; Martín-Díaz, M.L. Environmental risk assessment of effluents as a whole emerging contaminant: Efficiency of alternative tertiary treatments for wastewater depuration. Water Res. 2017, 119, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. ISWCR 2015, 3, 57–65. [Google Scholar]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Emerging contaminants: Here today, there tomorrow! Environ. Nanotechnol. Monit. Manag. 2018, 10, 122–126. [Google Scholar] [CrossRef]
- Tornero, V.; Hanke, G. Potential Chemical Contaminants in the Marine Environment: An Overview of Main Contaminant Lists; EUR 28925; Publications Office of the European Union: Luxembourg, 2017; ISBN 978-92-79-77045-6. [Google Scholar]
- Zhang, B.T.; Zhang, Y.; Teng, Y.; Fan, M. Sulfate radical and its application in decontamination technologies. Crit. Rev. Env. Sci. Technol. 2015, 45, 1756–1800. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Machulek, A.; Oliveira, S.; Osugi, M.; Ferreira, V.; Quina, F.; Dantas, R.; Oliveira, S.; Casagrande, G.; Anaissi, F.; Silva, V.; et al. Application of Different Advanced Oxidation Processes for the Degradation of Organic Pollutants; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Shukla, P.; Sun, H.; Wang, S.; Ang, H.M.; Tad´e, M.O. Co-SBA-15 for heterogeneous oxidation of phenol with sulfate radical for wastewater treatment. Catal. Today 2011, 175, 380–385. [Google Scholar] [CrossRef]
- Zhao, Q.; Mao, Q.; Zhou, Y.; Wei, J.; Liu, X.; Yang, J.; Luo, L.; Zhang, J.; Chen, H.; Chen, H.; et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: A review on heterogeneous catalysts and applications. Chemosphere 2017, 189, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Bruell, C.J. Thermally activated persulfate oxidation of trichloroethylene: Experimental investigation of reaction orders. Ind. Eng. Chem. Res. 2008, 47, 2912–2918. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Deng, Y.; Ezyske, C.M. Sulfate radical-advanced oxidation process (SR-AOP) for simultaneous removal of refractory organic contaminants and ammonia in landfill leachate. Water Res. 2011, 45, 6189–6194. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Yu, M.; Teel, A.L.; Watts, R.J. Activation of peroxymonosulfate by subsurface minerals. J. Contam. Hydrol. 2016, 191, 33–43. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Amor, C.; Silva, T.; Dionysiou, D.D.; Li puma, G.; Lucas, M.S.; Peres, J.A. Treatment of winery wastewater by sulphate radicals: HSO5−/transition metal/UV-A LEDs. Chem. Eng. J. 2017, 310, 473–483. [Google Scholar] [CrossRef]
- Marjanovic, M.; Giannakis, S.; Grandjean, D.; de Alencastro, L.F.; Pulgarin, C. Effect of μM fe addition, mild heat and solar UV on sulfate radical-mediated inactivation of bacteria, viruses, and micropollutant degradation in water. Water Res. 2018, 140, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xu, T.; Chen, W.; Lu, W. Synergistic multiple active species for the photocatalytic degradation of contaminants by imidazole-modified g-C3N4 coordination with iron phthalocyanine in the presence of peroxymonosulfate. Chem. Eng. J. 2019, 357, 198–208. [Google Scholar] [CrossRef]
- Oh, W.; Dong, Z.; Lim, T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Degradation of carbamazepine by radiation-induced activation of peroxymonosulfate. Chem. Eng. J. 2018, 336, 595–601. [Google Scholar] [CrossRef]
- Alkhuraiji, T.S.; Boukari, S.O.B.; Alfadhl, F.S. Gamma irradiation-induced complete degradation and mineralization of phenol in aqueous solution: Effects of reagent. J. Hazard. Mater. 2017, 328, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, S.; Kumar, R.; Khan, M.A.; Paeng, K.J.; Kurade, M.B.; Kim, S.J.; Jeon, B.H. Aqueous phase degradation of methyl paraben using UV-activated persulfate method. Chem. Eng. J. 2017, 321, 11–19. [Google Scholar] [CrossRef]
- Ghauch, A.; Baalbaki, A.; Amasha, M.; El Asmar, R.; Tantawi, O. Contribution of persulfate in UV-254nm activated systems for complete degradation of chloramphenicol antibiotic in water. Chem. Eng. J. 2017, 317, 1012–1025. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, Y.; Gao, N.; Chu, W.; Shen, X.; Lu, X.; Chen, J.; Zhu, Y. Degradation kinetics and mechanism of 2,4-di-tert-butylphenol with UV/persulfate. Chem. Eng. J. 2016, 304, 201–208. [Google Scholar] [CrossRef]
- Hou, S.; Ling, L.; Shang, C.; Guan, Y.; Fang, J. Degradation kinetics and pathways of haloacetonitriles by the UV/persulfate process. Chem. Eng. J. 2017, 320, 478–484. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, X.; Jiang, J.; Ma, J.; Liu, G.; Cao, Y.; Liu, W.; Li, J.; Pang, S.; Kong, X.; et al. Degradation of sulfamethoxazole by UV, UV/H2O2 and UV/persulfate (PDS): Formation of oxidation products and effect of bicarbonate. Water Res. 2017, 118, 196–207. [Google Scholar] [CrossRef]
