Chemical Weathering and Riverine Carbonate System Driven by Human Activities in a Subtropical Karst Basin, South China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Measurements
2.3. Data Processing
3. Results
3.1. Physical and Chemical Parameters
3.2. Cations and Anions
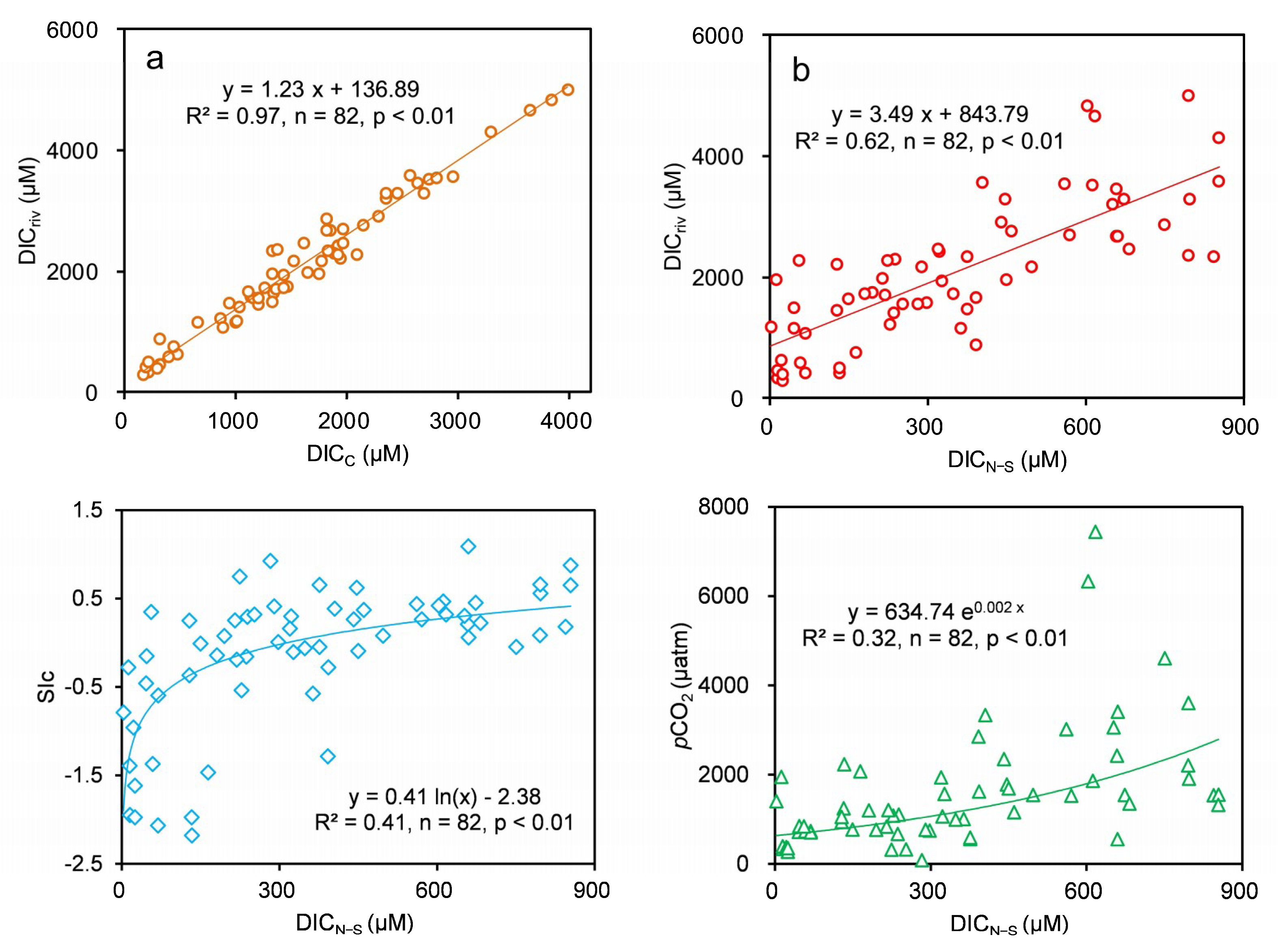
3.3. Spatial Patterns of pCO2, SIc and DIC
4. Discussions
4.1. Material Sources of River Dissolved Loads
4.1.1. Sea Salt Precipitation
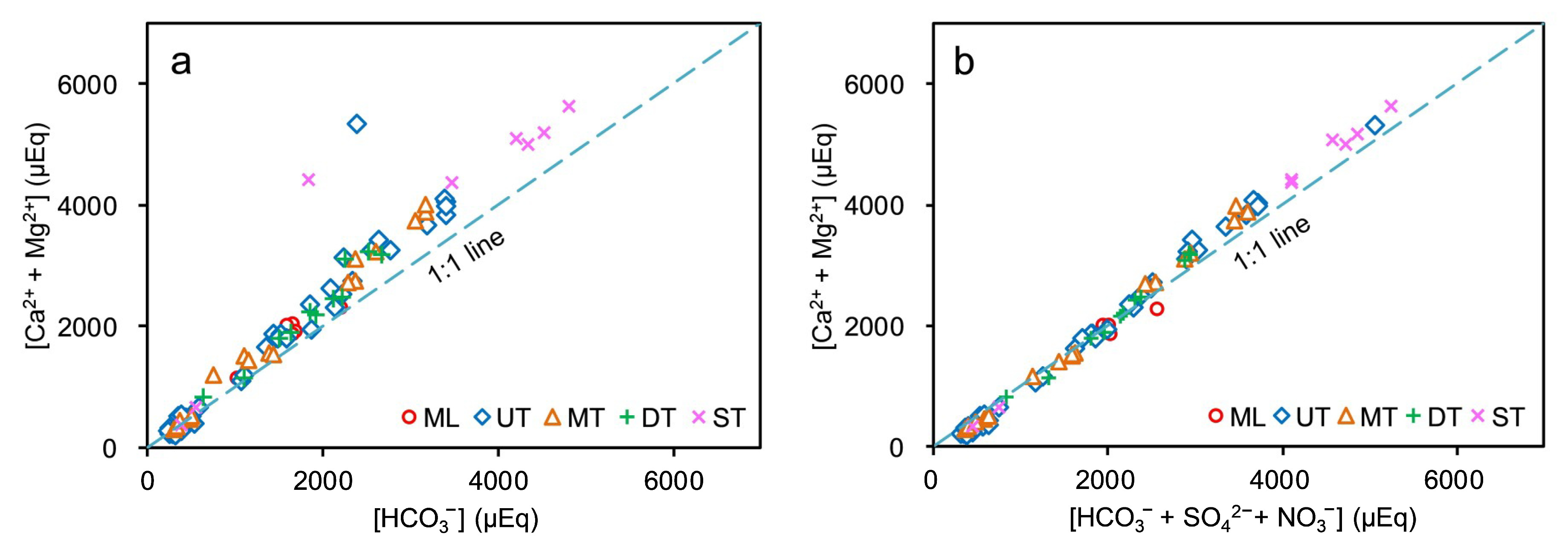
4.1.2. Carbonate Chemical Weathering
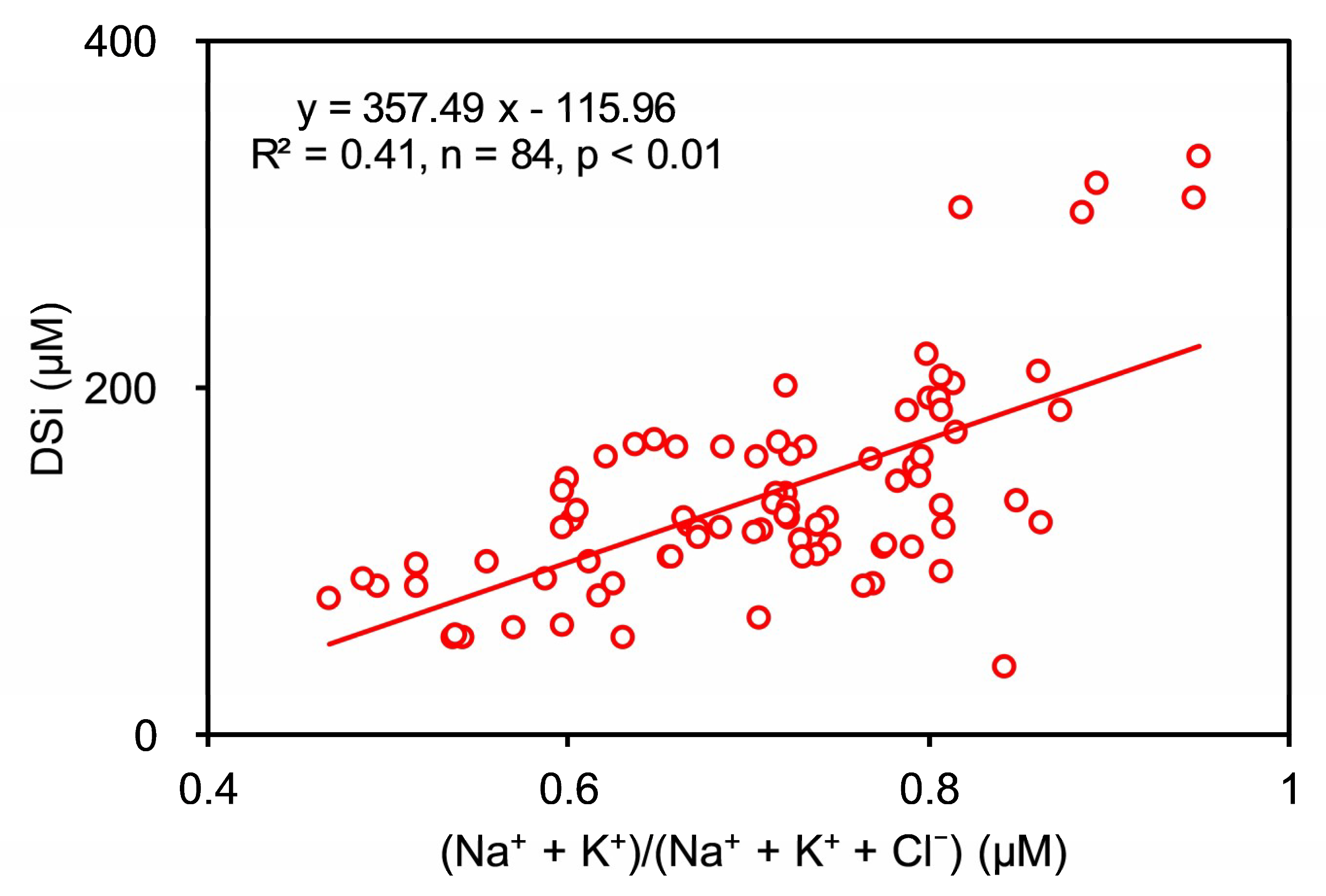
4.1.3. Chemical Weathering of Silicates
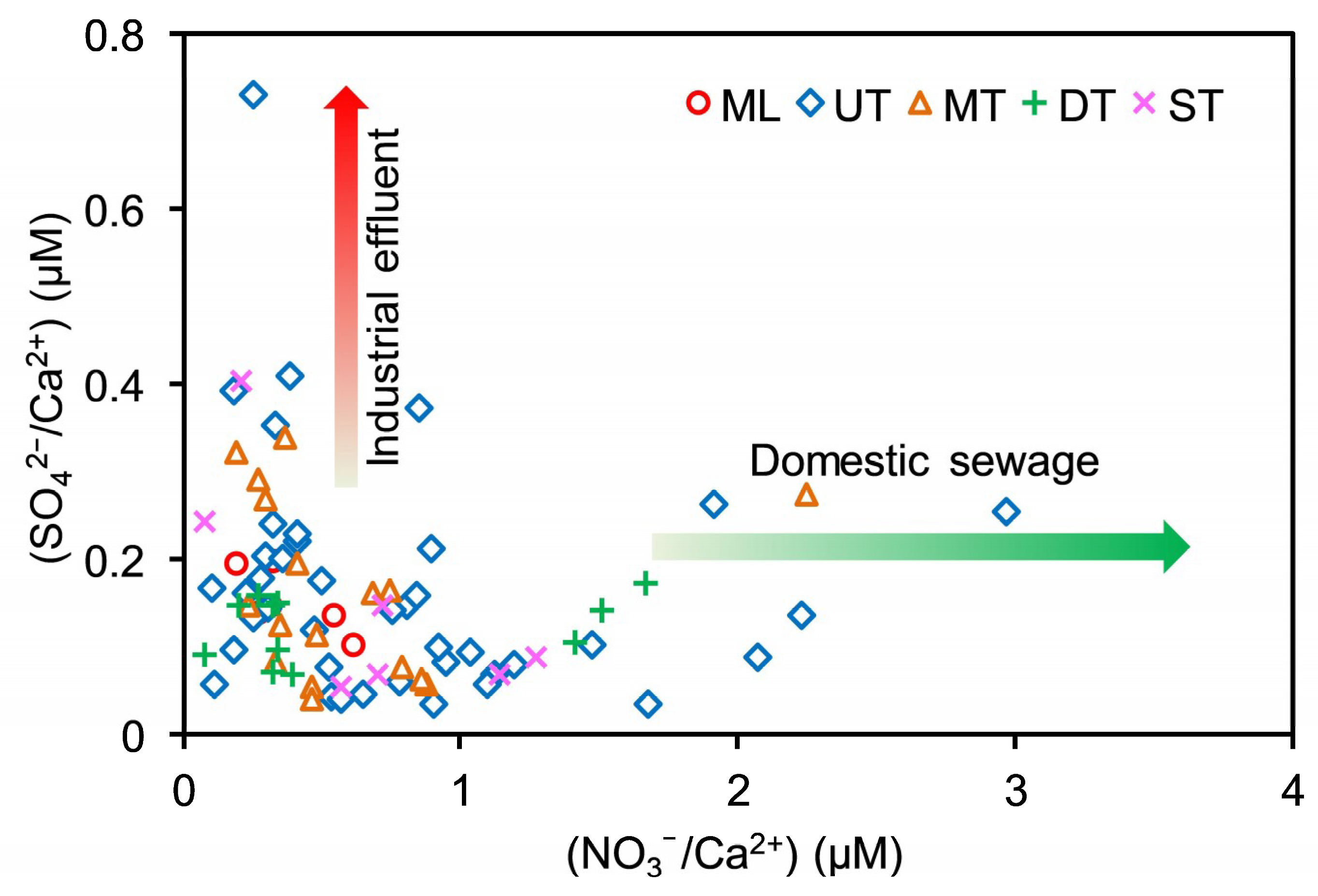
4.1.4. Human Activities
4.2. Evidence of Chemical Weathering Associated with Sulfuric and Nitric Acids
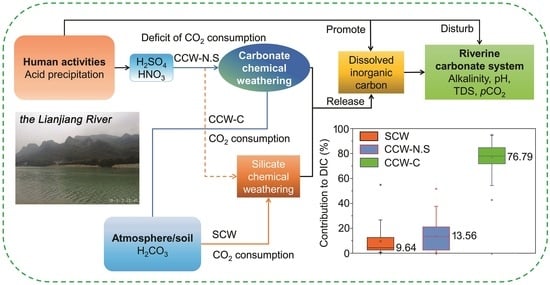
4.3. Chemical Weathering and the Related CO2 Consumption Driven by Anthropogenic Acids
4.3.1. Influence from Sea Salt Precipitation and SCW
4.3.2. CO2 Consumption Deficit Related with Anthropogenic Acid-Involved CCW
4.4. Response of Riverine Carbonate System to Human Activities
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hedges, J.I.; Clark, W.A.; Quay, P.D.; Richey, J.E.; Devol, A.H.; Santos, U.D.M. Compositions and fluxes of particulate organic material in the Amazon River. Limnol. Oceanogr. 1986, 31, 717–738. [Google Scholar] [CrossRef]
- Richey, J.E.; Hedges, J.I.; Devol, A.H.; Quay, P.D.; Victoria, R.; Martinelli, L.; Forsberg, B.R. Biogeochemistry of carbon in the Amazon River. Limnol. Oceanogr. 1990, 35, 352–371. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allègre, C.J. Global silicate chemical weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 171–184. [Google Scholar] [CrossRef]
- Tao, F.X.; Liu, C.Q.; Li, S.L. Source and flux of POC in two subtropical karstic tributaries with contrasting land use practice in the Yangtze River Basin. Appl. Geochem. 2009, 24, 2102–2112. [Google Scholar] [CrossRef]
- Liu, Z.H.; Dreybrodt, W.; Liu, H. Atmospheric CO2 sink: Silicate chemical weathering or carbonate chemical weathering? Appl. Geochem. 2011, 26, 292–294. [Google Scholar] [CrossRef]
- Liu, Z.H.; Dreybrodt, W.; Wang, H.J. A new direction in effective accounting for the atmospheric CO2 budget: Considering the combined action of carbonate dissolution, the global water cycle and photosynthetic uptake of DIC by aquatic organisms. Earth-Sci. Rev. 2010, 99, 162–172. [Google Scholar] [CrossRef]
- Yang, R.; Chen, B.; Liu, H.; Liu, Z.; Yan, H. Carbon sequestration and decreased CO2, emission caused by terrestrial aquatic photosynthesis: Insights from diel hydrochemical variations in an epikarst spring and two spring-fed ponds in different seasons. Appl. Geochem. 2015, 63, 248–260. [Google Scholar] [CrossRef]
- Chen, B.; Yang, R.; Liu, Z.H.; Sun, H.L.; Yan, H.; Zeng, Q.R.; Zeng, S.B.; Zeng, C.; Zhao, M. Coupled control of land uses and aquatic biological processes on the diurnal hydrochemical variations in the five ponds at the Shawan Karst Test Site, China: Implications for the carbonate chemical weathering-related carbon sink. Chem. Geol. 2017, 456, 58–71. [Google Scholar] [CrossRef]
- Liu, M.X.; Xu, X.L.; Wang, D.B.; Sun, A.Y.; Wang, K.L. Karst catchments exhibited higher degradation stress from climate change than the non-karst catchments in southwest China: An ecohydrological perspective. J. Hydrol. 2016, 535, 173–180. [Google Scholar] [CrossRef]
- Suchet, P.A.; Probst, A.; Probst, J.L. Influence of acid rain on CO2 consumption by rock weathering: Local and global scales. Water Air Soil Poll. 1995, 85, 1563–1568. [Google Scholar] [CrossRef]
- Han, G.L.; Liu, C.Q. Water geochemistry controlled by carbonate dissolution: A study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chem. Geol. 2004, 204, 1–21. [Google Scholar] [CrossRef]
- Perrin, A.S.; Probst, A.; Probst, J.L. Impact of nitrogenous fertilizers on carbonate dissolution in small agricultural catchments: Implications for weathering CO2 uptake at regional and global scales. Geochim. Cosmochim. Acta 2008, 72, 3105–3123. [Google Scholar] [CrossRef]
