3.1. Vertical Distribution of Trace Elements in Soil
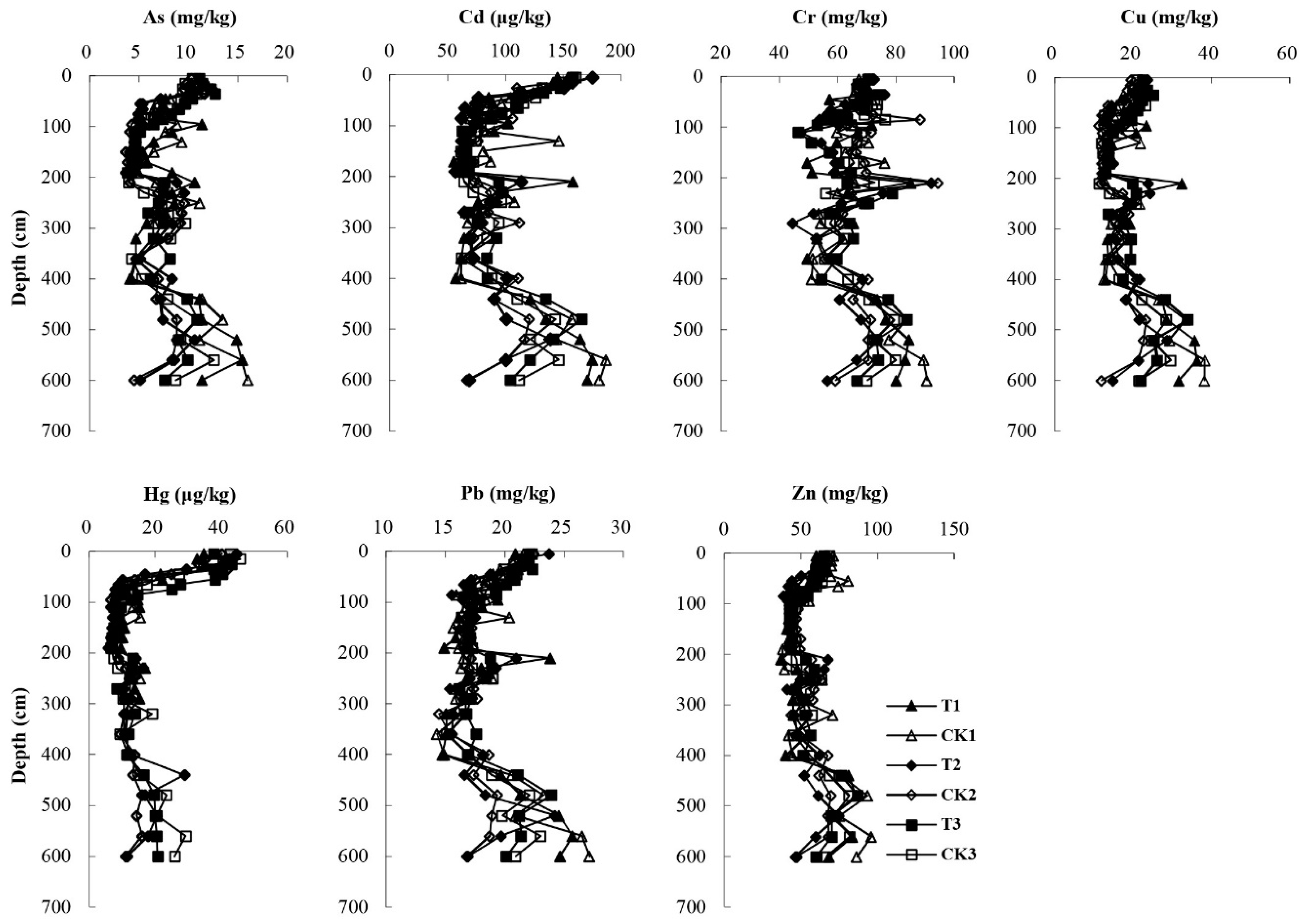
The vertical distribution of trace elements could help describe the soil contamination level and characteristics. The vertical distribution of As, Cd, Cr, Cu, Hg, Pb and Zn concentrations along the 0–600 cm soil profiles under three irrigation times and the three corresponding control cases is illustrated in
Figure 2. For all samples, the highest concentrations were obtained for soil Cr, Zn and Cu. For the weighted average of 0–600 cm profiles, Cr, Zn and Cu concentrations of the treatment and control groups were in the range of 62.38–68.58 mg/kg (66.54 mg/kg), 53.07–60.86 mg/kg (58.06 mg/kg) and 16.80–22.26 mg/kg (19.88 mg/kg), respectively, which were higher than the reported background concentrations in Beijing (29.8 mg/kg for Cr, 57.5 mg/kg for Zn, 18.7 mg/kg for Cu) [
23]. The trace element concentrations in soils in descending order were as follows: Cr > Zn > Cu > Pb > As > Cd > Hg. Comparing with the previous studies, the contents of seven trace elements in soils of the control and treatment groups were close to or even lower than that in agricultural soils irrigated without sewage or reclaimed water in other regions of China. In Lu et al.’s [
26] survey of agricultural soils in Shunyi, Beijing, the mean values of the trace elements were 7.85, 0.136, 22.4, 0.073, 20.4 and 69.8 for As, Cd, Cu, Hg, Pb and Zn, respectively, slightly higher than the contents of the present study. And in Li et al.’s [
27] research, the mean Cd, Pb, Cu and Zn concentrations in the 0–20 cm soil of Shenyang were 0.10, 21.60, 16.88 and 46.46 mg/kg, respectively, which showed a slightly lower values.
In general, the As, Cd, Cu, Hg, Pb and Zn contents decreased with increasing depth on the top 100 cm soil layer, suggesting the strong adsorption and fixation capacity of the surface soil. Among them, Hg decreased the most. The concentration of Hg decreased sharply from 33.86–43.00 µg/kg in top 30 cm profiles to 6.84–10.92 µg/kg at 100 cm depth (68–83% decrease) and did not change substantially in the 100–600 cm soil profiles. The results showed the strong adsorption effect of the topsoil on Hg. Besides the clay fraction, atmospheric deposition might play an important role in the accumulation of Hg in topsoil [