- Ao, X.; Liu, W. Degradation of sulfamethoxazole by medium pressure UV and oxidants: Peroxymonosulfate, persulfate, and hydrogen peroxide. Chem. Eng. J. 2017, 313, 629–637. [Google Scholar] [CrossRef]
- Mahdi-Ahmed, M.; Chiron, S. Ciprofloxacin oxidation by UV-C activated peroxymonosulfate in wastewater. J. Hazard. Mater. 2014, 265, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, Z.; Wang, Y.; Zhang, H. The UV/peroxymonosulfate process for the mineralization of artificial sweetener sucralose. Chem. Eng. J. 2017, 317, 561–569. [Google Scholar] [CrossRef]
- Wangc, C.; Liang, C. Oxidative degradation of TMAH solution with UV persulfate activation. Chem. Eng. J. 2014, 254, 472–478. [Google Scholar] [CrossRef]
- Cui, C.; Jin, L.; Jiang, L.; Han, Q.; Lin, K.; Lu, S.; Zhang, D.; Cao, G. Removal of trace level amounts of twelve sulfonamides from drinking water by UV-activated PMS. Sci. Total Environ. 2016, 572, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Wacławek, S.; Grübel, K.; Černík, M. Simple spectrophotometric determination of monopersulfate. Spectrochim. Acta Part A 2015, 149, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; He, X.; Wang, D.; Mezyk, S.P.; Otto, S.C.; Marfil-Vega, R.; Mills, M.A.; Dionysiou, D.D. Decomposition of iodinated pharmaceuticals by UV-254 nm-assisted advanced oxidation processes. J. Hazard. Mater. 2017, 323, 489–499. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Xie, Y.; Zeng, Y.; Li, P.; Xie, T.; Wang, Y. Ultrasonic-enhanced fenton-like degradation of bisphenol A using a bio-synthesized schwertmannite catalyst. J. Hazard. Mater. 2018, 344, 689–697. [Google Scholar] [CrossRef]
- Ayare, S.D.; Gogate, P.R. Sonocatalytic treatment of phosphonate containing industrial wastewater intensified using combined oxidation approaches. Ultrason. Sonochem. 2019, 51, 69–76. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; El-taliawy, H.; Durán, A.; Caro, G.; Bester, K. Sono-activated persulfate oxidation of diclofenac: Degradation, kinetics, pathway and contribution of the different radicals involved. J. Hazard. Mater. 2018, 357, 457–465. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Lin, K.; Zhang, W.; Lu, S.; Luo, Q. Removal of 1,1,1-trichloroethane from aqueous solution by a sono-activated persulfate process. Ultrason. Sonochem. 2013, 20, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shao, Y.; Gao, N.; Lu, X.; An, N. Degradation of diethyl phthalate (DEP) by UV/persulfate: An experiment and simulation study of contributions by hydroxyl and sulfate radicals. Chemosphere 2018, 193, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Shao, Y.; Gao, N.; Xia, S.; Tan, C.; Zhou, S.; Hu, X. Degradation of the antiepileptic drug carbamazepine upon different UV-based advanced oxidation processes in water. Chem. Eng. J. 2013, 222, 150–158. [Google Scholar] [CrossRef]
- Verma, S.; Nakamura, S.; Sillanpää, M. Application of UV-C LED activated PMS for the degradation of anatoxin-a. Chem. Eng. J. 2016, 284, 122–129. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; García-Cañibano, C.; Lepistö, R.J.; Encinas, Á.; Pellinen, J.; Marugán, J. Intensification of UV-C tertiary treatment: Disinfection and removal of micropollutants by sulfate radical based Advanced Oxidation Processes. J. Hazard. Mater. 2018, in press. [Google Scholar] [CrossRef]
- Li, B.; Zhu, J. Simultaneous degradation of 1,1,1-trichloroethane and solvent stabilizer 1,4-dioxane by a sono-activated persulfate process. Chem. Eng. J. 2016, 284, 750–763. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, N. Removal of carbamazepine from aqueous solution using sono-activated persulfate process. Ultrason. Sonochem. 2016, 29, 156–162. [Google Scholar] [CrossRef]
- Ferkous, H.; Merouani, S.; Hamdaoui, O.; Pétrier, C. Persulfate-enhanced sonochemical degradation of naphthol blue black in water: Evidence of sulfate radical formation. Ultrason. Sonochem. 2017, 34, 580–587. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Zhou, X.; Wu, Q.; Zheng, H.; Chang, J.; Ren, N. Enhanced peroxymonosulfate activation for sulfamethazine degradation by ultrasound irradiation: Performances and mechanisms. Chem. Eng. J. 2018, 335, 145–153. [Google Scholar] [CrossRef]
- Feng, Y.; Song, Q.; Lu, W.; Liu, G. Degradation of ketoprofen by sulfate radical-based advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Chemosphere 2017, 189, 643–651. [Google Scholar] [CrossRef]
