Abstract
We examined the food preference of Chinese mitten crabs, Eriocheir sinensis (H. Milne Edwards, 1853), under food shortage, habitat choice in the presence of predators, and cannibalistic behavior by comparing their response to the popular culture plant Elodea nuttallii and the structurally more complex Myriophyllum verticillatum L. in a series of mesocosm experiments. Mitten crabs were found to consume and thus reduce the biomass of Elodea, whereas no negative impact on Myriophyllum biomass was recorded. In the absence of adult crabs, juveniles preferred to settle in Elodea habitats (appearance frequency among the plants: 64.2 ± 5.9%) but selected for Myriophyllum instead when adult crabs were present (appearance frequency among the plants: 59.5 ± 4.9%). The mortality rate of mitten crabs in the absence of plant shelter was higher under food shortage, primarily due to cannibalism. The proportion of molting crabs dying in the structurally more complex Myriophyllum habitats was significantly lower than in the less complex Elodea habitats, indicating that Myriophyllum provides better protection from cannibalistic behavior, likely due to its structurally more complex canopy. Stable isotope analyses of crab samples revealed a trophic shift in both δ13C and δ15N (Δδ13C: 2.2–4.0‰; Δδ15N: 1.5–2.8‰) during the experimental period. Significant positive correlations between body mass and δ13C and δ15N were recorded, suggesting that cannibalistic feeding might further increase crab growth and lead to ontogenetic increases in trophic position with increasing size. Our study overall demonstrates that a combination of submerged aquatic vegetation functioning as a highly suitable food with other less palatable plant species acting as efficient refuges against predators may be the optimal method of plant stocking in mitten crab aquacultures to ensure both high crab growth and a high survival rate.
1. Introduction
Cannibalism occurs frequently in natural and cultured populations of decapod crustaceans [1,2] and inter-cohort cannibalism plays a major role in controlling population size and structure [3]. Various factors may influence the extent and rate of cannibalism, including: (1) food availability [4,5], (2) crustacean density [6,7,8,9], (3) habitat structural complexity [2,10], and (4) prey vulnerability [7,11]. During ecdysis, crustaceans are especially vulnerable to predation and/or cannibalism [11,12]. Numerous predator-prey studies have demonstrated that habitat complexity significantly reduces the effects of cannibalism [2,6,7,13,14] and the presence of multiple shelters has been shown to proportionally increase the survival of juvenile blue-swimmer crabs, Portunus pelagicus [1]. Furthermore, both blue king crab, Paralithodes platypus, and red king crab, Paralithodes camtschaticus, have demonstrated a preference for complex habitats [6,7,15]. Moreover, investigations have revealed that the rates of cannibalism of Age-1 red king crab on Age-0 specimens decreased in macroalgae refuges [13,14]. A high refuge effect seems to, therefore, reduce predator movement and aggressive activity, increase the duration of resting, and allow the prey to move vertically and horizontally to avoid predation [7,16,17].
Chinese mitten crab, Eriocheir sinensis (H. Milne Edwards 1853), is an invasive species in North America and Europe, and due to the generally low concentration of heavy metals in these areas, it is intensively fished for consumption, which contributes to the control of populations. In China, Japan, Korea, and other parts of the world, mitten crab is regarded as a delicacy because of its high quality and tasty flesh, and it is, therefore, of great commercial value [18]. In China, the yield and value of the species have grown steeply from 1991 to 2015, from 8.4 × 103 t in 1991 to 8.2 × 105 t in 2015, with an estimated value of 6.0 × 107 United States Dollars (USD) in 1991 to 6.5 × 109 USD in 2015 [19] (Figure 1). However, extensive mortality occurring in the period from stocking to harvesting, especially during the early stages of development, has diminished the yields of cultivated populations [2,20], and field studies have shown that cannibalism is a major problem in the commercial production of Chinese mitten crabs. Aquaculture systems are characterized by high stocking densities, lack of alternative live food, and absence of natural refuges [21], all conditions that are likely to promote cannibalism. Many studies of marine crabs suggest that the presence of suitable shelters for the intermolt and/or newly molted crabs may decrease the cannibalism [1,2]. Yet, so far, only a few studies have specifically addressed the cannibalistic behavior of freshwater mitten crab and how submerged vegetation affects interspecific interactions [18,20,22].
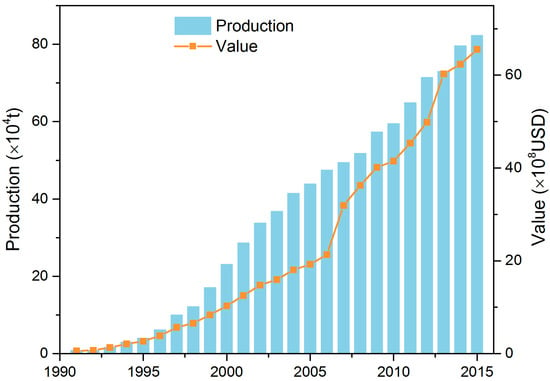
Figure 1.
Trends in the production (×104 t) and value (×108 United States Dollars (USD)) of Chinese mitten crab cultures from 1991 to 2015 in China (based on data from FAO (Food and Agriculture Organization of the United Nations) 2016 and China fishery statistics yearbooks from 2012 to 2016).
Macrophytes serve as both a natural food source and a refuge for mitten crabs during molting [18]. At present, Elodea nuttallii is usually planted in the middle and lower reaches of the Yangtze River for mitten crab production due to its high colonization ability and faster growth under eutrophic conditions [23,24]. Myriophyllum verticillatum L. is used less frequently in crab aquacultures; its more finely dissected leaves compared to those of Elodea may increase the structural complexity of the habitat and thus act as a refuge, more than Elodea, for young crabs from cannibalism [25]. However, it is not well known how the two plants affect the intraspecific interaction and habitat choice of the crabs.