- Li, S.L.; Calmels, D.; Han, G.L.; Gaillardet, J.; Liu, C.Q. Sulfuric acid as an agent of carbonate chemical weathering constrained by δ13CDIC: Examples from Southwest China. Earth Planet. Sci. Lett. 2008, 270, 189–199. [Google Scholar] [CrossRef]
- Ali, H.N.; Atekwana, E.A. The effect of sulfuric acid neutralization on carbonate and stable carbon isotope evolution of shallow groundwater. Chem. Geol. 2011, 284, 217–228. [Google Scholar] [CrossRef]
- Jiang, Y.J. The contribution of human activities to dissolved inorganic carbon fluxes in a karst underground river system: Evidence from major elements and δ¹³CDIC in Nandong, Southwest China. J. Contam. Hydrol. 2013, 152, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.J.; Tang, C.Y.; Cao, G.M.; Wang, X.Z. Hydrochemical zoning: Natural and anthropogenic origins of the major elements in the surface water of Taizi River Basin, Northeast China. Environ. Earth Sci. 2016, 75, 811. [Google Scholar] [CrossRef]
- Ding, H.; Liu, C.Q.; Zhao, Z.Q.; Li, S.L.; Lang, Y.C.; Li, X.D.; Hu, J.; Liu, B.J. Geochemistry of the dissolved loads of the Liao River Basin in Northeast China under anthropogenic pressure: Chemical weathering and controlling factors. J. Asian Earth Sci. 2016, 138, 657–671. [Google Scholar] [CrossRef]
- Li, X.D.; Liu, C.Q.; Liu, X.L.; Bao, L.R. Identification of dissolved sulfate sources and the role of sulfuric acid in carbonate chemical weathering using dual-isotopic data from the Jialing River, Southwest China. J. Asian Earth Sci. 2011, 42, 370–380. [Google Scholar] [CrossRef]
- Etchanchu, D.; Probst, J.L. Evolution of the chemical composition of the Garonne River water during the period 1971–1984. Int. Assoc. Sci. Hydrol. Bull. 1988, 33, 243–256. [Google Scholar] [CrossRef]
- Semhi, K.; Suchet, P.A.; Clauer, N.; Probst, J.L. Impact of nitrogen fertilizers on the natural weathering-erosion processes and fluvial transport in the Garonne Basin. Appl. Geochem. 2000, 15, 865–878. [Google Scholar] [CrossRef]
- Anderson, S.P.; Drever, J.I.; Frost, C.D.; Holden, P. Chemical weathering in the foreland of a retreating glacier. Geochim. Cosmochim. Acta 2000, 64, 1173–1189. [Google Scholar] [CrossRef]
- Martin, J.B. Carbonate minerals in the global carbon cycle. Chem. Geol. 2016, 449, 58–72. [Google Scholar] [CrossRef]
- Li, S.L.; Chetelat, B.; Yue, F.J.; Zhao, Z.Q.; Liu, C.Q. Chemical weathering processes in the Yalong River draining the eastern Tibetan Plateau, China. J. Asian Earth Sci. 2014, 88, 74–84. [Google Scholar] [CrossRef]
- Torres, M.A.; West, A.J.; Li, G.J. Sulphide oxidation and carbonate dissolution as a source of CO2 over geological timescales. Nature 2014, 507, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Shi, C.; Xu, Z.F.; Zhao, T.; Jiang, H.; Liang, C.S.; Zhang, X.; Zhou, L.; Yu, C. Water geochemistry of the Qiantangjiang River, East China: Chemical weathering and CO2 consumption in a basin affected by severe acid deposition. J. Asian Earth Sci. 2016, 127, 246–256. [Google Scholar] [CrossRef]
- Jiang, Z.C.; Lian, Y.Q.; Qin, X.Q. Carbon cycle in the epikarst systems and its ecological effects in South China. Environ. Earth Sci. 2013, 68, 151–158. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, Z.H.; Li, H.C.; Zeng, C.; Yang, R.; Chen, B.; Yan, H. Response of dissolved inorganic carbon (DIC) and δ13CDIC, to changes in climate and land cover in SW China karst catchments. Geochim. Cosmochim. Acta 2015, 165, 123–136. [Google Scholar] [CrossRef]
- Kump, L.R.; Brantley, S.L.; Arthur, M.A. Chemical weathering, atmospheric CO2 and climate. Annu. Rev. Earth Planet. Sci. 2000, 28, 611–667. [Google Scholar] [CrossRef]
- Hartmann, J.; Jansen, N.; Dürr, H.H.; Kempe, S.; Köhler, P. Global CO2 consumption by chemical weathering: What is the contribution of highly active weathering regions? Glob. Planet. Chang. 2009, 69, 185–194. [Google Scholar] [CrossRef]
- Moon, S.; Chamberlain, C.P.; Hilley, G.E. New estimates of silicate weathering rates and their uncertainties in global rivers. Geochim. Cosmochim. Acta 2014, 134, 257–274. [Google Scholar] [CrossRef]
