Effects of Commonly Occurring Metal Ions on Hydroxyapatite Crystallization for Phosphorus Recovery from Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus and Design
2.2. Analytical Methods
3. Results and Discussion
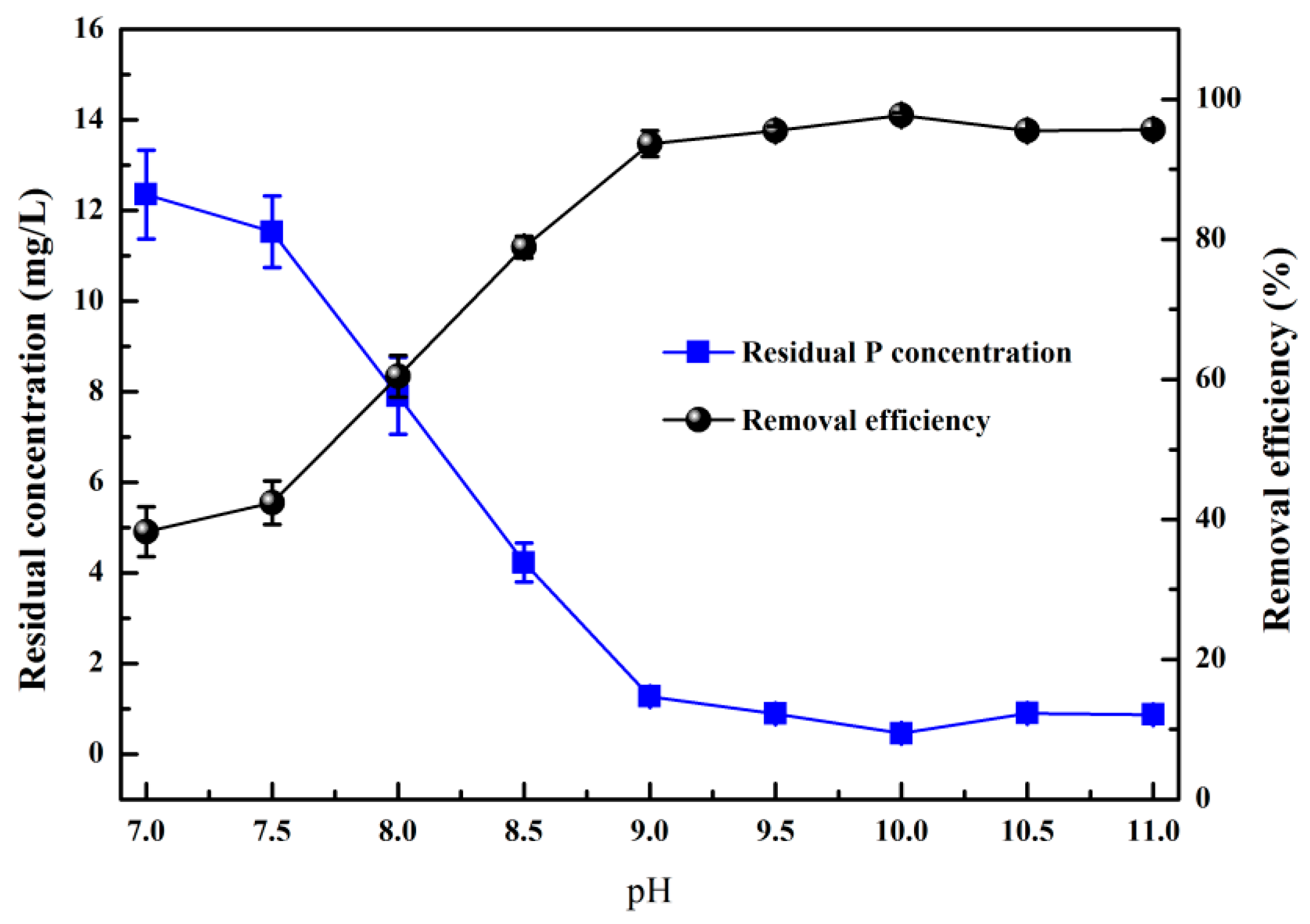
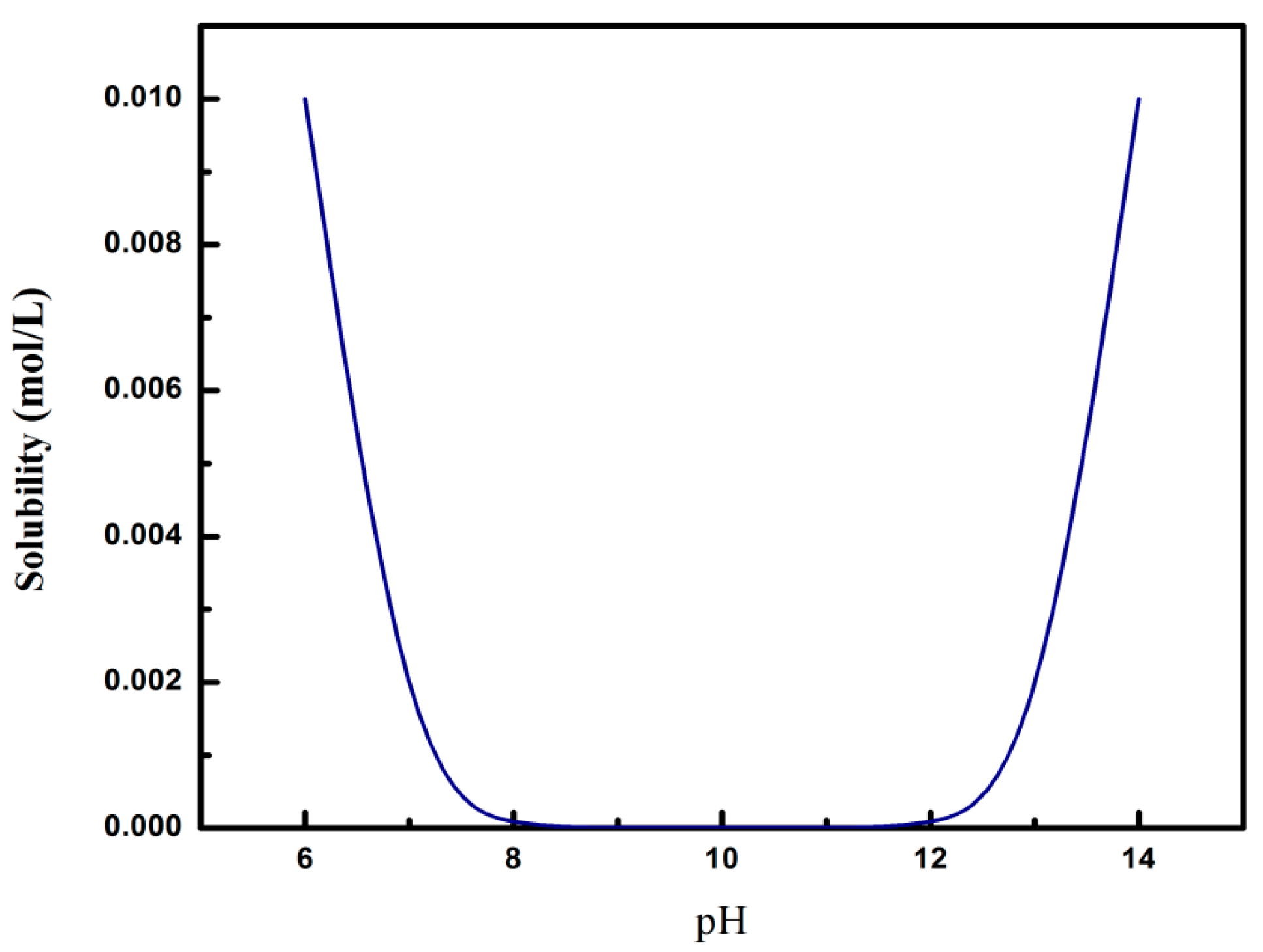
3.1. Effects of Ph on HAP Crystallization
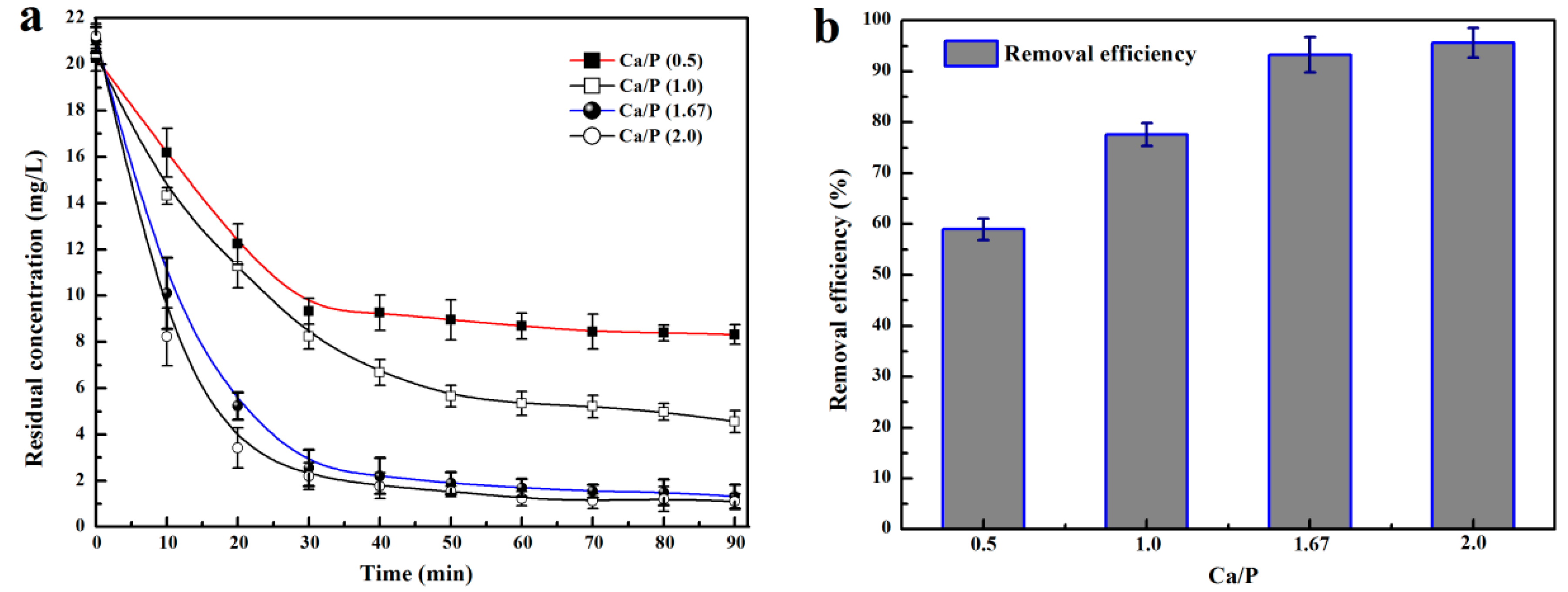
3.2. Effects of Ca/P on HAP Crystallization
3.3. Effects of Metal Ions on the HAP Crystallization
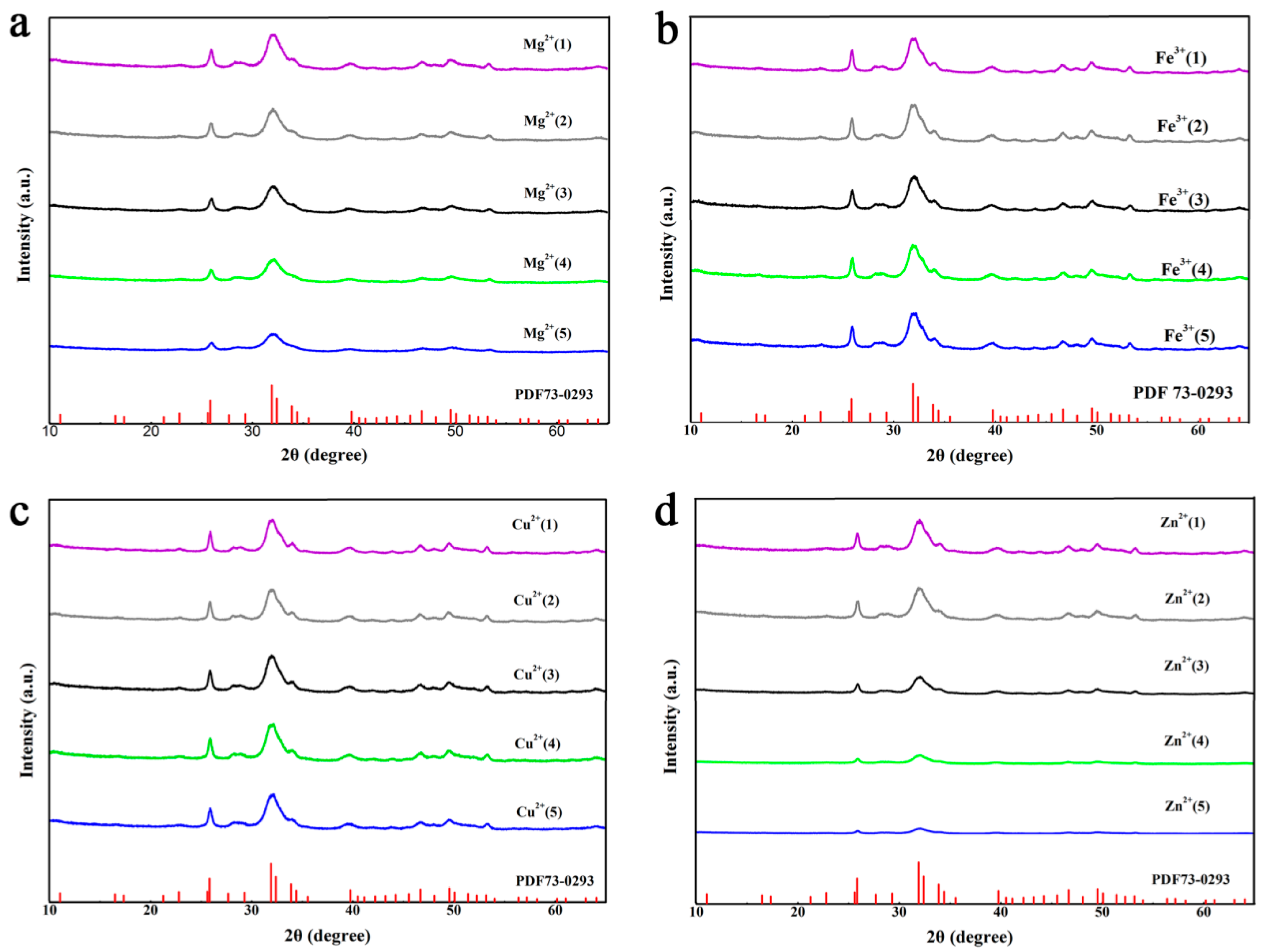
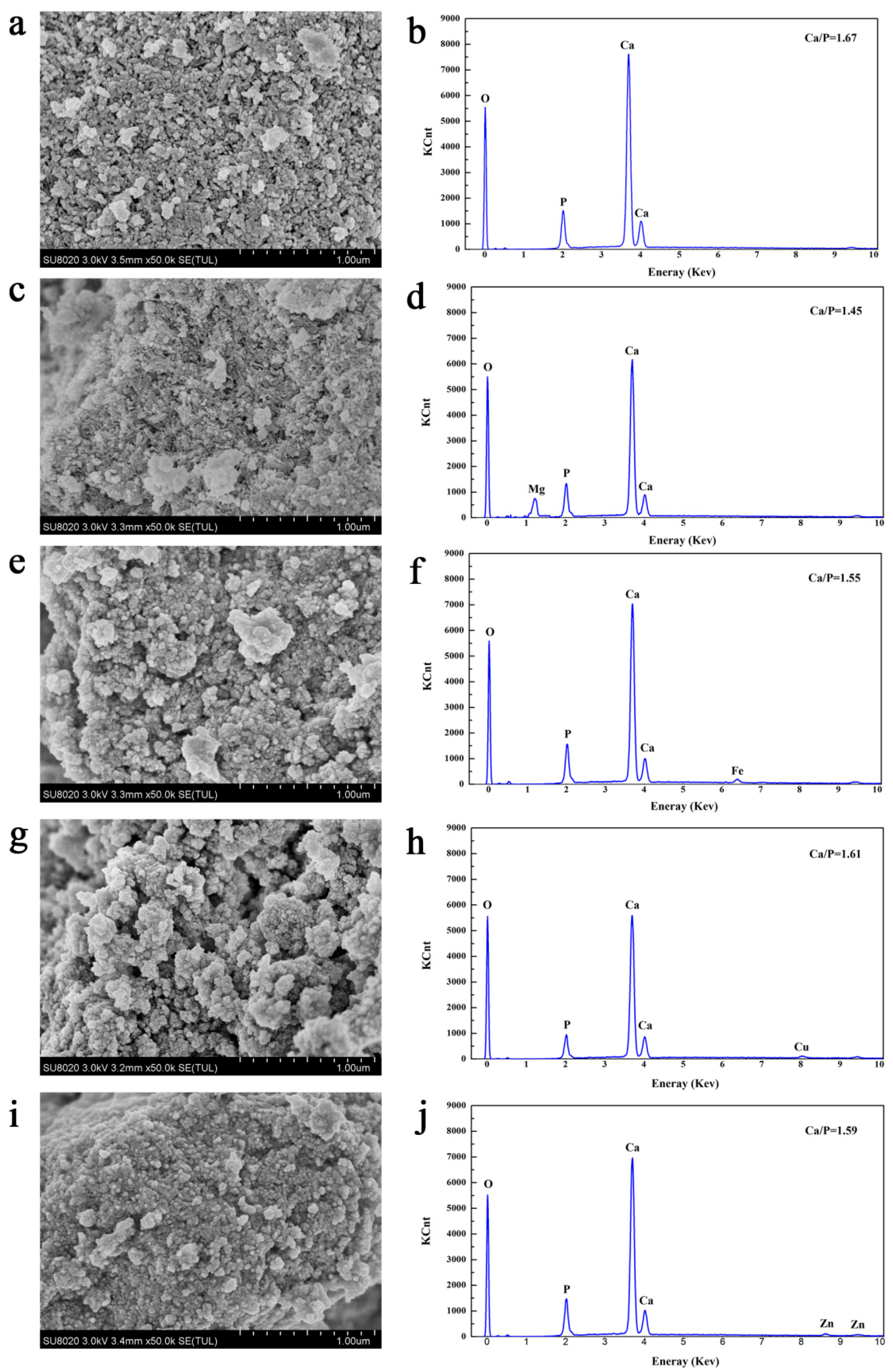
3.4. Effects of Metal Ions on the Characteristics of Crystallized Products
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shepherd, J.G.; Sohi, S.P.; Heal, K.V. Optimizing the recovery and reuse of phosphorus from wastewater effluent for sustainable fertilizer development. Water Res. 2016, 94, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Lu, X.; Peng, Y.; Yang, Z.; Zhu, H. Effects of supersaturation control strategies on hydroxyapatite (HAP) crystallization for phosphorus recovery from wastewater. Environ. Sci. Pollut. Res. 2017, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.K.; Baker, L.A.; Boyer, T.H.; Drechsel, P.; Gifford, M.; Hanjra, M.A.; Parameswaran, P.; Stoltzfus, J.; Westerhoff, P.; Rittmann, B.E. Total value of phosphorus recovery. Environ. Sci. Technol. 