In this study, we examined whether mitten crabs would select for Elodea under conditions of food shortage in cultures also including Myriophyllum and how predator presence altered the habitat choice of juvenile crabs. Moreover, a relatively long-term interaction experiment using stable isotope analysis was conducted to determine how the two plant species affected the growth, survival, and cannibalistic behavior of the juveniles. We hypothesized that the mitten crabs would prefer Elodea over Myriophyllum as a food source, but that juvenile crabs would preferentially use the structurally complex Myriophyllum to avoid cannibalism when threatened by adult crabs or when occurring in high densities.
2. Materials and Methods
2.1. Experimental Setup
This study comprised three elements: (1) a herbivory selection experiment, (2) a refuge selection experiment, and (3) a cannibalism experiment (Figure 2). All experiments were conducted in 2015 at Guchenghu Lake Aquaculture Research Center, located in the reclamation area of Guchenghu Lake (31.2287–31.3148 N, 118.8794–118.9655 E), Jiangsu Province, China.
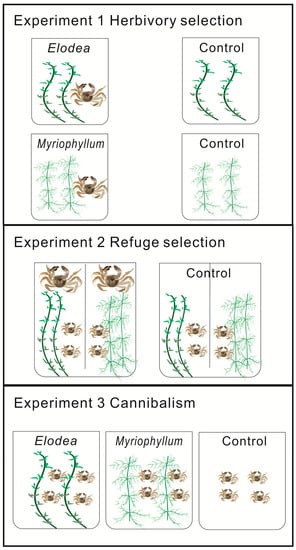
Figure 2.
Experimental design. In all experiments, each treatment consisted of three replicates. Experiment 1: One adult crab was put in each tank for 10 days. Tanks without adult crabs served as controls. Experiment 2: One adult crab and five juveniles were put in each side of the tank for 7 days. Tanks without adult crabs served as controls. Experiment 3: Twenty juveniles were put in each tank for 60 days. Tanks without plants served as controls.
All crabs were collected from a local mitten crab farm in the reclamation area of Guchenghu Lake. Prior to the experiment, the crabs were acclimated separately to tank culture conditions in 64 μm filtered pond water for 1 week during which they were fed with a commercial feed (36% crude protein, 6% crude lipid from Shuaifeng, Nanjing, China) once daily at 17:00. (in an amount equivalent to 8% of their body weight). Only active and similar-sized crabs were used in the experiments. Large male adults (70.3 ± 0.03 mm in carapace width) were used as predators as they have a larger and stronger chela than the smaller juveniles [26]. Coin-sized crabs (generally 15–40 mm in carapace width) were chosen as juveniles (20.9 ± 0.04 mm in carapace width) [27]. The experimental plants, Elodea nuttallii and Myriophyllum spicatum L., were removed by hand as whole plants from local aquaculture ponds. All plant materials (dead leaves and stems) were rinsed with tap water prior to the experiment, and the plants were acclimated for 24 h in a container filled with 64 μm filtered pond water.
The experiments were conducted in 500 L outdoor polypropylene tanks (diameter = 110 cm, height = 125 cm) with moderate aeration. High water quality was maintained by daily exchange of 50% of the water volume with fresh 64 μm filtered pond water. The water level in the tanks was set to 75 cm, leaving adequate space above the water to prevent the crabs from escaping. Mixed tap water-rinsed sands and stones in a 2 cm layer were used to root the plants. During the experiment, the water pH varied between 8.05 and 8.15, dissolved oxygen was >5 mg L−1, ammonia nitrogen ranged from 0.12 to 0.21 mg L−1 and phosphate phosphorus from 0.04 to 0.05 mg L−1. Uneaten feed and feces were removed once a day. Temperature, pH, and dissolved oxygen were measured in situ using a Yellow Springs Instruments (YSI, Inc., Yellow Springs, OH, USA) 6600 multi-sensor sonde. Ammonia nitrogen, and phosphate phosphorus concentrations were determined using standard methods [28].
2.2. Herbivory Selection Experiment
To assess whether the crabs exhibited a feeding preference for one of the two plant species, we randomly picked one adult crab from the acclimation container and placed it in the Elodea and Myriophyllum tanks, respectively (Figure 2). Tanks without crabs served as controls (all in three replicates). The experiments were run from 4 to 13 February 2015. A thermostat-regulated immersion heater was used to maintain a constant temperature (14.3 ± 1.5 °C). During the experimental period, the crabs were not fed. A total of 20 g wet weight of Elodea was randomly selected and added to six tanks, and the same was done for Myriophyllum. After ten days, all plant material was removed from the tanks, and wet weight was determined after absorbing the surface water with absorbent paper for 30 s. The percentage change in wet weight was calculated as 100 × (final − initial)/initial.
2.3. Refuge Selection Experiment
Experiment 2 aimed to explain whether juveniles still preferred Elodea over Myriophyllum in the presence of an adult crab (Figure 2). The experiments were run from 26 February to 4 March 2015 at a temperature of 14.3 ± 1.5 °C. Six 500 L tanks were divided into two similar-sized halves with plastic lattice (diameter = 6 mm mesh). A small hole (diameter = 50 mm) was cut under the plastic lattice, allowing the juveniles, but not the adult crab, to move freely through the partition. A total of 50 g of Elodea and 50 g of Myriophyllum were grown in opposite sides of each tank. Ten juvenile crabs were placed in each tank, five on each side. In three tanks, one adult crab was placed in each side of the tank, whereas the remaining three tanks held no adult crabs and served as controls. During the experiment, the number of juveniles on each side of the tanks was counted five times a day for a period of seven days. No mortality was recorded during the experiment. The appearance frequency among the plants was calculated as 100 × (∑i Ii/10)/7, where Ii is the average juvenile crab number among plants per side per day. Juveniles hiding in the sand and not among the plants were not included in the data. During the experiment, the crabs were fed with a commercial feed once a day (with an amount equivalent to 8% of their body weight).