- Zhang, G.W.; Guo, A.L.; Wang, Y.J.; Li, S.Z.; Dong, Y.P.; Liu, S.F.; He, D.F.; Cheng, S.Y.; Lu, R.K.; Yao, A.P. Tectonics of South China continent and its implications. Sci. China Ser. D 2013, 56, 1804–1828. (In Chinese) [Google Scholar] [CrossRef]
- Lin, Q.; Xing, Y.Q. Study on Paleomagnetism in the ε-type Tectonic System in Northern Guangdong. Acta Geolo. Sin. 1983, 1, 63–71. (In Chinese) [Google Scholar]
- Wang, W.X.; Xu, P.J. Research progress in precipitation chemistry in China. Prog. Chem. 2009, 21, 266–281. (In Chinese) [Google Scholar]
- Wang, W.X.; Liang, J.Y.; Chen, Y.Z. Regional sources of acid deposition in South China. Acta. Sci. Circum. 1992, 12, 1–6. (In Chinese) [Google Scholar]
- Tao, Z.Y.; Zheng, Y.G.; Zhang, X.L. Southern China quasi-stationary front during ice-snow disaster of January 2008. Acta Meteor. Sin. 2008, 66, 850–854. (In Chinese) [Google Scholar] [CrossRef]
- Qin, P.; Du, Y.D.; Liu, J.L.; Song, L.L.; Liu, A.J.; Wang, Q.Q. Distributional characteristics of acid rain and its affecting factors in Guangdong Province. J. Trop. Meteor. 2006, 22, 297–300. (In Chinese) [Google Scholar] [CrossRef]
- Gran, G. Determination of the equivalence point in potentiometric titrations, Part II, Section3: Electrical Methods. Analyst 1952, 77, 661–671. [Google Scholar] [CrossRef]
- Geospatial Data Cloud. Available online: http://www.gscloud.cn/search (accessed on 11 December 2017).
- Meybeck, M. Global analysis of river systems: From earth system controls to Anthropocene syndromes. Philos. T. Roy. Soc. B 2003, 358, 1935–1955. [Google Scholar] [CrossRef] [PubMed]
- Dürr, H.H.; Meybeck, M.; Hartmann, J.; Laruelle, G.G. Global spatial distribution of natural riverine silica inputs to the coastal zone. Biogeosciences 2011, 8, 597–620. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Tao, Z.; Ma, Z.W.; Tang, W.K.; Gao, Q.Z.; Xu, P.; Ding, J.; Liu, Z.F.; Lin, Y.W.; Su, D.; et al. Influences of anthropogenic activities on dissolved silica migration in a granite-hosted basin, Hainan Island, China. Quat. Int. 2017, 440, 99–110. [Google Scholar] [CrossRef]
- NOAA. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/gl_trend.html (accessed on 21 June 2018).
- Aufdenkampe, A.K.; Mayorga, E.; Raymond, P.A.; Melack, J.M.; Doney, S.C.; Alin, S.R.; Aalto, R.E.; Yoo, K. Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere. Front. Ecol. Environ. 2011, 9, 53–60. [Google Scholar] [CrossRef]
- Viers, J.; Dupre, B.; Braun, J.J.; Freydier, R.; Greenberg, S.; Ngoupayou, J.N.; Nkamdjou, L.S. Evidence for non-conservative behaviour of chlorine in humid tropical environments. Aquat. Geochem. 2001, 7, 127–154. [Google Scholar] [CrossRef]
- Suchet, P.A.; Probst, J.L. Modelling of atmospheric CO2 consumption by chemical weathering of rocks: Application to the Garonne, Congo and Amazon basins. Chem. Geol. 1993, 107, 205–210. [Google Scholar] [CrossRef]
- Roy, S.; Gaillardet, J.; Allegre, C.J. Geochemistry of dissolved and suspended loads of the Seine River, France: Anthropogenic impact, carbonate and silicate weathering. Geochim. Cosmochim. Acta 1999, 63, 1277–1292. [Google Scholar] [CrossRef]
- Grosbois, C.; Négrel, P.; Grimaud, D.; Fouillac, C. An overview of dissolved and suspended matter fluxes in the Loire River Basin: Natural and anthropogenic inputs. Aquat. Geochem. 2001, 7, 81–105. [Google Scholar] [CrossRef]
- Yuan, D.X.; Zhang, C. Karst dynamics theory in China and its practice. Acta Geo. Sin. 2008, 29, 355–365. (In Chinese) [Google Scholar] [CrossRef]
- Williams, E.L.; Szramek, K.J.; Jin, L.X.; Ku, T.C.W.; Walter, L.M. The carbonate system geochemistry of shallow groundwater-surface water systems in temperate glaciated watersheds (Michigan, USA): Significance of open-system dolomite weathering. Geol. Soc. Am. Bull. 2007, 119, 515–528. [Google Scholar] [CrossRef]
