FDOM Conversion in Karst Watersheds Expressed by Three-Dimensional Fluorescence Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
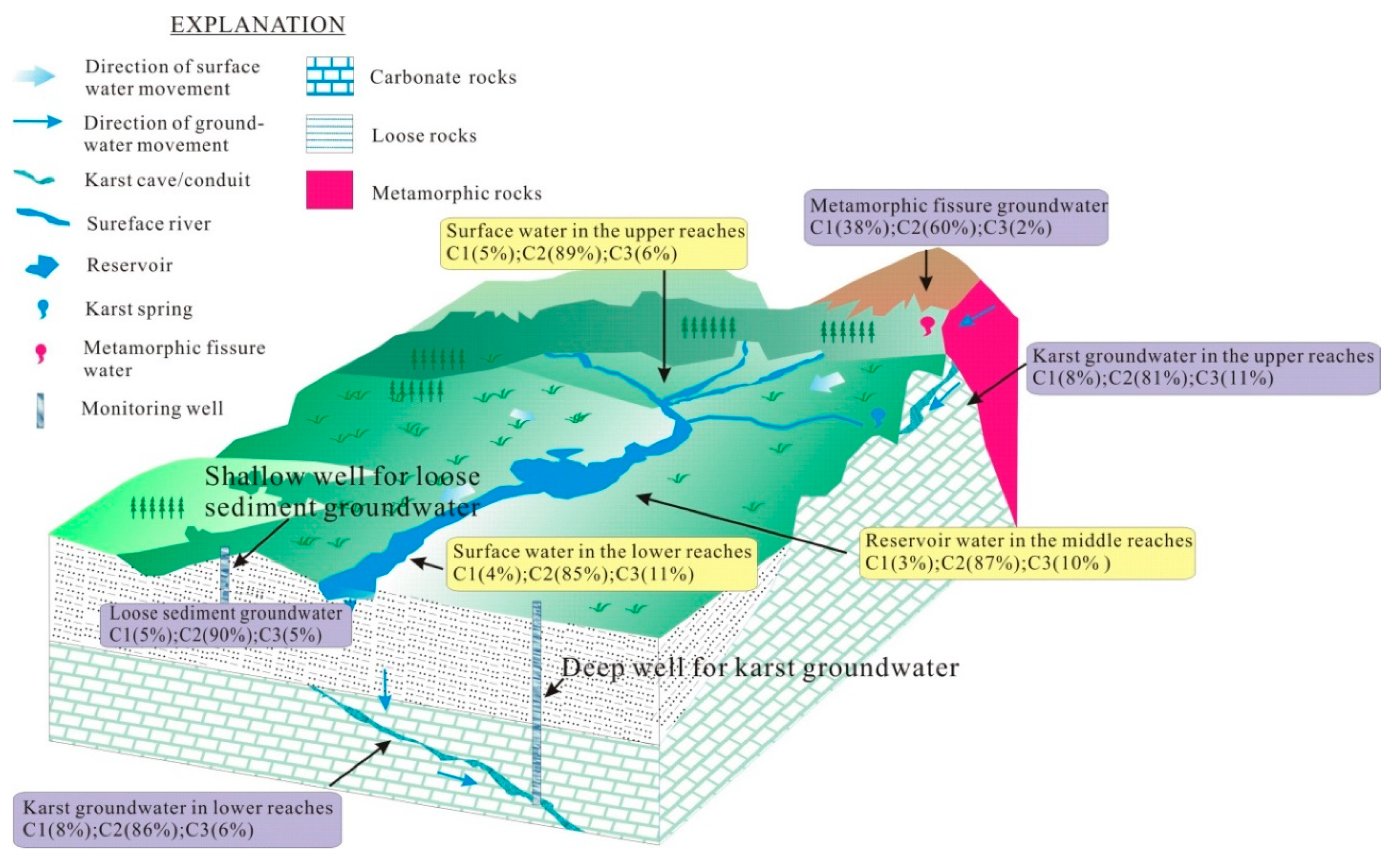
2.1. Field Site
2.2. Sample Collection
2.3. Optical Measurement and Analysis
2.4. The PARAFAC Model
3. Results
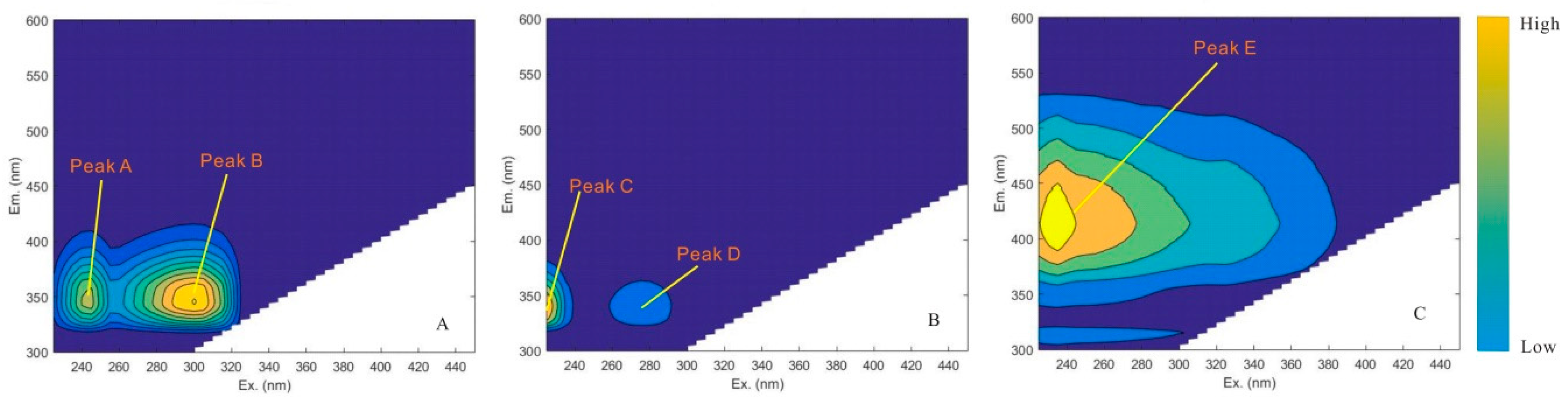
3.1. Component Extraction by PARAFAC
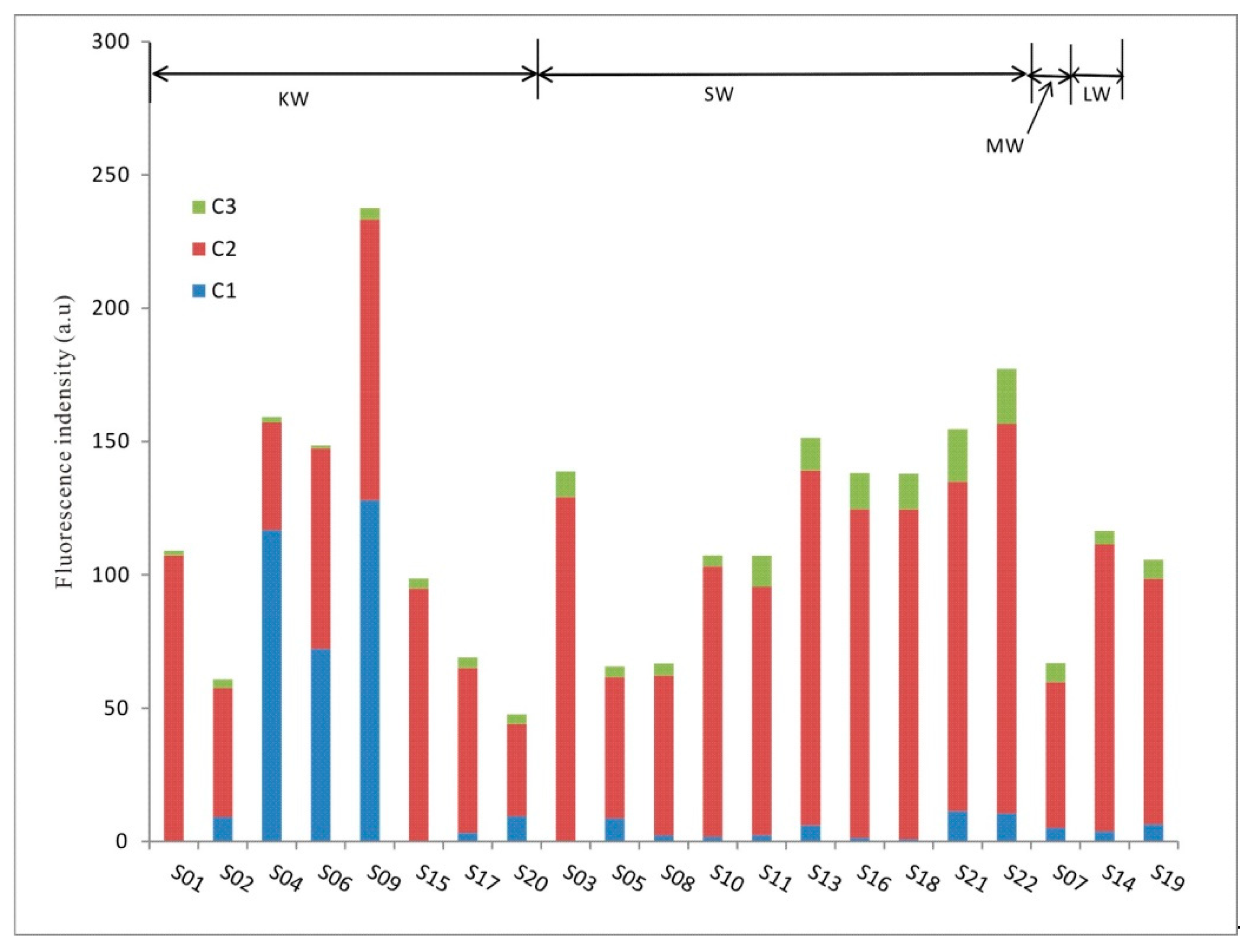
3.2. Spatial Distribution of FDOM Fluorescent Components
4. Discussion
4.1. DOM Source Tracing
4.2. Differentiation of FDOM Fluorescence Signal
4.3. DOM Fluorescent Component Conversion in the Surface and Ground Water
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, D. Karst in China; Geological Publishing House: Beijing, China, 1993. [Google Scholar]
- Wang, R.; Yao, C.; Jiang, Z.; Pei, J. Characteristics, formation, and control of rocky desertification in Liupanshui City, Guizhou province. Carsol. Sin. 2001, 20, 211–216. [Google Scholar]