- Aimer, Y.; Benali, O.; Serrano, K.G. Study of the degradation of an organophosphorus pesticide using electrogenerated hydroxyl radicals or heat-activated persulfate. Sep. Purif. Technol. 2019, 208, 27–33. [Google Scholar] [CrossRef]
- Qian, Y.; Xue, G.; Chen, J.; Luo, J.; Zhou, X.; Gao, P.; Wang, Q. Oxidation of cefalexin by thermally activated persulfate: Kinetics, products, and antibacterial activity change. J. Hazard. Mater. 2018, 354, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Ji, Y.; Shi, Y.; Chen, J.; Cai, T. Sulfate radical-based oxidation of fluoroquinolone antibiotics: Kinetics, mechanisms and effects of natural water matrices. Water Res. 2016, 106, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Zrinyi, N.; Pham, A.L. Oxidation of benzoic acid by heat-activated persulfate: Effect of temperature on transformation pathway and product distribution. Water Res. 2017, 120, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, L.; Zhang, S.; Ou, L.H.; Liu, C.B.; Zheng, L.Y.; Yang, Y.F.; Ying, G.G.; Luo, S.L. Degradation of azole fungicide fluconazole in aqueous solution by thermally activated persulfate. Chem. Eng. J. 2017, 321, 113–122. [Google Scholar] [CrossRef]
- Gao, H.; Chen, J.; Zhang, Y.; Zhou, X. Sulfate radicals induced degradation of triclosan in thermally activated persulfate system. Chem. Eng. J. 2016, 306, 522–530. [Google Scholar] [CrossRef]
- Ji, Y.; Shi, Y.; Dong, W.; Wen, X.; Jiang, M.; Lu, J. Thermo-activated persulfate oxidation system for tetracycline antibiotics degradation in aqueous solution. Chem. Eng. J. 2016, 298, 225–233. [Google Scholar] [CrossRef]
- Xu, X.; Pliego, G.; Zazo, J.A.; Casas, J.A.; Rodriguez, J.J. Mineralization of naphtenic acids with thermally-activated persulfate: The important role of oxygen. J. Hazard. Mater. 2016, 318, 355–362. [Google Scholar] [CrossRef]
- Ike, I.A.; Orbell, J.D.; Duke, M. Feasibility, mechanisms, and optimisation of organic pollutant degradation by thermally activated persulphate. Chem. Eng. Res. Des. 2018, 136, 304–314. [Google Scholar] [CrossRef]
- Fernandes, A.; Makoś, P.; Boczkaj, G. Treatment of bitumen post oxidative effluents by sulfate radicals based advanced oxidation processes (S-AOPs) under alkaline pH conditions. J. Clean. Prod. 2018, 195, 374–384. [Google Scholar] [CrossRef]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Sulfate radical-based ferrous–peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B Environ. 2009, 85, 171–179. [Google Scholar] [CrossRef]
- Pan, X.; Yan, L.; Qu, R.; Wang, Z. Degradation of the UV-filter benzophenone-3 in aqueous solution using persulfate activated by heat, metal ions and light. Chemosphere 2018, 196, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Dionysiou, D.D. Transition metal/UV-based advanced oxidation technologies for water decontamination. Appl. Catal. B Environ. 2004, 54, 155–163. [Google Scholar] [CrossRef]
- Ismail, L.; Ferronato, C.; Fine, L.; Jaber, F.; Chovelon, J. Elimination of sulfaclozine from water with SO4− radicals: Evaluation of different persulfate activation methods. Appl. Catal. B Environ. 2017, 201, 573–581. [Google Scholar] [CrossRef]
- Rodriguez, S.; Vasquez, L.; Costa, D.; Romero, A.; Santos, A. Oxidation of orange G by persulfate activated by Fe(II), Fe(III) and zero valent iron (ZVI). Chemosphere 2014, 101, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, P.; Zeng, Y.; Li, X.; Xiao, Y.; Wang, Y.; Zhang, Y. Thermally treated fungal manganese oxides for bisphenol A degradation using sulfate radicals. Chem. Eng. J. 2018, 335, 728–736. [Google Scholar] [CrossRef]
- Li, C.; Wu, J.; Peng, W.; Fang, Z.; Liu, J. Peroxymonosulfate activation for efficient sulfamethoxazole degradation by Fe3O4/β-FeOOH nanocomposites: Coexistence of radical and non-radical reactions. Chem. Eng. J. 2019, 356, 904–914. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, G.; Liu, M.; Wang, Q.; Dong, S.; Wang, P. Application of nickel foam-supported Co3O4-Bi2O3 as a heterogeneous catalyst for BPA removal by peroxymonosulfate activation. Sci. Total Environ. 2019, 647, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Sui, M.; Yuan, B.; Wang, J.; Lu, Y. Efficient degradation of nitrobenzene by Cu-Co-Fe-LDH catalyzed peroxymonosulfate to produce hydroxyl radicals. Chem. Eng. J. 2019, 357, 140–149. [Google Scholar] [CrossRef]
- Ding, Y.; Nie, W.; Li, W.; Chang, Q. Co-doped NaBiO3 nanosheets with surface confined co species: High catalytic activation of peroxymonosulfate and ultra-low co leaching. Chem. Eng. J. 2019, 356, 359–370. [Google Scholar] [CrossRef]