- Zavadlav, S.; Kanduč, T.; Mclntosh, J.; Lojen, S. Isotopic and chemical constraints on the biogeochemistry of dissolved inorganic carbon and chemical weathering in the karst watershed of Krka River (Slovenia). Aquat. Geochem. 2013, 19, 209–230. [Google Scholar] [CrossRef]
- Lang, Y.C.; Liu, C.Q.; Li, S.L.; Zhao, Z.Q.; Zhou, Z.H. Tracing natural and anthropogenic sources of dissolved sulfate in a karst region by using major ion chemistry and stable sulfur isotopes. Appl. Geochem. 2011, 26, S202–S205. [Google Scholar] [CrossRef]
- Huang, Q.B.; Qin, X.Q.; Liu, P.Y.; Zhang, L.K.; Su, C.T. Impact of sulfuric and nitric acids on carbonate dissolution, and the associated deficit of CO2 uptake in the upper-middle reaches of the Wujiang River, China. J. Contam. Hydrol. 2017, 203, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Bögli, A. Karst Hydrology and Physical Speleology; Springer: Berlin/Heidelberg, Germany, 1980; pp. 73–76. ISBN 978-3-642-67671-0. [Google Scholar]
- Song, L.H.; Fang, J.F.; Deng, Z.M.; Liu, H. Fractal and geometry of karst depressions in south China. Geog. Res. 1995, 14, 8–16. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Z.H.; Wu, K.Y.; Wang, J.L.; Li, Q.; Sun, H.L.; Han, J. In situ precise measurement of erosion rates of carbonate rock blocks under flowing non-karst water using micro-erosion meter and the rate-determining factors. Chin. J. Geochim. 2006, 35, 103–110. (In Chinese) [Google Scholar]
- Ford, D.C.; William, P.W. Karst Hydrogeology and Geomorphology; John Wiley & Sons Ltd.: Chichester, UK, 2007; pp. 1–562. ISBN 978-0-470-84996-5. [Google Scholar]
- Strandmann, P.A.E.P.V.; Desrochers, A.; Murphy, M.J.; Finlay, A.J.; Selby, D.; Lenton, T.M. Global climate stabilisation by chemical weathering during the Hirnantian glaciation. Geochem. Perspect. Lett. 2017, 3, 230–237. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Conceição, F.T.D.; Junior, E.P.S.; Sardinha, D.D.S.; Mortatti, J. Chemical weathering rates and atmospheric/soil CO2 consumption of igneous and metamorphic rocks under tropical climate in Southeastern Brazil. Chem. Geol. 2016, 443, 54–66. [Google Scholar] [CrossRef]
- Fontorbe, G.; Rocha, C.L.D.L.; Chapman, H.J.; Bickle, M.J. The silicon isotopic composition of the Ganges and its tributaries. Earth. Planet. Sci. Lett. 2013, 381, 21–30. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.J.; Cai, W.J.; Wang, B.S.; Yu, Z.G. Consumption of atmospheric CO2 via chemical weathering in the Yellow River Basin: The Qinghai–Tibet Plateau is the main contributor to the high dissolved inorganic carbon in the Yellow River. Chem. Geol. 2016, 430, 34–44. [Google Scholar] [CrossRef]
- An, Y.L.; Hou, Yi.L.; Wu, Q.X.; Lin, Q.; Li, L.B. Chemical weathering and CO2 consumption of a high-erosion-rate karstic river: A case study of the Sanchahe River, Southwest China. Chin. J. Geochem. 2015, 34, 601–609. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Reynolds, B.C.; Prokushkin, A.S.; Schott, J.; Viers, J. Silicon isotope variations in central Siberian Rivers during basalt weathering in permafrost-dominated larch forests. Chem. Geol. 2013, 355, 103–116. [Google Scholar] [CrossRef]
- Chen, J.S.; He, D.W. Chemical characteristics and genesis of major ions in the Pearl River Basin. Acta Sci. Nat. Univ. Pekinensis 1999, 35, 786–793. (In Chinese) [Google Scholar] [CrossRef]
- Wu, W.H. Hydrochemistry of inland rivers in the north Tibetan Plateau: Constraints and weathering rate estimation. Sci. Total Environ. 2016, 541, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Chetelat, B.; Liu, C.Q.; Zhao, Z.Q.; Wang, Q.L.; Li, S.L.; Li, J.; Wang, B.L. Geochemistry of the dissolved load of the Changjiang Basin rivers: Anthropogenic impacts and chemical weathering. Geochim. Cosmochim. Acta 2008, 72, 4254–4277. [Google Scholar] [CrossRef]
- Wu, W.H.; Zheng, H.B.; Yang, J.D.; Luo, C.; Zhou, B. Chemical weathering, atmospheric CO2 consumption, and the controlling factors in a subtropical metamorphic-hosted watershed. Chem. Geol. 2013, 356, 141–150. [Google Scholar] [CrossRef]