2.4. Cannibalism Experiment
The third and longest experiment served to test whether different kinds of plants may help diminish the cannibalistic behavior of juvenile crabs (Figure 2). The experiments were run from 9 March to 7 May 2015 at a temperature of 18.8 ± 1.5 °C. A total of 180 juveniles were randomly assigned to three treatment groups: Elodea, Myriophyllum, and no plants. There were 60 individuals per treatment (at a density of 21 ind. m−2), equally divided between three tanks. A total of 100 g of Elodea and 100 g of Myriophyllum were grown in each tank in the Elodea and Myriophyllum treatments, respectively. During the experiment, crabs were fed with a commercial feed once a day (in an amount equivalent to 2% of their body weight).
The number of dead juveniles was counted at 18:00 each day, and total crab mortality was calculated after a period of 15 days. The mortality rate was obtained using an exponential loss model, p = e−mt, where p is the probability of death, m is the mortality rate expressed per day (d−1), and t is time (d) [7]. Uneaten feed and feces were removed once a day and dead crabs every second day to ensure that the live juveniles had sufficient time for consumption. Crab molting was observed from Day 18 to 40. The number of dead juveniles in the molting stages was recorded for two separate periods (Day 15–30 and Day 30–45). The proportion of dead crabs in the molting stages was obtained using the formula:
death due to molting (%) = dead juvenile individuals in the molting stages/total experimental crabs × 100
In order to maintain calcium and phosphorus levels during crab molting, a mixed solution of 5 g of CaCl2 and 2.5 g of Na2HPO4 was added to each tank twice a week. The nutrient levels chosen were similar to those applied by local aquacultures. The wet weight of all the plant material at the end of the experiment was evaluated according to the method in Section 2.2.
In the beginning and then every 15 days, one crab from each tank (3 replicates per treatment) was randomly sampled for recording of carapace width, wet body mass, and δ13C and δ15N signatures. Crab growth rates were expressed as specific growth rates (SGR) based on the formula, SGR = [(ln Wf − ln Wi) × 100] / T, where Wf is final weight (g), Wi is initial weight (g), and T is experiment time (days). Pereiopods, chelipeds, and ventral muscle tissue were gently removed for stable isotope analysis. All the samples were freeze-dried and ground into powder. Carbon and nitrogen isotope ratios were determined at Nanjing Institute of Geography and Limnology using a Flash EA1112 elemental analyzer coupled to a Thermo Finnigan MAT Delta plus dual-inlet continuous flow isotope ratio mass spectrometer. Stable isotope ratios were expressed in conventional δ notation as parts per mill (‰) according to the following equation:
where X is 13C or 15N and Rsample is the corresponding ratio 13C/12C or 15N/14N. Rstandard values were based on Vienna Pee Dee Belemnite (VPDB) for carbon and atmospheric N2 for nitrogen. The precision of the isotope analyses was 0.1‰ for both carbon and nitrogen.
δX = [(Rsample/Rstandard) − 1] × 100
2.5. Statistical Analysis
The change in plant wet weight (Experiment 1) and frequency of crab presence among plants (Experiment 2) were analyzed with two-way ANOVA and simple main effects analysis (Experiment 2) where the independent variables were plant species and crab presence. Two-way ANOVA was also applied to test for the effects of treatment (Fixed factor, 3 levels: Elodea, Myriophyllum, and no plant) and time (Fixed factor, 5 levels: Day 1, 15, 30, 45, and 60) on crab mortality, body mass, and δ15N (Experiment 3). Univariate tests were used to analyze the main difference between plants for each time or treatment. One-way ANOVA was used to determine whether carapace width and the δ13C signatures of the juvenile crab samples differed between treatments. In order to test whether a relationship could be detected between crab body mass and δ15N, Pearson correlations were performed on data pooled across all sampling days. The level of significance, α, was set to 0.05 for all analyses. Homogeneity of variance was tested using Levene’s tests. Tukey’s HSD (Honest Significant Difference) test was used for post hoc analysis. Crab body weights were log10-transformed to meet the assumptions of a two-way ANOVA. All statistical tests were performed using SPSS for Windows, version 18.
3. Results
In the absence of food, adult crabs had a more significant herbivore effect on Elodea than on Myriophyllum (F(1,8) = 19.080, p = 0.002, Table 1). Elodea biomass was, on average, 18.7 ± 5.4% lower in the presence than in the absence of an adult crab, where the biomass increased by 20.5 ± 5.9%. Myriophyllum biomass increased by 8.9–16.8% and was independent of crab presence/absence (Figure 3A).

Table 1.
Summary of two-way ANOVA results in the herbivory selection experiment.
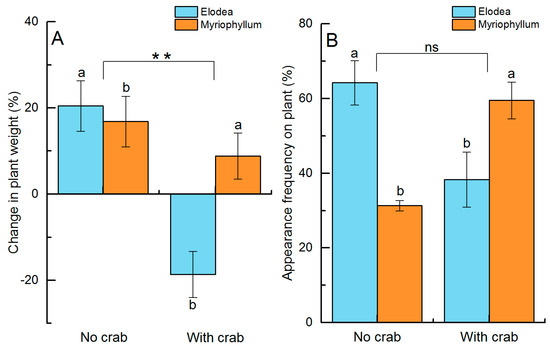
Figure 3.