28]. In the 200–300 cm and 450–550 cm soil layers As, Cd, Cu, Pb and Zn showed an increase in concentration, which was because of the high clay content in these soil layers. The increase in the adsorption efficiency can be explained by the increasing surface area where the adsorption takes place [
29]. The Pb concentration showed little variation in the 100–400 cm profile and had an increasing tendency in the 400–600 cm profile. In an alkaline medium (pH > 7), the soil negative charge increases with the increasing pH and clay content, as in the 400–600 cm profiles, thus resulting in increased adsorption of Pb [
30]. The Cr content had a small fluctuation in the 0–200 cm soil layers and showed an increase with increasing depth in the 400–600 cm profiles. The mobility of Cr in soil depends on its oxidation state [
31]. Cr added to the soil tends to be oxidized to its mobile state, Cr(VI), generally in the anionic form, because of the well-aerated soil in the surface layer. The alkaline environment and the negative charge of soil particles enhanced the mobility of Cr(VI) in soils. With increasing depth, the soil conditions gradually changed to reducing conditions where Cr(VI) might be converted to the stable form Cr(III). The trace elements concentrations were relatively low at 300–400 cm but relatively high at 400–600 cm, which might be attributed to long-term leaching.
The vertical distribution of trace elements in soil profiles of other regions have been discussed in many previous studies. Huang et al. [
32] found that the heavy metals showed a slight enrichment at the surface or subsurface and gradually decreased with depth in 0–200 cm agricultural soils. This trend was similar as the distribution of the trace elements in 0–200 cm profiles in this study. By subtracting the heavy metals which included the one brought by wastewater irrigation and the one in original soil, Li et al. [
27] found that the upper 0–20 cm soil layer have accumulated most of the exogenous heavy metals than 20–40 cm soil layer, indicating that the atmospheric precipitation was a significant contributor to heavy metals in topsoil. It was likely that the reclaimed water irrigation and the atmospheric precipitation were the main reason of relatively high concentration of trace elements in the 0–30 cm soil layer.