- Zhang, H.B.; Hu, A.Q.; Lu, C.Z.; Zhang, G.X. Sulfur isotopic composition of acid deposition in South China Regions and its environmental significance. China Environ. Sci. 2002, 22, 165–169. (In Chinese) [Google Scholar] [CrossRef]
- Spence, J.; Telmer, K. The role of sulfur in chemical weathering and atmospheric CO2 fluxes: Evidence from major ions, δ13CDIC, and δ34SSO4 in rivers of the Canadian Cordillera. Geochim. Cosmochim. Acta 2005, 69, 5441–5458. [Google Scholar] [CrossRef]
- Gao, Q.Z.; Wang, Z.G. Dissolved inorganic carbon in the Xijiang River: Concentration and stable isotopic composition. Environ. Earth Sci. 2015, 73, 253–266. [Google Scholar] [CrossRef]
- Dreybrodt, W. Processes in Karst Systems: Physics, Chemistry, and Geology; Springer: Berlin/Heidelberg, Germany, 1988; pp. 1–283. ISBN 978-3-642-83354-0. [Google Scholar]
- Mortatti, J.; Probst, J.L. Silicate rock weathering and atmospheric/soil CO2 uptake in the Amazon Basin estimated from river water geochemistry: Seasonal and spatial variations. Chem. Geol. 2003, 197, 177–196. [Google Scholar] [CrossRef]
- Zhao, H.J.; Xiao, Q.; Wu, X.; Liu, F.; Miao, Y.; Jiang, Y.J. Impact of human activities on water-rock interactions in surface water of Lijiang River. J. Environ. Sci. 2017, 38, 4108–4119. (In Chinese) [Google Scholar]
- Zhang, X.B.; Jiang, Y.J.; Qiu, S.L.; Cao, M.; Hu, Y.J. Agricultural activities and carbon cycling in Karst areas in Southwest China: Dissolving carbonate rocks and CO2 sink. Adv. Earth Sci. 2012, 27, 466–476. (In Chinese) [Google Scholar]
- Raymond, P.A.; Cole, J.J. Increase in the export of alkalinity from North America’s largest river. Science 2003, 301, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.H.; Hu, B.; Groves, C.; Huang, F.; Yang, H.; Zhang, C.L. Karst dynamic system and the carbon cycle. Z. Geomorphol. Suppl. Issues 2016, 60, 35–55. [Google Scholar] [CrossRef]
- Fan, M.; Jiang, X.Q.; Liu, W.X.; Zhang, J.Y.; Chen, H.Y. Dissolution of carbonate rocks in CO2 solution under the different temperatures. Acta Sedim. Sin. 2007, 25, 825–830. (In Chinese) [Google Scholar] [CrossRef]
- Baker, A.; Cumberland, S.; Hudson, N. Dissolved and total organic and inorganic carbon in some British rivers. Area 2008, 40, 117–127. [Google Scholar] [CrossRef]
- Barnes, R.T.; Raymond, P.A. The contribution of agriculture and urban activities to inorganic carbon fluxes within temperate watersheds. Chem. Geol. 2009, 266, 318–327. [Google Scholar] [CrossRef]
- Zhai, W.D.; Dai, M.H.; Guo, X.H. Carbonate system and CO2 degassing fluxes in the inner estuary of Changjiang (Yangtze) River, China. Mar. Chem. 2007, 107, 342–356. [Google Scholar] [CrossRef]
- Johnson, M.S.; Lehmann, J.; Riha, S.J.; Krusche, A.V.; Richey, J.E.; Ometto, J.P.H.B.; Couto, E.G. CO2 efflux from Amazonian headwater streams represents a significant fate for deep soil respiration. Geophys. Res. Lett. 2008, 35, L17401. [Google Scholar] [CrossRef]
- Abril, G.; Martinez, J.M.; Artigas, L.F.; Moreira-turcq, P.; Benedetti, M.F.; Vidal, L.; Meziane, T.; Kim, J.H.; Bernardes, M.C.; Savoye, N.; et al. Amazon River carbon dioxide outgassing fuelled by wetlands. Nature 2014, 505, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Teodoru, C.R.; Giorgio, P.A.D.; Prairie, Y.T.; Camire, M. Patterns in pCO2 in boreal streams and rivers of Northern Quebec, Canada. Glob. Biogeochem. Cycles 2009, 23, GB2012. [Google Scholar] [CrossRef]
- Sawakuchi, H.O.; Bastviken, D.; Sawakuchi, A.O.; Ward, N.D.; Borges, C.D.; Tsai, S.M.; Richey, J.E.; Ballester, M.V.; Krusche, A.V. Oxidative mitigation of aquatic methane emissions in large Amazonian rivers. Glob. Chang. Biol. 2016, 22, 1075–1085. [Google Scholar] [CrossRef] [PubMed]



| Catchment | Sample No. | T (°C) | pH | TDS (mg·L−1) | Ca2+ | Mg2+ | Na+ | K+ | HCO3− | Cl− | SO42− | NO3− | DSi | TZ+ | TZ− | NICB (%) | Location b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (μM) a | (μM) | (μM) | (μM) | (μM) | (μM) | (μM) | (μM) | (μM) | (μEq) a | (μEq) | |||||||
| LR | 1 | 15.2 | 7.9 | 138 | 780 | 155 | 280 | 65 | 1660 | 107 | 153 | 53 | 86 | 2214 | 2126 | 3.97 | ML |