Differences in herbivory selection and refuge selection by experimental crabs between Elodea and Myriophyllum. Error bars represent standard deviation (n = 3, %). Different letters above the bars denote significant differences between the two plants at p < 0.05. ** indicates significant differences between crab treatments at p < 0.01, while ns indicates no significance.
There was a significant interaction effect between the occurrence frequency of juveniles among the two plant species and adult crab presence (F(1,8) = 75.832, p < 0.001, Table 2). Simple main effects analysis showed that the plant selection by juveniles differed significantly between the two plants depending on whether the predator was present or not (p < 0.001 in the no crab treatment and p = 0.001 in the plus crab treatment). The juveniles preferred to settle among the Elodea in the absence of an adult crab (appearance frequency among the plants: 64.2 ± 5.9%) but selected for Myriophyllum in the presence of an adult crab (appearance frequency among the plants: 59.5 ± 4.9%, Figure 3B).

Table 2.
Summary of two-way ANOVA results in the refuge selection experiment.
During the two-month experiment, a linear increase in mean carapace width (Figure 4A) and an exponentially increasing trend in mean body mass (Figure 4B) with time occurred in all treatments. The crabs in the no plant treatment were heavier than those in the Elodea and Myriophyllum treatments (F(2,30) = 10.196, p < 0.001, Table 3). However, no significant differences in crab body weight were found between the two plant treatments (Tukey p = 0.939). The number of dead crabs recorded at 15-day intervals did not differ significantly between the plant treatments (Table 3), and most of the crabs died during the first 30 days (Figure 4C). However the proportion of crabs dying when entering the molting stage differed significantly between the two plants (F(2,12) = 9.320, p = 0.004, Table 3). Post hoc comparison revealed that the mortality rates in the molting stages were significantly lower in the Myriophyllum treatment than in the Elodea treatment (Tukey p = 0.022) and the control (Tukey p = 0.004) (Figure 5). The total estimated mortality rate in the treatments without plants was 0.034 ± 0.003 day−1 (R2 = 0.98, p < 0.001), which was considerably higher than in the Elodea (0.025 ± 0.002 day−1, R2 = 0.97, p < 0.001) and Myriophyllum (0.022 ± 0.001 day−1, R2 = 0.98, p < 0.001) treatments (Figure 4D).
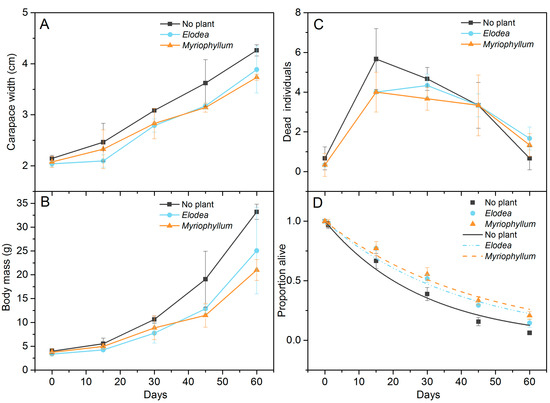
Figure 4.
Temporal trends of carapace width (cm) (A) and wet body mass (g) (B) of the experimental crabs and crab mortality (individual) (C) and alive proportion (D) in the cannibalism experiment. Error bars represent standard deviation (n = 3). The lines illustrate the preferred exponential loss model (p = e−mt) (D).

Table 3.
Summary of two-way ANOVA results in the cannibalism experiment.
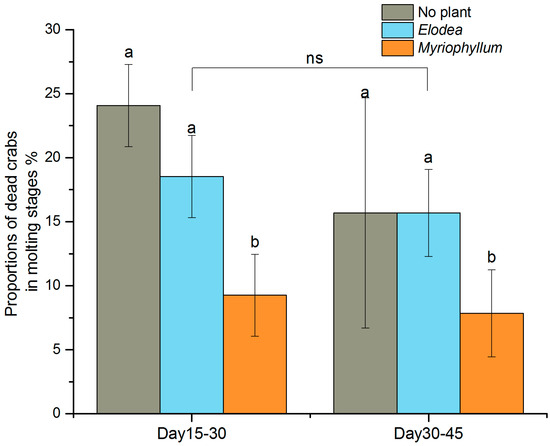
Figure 5.
Influence of plants on the proportion of dead juvenile crabs in the molting stages during the two experimental periods. Error bars represent standard deviation (n = 3, %). Different letters above the bars denote significant differences between the treatments for each experimental period at p < 0.05, while ns indicates no significant differences between the two experimental periods.
Both the δ13C and δ15N content of the crabs gradually increased during the experimental period (Figure 6A,B). δ13C varied between −25.8 ± 1.0‰ and −20.4 ± 2.1‰, while δ15N ranged from 6.3 ± 0.3‰ to 9.3 ± 0.3‰. No significant difference in δ13C was detected among the treatments (one-way ANOVA, F = 0.471, p = 0.628), while δ15N became significantly more enriched with time and differed markedly among the treatments (Table 3). Post hoc comparisons revealed no significant difference in δ15N between the no plant and the Elodea treatments (Tukey, p = 0.349), but δ15N in the Myriophyllum treatment was significantly more enriched than in the Elodea (Tukey, p < 0.006) and the no plant (Tukey, p < 0.001) treatments. Throughout the entire experimental period, significant positive correlations existed between δ13C and δ15N and body mass in all treatments (Pearson correlations, p < 0.01, Figure 6C,D).