The trace element concentrations in different soil layers of the treatment group (T1, T2 and T3) and the control group (CK1, CK2 and CK3) and the percentage increase in the concentration of the treatment group when compared with the control group are shown in
Table 4. On average, after long-term reclaimed water irrigation, the concentrations of As, Cd, Cr, Cu, Hg and Pb in top 30 cm soils increased by 10%, 6%, 2%, 10%, 4% and 2%, respectively; the Zn contents decreased by 2%. The trace elements showed an obvious accumulation in the 0–30 cm soil layer. At the depth of 30–100 cm, the concentrations of As, Cu, Hg and Pb increased by 8%, 14%, 15% and 1%, respectively and the Cd, Cr and Zn contents decreased by 6%, 7% and 0.4%, respectively. In the 100–200 cm soil layers, the As, Cu, Hg contents increased by 5%, 7% and 7%, respectively, whereas the Cd, Cr, Pb, Zn contents declined by 13%, 15%, 2% and 8%, respectively. The concentrations of the seven trace elements all increased in the 200–300 cm profiles, by 2% for As, 7% for Cd, 3% for Cr, 24% for Cu, 20% for Hg, 5% for Pb and 4% for Zn. At 300–600 cm depth, the contents of Cd, Cu, Hg and Pb increased by 0.4%, 4%, 5% and 2%, respectively and the As, Cr, Zn contents decreased by 0.1%, 2% and 5%, respectively.
According to the change in trace element contents in soil profiles after long-term reclaimed water irrigation, the top 30 cm and 200–300 cm soil layers were more likely to accumulate trace elements especially As, Cu and Hg in the study area, which might be explained by the higher clay and OM contents of these two soil layers (
Table 3). Similarly, Bao et al. [
33] studied the accumulation of heavy metals in soil profiles with long-term sewage irrigation and found that the irrigation area showed the greatest accumulation of Hg, Pb and Cu in topsoil (0–30 cm) due to the greatest topsoil OM contents. Present and previous studies indicated that the reclaimed water irrigation could contribute to the trace elements accumulation in soil which was similar to sewage irrigation [
34,
35]. However, the increases in trace element concentrations in soils of the treatment groups relative to those of the control groups in this study were less than 10% in the 0–30 cm profiles and less than 7% in the 200–300 cm profiles except for Cu (20%) and Hg (20%). Overall, comparing the three pairs of treatment and control groups, no obvious accumulation was observed in the 0–600 cm soil profiles with the increase in years of reclaimed water irrigation.
3.2. Effects of Soil Properties on Trace Element Distribution
The distribution of trace elements in the soil profile is closely related to soil properties, trace element species and sources [
36]. Understanding the relationship between the trace elements and the soil parameters could help clarify the behavior of these elements in soil. Pearson correlation analyses between trace element concentrations and soil properties (clay, OM and Eh) of the treatment group and control group soils and among trace elements were conducted in this study (
Table 5).
The clay content was significantly positively correlated with the seven trace elements in all treatments T1–T3 (
Table 5;
p < 0.01 except for Hg in T2 where
p < 0.05). The clay in CK1 showed a significant positive correlation with the contents of Cd, Cu, Pb and Zn (
p < 0.05). In CK2, clay was significantly positively correlated with As, Cu and Zn (
p < 0.01) and with Cr, Hg and Pb (
p < 0.05). In CK3, clay was significantly positively correlated with As, Cd, Cu, Pb and Zn (
p < 0.01) and with Cr and Hg (
p < 0.05). Positive correlations were observed between trace elements and soil clay fraction in many different researches [
37,
38]. The correlations in treatment groups were more significant than that in control groups, suggesting that the trace elements in soils of control groups have not reached their saturation values. The clay was considered as the main soil component associated with trace elements because of the high affinity of the trace elements for clay minerals [
39]. The high correlation coefficients shown in
Table 5 confirmed the results obtained in Bradl’s [
40] study that the fine-grained soil fraction exhibited higher tendency for trace element adsorption than coarse-grained soils as it contains soil particles with large surface areas such as clay minerals. This might explain the high content of trace elements in the 250–300 and 450–550 cm soil layers in this study.
OM was positively correlated with the seven trace elements (
Table 5). Trace elements tend to adsorb on OM and form stable organic-metallic complexes. Therefore, soils rich in OM can reduce the migration and biological activity of trace elements [
41,
42,
43]. OM and clay content were found to be significantly correlated [
44]. Clay particles are more likely to adsorb OM and form clay-organic complexes because of their higher surface area and charge density. The small pores and poor ventilation of soil with high clay contents are responsible for inhibiting the activity of aerobic microbes, by extension, weakening the decomposition of OM and benefiting its accumulation. Therefore, soil with high clay contents has relatively high OM contents. Results showed that the correlation between trace element concentration and clay fraction is consistent with that of trace element concentration and OM. Moreover, organic carbon (OC) content, as the amount of carbon found in OM, is also found to correlate positively with trace elements by many researchers [
39,
45].