| 2 | 16.4 | 8.18 | 150 | 960 | 190 | 184 | 62 | 2206 | 87 | 131 | 100 | 121 | 2547 | 2656 | −4.26 | ML | |
| 3 | 15.6 | 7.75 | 142 | 840 | 167 | 285 | 78 | 1580 | 141 | 166 | 92 | 127 | 2377 | 2144 | 9.80 | ML | |
| XZHR | 4 | 9.3 | 7.89 | 76 | 513 | 53 | 77 | 25 | 1020 | 39 | 52 | 47 | 130 | 1233 | 1210 | 1.83 | ML |
| 5 | 13.5 | 7.97 | 128 | 853 | 162 | 129 | 50 | 1652 | 54 | 125 | 40 | 86 | 2207 | 1996 | 9.57 | ML | |
| 6 | 15 | 8.1 | 130 | 803 | 154 | 160 | 57 | 1696 | 90 | 113 | 42 | 67 | 2130 | 2053 | 3.64 | ML | |
| BAHR | 7 | 11.6 | 7.77 | 75 | 433 | 111 | 98 | 26 | 1062 | 42 | 42 | 18 | 110 | 1211 | 1206 | 0.45 | UT |
| 8 | 12.4 | 7.71 | 39 | 182 | 54 | 87 | 24 | 484 | 39 | 35 | 35 | 105 | 583 | 630 | −8.04 | MT | |
| 9 | 11.2 | 7.36 | 45 | 184 | 57 | 104 | 27 | 474 | 45 | 38 | 31 | 125 | 611 | 625 | −2.18 | UT | |
| CTHR | 10 | 11.8 | 8 | 241 | 1540 | 483 | 94 | 43 | 3402 | 110 | 105 | 105 | 100 | 4184 | 3827 | 8.53 | UT |
| 11 | 10.2 | 8.05 | 236 | 1505 | 438 | 160 | 71 | 3179 | 146 | 173 | 76 | 100 | 4115 | 3747 | 8.95 | MT | |
| 12 | 10.1 | 8.1 | 194 | 1285 | 306 | 413 | 123 | 2679 | 96 | 116 | 32 | 134 | 3718 | 3038 | 18.29 | DT | |
| 13 | 10.2 | 8.08 | 161 | 1250 | 115 | 170 | 45 | 2346 | 54 | 73 | 18 | 194 | 2944 | 2563 | 12.94 | UT | |
| DBHR | 14 | 9.4 | 7.82 | 41 | 155 | 20 | 217 | 54 | 417 | 39 | 31 | 77 | 188 | 620 | 597 | 3.78 | UT |
| 15 | 11.8 | 7.86 | 110 | 655 | 123 | 196 | 77 | 1391 | 101 | 82 | 68 | 112 | 1829 | 1725 | 5.68 | MT | |
| 16 | 15.1 | 8.42 | 122 | 765 | 134 | 197 | 69 | 1511 | 99 | 116 | 66 | 103 | 2064 | 1907 | 7.57 | DT | |
| 17 | 11.6 | 7.97 | 197 | 1303 | 307 | 183 | 117 | 2608 | 115 | 101 | 144 | 125 | 3519 | 3069 | 12.79 | MT | |
| 18 | 11.2 | 7.68 | 28 | 109 | 66 | 73 | 14 | 295 | 21 | 38 | 24 | 120 | 436 | 416 | 4.42 | UT | |
| 19 | 11.7 | 7.78 | 114 | 628 | 128 | 190 | 53 | 1100 | 68 | 213 | 69 | 146 | 1753 | 1662 | 5.21 | MT | |
| 20 | 13.3 | 7.45 | 38 | 106 | 135 | 77 | 13 | 374 | 45 | 77 | 19 | 121 | 572 | 593 | −3.58 | UT | |
| 21 | 11.9 | 7.72 | 27 | 96 | 79 | 67 | 13 | 328 | 13 | 31 | 12 | 123 | 429 | 415 | 3.32 | MT | |
| SJSR | 22 | 12.4 | 7.72 | 26 | 102 | 57 | 73 | 9 | 257 | 22 | 42 | 27 | 108 | 398 | 390 | 2.19 | UT |
| 23 | 13.2 | 7.69 | 39 | 189 | 71 | 99 | 17 | 497 | 34 | 51 | 29 | 109 | 636 | 662 | −4.09 | MT | |
| 24 | 15.8 | 7.83 | 155 | 895 | 219 | 321 | 78 | 1848 | 206 | 132 | 65 | 166 | 2627 | 2383 | 9.28 | DT | |
| 25 | 12.1 | 7.53 | 46 | 152 | 45 | 232 | 59 | 468 | 65 | 36 | 74 | 304 | 686 | 680 | 0.85 | UT | |
| 26 | 11.8 | 7.5 | 29 | 78 | 27 | 188 | 46 | 290 | 28 | 31 | 33 | 318 | 444 | 412 | 7.23 | UT | |
| 27 | 17.2 | 7.84 | 194 | 1363 | 265 | 87 | 42 | 2779 | 68 | 77 | 95 | 103 | 3383 | 3096 | 8.48 | UT | |
| 28 | 12.2 | 7.95 | 358 | 2185 | 479 | 362 | 216 | 2395 | 293 | 1123 | 419 | 125 | 5906 | 5353 | 9.36 | UT | |
| 29 | 13.3 | 7.76 | 32 | 145 | 68 | 71 | 12 | 371 | 20 | 42 | 19 | 94 | 510 | 494 | 3.11 | MT | |
| DGSR | 30 | 11.8 | 7.78 | 38 | 213 | 29 | 107 | 33 | 430 | 34 | 38 | 53 | 195 | 623 | 592 | 4.96 | UT |
| 31 | 13.4 | 7.88 | 132 | 780 | 169 | 238 | 55 | 1641 | 121 | 123 | 65 | 118 | 2190 | 2073 | 5.38 | DT | |
| 32 | 11.6 | 7.59 | 23 | 96 | 23 | 100 | 32 | 262 | 21 | 21 | 41 | 210 | 370 | 366 | 1.05 | UT | |
| 33 | 14 | 7.94 | 82 | 498 | 93 | 75 | 22 | 1102 | 51 | 42 | 71 | 103 | 1278 | 1307 | −2.23 | UT | |
| 34 | 13 | 8.05 | 115 | 683 | 141 | 74 | 41 | 1359 | 42 | 100 | 60 | 166 | 1763 | 1660 | 5.81 | UT | |
| 35 | 11.9 | 8.15 | 119 | 708 | 227 | 98 | 48 | 1429 | 56 | 150 | 87 | 201 | 2014 | 1873 | 7.01 | UT | |
| 36 | 12.6 | 7.99 | 189 | 1213 | 343 | 150 | 86 | 2377 | 144 | 200 | 111 | 160 | 3345 | 3032 | 9.38 | MT | |
| GBHR | 37 | 11.1 | 7.85 | 35 | 208 | 48 | 83 | 28 | 499 | 25 | 25 | 39 | 174 | 622 | 613 | 1.48 | UT |
| 38 | 17.1 | 8.61 | 150 | 915 | 327 | 75 | 30 | 2216 | 42 | 64 | 29 | 133 | 2589 | 2415 | 6.72 | DT | |
| QGHR | 39 | 15 | 7.52 | 216 | 1573 | 142 | 70 | 79 | 2638 | 99 | 125 | 84 | 124 | 3578 | 3071 | 14.18 | UT |
| 40 | 17 | 7.78 | 193 | 1288 | 261 | 172 | 82 | 2251 | 107 | 181 | 260 | 160 | 3352 | 2980 | 11.09 | DT | |