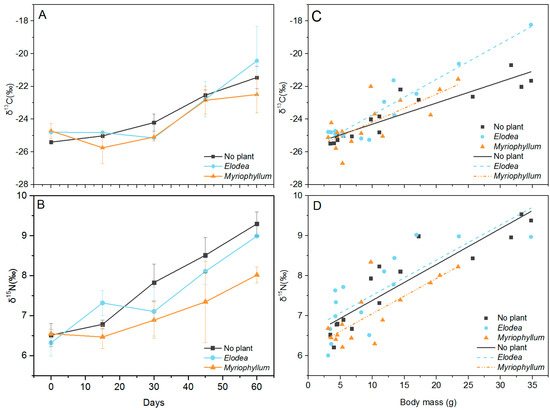
Figure 6.
Temporal trend of δ13C (‰) (A) and δ15N (‰) (B) of crabs and the relationship between body mass, δ13C (‰) (C) and δ15N (‰) (D) of crabs throughout the experimental period in the cannibalism experiment. Error bars represent standard deviation (n = 3). Lines represent linear fits for body mass versus δ13C (‰) and body mass versus δ15N (‰). Correlations were significant at p = 0.01.
4. Discussion
As in earlier studies of the feeding behavior of Chinese mitten crab, we found a preference for Elodea [29,30,31]. In the first experiment, the crabs had reduced the biomass of Elodea by 18.7% after 10 days of feeding, while Myriophyllum biomass increased irrespective of the absence/presence of adult crabs. The higher concentrations of lignin and polyphenols found in Myriophyllum may help to defend it against aquatic herbivores [25,32]. Moreover, Myriophyllum tissue is less nutritious, as also indicated by the overall low growth and survival of herbivorous arthropods and gastropods feeding on a Myriophyllum diet, recorded in other investigations [33,34]. Accordingly, in our study, starving mitten crabs had no negative effect on Myriophyllum growth, and Myriophyllum actually exhibited an 8.9–16.8% biomass increase during the experiment. Similarly, Valinoti et al. found a Myriophyllum biomass increased of 4–10% regardless of whether the shrimp Palaemonetes was present or not, which they attributed to limited grazing [25].
In our study, the presence of an adult crab significantly impacted the habitat choice of juvenile crabs. We found that juveniles preferred Elodea (64.2% appearance frequency among the plants) over Myriophyllum in the absence of predators, whereas more crabs (59.5% appearance frequency among the plants) remained in Myriophyllum habitats in the presence of an adult crab. Myriophyllum is much more structurally complex than Elodea [35] and this complexity can reduce predation through interference with predator movement, thus allowing the prey an opportunity to escape predation [16]. Even a very sparse vegetation cover may notably mitigate the cannibalistic pressure [10]. Studies of other species of crab have shown that cannibalism is exerted by older and larger crabs on smaller individuals; being smaller made them easier to capture and consume [7,36]. In Experiment 2, the juveniles had a greater affinity for Myriophyllum in the presence of adult crabs, likely seeking escape from capture in the complex habitat.
Habitat significantly affected the survival of juveniles in the third, relatively long-term, experiment. Crab mortality rate was highest in the habitat without shelter and decreased in the habitats containing aquatic macrophytes. Similar to the results obtained for other species of crab, we observed that a complex habitat, regardless of type, is effective in augmenting the survival of mitten crab [7,12]. Mortality was also inversely affected by stocking density [9]. In Experiment 3, 20 crabs were placed in each tank with a stocking density of 21 crabs m−2. In typical aquaculture, the average stocking density is 1.5 crabs m−2. High crab density increases cannibalism when the food is scarce, especially under conditions of shelter shortage [8,12] as crab flesh may be more attractive for energetic or nutritional requirements than plants [36]. We found occasional grazing by juveniles on Elodea but still not on Myriophyllum. The biomass of the two plants in Experiment 3 did not differ significantly (Elodea: 182.3 ± 8.2 g, Myriophyllum: 212.3 ± 8.0 g; t = −2.690, p = 0.115). On the other hand, we found that dead or injured crabs were found more frequently during or immediately after molting. The proportion of crabs dying when entering the molting stages during Day 15–30 and Day 30–45 period varied from 7.8% to 24.1%. In addition, the crab mortality rates in the molting-related stages differed significantly between the Elodea and Myriophyllum treatments. Molting crabs are highly susceptible to cannibalism because they are soft and less mobile and thus may attract the attention of other active animals nearby [1]. Therefore, hiding among aquatic macrophytes likely improved juvenile survival, and it thus seems that the quantity and quality of shelter are important for reducing cannibalism.
δ13C and δ15N are useful tools for estimating the trophic position of consumers in food webs. The stable isotope ratio in the consumer’s tissues reflects the corresponding ratio in the diet that is actually assimilated. Increases in δ15N between diet and consumer (Δδ15N), also referred to as trophic fractionation, can be used to indicate the relative trophic positions of sets of individuals. When cannibalism occurs, the cannibalistic individuals will have higher δ15N than the non-cannibalistic ones, reflecting a higher trophic level [37]. In the extreme case where crabs gain all their nutrition by consuming conspecifics, the cannibalistic crabs occupy a trophic level one step higher (i.e., a 3–4‰ higher δ15N value) than that of non-cannibalistic crabs. Therefore, δ15N is potentially a good indicator of cannibalistic behavior [38]. We found that both the δ13C and δ15N in crab muscles increased constantly over time. The shift in δ15N with time indicated that the crabs had a higher trophic position than that of their conspecifics, most likely reflecting cannibalism. Although the Δδ13C of 2.2–4.0‰ and the Δδ15N of 1.5–2.8‰ measured here from the beginning to the end of the experiment deviated from commonly cited Δ-values in the literature (0 to 1‰ for Δδ13C and ~3‰ for Δδ15N, according to McCutchan et al. [39]), they are, in fact, consistent with previous reports showing that Δδ13C usually ranges from 2 to 4‰ [40,41] and Δδ15N from 1.7 to 2.2‰ [38,42,43]. The high protein quality and the high quantity of the diet contributed to the low Δδ15N values of the predator [41,44].