- Oh, W.; Lim, T. Design and application of heterogeneous catalysts as peroxydisulfate activator for organics removal: An overview. Chem. Eng. J. 2019, 358, 110–133. [Google Scholar] [CrossRef]
- Han, Z.; Li, J.; Han, X.; Ji, X.; Zhao, X. A comparative study of iron-based PAN fibrous catalysts for peroxymonosulfate activation in decomposing organic contaminants. Chem. Eng. J. 2019, 358, 176–187. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, X.; Yan, Y.; Sun, C.; Wu, H.; He, J.; Wang, D. Heterogeneous activation of peroxymonosulfate by different ferromanganese oxides for tetracycline degradation: Structure dependence and catalytic mechanism. Chem. Eng. J. 2018, 348, 263–270. [Google Scholar] [CrossRef]
- Nie, W.; Mao, Q.; Ding, Y.; Hu, Y.; Tang, H. Highly efficient catalysis of chalcopyrite with surface bonded ferrous species for activation of peroxymonosulfate toward degradation of bisphenol A: A mechanism study. J. Hazard. Mater. 2019, 364, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tian, X.; Nie, Y.; Yang, C.; Wang, Y. Enhanced peroxymonosulfate activation for phenol degradation over MnO2 at pH 3.5–9.0 via Cu(II) substitution. J. Hazard. Mater. 2018, 360, 303–310. [Google Scholar] [CrossRef]
- Kang, Y.; Yoon, H.; Lee, W.; Kim, E.; Chang, Y. Comparative study of peroxide oxidants activated by nZVI: Removal of 1,4-dioxane and arsenic(III) in contaminated waters. Chem. Eng. J. 2018, 334, 2511–2519. [Google Scholar] [CrossRef]
- Dong, H.; He, Q.; Zeng, G.; Tang, L.; Zhang, L.; Xie, Y.; Zeng, Y.; Zhao, F. Degradation of trichloroethene by nanoscale zero-valent iron (nZVI) and nZVI activated persulfate in the absence and presence of EDTA. Chem. Eng. J. 2017, 316, 410–418. [Google Scholar] [CrossRef]
- Wei, X.; Gao, N.; Li, C.; Deng, Y.; Zhou, S.; Li, L. Zero-valent iron (ZVI) activation of persulfate (PS) for oxidation of bentazon in water. Chem. Eng. J. 2016, 285, 660–670. [Google Scholar] [CrossRef]
- Li, H.; Wan, J.; Ma, Y.; Huang, M.; Wang, Y.; Chen, Y. New insights into the role of zero-valent iron surface oxidation layers in persulfate oxidation of dibutyl phthalate solutions. Chem. Eng. J. 2014, 250, 137–147. [Google Scholar] [CrossRef]
- Xiao, R.; Luo, Z.; Wei, Z.; Luo, S.; Spinney, R.; Yang, W.; Dionysiou, D.D. Activation of peroxymonosulfate/persulfate by nanomaterials for sulfate radical-based advanced oxidation technologies. Curr. Opin. Chem. Eng. 2018, 19, 51–58. [Google Scholar] [CrossRef]
- Pan, X.; Chen, J.; Wu, N.; Qi, Y.; Xu, X.; Ge, J.; Wang, X.; Li, C.; Qu, R.; Sharma, V.K.; et al. Degradation of aqueous 2,4,4′-trihydroxybenzophenone by persulfate activated with nitrogen doped carbonaceous materials and the formation of dimer products. Water Res. 2018, 143, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zeng, Z.; Li, Y.; Huang, Z.; Cui, Y. In-situ sulfur-doped carbon as a metal-free catalyst for persulfate activated oxidation of aqueous organics. Catal. Today 2018, 307, 12–19. [Google Scholar] [CrossRef]
- Liu, H.; Sun, P.; Feng, M.; Liu, H.; Yang, S.; Wang, L.; Wang, Z. Nitrogen and sulfur co-doped CNT-COOH as an efficient metal-free catalyst for the degradation of UV filter BP-4 based on sulfate radicals. Appl. Catal. B Environ. 2016, 187, 1–10. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, L.; Xue, Y.; Lv, J.; Yu, Q.; Yuan, X. Nitrogen-doped carbon material as a catalyst for the degradation of direct red23 based on persulfate oxidation. Sep. Purif. Technol. 2017, 184, 213–219. [Google Scholar] [CrossRef]
- Chen, J.; Hong, W.; Huang, T.; Zhang, L.; Li, W.; Wang, Y. Activated carbon fiber for heterogeneous activation of persulfate: Implication for the decolorization of azo dye. Environ. Sci. Pollut. Res. 2016, 23, 18564–18574. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Sun, H.; Tade, M.; Wang, S. Metal-free activation of persulfate by cubic mesoporous carbons for catalytic oxidation via radical and nonradical processes. Catal. Today 2018, 307, 140–146. [Google Scholar] [CrossRef]
- Wei, M.; Gao, L.; Li, J.; Fang, J.; Cai, W.; Li, X.; Xu, A. Activation of peroxymonosulfate by graphitic carbon nitride loaded on activated carbon for organic pollutants degradation. J. Hazard. Mater. 2016, 316, 60–68. [Google Scholar] [CrossRef]