2016, 50, 6606–6620. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Li, J.S.; Guo, M.Z.; Cheeseman, C.R.; Tsang, D.C.; Donatello, S.; Poon, C.S. Phosphorus recovery and leaching of trace elements from incinerated sewage sludge ash (ISSA). Chemosphere 2018, 193, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, B.; Konieczka, P. A review of phosphorus recovery methods at various steps of wastewater treatment and sewage sludge management. The concept of “no solid waste generation” and analytical methods. J. Clean. Prod. 2017, 142, 1728–1740. [Google Scholar] [CrossRef]
- Yuan, Z.; Pratt, S.; Batstone, D.J. Phosphorus recovery from wastewater through microbial processes. Curr. Opin. Biotechnol. 2012, 23, 878–883. [Google Scholar] [CrossRef] [PubMed]
- De-Bashan, L.E.; Bashan, Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Law, Y.M.; Das, S.; Ting, Y.P. Direct and complete phosphorus recovery from municipal wastewater using a hybrid microfiltration-forward osmosis membrane bioreactor process with seawater brine as draw solution. Environ. Sci. Technol. 2015, 49, 6156–6163. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Lu, X.; Peng, Y.; Zou, H.; Jing, S. An efficient approach for phosphorus recovery from wastewater using series-coupled air-agitated crystallization reactors. Chemosphere 2016, 165, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; Uemoto, M.; Kagami, J.; Miura, K.; Aketo, T.; Toda, M.; Honda, K.; Ohtake, H. Novel technique for phosphorus recovery from aqueous solutions using amorphous calcium silicate hydrates (A-CSHs). Water Res. 2013, 47, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Egle, L.; Rechberger, H.; Krampe, J.; Zessner, M. Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of p recovery technologies. Sci. Total Environ. 2016, 571, 522–542. [Google Scholar] [CrossRef] [PubMed]
- Güney, K.; Weidelener, A.; Krampe, J. Phosphorus recovery from digested sewage sludge as MAP by the help of metal ion separation. Water Res. 2008, 42, 4692–4698. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dai, Y.; Hu, Q.; Yu, X.; Qian, F. Effects of three kinds of organic acids on phosphorus recovery by magnesium ammonium phosphate (MAP) crystallization from synthetic swine wastewater. Chemosphere 2014, 101, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Corre, K.S.L.; Valsamijones, E.; Hobbs, P.; Parsons, S.A. Phosphorus recovery from wastewater by struvite crystallization: A review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Pastor, L.; Marti, N.; Bouzas, A.; Seco, A. Sewage sludge management for phosphorus recovery as struvite in EBPR wastewater treatment plants. Bioresour. Technol. 