| 41 | 18.1 | 7.78 | 225 | 1683 | 234 | 39 | 16 | 3400 | 56 | 60 | 65 | 85 | 3888 | 3642 | 6.34 | UT | |
| 42 | 14.2 | 8.06 | 121 | 853 | 85 | 63 | 48 | 1525 | 51 | 122 | 47 | 166 | 1986 | 1866 | 6.01 | UT | |
| 43 | 14 | 8.11 | 146 | 978 | 180 | 87 | 51 | 2141 | 54 | 45 | 56 | 139 | 2452 | 2341 | 4.54 | UT | |
| 44 | 11.6 | 7.75 | 44 | 185 | 38 | 169 | 56 | 511 | 56 | 33 | 47 | 219 | 672 | 681 | −1.43 | UT | |
| 45 | 13.6 | 7.94 | 74 | 283 | 49 | 88 | 37 | 590 | 48 | 45 | 74 | 161 | 788 | 801 | −1.71 | UT | |
| 46 | 14.3 | 7.92 | 225 | 1280 | 283 | 142 | 212 | 2242 | 163 | 173 | 316 | 119 | 3479 | 3068 | 11.82 | UT | |
| 47 | 19.1 | 8.03 | 212 | 1695 | 135 | 48 | 14 | 3188 | 70 | 59 | 44 | 79 | 3723 | 3421 | 8.12 | UT | |
| QLSR | 48 | 14.3 | 7.48 | 31 | 118 | 11 | 215 | 37 | 385 | 14 | 20 | 21 | 310 | 510 | 460 | 9.77 | UT |
| 49 | 15.7 | 7.99 | 102 | 673 | 95 | 112 | 30 | 1445 | 34 | 55 | 37 | 132 | 1676 | 1626 | 2.96 | MT | |
| 50 | 16.8 | 8.13 | 161 | 950 | 137 | 120 | 59 | 1921 | 76 | 93 | 40 | 117 | 2354 | 2223 | 5.56 | DT | |
| 51 | 14.6 | 7.47 | 26 | 88 | 10 | 190 | 23 | 315 | 11 | 14 | 43 | 334 | 408 | 398 | 2.61 | UT | |
| 52 | 17.4 | 7.32 | 42 | 173 | 18 | 234 | 47 | 534 | 37 | 23 | 58 | 301 | 662 | 674 | −1.88 | UT | |
| 53 | 14.5 | 8.07 | 121 | 758 | 143 | 122 | 55 | 1593 | 42 | 110 | 37 | 187 | 1978 | 1893 | 4.31 | UT | |
| 54 | 17.6 | 8.36 | 152 | 1213 | 140 | 94 | 19 | 2284 | 45 | 49 | 44 | 139 | 2817 | 2470 | 12.30 | MT | |
| 55 | 15.1 | 8.06 | 151 | 1138 | 117 | 114 | 42 | 2216 | 62 | 46 | 65 | 169 | 2665 | 2435 | 8.64 | UT | |
| 56 | 15.5 | 8.55 | 221 | 1740 | 309 | 85 | 53 | 3385 | 79 | 106 | 66 | 168 | 4236 | 3743 | 11.65 | UT | |
| 57 | 17.5 | 7.82 | 245 | 1798 | 192 | 102 | 64 | 3398 | 144 | 139 | 53 | 56 | 4144 | 3872 | 6.56 | UT | |
| SBHR | 58 | 13.6 | 7.88 | 172 | 1180 | 131 | 97 | 123 | 2084 | 144 | 103 | 200 | 129 | 2842 | 2634 | 7.33 | UT |
| 59 | 13.9 | 7.78 | 164 | 588 | 124 | 139 | 104 | 1157 | 118 | 95 | 95 | 118 | 1665 | 1560 | 6.33 | MT | |
| 60 | 13.6 | 7.64 | 89 | 475 | 100 | 230 | 75 | 1099 | 79 | 70 | 74 | 149 | 1454 | 1391 | 4.32 | DT | |
| 61 | 14.1 | 7.79 | 160 | 1055 | 122 | 122 | 139 | 1858 | 175 | 107 | 179 | 147 | 2615 | 2426 | 7.23 | UT | |
| 62 | 13.4 | 7.44 | 23 | 69 | 39 | 69 | 41 | 253 | 29 | 16 | 28 | 154 | 326 | 342 | −4.78 | UT | |
| 63 | 11.6 | 7.36 | 31 | 103 | 29 | 85 | 81 | 234 | 45 | 38 | 72 | 188 | 430 | 426 | 0.85 | UT | |
| DTHR | 64 | 15.2 | 7.74 | 124 | 720 | 250 | 128 | 44 | 1865 | 39 | 31 | 68 | 203 | 2113 | 2035 | 3.70 | UT |
| 65 | 16.6 | 7.85 | 156 | 1220 | 147 | 122 | 45 | 2364 | 51 | 67 | 56 | 159 | 2902 | 2604 | 10.25 | MT | |
| 66 | 17 | 8.21 | 145 | 1053 | 169 | 125 | 51 | 2118 | 51 | 75 | 40 | 109 | 2619 | 2359 | 9.92 | DT | |
| 67 | 17.5 | 7.99 | 220 | 1723 | 275 | 75 | 52 | 3178 | 110 | 109 | 65 | 57 | 4122 | 3571 | 13.36 | MT | |
| JTHR | 68 | 15.2 | 6.99 | 38 | 216 | 50 | 34 | 33 | 379 | 56 | 55 | 100 | 56 | 598 | 646 | −7.98 | UT |
| 69 | 18.1 | 7.2 | 82 | 515 | 75 | 44 | 37 | 759 | 76 | 141 | 98 | 86 | 1260 | 1215 | 3.62 | MT | |
| 70 | 18.5 | 7.65 | 198 | 1385 | 224 | 86 | 82 | 2526 | 158 | 146 | 121 | 99 | 3387 | 3096 | 8.57 | DT | |
| 71 | 17.1 | 7.79 | 192 | 1470 | 152 | 69 | 67 | 2559 | 85 | 140 | 71 | 80 | 3380 | 2994 | 11.44 | UT | |
| ZTHR | 72 | 20.5 | 9.02 | 120 | 768 | 133 | 69 | 46 | 1488 | 121 | 76 | 63 | 90 | 1916 | 1824 | 4.77 | UT |
| 73 | 16.6 | 7.77 | 243 | 1675 | 194 | 230 | 143 | 3057 | 262 | 96 | 202 | 89 | 4111 | 3712 | 9.71 | MT | |
| 74 | 16.4 | 7.26 | 60 | 353 | 62 | 42 | 29 | 643 | 48 | 61 | 69 | 63 | 899 | 883 | 1.83 | DT | |
| 75 | 16.5 | 7.22 | 53 | 206 | 50 | 31 | 25 | 358 | 42 | 54 | 60 | 62 | 568 | 568 | −0.09 | UT | |
| Small | 76 | 11.4 | 8.09 | 267 | 1978 | 207 | 70 | 64 | 3478 | 65 | 293 | 50 | 114 | 4502 | 4179 | 7.19 | ST |
| tributaries | 77 | 11.2 | 8.24 | 293 | 2020 | 521 | 131 | 57 | 4199 | 113 | 142 | 92 | 87 | 5270 | 4687 | 11.06 | ST |
| 78 | 10.9 | 7.51 | 25 | 138 | 11 | 65 | 24 | 301 | 17 | 20 | 15 | 40 | 387 | 373 | 3.38 | MT | |