- Duan, X.; Su, C.; Zhou, L.; Sun, H.; Suvorova, A.; Odedairo, T.; Zhu, Z.; Shao, Z.; Wang, S. Surface controlled generation of reactive radicals from persulfate by carbocatalysis on nanodiamonds. Appl. Catal. B Environ. 2016, 194, 7–15. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I.; Gurmen, S.; Gafarli, I.; Khoei, S.; Safaltin, S.; Ozcelik, D.Y. Oxidative degradation of bisphenol A by carbocatalytic activation of persulfate and peroxymonosulfate with reduced graphene oxide. J. Hazard. Mater. 2018, 360, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Weon, S.; Choi, W.; Hwang, Y.S.; Seo, J.; Lee, C.; Kim, J.H. Activation of persulfates by graphitized nanodiamonds for removal of organic compounds. Environ. Sci. Technol. 2016, 50, 10134–10142. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Carroll, K.C. Metal-free catalysis of persulfate activation and organic-pollutant degradation by nitrogen-doped graphene and aminated graphene. Environ. Pollut. 2016, 215, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Jiang, J.; Luo, C.; Pang, S.; Jiang, C.; Ma, J.; Jin, Y.; Li, J. Transformation of iodide by carbon nanotube activated peroxydisulfate and formation of iodoorganic compounds in the presence of natural organic matter. Environ. Sci. Technol. 2017, 51, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.; Moon, G.; Lee, H.; Jeon, T.H.; Lee, C.; Choi, W.; Lee, J. Oxidation of organic pollutants by peroxymonosulfate activated with low-temperature-modified nanodiamonds: Understanding the reaction kinetics and mechanism. Appl. Catal. B Environ. 2018, 237, 432–441. [Google Scholar] [CrossRef]
- Sun, P.; Liu, H.; Zhai, Z.; Zhang, X.; Fang, Y.; Tan, J.; Wu, J. Degradation of UV filter BP-1 with nitrogen-doped industrial graphene as a metal-free catalyst of peroxymonosulfate activation. Chem. Eng. J. 2019, 356, 262–271. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Huang, T.; Li, W.; Wang, Y.; Wang, Z. Decolorization of azo dye by peroxymonosulfate activated by carbon nanotube: Radical versus non-radical mechanism. J. Hazard. Mater. 2016, 320, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Jiang, J.; Luo, C.; Pang, S.; Yang, Y.; Wang, Z.; Ma, J.; Yu, J.; Zhao, X. Oxidation of bromophenols by carbon nanotube activated peroxymonosulfate (PMS) and formation of brominated products: Comparison to peroxydisulfate (PDS). Chem. Eng. J. 2018, 337, 40–50. [Google Scholar] [CrossRef]
- Barzegar, G.; Jorfi, S.; Zarezade, V.; Khatebasreh, M.; Mehdipour, F.; Ghanbari, F. 4-chlorophenol degradation using ultrasound/peroxymonosulfate/nanoscale zero valent iron: Reusability, identification of degradation intermediates and potential application for real wastewater. Chemosphere 2018, 201, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Li, D.; Xu, H.; Zan, J.; Sun, L.; Li, Q.; Zhang, B.; Wang, Y.; Xia, D. Effect of electronic migration of MIL-53(Fe) on the activation of peroxymonosulfate under visible light. Chem. Phys. Lett. 2018, 706, 694–701. [Google Scholar] [CrossRef]
- Ling, L.; Zhang, D.; Fang, J.; Fan, C.; Shang, C. A novel Fe(II)/citrate/UV/peroxymonosulfate process for micropollutant degradation: Optimization by response surface methodology and effects of water matrices. Chemosphere 2017, 184, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Chakma, S.; Praneeth, S.; Moholkar, V.S. Mechanistic investigations in sono-hybrid (ultrasound/Fe2+/UVC) techniques of persulfate activation for degradation of azorubine. Ultrason. Sonochem. 2017, 38, 652–663. [Google Scholar] [CrossRef]
- Liu, F.; Yi, P.; Wang, X.; Gao, H.; Zhang, H. Degradation of acid orange 7 by an ultrasound/ZnO-GAC/persulfate process. Sep. Purif. Technol. 2018, 194, 181–187. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, N.; Wang, W.; Kang, S.; Xu, J.; Xiang, H.; Yin, D. Ultrasound-assisted heterogeneous activation of persulfate by nano zero-valent iron (nZVI) for the propranolol degradation in water. Ultrason. Sonochem. 2018, 49, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Arellano, M.; Sanromán, M.A.; Pazos, M. Electro-assisted activation of peroxymonosulfate by iron-based minerals for the degradation of 1-butyl-1-methylpyrrolidinium chloride. Sep. Purif. Technol. 2019, 208, 34–41. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Ding, Z.; Zhao, Z.; Xu, X.; Fang, Z. Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4 for degradation of azo dye. Ultrason. Sonochem. 2017, 34, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chueca, J.; Moreira, S.I.; Lucas, M.S.; Fernandes, J.R.; Tavares, P.B.; Sampaio, A.; Peres, J.A. Disinfection of simulated and real winery wastewater using sulphate radicals: Peroxymonosulphate/transition metal/UV-A LED oxidation. J. Clean. Prod. 2017, 149, 805–817. [Google Scholar] [CrossRef]