The Eh was not significantly correlated with trace elements (
Table 5). Eh is an important indicator reflecting the soil oxidation state, as such, Eh could induce a serious of complex chemical changes in soil pH, OM contents and mineral components, which directly or indirectly affect the environmental behavior of trace elements [
46]. These complex chemical changes could make it difficult to quantify the effects of Eh on trace elements.
Correlation analysis was also carried out between trace elements (
Table 6). Inter-elements relationships provide information on trace element sources and pathways. According to the Pearson correlation coefficient values, all elements were highly correlated (
p < 0.01) and the high correlations suggest that the trace elements have a similar origin [
47,
48].
Results of the statistical analyses have shown the various and complex relationships between trace elements and soil properties. Some of the correlation coefficients were low, although significant, suggesting a weak linear dependence. Nevertheless, the main relationships described in the study have been observed in many previous researches of trace elements [
39,
48].
3.3. Potential Ecological Risk Assessment
The contents of As, Cd, Cr, Cu, Hg, Pb and Zn in the soil of control groups were 0.95–1.30 (mean 1.10), 0.77–0.94 (0.85), 2.25–2.30 (2.28), 0.90–1.15 (1.02), 0.23–0.30 (0.27), 0.72–0.78 (0.75) and 0.99–1.06 (0.99) times those of the background values of trace elements in Beijing, respectively. The contents of As, Cd, Cr, Cu, Hg, Pb and Zn in the soil of treatment groups were 1.00–1.27 (1.13), 0.75–0.89 (0.84), 2.09–2.24 (2.19), 0.99–1.19 (1.10), 0.26–0.34 (0.30), 0.73–0.78 (0.77) and 0.92–1.03 (1.03) times those of the background values, respectively. The contamination factors followed the order of Cr > As > Cu > Zn > Cd > Pb > Hg. The results revealed that the study area already has some accumulation of Cr, As and Cu and that the Zn concentration was close to the background value.
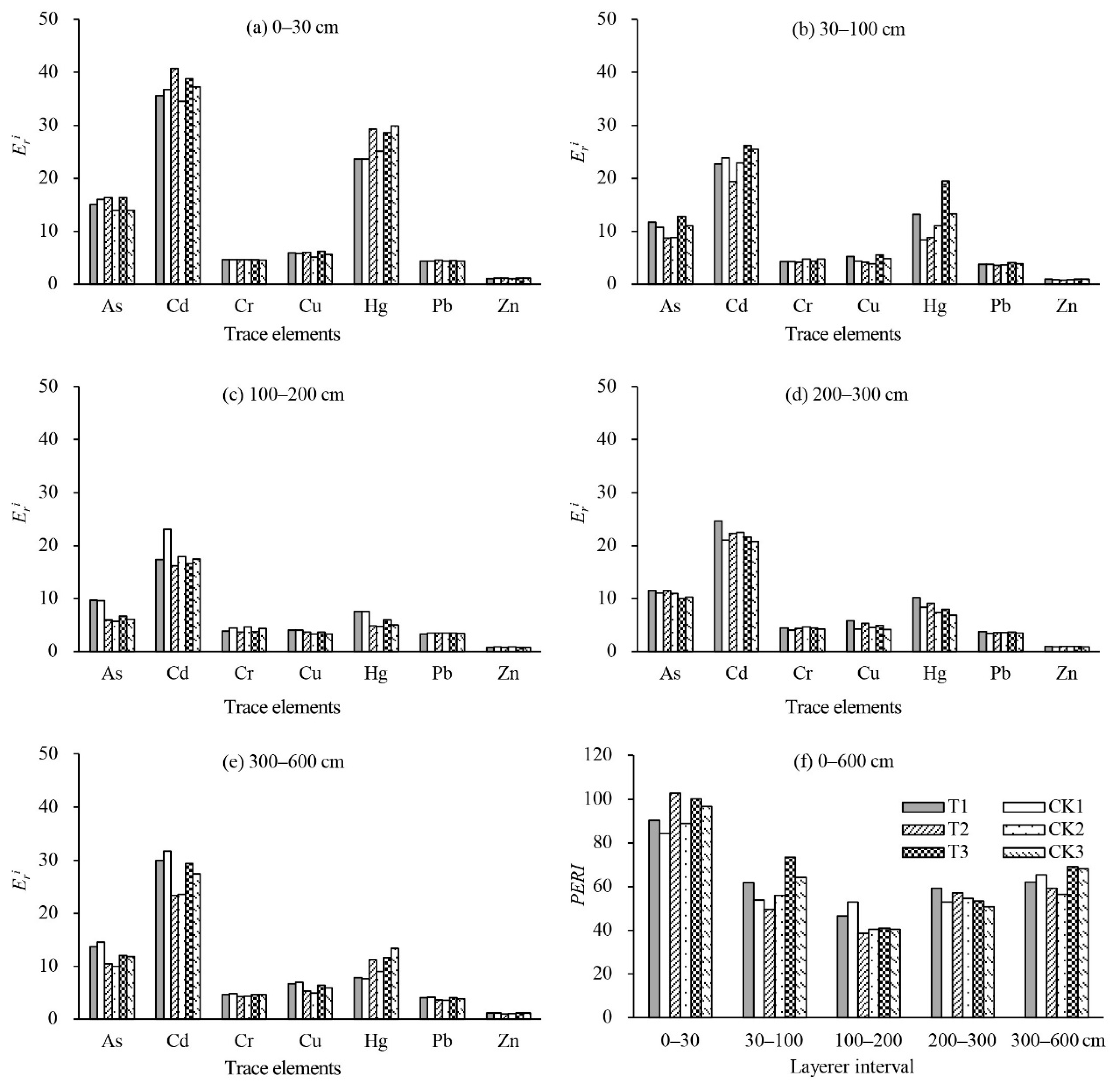
The results of the potential ecological risk factor of single elements (
) and the comprehensive potential ecological risk index (
PERI) for the seven trace elements in different soil layers are depicted in
Figure 3. The
values of all trace elements were < 40 in the 0–600 cm soil profiles except for the