Both δ13C and δ15N gradually increased with body weight, indicating that the crabs require a substantial amount of time for complete tissue turnover rate, and hence, to reflect an increase in the assimilation of 13C- and 15N-enriched food sources. The shift in the isotopic composition of tissues after a change in diet depends on the turnover rate of the particular tissue [45,46]. In general, turnover rates are higher in smaller organisms with higher metabolic rates than in larger-sized organisms [43,47]; in accordance with this, during Experiment 3, juveniles were chosen and we observed a crab body mass increase of 5.6–8.3 times after 60 days. The specific growth rates in the No plant, Elodea, and Myriophyllum treatments were 3.55 ± 0.31, 3.27 ± 0.53, and 2.89 ± 0.43% day−1, respectively. Most studies have concluded that growth explains most of the carbon and nitrogen turnover [43,46,47]. Cannibalism likely increases growth, and this may explain the positive correlation between size and δ15N also observed by Møller et al. in cannibalistic crabs [38].
For crustaceans, the muscle turnover rate could be influenced by the molting cycle, especially in juvenile individuals [38]. In Experiment 3, most of the juveniles molted once. The proportion of crabs dying when entering the molting stage differed significantly between the two plants. The impact of this on the stable isotope signature variation deserves more attention because molting affects protein synthesis and degradation. However, deVries et al. reported from an experiment that sex and molting did not affect the incorporation rates of mantis shrimp [41]. In addition, the influence of molting on incorporation rates needs further study.
The δ15N of crabs in the Myriophyllum treatment became significantly lower with time than that in the Elodea treatment, also suggesting that Myriophyllum provides a better shelter for juveniles against cannibalistic feeding. We cannot exclude the possibility that the closed experimental conditions led to higher consumption of crab remains due to agonistic encounters when the crabs got larger, which may also have affected the δ15N ratios.
5. Conclusions
This study demonstrated how Myriophyllum and Elodea differentially affected the feeding preference, habitat choice, growth, and survival of Chinese mitten crab. When food was scarce, the mitten crabs preferred Elodea as their food source, but in the presence of predators, most of the crabs settled among the Myriophyllum, probably using them as a refuge against predation. We found that, overall, the crab mortality rate was lower in the structurally more complex Myriophyllum treatment than in the Elodea treatment. Stable isotope analyses indicated that cannibalistic feeding might further increase growth, producing ontogenetic increases in trophic position with size. Our study demonstrates that a combination of submerged aquatic vegetation suitable as food combined with other plant species acting as efficient refuges against predators may be the optimal plant stocking method in mitten crab cultures to ensure both high crab survival and high growth.
Author Contributions
Q.Z. conceived and designed the experiments; Z.M. performed the experiments; H.C. and Q.Z. analyzed the data; E.J. and X.G. contributed reagents/materials/analysis tools; Q.Z. wrote the paper.
Funding
This work was supported by the National “Twelfth Five-Year” Plan for Science and Technology Support (No. 2015BAD13B06), the Science and Technology Service Network Initiative (No. KFJ-SW-STS-145), and the Natural Science Foundation of China (No. 31400399).
Acknowledgments
We thank Anne Mette Poulsen for critical editorial assistance. Erik Jeppesen was supported by the projects MARS (Managing Aquatic ecosystems and water Resources under multiple Stress) funded under the 7th EU Framework Programme, Contract No.: 603378 (http://www.mars-project.eu) and the Horizon2020 project AQUACOSM (Network of Leading European AQUAtic MesoCOSM Facilities Connecting Mountains to Oceans from the Arctic to the Mediterranean).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marshall, S.; Warburton, K.; Paterson, B.; Mann, D. Cannibalism in juvenile blue-swimmer crabs Portunus pelagicus (Linnaeus, 1766): Effects of body size, moult stage and refuge availability. Appl. Anim. Behav. Sci. 2005, 90, 65–82. [Google Scholar] [CrossRef]
- Januario, S.M.; Navarrete, S.A. Cannibalism and inter-specific predation in early stages of intertidal crab species that compete for refuges. J. Exp. Mar. Biol. Ecol. 2013, 446, 36–44. [Google Scholar] [CrossRef]
- Amarasekare, P. Coexistence of intraguild predators and prey in resource-rich environments. Ecology 2008, 89, 2786–2797. [Google Scholar] [CrossRef] [PubMed]
- Amaral, V.; Paula, J.; Hawkins, S.; Jenkins, S. Cannibalistic interactions in two cooccurring decapod species: Effects of density, food, alternative prey and habitat. J. Exp. Mar. Biol. Ecol. 2009, 368, 88–93. [Google Scholar] [CrossRef]
- Duarte, C.; Jaramillo, E.; Contreras, H.; Acuña, K. Cannibalism and food availability in the talitrid amphipod Orchestoidea tuberculata. J. Sea Res. 2010, 64, 417–421. [Google Scholar] [CrossRef]
- Long, W.C.; Popp, J.; Swiney, K.M.; Van Sant, S.B. Cannibalism in red king crab, Paralithodes camtschaticus (Tilesius, 1815): Effects of habitat type and predator density on predator functional response. J. Exp. Mar. Biol. Ecol. 2012, 422, 101–106. [Google Scholar] [CrossRef]