| 79 | 16.3 | 7.62 | 315 | 2243 | 351 | 176 | 84 | 4530 | 141 | 120 | 100 | 171 | 5446 | 5010 | 8.01 | ST | |
| 80 | 18 | 7.9 | 332 | 1890 | 925 | 77 | 48 | 4814 | 73 | 169 | 97 | 56 | 5755 | 5322 | 7.52 | ST | |
| 81 | 17.9 | 7.54 | 303 | 2098 | 402 | 89 | 40 | 4337 | 87 | 144 | 102 | 141 | 5128 | 4813 | 6.13 | ST | |
| 82 | 20.1 | 7.47 | 281 | 1705 | 504 | 92 | 58 | 1837 | 101 | 1115 | 48 | 119 | 4568 | 4216 | 7.71 | ST | |
| 83 | 15.4 | 7.63 | 51 | 228 | 100 | 111 | 32 | 546 | 37 | 92 | 23 | 160 | 798 | 788 | 1.22 | ST | |
| 84 | 15.9 | 6.79 | 32 | 110 | 67 | 106 | 43 | 388 | 35 | 27 | 8 | 207 | 502 | 485 | 3.51 | ST |
| Parameter | ML | UT | MT | DT | ||||
|---|---|---|---|---|---|---|---|---|
| Range | Average | Range | Average | Range | Average | Range | Average | |
| T (°C) | 9.3–16.4 | 14.2 ± 2.3 | 9.4–20.5 | 13.9 ± 2.5 | 10.2–18.1 | 13.9 ± 2.5 | 10.1–18.5 | 15.5 ± 2.3 |
| EC(μS·cm−1) | 116–228 | 194 ± 36 | 35–517 | 169 ± 122 | 38–360 | 188 ± 109 | 94–299 | 220 ± 61 |
| pH | 7.75–8.18 | 7.97 ± 0.14 | 6.99–9.02 | 7.79 ± 0.35 | 7.2–8.36 | 7.82 ± 0.24 | 7.26–8.61 | 7.95 ± 0.37 |
| pCO2 (μatm) | 712–1624 | 1025 ± 313 | 88–4609 | 1177 ± 930 | 349–3063 | 1242 ± 798 | 323–3416 | 1394 ± 87 |
| CO32− (mg·L−1) | 0.15–0.77 | 0.37 ± 0.21 | 0.01–3.9 | 0.38 ± 0.7 | 0.02–1.22 | 0.32 ± 0.32 | 0.03–2.08 | 0.57 ± 0.55 |
| SIc | −0.59–0.35 | −0.11 ± 0.29 | −2.4–1.09 | −0.74 ± 1.09 | −2–0.65 | −0.51 ± 0.9 | −1.46–0.75 | −0.03 ± 0.59 |
| DIC (μM) | 1062–2256 | 1690 ± 346 | 263–3549 | 1461 ± 1107 | 328–3275 | 1645 ± 1063 | 735–2753 | 1932 ± 587 |
| Ca2+ | Mg2+ | Na+ | K+ | HCO3− | Cl− | SO42− | NO3− | DSi | TDS | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | 1 | ** | ** | ** | ** | ** | ** | ** | ** | |
| Mg2+ | 0.764 | 1 | ** | ** | ** | ** | ** | ** | ** | |
| Na+ | 0.091 | 0.112 | 1 | ** | ** | * | ** | ** | ||
| K+ | 0.454 | 0.34 | 0.539 | 1 | ** | ** | ** | ** | ** | ** |
| HCO3− | 0.961 | 0.793 | 0.059 | 0.342 | 1 | ** | * | ** | ** | |
| Cl− | 0.632 | 0.483 | 0.472 | 0.813 | 0.53 | 1 | ** | ** | * | ** |
| SO42− | 0.462 | 0.497 | 0.249 | 0.46 | 0.252 | 0.509 | 1 | ** | ** | |
| NO3− | 0.456 | 0.386 | 0.298 | 0.823 | 0.333 | 0.744 | 0.493 | 1 | ** | |
| DSi | −0.352 | −0.309 | 0.33 | −0.031 | −0.338 | −0.274 | −0.145 | −0.105 | 1 | ** |
| TDS | 0.981 | 0.834 | 0.156 | 0.528 | 0.94 | 0.688 | 0.534 | 0.522 | −0.348 | 1 |
| T | TDS | pH | pCO2 | Alk. | SIc | DICN−S | DICC | DICriv | |
|---|---|---|---|---|---|---|---|---|---|
| T | 1 | ** | * | ||||||
| TDS | 0.213 | 1 | ** | ** | ** | ** | ** | ** | |
| pH | 0.001 | 0.239 | 1 | ** | * | ** | * | * | |
| pCO2 | 0.381 | 0.624 | −0.446 | 1 | ** | ** | ** | ** | |
| Alk. | 0.234 | 0.974 | 0.284 | 0.606 | 1 | ** | ** | ** | ** |
| SIc | 0.231 | 0.796 | 0.706 | 0.197 | 0.813 | 1 | ** | ** | ** |
| DICN−S | 0.141 | 0.853 | 0.112 | 0.517 | 0.785 | 0.615 | 1 | ** | ** |
| DICC | 0.249 | 0.936 | 0.306 | 0.587 | 0.985 | 0.804 | 0.668 | 1 | ** |
| DICriv | 0.244 | 0.975 | 0.259 | 0.633 | 0.999 | 0.8 | 0.787 | 0.984 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, X.; Tao, Z.; Gao, Q.; Peng, H.; Zhou, M. Chemical Weathering and Riverine Carbonate System Driven by Human Activities in a Subtropical Karst Basin, South China. Water 2018, 10, 1524. https://doi.org/10.3390/w10111524
Lyu X, Tao Z, Gao Q, Peng H, Zhou M. Chemical Weathering and Riverine Carbonate System Driven by Human Activities in a Subtropical Karst Basin, South China. Water. 2018; 10(11):1524. https://doi.org/10.3390/w10111524
Chicago/Turabian StyleLyu, Xiaoxi, Zhen Tao, Quanzhou Gao, Haixia Peng, and Mei Zhou. 2018. "Chemical Weathering and Riverine Carbonate System Driven by Human Activities in a Subtropical Karst Basin, South China" Water 10, no. 11: 1524. https://doi.org/10.3390/w10111524
APA StyleLyu, X., Tao, Z., Gao, Q., Peng, H., & Zhou, M. (2018). Chemical Weathering and Riverine Carbonate System Driven by Human Activities in a Subtropical Karst Basin, South China. Water, 10(11), 1524. https://doi.org/10.3390/w10111524