value of Cd, which was close to 40 in top 30 cm soils, implying a low ecological risk. The degree of pollution from the seven trace elements decreased in the following sequence: Cd > Hg > As > Cu > Cr > Pb > Zn. Although the contents of Cd and Hg were lower than the soil background values, their biological toxicity factor was higher apparently than that of the other elements (Cd = 30, Hg = 40) resulting in a higher degree of ecological risk. Cd and Hg were also revealed as the main contribution to the potential ecological risk in previous studies [
49,
50]. Cd and Hg are highly toxic and can enter the human body through the food chain and seriously endanger human health. The accumulation of Cd and Hg in soil should be strictly controlled as they are priority pollutants in the study area. In addition, the
PERI values were all < 150, which was classified as low potential ecological risk. Comparing the
and
PERI of different soil layers, the top 30 cm soil layer had the highest values. The results suggested that the trace element concentrations in the 0–30 cm soil layers should be monitored for pollution prevention.
To study the impact of reclaimed water irrigation, the difference in soil
PERI between the treatment and control groups was analyzed using paired sample
t-test (
Table 7). All the
P-values of T1-CK1, T2-CK2 and T3-CK3 pairs were > 0.05, except 0.040 of T2-CK2 and 0.005 of T3-CK3 in the 30–100 cm soil profiles, which showed no significant difference at a level of 0.05. It was concluded that the long-term reclaimed water irrigation experiment in the study area did not result in a significantly increased
PERI of trace elements in the profiles.
Compared with previous studies about the impact of long-term reclaimed water irrigation on soil [
15], the trace element concentration of Dalong River reclaimed water in this experiment was very low. Because many industries creating trace element pollution have been moved out of the city in recent years and discharges of industrial wastewater are well regulated, the trace elements concentration in reclaimed water in Beijing is largely negligible. In addition, trace elements can be effectively removed from wastewater treatment processes and be concentrated in the sludge fraction. The trace element input from reclaimed water is generally small in comparison to atmospheric deposition and agrochemicals [
51,
52]. It is likely that the low trace element concentration of reclaimed water might be one of the main reasons for little accumulation of trace elements in the soils in this study.