2008, 99, 4817–4824. [Google Scholar] [CrossRef] [PubMed]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resour. Conserv. Recycl. 2016, 107, 142–156. [Google Scholar] [CrossRef]
- Kumar, R.; Prakash, K.H.; Cheang, P.; Khor, K.A. Temperature driven morphological changes of chemically precipitated hydroxyapatite nanoparticles. Langmuir 2004, 20, 5196–5200. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Weidler, P.G.; Berg, U.; Nüesch, R.; Donnert, D. Calcite-seeded crystallization of calcium phosphate for phosphorus recovery. Chemosphere 2006, 63, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Liu, Y.; Sun, X. O3 and UV/O3 oxidation of organic constituents of biotreated municipal wastewater. Water Res. 2008, 42, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Shih, K. Effects of calcium and ferric ions on struvite precipitation: A new assessment based on quantitative X-ray diffraction analysis. Water Res. 2016, 95, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Ottosen, L.M.; Kirkelund, G.M.; Jensen, P.E. Extracting phosphorous from incinerated sewage sludge ash rich in iron or aluminum. Chemosphere 2013, 91, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhao, W.; Li, Y.; Wang, W.; Zhu, X. Heavy metal removal and speciation transformation through the calcination treatment of phosphorus-enriched sewage sludge ash. J. Hazard. Mater. 2015, 283, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Madsen, H.E.L. Influence of foreign metal ions on crystal growth and morphology of brushite (CaHPO4·2H2O) and its transformation to octacalcium phosphate and apatite. J. Cryst. Growth 2008, 310, 2602–2612. [Google Scholar] [CrossRef]
- Muryanto, S.; Bayuseno, A. Influence of Cu2+ and Zn2+ as additives on crystallization kinetics and morphology of struvite. Powder Technol. 2014, 253, 602–607. [Google Scholar] [CrossRef]
- Dai, H.; Lu, X.; Peng, Y.; Yang, Z.; Zhu, H. Characteristics of metastable zone in the crystallization process: A case study of sparingly soluble hydroxyapatite. Desalin. Water Treat. 2017, 62, 192–199. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water & Wastewater, 21st ed.; American Public Health Association, American Water Works Association and Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Monballiu, A.; Desmidt, E.; Ghyselbrecht, K.; Meesschaert, B. Phosphate recovery as hydroxyapatite from nitrified UASB effluent at neutral pH in a CSTR. J. Environ. Chem. Eng. 2018, 6, 4413–4422. [Google Scholar] [CrossRef]