- Xia, D.; Li, Y.; Huang, G.; Yin, R.; An, T.; Li, G.; Zhao, H.; Lu, A.; Wong, P.K. Activation of persulfates by natural magnetic pyrrhotite for water disinfection: Efficiency, mechanisms, and stability. Water Res. 2017, 112, 236–247. [Google Scholar] [CrossRef]
- Wordofa, D.N.; Walker, S.L.; Liu, H. Sulfate radical-induced disinfection of pathogenic Escherichia coli O157:H7 via iron-activated persulfate. Environ. Sci. Technol. Lett. 2017, 4, 154–160. [Google Scholar] [CrossRef]
- Garkusheva, N.; Matafonova, G.; Tsenter, I.; Beck, S.; Batoev, V.; Linden, K. Simultaneous atrazine degradation and E. coli inactivation by simulated solar photo-Fenton-like process using persulfate. J. Environ. Sci. Health. Part A Toxic. Hazard. Subst. Environ. Eng. 2017, 52, 849–855. [Google Scholar] [CrossRef]
- Xia, D.; He, H.; Liu, H.; Wang, Y.; Zhang, Q.; Li, Y.; Lu, A.; He, C.; Wong, P.K. Persulfate-mediated catalytic and photocatalytic bacterial inactivation by magnetic natural ilmenite. Appl. Catal. B Environ. 2018, 238, 70–81. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Silva, T.; Fernandes, J.R.; Lucas, M.S.; Li Puma, G.; Peres, J.A.; Sampaio, A. Inactivation of pathogenic microorganisms in freshwater using HSO5−/UV-A LED and HSO5−/Mn+/UV-A LED oxidation processes. Water Res. 2017, 12, 113–123. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21th ed.; Amer Public Health Assn: Washington, DC, USA, 2009. [Google Scholar]
- Wen, G.; Xu, X.; Zhu, H.; Huang, T.; Ma, J. Inactivation of four genera of dominant fungal spores in groundwater using UV and UV/PMS: Efficiency and mechanisms. Chem. Eng. J. 2017, 328, 619–628. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, H.; Ding, A.; Zhu, X.; Tang, X.; Gan, Z.; Xing, J.; Wu, D.; Li, G. Application of Fe(II)/peroxymonosulfate for improving ultrafiltration membrane performance in surface water treatment: Comparison with coagulation and ozonation. Water Res. 2017, 124, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wu, C.; Yu, H.; Gao, S.; Li, G.; Cui, F.; Qu, F. Applying ultraviolet/persulfate (UV/PS) pre-oxidation for controlling ultrafiltration membrane fouling by natural organic matter (NOM) in surface water. Water Res. 2018, 132, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Liang, H.; Ding, A.; Tang, X.; Liu, B.; Zhu, X.; Gan, Z.; Wu, D.; Li, G. Ferrous iron/peroxymonosulfate oxidation as a pretreatment for ceramic ultrafiltration membrane: Control of natural organic matter fouling and degradation of atrazine. Water Res. 2017, 113, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wu, D.; Liang, H.; Zhu, X.; Tang, X.; Gan, Z.; Xing, J.; Luo, X.; Li, G. Effect of sulfate radical-based oxidation pretreatments for mitigating ceramic UF membrane fouling caused by algal extracellular organic matter. Water Res. 2018, 145, 39–49. [Google Scholar] [CrossRef]
- Wang, Z.; Wan, Y.; Xie, P.; Zhou, A.; Ding, J.; Wang, J.; Zhang, L.; Wang, S.; Zhang, T.C. Ultraviolet/persulfate (UV/PS) pretreatment of typical natural organic matter (NOM): Variation of characteristics and control of membrane fouling. Chemosphere 2019, 214, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chueca, J.; Laski, E.; García-Cañibano, C.; Martín de Vidales, M.J.; Encinas, Á.; Kuch, B.; Marugán, J. Micropollutants removal by full-scale UV-C/sulfate radical based advanced oxidation processes. Sci. Total Environ. 2018, 630, 1216–1225. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Varella della Giustina, S.; Rocha, J.; Fernandes, T.; Pablos, C.; Encinas, Á.; Barceló, D.; Rodríguez-Mozaz, S.; Manaia, C.M.; Marugán, J. Assessment of full-scale tertiary wastewater treatment by UV-C based-AOPs: Removal or persistence of antibiotics and antibiotic resistance genes? Sci. Total Environ. 2019, 652, 1051–1061. [Google Scholar] [CrossRef]
- Fernandes, A.; Makoś, P.; Khan, J.A.; Boczkaj, G. Pilot scale degradation study of 16 selected volatile organic compounds by hydroxyl and sulfate radical based advanced oxidation processes. J. Clean. Prod. 2019, 208, 54–64. [Google Scholar] [CrossRef]
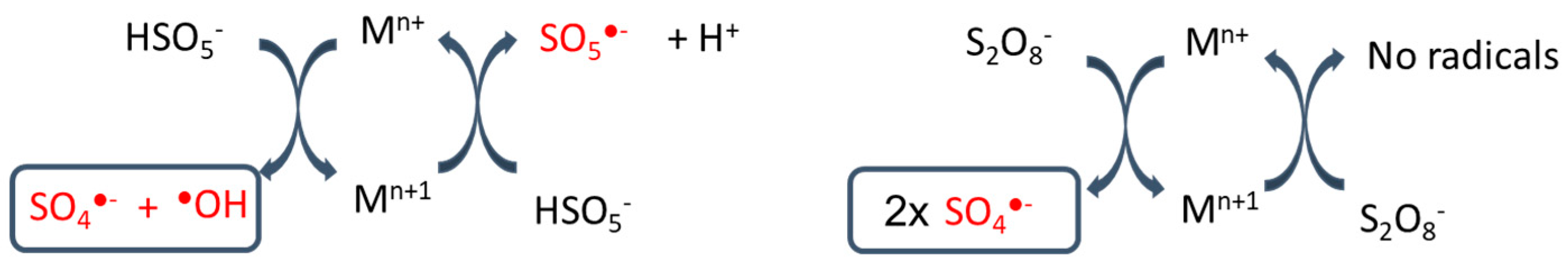

| Oxidant | Oxidation Potential (V) |
|---|---|
| Fluorine [F2] | 3.0 |
| Hydroxyl radical [HO˙] | 2.8 |
| Sulfate radical [SO4˙−] | 2.5–3.1 |
| Ozone [O3] | 2.1 |
| Persulfate [S2O82−] | 2.1 |
| Peroxymonosulfate [HSO5−] | 1.8 |
| Hydrogen peroxide [H2O2] | 1.8 |
| Permanganate [MnO4−] | 1.7 |
| Chlorine dioxide [ClO2] | 1.5 |
| Chlorine [Cl2] | 1.4 |
| PMS | PS | |
|---|---|---|
| Formula | HSO5− | S2O82− |
| Structure |  | 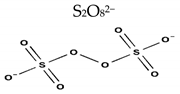 |
| Molecular weight [g·mol−1] | 113.07 | 192.12 |