- Long, W.C.; Van Sant, S.B.; Haaga, J.A. Habitat, predation, growth, and coexistence: Could interactions between juvenile red and blue king crabs limit blue king crab productivity? J. Exp. Mar. Biol. Ecol. 2015, 464, 58–67. [Google Scholar] [CrossRef]
- Takeshita, F.; Tamura, R. Optimal stocking density of juvenile red king crabs Paralithodes camtschaticus under cannibalism consideration. Fish. Sci. 2014, 80, 775–783. [Google Scholar] [CrossRef]
- Waiho, K.; Mustaqim, M.; Fazhan, H.; Norfaizza, W.I.W.; Megat, F.H.; Ikhwanuddin, M. Mating behavior of the orange mud crab, Scylla olivacea: The effect of sex ratio and stocking density on mating success. Aquac. Rep. 2015, 2, 50–57. [Google Scholar] [CrossRef]
- Almeida, M.J.; González-Gordillo, J.I.; Flores, A.A.V.; Queiroga, H. Cannibalism, post-settlement growth rate and size refuge in a recruitment-limited population of the shore crab Carcinus maenas. J. Exp. Mar. Biol. Ecol. 2011, 410, 72–79. [Google Scholar] [CrossRef]
- Sotelano, M.P.; Lovrich, G.A.; Romero, M.C.; Tapella, F. Cannibalism during intermolt period in early stages of the Southern King Crab Lithodes santolla (Molina 1872): Effect of stage and predator-prey proportions. J. Exp. Mar. Biol. Ecol. 2012, 411, 52–58. [Google Scholar] [CrossRef]
- Daly, B.; Swingle, J.S.; Eckert, G.L. Effects of diet, stocking density, and substrate on survival and growth of hatchery-cultured red king crab (Paralithodes camtschaticus) juveniles in Alaska, USA. Aquaculture 2009, 293, 68–73. [Google Scholar] [CrossRef]
- Stoner, A.W.; Ottmar, M.L.; Haines, S.A. Temperature and habitat complexity mediate cannibalism in red king crab: Observations on activity, feeding, and prey defense mechanisms. J. Shellfish Res. 2010, 29, 1005–1012. [Google Scholar] [CrossRef]
- Long, W.C.; Whitefleet-Smith, L. Cannibalism in red king crab: Habitat, ontogeny, and the predator functional response. J. Exp. Mar. Biol. Ecol. 2013, 449, 142–148. [Google Scholar] [CrossRef]
- Stevens, B.G. Settlement, substratum preference, and survival of red king crab Paralithodes camtschaticus (Tilesius, 1815) glaucothoe on natural substrata in the laboratory. J. Exp. Mar. Biol. Ecol. 2003, 283, 63–78. [Google Scholar] [CrossRef]
- Bartholomew, A.; Diaz, R.; Cicchetti, G. New dimensionless indices of structural habitat complexity: Predicted and actual effects on a predator’s foraging success. Mar. Ecol. Prog. Ser. 2000, 206, 45–58. [Google Scholar] [CrossRef]
- Hossie, T.J.; Murray, D.L. You can’t run but you can hide: Refuge use in frog tadpoles elicits density-dependent predation by dragonfly larvae. Oecologia 2010, 163, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.D.; Liu, J.S.; Zhang, S.Y.; Lian, Y.X.; Ding, H.Y.; Du, X.; Li, Z.J.; De Silva, S.S. Sustainable farming practices of the Chinese mitten crab (Eriocheir sinensis) around Hongze Lake, lower Yangtze River Basin, China. Ambio 2016, 45, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Fish Stat J: A Tool for Fishery Statistical Analysis. 2016. Available online: http://www.fao.org/fishery/statistics/software/fishstatj/en (accessed on 21 July 2016).
- Wang, W.; Li, Y.S. Chinese Mitten Crab Ecological Aquaculture; China Agriculture Press: Beijing, China, 2010. (In Chinese) [Google Scholar]
- Smith, C.; Reay, P. Cannibalism in teleost fish. Rev. Fish Biol. Fish. 1991, 1, 41–64. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Liang, X.; Cui, Y. Stocking models of Chinese mitten crab (Eriocheir japonica sinensis) in Yangtze lakes. Aquaculture 2006, 255, 456–465. [Google Scholar] [CrossRef]
- Josefsson, M. NOBANIS—Invasive Species Fact Sheet—Elodea canadensis, Elodea nuttallii and Elodea callitrichoides—From: Online Database of the European Network on Invasive Alien Species—NOBANIS. 2011. Available online: https://www.nobanis.org/fact-sheets/ (accessed on 29 October 2018).
- Kong, L.; Cai, C.F.; Ye, Y.T.; Chen, D.X.; Wu, P.; Li, E.C.; Chen, L.Q.; Song, L. Comparison of non-volatile compounds and sensory characteristics of Chinese mitten crabs (Eriocheir sinensis) reared in lakes and ponds: Potential environmental factors. Aquaculture 2012, 364–365, 96–102. [Google Scholar] [CrossRef]
- Valinoti, C.E.; Ho, C.K.; Armitage, A.R. Native and exotic submerged aquatic vegetation provide different nutritional and refuge values for macroinvertebrates. J. Exp. Mar. Biol. Ecol. 2011, 409, 42–47. [Google Scholar] [CrossRef]
- Dutil, J.D.; Munro, J.; Pe´loquin, M. Laboratory study of the influence of prey size on vulnerability to cannibalism in snow crab (Chionoecetes opilio O.; Fabricius, 1780). J. Exp. Mar. Biol. Ecol. 1997, 212, 81–94. [Google Scholar] [CrossRef]
- Tian, Z.; Kang, X.; Mu, S. The molt stages and the hepatopancreas contents of lipids, glycogen and selected inorganic elements during the molt cycle of the Chinese mitten crab Eriocheir sinensis. Fish. Sci. 2012, 78, 67–74. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 9th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Czerniejewski, P.; Rybczyk, A.; Wawrzyniak, W. Diet of the Chinese mitten crab, Eriocheir sinensis H. Milne Edwards, 1853, and potential effects of the crab on the aquatic community in the river Odra/Oder Estuary (N.-W. Poland). Crustaceana 2010, 83, 195–205. [Google Scholar] [CrossRef]
- Stark, R. The Impacts of the Chinese Mitten Crab on the San Francisco Bay. 2015. ENVS 190. p. 7. Available online: http://www.csus.edu/envs/documents/theses/spring%202015/855.2015.spring.pdf (accessed on 18 May 2015).