- Kabdaszli, I.; Parsons, S.A.; Tünaya, O. Effect of major ions on induction time of struvite precipitation. Croat. Chem. Acta 2006, 9, 243–251. [Google Scholar]
- Zheng, X.; Sun, P.; Lou, J.; Fang, Z.; Guo, M.; Song, Y.; Tang, X.; Jiang, T. The long-term effect of nitrite on the granule-based enhanced biological phosphorus removal system and the reversibility. Bioresour. Technol. 2013, 132, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.G.; Wilkie, A.C.; Cao, X.; Sirengo, R. Bench-scale recovery of phosphorus from flushed dairy manure wastewater. Bioresour. Technol. 2008, 99, 3036–3043. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties anduses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [PubMed]
- Karapinar, N.; Hoffmann, E.; Hahn, H.H. P-recovery by secondary nucleation and growth of calcium phosphates on magnetite mineral. Water Res. 2006, 40, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Hosni, K.; Moussa, S.B.; Chachi, A.; Amor, M.B. The removal of PO43−, by calcium hydroxide from synthetic wastewater: Optimisation of the operating conditions. Desalination 2008, 223, 337–343. [Google Scholar] [CrossRef]
- Luedecke, C.; Hermanowicz, S.W.; Jenkins, D. Precipitation of ferric phosphate in activated sludge: A chemical model and its verification. Water Sci. Technol. 1989, 21, 325–337. [Google Scholar] [CrossRef]
- Sangwal, K.; Mielniczek-Brzóska, E. Effect of impurities on metastable zone width for the growth of ammonium oxalate monohydrate crystals from aqueous solutions. J. Cryst. Growth 2004, 267, 662–675. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, H.; Tan, X.; Zhu, H.; Sun, T.; Wang, X. Effects of Commonly Occurring Metal Ions on Hydroxyapatite Crystallization for Phosphorus Recovery from Wastewater. Water 2018, 10, 1619. https://doi.org/10.3390/w10111619
Dai H, Tan X, Zhu H, Sun T, Wang X. Effects of Commonly Occurring Metal Ions on Hydroxyapatite Crystallization for Phosphorus Recovery from Wastewater. Water. 2018; 10(11):1619. https://doi.org/10.3390/w10111619
Chicago/Turabian StyleDai, Hongliang, Xinwei Tan, Hui Zhu, Tongshuai Sun, and Xingang Wang. 2018. "Effects of Commonly Occurring Metal Ions on Hydroxyapatite Crystallization for Phosphorus Recovery from Wastewater" Water 10, no. 11: 1619. https://doi.org/10.3390/w10111619
APA StyleDai, H., Tan, X., Zhu, H., Sun, T., & Wang, X. (2018). Effects of Commonly Occurring Metal Ions on Hydroxyapatite Crystallization for Phosphorus Recovery from Wastewater. Water, 10(11), 1619. https://doi.org/10.3390/w10111619