| Solubility in water at 25 °C [g·L−1] | >250 | 730 * |
| Redox potential (V) | 1.8 | 2.1 |
| O-O bond dissociation energy [kJ·mol−1] | 140–213 [26] | 140 [37] |
| O-O bond length (Å) | 1.453 | 1.497 |
| Oxidant | Contaminant | Wavelength (nm) | Operating Conditions | Efficiency | Ref. |
|---|---|---|---|---|---|
| PS | Diatrizoate thyroxine | 254 | PS 1 mM 1; pH 7.4; T 21 °C | 100% | [51] |
| PS | Chloroamiphenicol | 254 | PS 0.25 mM; T 20 °C | 100% (60 min) | [41] |
| PS | TMAH | 254 | PS 10 mM; T 24 °C | 100% (2 h) | [48] |
| PS | Methyl paraben | 254 | PS 1 mM; pH 6.5; Tamb 2 | 98.9% (90 min) | [40] |
| PS | Haloacetonitriles | 254 | PS 1 mM; pH 6; T 25 °C | 95% (10 min) | [43] |
| PS | Sulfonamides | 254 | PMS 1 mM; pH 7.5; T 25 °C | 95% (15 min) | [49] |
| PS | Diethyl phtalate | 254 | PS 0.2 mM; pH 5.7; T 20 °C | 92.6% (60 min) | [56] |
| PS | Sulfamethoxazole | 254 | PS 1 mM; pH 7–8; T 20 °C | 90% (30 min) | [44] |
| PS | 2,4-Di-tert-butylphenol | 254 | PS 1 mM; pH 7; T 25 °C | 85.64% (30 min) | [32] |
| PS | Carbamazepine | 254 | PS 1 mM; pH 3.5–5.5; T 25 °C | 76.2% (90 min) | [57] |
| PMS | Carbamazepine | 254 | PMS 1 mM; pH 4.5; T 25 °C | 98.9% (90 min) | [57] |
| PMS | Anatoxin-a | 260–290 | PMS 0.15 mM; pH 6.4; Tamb | 98.6% (10 min) | [58] |
| PMS | Criprofloxacin | 254 | PMS 1 mM; pH 7; T 25 °C | 97% (60 min) | [46] |
| PMS and PS | Various micropollutants | 254 | 5 mM PMS and PS; pH neutral; T 20 °C; Continuous flow rate | 24–100% (18 s) | [59] |
| PMS | Sucralose | 254 | PS 3.78 mM; pH 7; T 25 °C | 95% (60 min) | [47] |
| Oxidant | Contaminant | US Power (W) | Operating Conditions | Efficiency | Ref. |
|---|---|---|---|---|---|
| PS | Diclofenac | 700 | PS 0.44 mM; pH 6; T 30 °C | 97% (4 h) | [54] |
| PS | Naphthol Blue Black | 80 | PS 1.8 mg/L; pH 6; T 25 ° | 93% (20 min) | [63] |
| PS | 1,1,1-trichloroethane | 100 | PS 0.94 mM; pH 7; T 20 °C | 90% (25 min) | [55] |
| PS | Carbamazepine | 200 | PS 5 mM; pH 5; T 50 °C | 89.4% (2 h) | [60] |
| PS | 1,1,1-trichloroethane (TCA) | 100 | PS 1.5 mM; pH 7; T 15 °C | 100% TCA (2 h) | [61] |
| 1,4-dioxane | 60% dioxane (2 h) | ||||
| PMS | Sulfamethazine | 600 | PMS 1,95 mM; pH 7.5 | 97.5 % (20 min) | [62] |
| Oxidant | Contaminant | T Range | Operating Conditions | Efficiency | Ref. |
|---|---|---|---|---|---|
| PS | Tetracyclines | 40–70 °C | PS 2 mM; pH 7; T 70 °C | 100% (30 min) | [71] |
| PS | Triclosan | 50–80 °C | PS 0.155 mM; T 70 °C | 100% (2 h) | [61] |
| PS | Naphtenic acids | 40–97 °C | PS stoichometric dose; pH 8; T 80 °C | 100% | [72] |
| PS | Ketoprofen | 40–70 °C | PS 2 mM; pH 7; T 70 °C | 98% (10 min) | [64] |
| PS | Orange G | 20–100 °C | PS 10 mM; pH 6.8; T 90 °C | 97% (1 min) | [73] |
| PS | Ciprofloxacin | 40–70 °C | PS 2 mM; pH 7; T 70 °C | 92% (180 min) | [67] |
| PS | Cefalexin | 50–65 °C | PS 1.1 mM; pH 7; T 60 °C | 90.7 % (4 h) | [66] |
| PS | Fluconazole | 30–60 °C | PS 20 mM; pH 5; T 60 °C | 90% (4 h) | [69] |
| PS | Benzoic Acid | 22–70 °C | PS 1 mM; pH 7.5; T 70 °C | 80% (90 min) | [68] |
| PS | Bitumen | 25–70 °C | rox1 1.28; T 60 °C | 33% (182 min) | [74] |
| PMS | Dimethoate | 40–70 °C | PS 5 mM; T 60 °C | 100% (80 min) | [65] |
| PMS | Bitumen | 25–70 °C | rox 1.43; T 60 °C | 43% (182 min) | [73] |
| Process | Contaminant | [Catalyst] (g·L−1) | Operating Conditions | Efficiency | Ref. |
|---|---|---|---|---|---|
| PS/G-ND | phenol | 0.1 | PS 1 mM; pH 7 | 100% (10 min) | [104] |
| PS/CMK 1 | Phenol | 0.2 | PS 6.5 mM; T 25 °C | 100% (20 min) | [100] |
| PS/reduced GO | Bisphenol | 0.02 | PS 0.25 mM; pH 7; T 25 °C | 100% (30 min) | [103] |
| PS/ACS | 4-CP | 0.05 | PS 8 mM; T 25 °C | 100% (60 min) | [96] |
| PS/NH4NO3-CNT-OH | 2,4,4-HBP | 0.1 | PS 21.7 mM; pH 7; T 25 °C | 100% (2 h) | [95] |
| PS/N-GP | SMX | 0.05 | PS 1 mM; pH 6; T 25 °C | 99,9% (3 h) | [105] |
| PS/NNC 2 | DR23 | 0.2 | PS 0.5 mM; T 35 °C | 97% (2 h) | [98] |
| PS/CNT | Iodorganic compounds | 0.05 | PS 0.5 mM; pH 7; T 20 °C | 95% (15 min) | [106] |
| PS/NND | Phenol | 0.2 | PS 6.5 mM; pH 6; T 25 °C | 90% (90 min) | [102] |
| PS/AC Fiber | AO G | 0.3 | PS 1.76 mM; pH 7; T 25 °C | 90% (2 h) | [99] |
| PS/NH2-GP | SMX | 0.05 | PS 1 mM; pH 6; T 20 °C | 50% (10 h) | [103] |
| PMS/NS-CNT-COOH | BP-4 | 0.1 | PMS 3.25 mM; pH 7; T 25 °C | 100% (30 min) | [97] |
| PMS/ND/GO | 4-CP | 0.1 | PMS 1 mM; pH 7 | 100% (40 min) | [107] |
| PMS/N-IrGO3 | BP-1 | 0.05 | PMS 1.62 mM; T 25 °C | 100% (60 min) | [108] |
| PMS/g-C3N4/AC | AO7 | 0.2 | PMS 1.3 mM; pH 3.8; T 27 °C | 96,4% (10 min) | [101] |
| PMS/CNT | AO7 | 0.1 | PMS 1.14 mM; pH 7 | 95% (30 min) | [109] |