- Veilleux, E.; de Lafontaine, Y. Biological synopsis of the Chinese mitten crab (Eriocheir sinensis). Can. Manuscr. Rep. Fish. Aquat. Sci. 2007, 2812, 45. [Google Scholar]
- Smolders, A.J.P.; Vergeer, L.H.T.; van der Velde, G.; Roelofs, J.G. Phenolic contents of submerged, emergent, and floating leaves of aquatic and semi-aquatic macrophyte species: Why do they differ? Oikos 2000, 91, 307–310. [Google Scholar] [CrossRef]
- Choi, C.; Bareiss, C.; Walenciak, O.; Gross, E.M. Impact of polyphenols on growth of the aquatic herbivore Acentria ephemerella. J. Chem. Ecol. 2002, 28, 2223–2235. [Google Scholar] [CrossRef]
- Qiu, J.W.; Kwong, K.L. Effects of macrophytes on feeding and life-history traits of the invasive apple snail Pomacea canaliculata. Freshw. Biol. 2009, 54, 1720–1730. [Google Scholar] [CrossRef]
- Thomaz, S.M.; da Cunha, E.R. The role of macrophytes in habitat structuring in aquatic ecosystems: Methods of measurement, causes and consequences on animal assemblages’ composition and biodiversity. Acta Limnol. Bras. 2010, 22, 218–236. [Google Scholar] [CrossRef]
- Stevens, B.G.; Swiney, K.M. Post-settlement effects of habitat type and predator size on cannibalism of glaucothoe and juveniles of red king crab Paralithodes camtschaticus. J. Exp. Mar. Biol. Ecol. 2005, 321, 1–11. [Google Scholar] [CrossRef]
- Sogabe, A.; Hamaoka, H.; Fukuta, A.; Shibata, J.Y.; Shoji, J.; Omori, K. Application of stable isotope analysis for detecting filial cannibalism. Behav. Process. 2017, 140, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Møller, H.; Lee, S.Y.; Paterson, B.; Mann, D. Cannibalism contributes significantly to the diet of cultured sand crabs, Portunus pelagicus (L.): A dual stable isotope study. J. Exp. Mar. Biol. Ecol. 2008, 361, 75–82. [Google Scholar] [CrossRef]
- McCutchan, J.H.; Lewis, W.M.; Kendall, C.; McGrath, C.C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen and sulphur. Oikos 2003, 102, 378–390. [Google Scholar] [CrossRef]
- Gamboa-Delgado, J.; Peña-Rodríguez, A.; Ricque-Marie, D.; Cruz-Suárez, L.E. Assessment of nutrient allocation and metabolic turnover rate in Pacific white shrimp Litopenaeus vannamei co-fed Live macroalgae Ulva clathrata and inert feed: Dual stable isotope analysis. J. Shellfish Res. 2011, 30, 969–978. [Google Scholar] [CrossRef]
- deVries, M.S.; del Rio, C.M.; Tunstall, T.S.; Dawson, T.E. Isotopic incorporation rates and discrimination factors in mantis shrimp crustaceans. PLoS ONE 2015, 10, e0122334. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.J.; McDonald, R.A.; van Veen, F.J.F.; Kelly, S.D.; Rees, G.; Bearhop, S. Application of nitrogen and carbon stable isotopes (δ15N and δ13C) to quantify food chain length and trophic structure. PLoS ONE 2014, 9, e93281. [Google Scholar] [CrossRef] [PubMed]
- Kilham, S.S.; Hunte-Brown, M.; Verburg, P.; Pringle, C.M.; Whiles, M.R.; Lips, K.R.; Zandona, E. Challenges for interpreting stable isotope fractionation of carbon and nitrogen in tropical aquatic ecosystems. Verh. Int. Verein. Limnol. 2009, 30, 749–753. [Google Scholar] [CrossRef]
- Adams, T.S.; Sterner, R.W. The effect of dietary nitrogen content on trophic level 15N enrichment. Limnol. Oceanogr. 2000, 45, 601–607. [Google Scholar] [CrossRef]
- Gamboa-Delgado, J.; Cañavate, J.P.; Zerolo, R.; Le Vay, L. Natural carbon stable isotope ratios as indicators of the relative contribution of live and inert diets to growth in larval Senegalese sole (Solea senegalensis). Aquaculture 2008, 280, 190–197. [Google Scholar] [CrossRef]
- Vay, L.L.; Gamboa-Delgado, J. Naturally-occurring stable isotopes as direct measures of larval feeding efficiency, nutrient incorporation and turnover. Aquaculture 2011, 315, 95–103. [Google Scholar] [CrossRef]
- McIntyre, P.B.; Flecker, A.S. Rapid turnover of tissue nitrogen of primary consumers in tropical freshwaters. Oecologia 2006, 148, 12–21. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).