| PMS/CNT | Bromphenols | 50 | PMS 0.5 mM; pH 7; T 20 °C | 90% (60 min) | [110] |
| PMS/rGO | Bisphenol | 0.02 | PMS 0.5 mM; pH 7; T 25 °C | 83% (30 min) | [103] |
| Process | Contaminant | Operating Conditions | Efficiency | Ref. |
|---|---|---|---|---|
| US/PMS/nZVI | 4-Chlorophenol | pH 3; nZVI 0.4 g/L; PMS 1.25 mM | 95% (30 min) | [111] |
| PMS/Fe/UV | RhB | pH 5; PMS 0.6 mM; | 100% (20 min) | [112] |
| Fe2+/citrate/UV/PMS | carbamazepine | λ 1 = 254 nm; pH 7; Fe(II) 12.2 mM; PMS 100 mM; citrate 26.4 mM | 70% (20 min) | [113] |
| US/PS/UVC | Azorubine | pH 6.5; US = 1.2 W/cm2; λ = 254 | 92% (10 min) | [114] |
| US/ZnO-GAC/PS | Acid Orange 7 | PS 0.5 g/L; ZnO-GAC 0.5 g/L; T 30 °C; pH 3; US 60W; | 91% (60 min) | [115] |
| US/nZVI/PS | Propranolol | PS 0.1 mM; nZVI 0.15 g/L; US 250W; pH 4.5 | 94% (30 min) | [116] |
| PMS/Fe/electric field | C9H20ClN | PMS 10 mM; pyrite 1mM; Electric field 150 mA | 80% (90 min) | [117] |
| US/PMS/Fe3O4 | Acid Orange 7 | T 25 °C; pH 7.5; US 200W; PMS 3 mM; Fe3O4 0.4 g/L | 90% (30 min) | [118] |
| Process | Microorganism | Operating Conditions | Efficiency | Ref. |
|---|---|---|---|---|
| PS/NP 1 | E. coli | PS 1 mM; NP 1.25 g/L; pH 7; T 30 °C | 7 log (20 min) | [120] |
| PS/Ilmenite/vis 2 | E. coli | PS 0.5 mM; Ilmenite 1 g/L | 7 log (20 min) | [123] |
| PMS/UV-A/Fe2+ | E. coli | PMS 0.1 mM; Fe2+ 0.1 mM; pH 6.5 | 6.5 log (30 min) | [124] |
| PMS/UV-A/Co2+ | E. coli | PMS 0.1 mM; Co2+ 0.1 mM; pH 6.5 | 6.5 log (60 min) | [124] |
| PS/Fe2+/vis | E. coli | PS 150 mg/L; Fe2+ 5 mg/L | 6 log (45 min) | [122] |
| PMS/UV-A/ Fe2+ | E. coli | PMS 0.5 mM; Fe2+ 0.5 mM; pH 5 | 4 log (120 min) | [119] |
| PS/ Fe2+ | E. coli | PS 3 mM; Fe2+ 3 mM; pH 7 | 3.4 log (180 min) | [121] |
| PMS/UV-A/ Co2+ | E. coli | PMS 0.5 mM; Co2+ 0.5 mM; pH 5 | 1 log (120 min) | [119] |
| PS/NP | S. aurous | PS 1 mM; NP 1.25 g/L; pH 7; T 30 °C | 7 log (20 min) | [120] |
| PMS/UV-A/Co2+ | S. aurous | PMS 0.1 mM; Co2+ 0.1 mM; pH 6.5 | 6.1 log (120 min) | [124] |
| PMS/UV-A/Co2+ | S. aurous | PMS 0.5 mM; Co2+ 0.5 mM; pH 5 | 4.1 log (120 min) | [119] |
| PMS/UV-A/Fe2+ | S. aurous | PMS 0.5 mM; Fe2+ 0.5 mM; pH 5 | 3.5 log (120 min) | [119] |
| PMS/UV-A/Fe2+ | S. aurous | PMS 0.1 mM; Fe2+ 0.1 mM; pH 6.5 | 3.2 log (120 min) | [124] |
| PMS/UV-A/Co2+ | B. mycoides | PMS 0.1 mM; Co2+ 0.1 mM; pH 6.5 | 3.4 log (120 min) | [124] |
| PMS/UV-A/Fe2+ | B. mycoides | PMS 0.5 mM; Fe2+ 0.5 mM; pH 5 | 3.4 log (120 min) | [119] |
| PMS/UV-A/Fe2+ | B. mycoides | PMS 0.1 mM; Fe2+ 0.1 mM; pH 6.5 | 3.2 log (30 min) | [124] |
| PMS/UV-A/Co2+ | B. mycoides | PMS 0.5 mM; Co2+ 0.5 mM; pH 5 | 3.1 log (120 min) | [119] |
| PMS/UV-A/Co2+ | C. albicans | PMS 5 mM; Co2+ 2.5 mM; pH 6.5 | 5.3 log (30 min) | [124] |
| PMS/UV-A/Co2+ | C. albicans | PMS 10 mM; Co2+ 5 mM; pH 5 | 5 log (120 min) | [119] |
| PMS/UV-A/Fe2+ | C. albicans | PMS 5 mM; Fe2+ 2.5 mM; pH 6.5 | 5 log (60 min) | [124] |
| PMS/UV-A/Fe2+ | C. albicans | PMS 10 mM; Fe2+ 5 mM; pH 5 | 4.8 log (120 min) | [119] |
| PS/Fe3+/vis | Enterococcus sp. | PS 5 mM; Fe3+ 0.5 mM; pH 8; T 26 °C | 6 log (30 min) | [13] |
| PMS/UV-C | Acremonium sp. | PMS 0.1 mM; pH 7; T 20 °C | 5 log (6 min) | [126] |
| PS/UV-C | Acremonium sp. | PS 0.1 mM; pH 7; T 20 °C | 3.7 log (6 min) | [126] |
| PMS/UV-C | Cladosporium sp. | PMS 0.1 mM; pH 7; T 20 °C | 4.9 log (15 min) | [126] |
| PS/UV-C | Cladosporium sp. | PS 0.1 mM; pH 7; T 20 °C | 3.9 log (15 min) | [126] |
| PMS/UV-C | Penicillium sp. | PMS 0.1 mM; pH 7; T 20 °C | 6.2 log (9 min) | [126] |
| PS/UV-C | Penicillium sp. | PS 0.1 mM; pH 7; T 20 °C | 5.9 log (9 min) | [126] |
| PMS/UV-C | Trichoderma sp. | PMS 0.1 mM; pH 7; T 20 °C | 5.2 log (6 min) | [126] |
| PS/UV-C | Trichoderma sp. | PS 0.1 mM; pH 7; T 20 °C | 5 log (6 min) | [126] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra-Rodríguez, S.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review. Water 2018, 10, 1828. https://doi.org/10.3390/w10121828
Guerra-Rodríguez S, Rodríguez E, Singh DN, Rodríguez-Chueca J. Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review. Water. 2018; 10(12):1828. https://doi.org/10.3390/w10121828
Chicago/Turabian StyleGuerra-Rodríguez, Sonia, Encarnación Rodríguez, Devendra Narain Singh, and Jorge Rodríguez-Chueca. 2018. "Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review" Water 10, no. 12: 1828. https://doi.org/10.3390/w10121828
APA StyleGuerra-Rodríguez, S., Rodríguez, E., Singh, D. N., & Rodríguez-Chueca, J. (2018). Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review. Water, 10(12), 1828. https://doi.org/10.3390/w10121828