- Goldscheider, N. Karst groundwater vulnerability mapping: Application of a new method in the Swabian Alb, Germany. Hydrogeol. J. 2005, 13, 555–564. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Qi, S.; Li, X.; Kong, X.; Yuan, D.; Theodore, O.I. Distribution and potential sources of organochlorine pesticides in the karst soils of a tiankeng in southwest China. Environ. Earth Sci. 2013, 70, 2873–2881. [Google Scholar] [CrossRef]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Zhang, R.; Shu, L.; Zhu, J.; Yu, Z.; Jiang, P. Storage and drainage characteristics of a highly heterogeneous karst aquifer in Houzhai Basin. Groundwater 2016, 54, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.W. The role of the subcutaneous zone in karst hydrology. J. Hydrol. 1983, 61, 45–67. [Google Scholar] [CrossRef]
- Yang, P.; Kuang, Y.; Yuan, W.; Jia, P.; He, Q.; Lin, Y. Substance transport in an underground river of typical karst watershed during storm events. Environ. Sci. 2009, 30, 3249–3255. [Google Scholar]
- He, S.; Zhu, L.; Dong, Z.; Zhang, Y.; Yu, X. Study on geochemical susceptivity of groundwater system in representative karstic regions. Environ. Sci. 2010, 31, 1176–1182. [Google Scholar]
- Butscher, C.; Huggenberger, P. Enhanced vulnerability assessment in karst areas by combining mapping with modeling approaches. Sci. Total Environ. 2009, 407, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.R.P.; Ávila, J.P.; Ming, Y.; Sansores, A.C. Groundwater quality: Analysis of its temporal and spatial variability in a karst aquifer. Groundwater 2018, 56, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y. The impact of land use on soil properties in a karst agricultural region of Southwest China: A case study of Xiaojiang watershed, Yunnan. J. Geogr. Sci. 2006, 16, 69–77. [Google Scholar] [CrossRef]
- Yuan, D. The situation and tasks for northern karst research of our country. Carsol. Sin. 2010, 29, 219–221. [Google Scholar]
- Candler, R.; Zech, W.; Alt, H. Characterization of water-soluble organic substances from a Typic Dystrochrept under spruce using GPC, IR, 1H NMR, and 13C NMR spectroscopy. Soil Sci. 1988, 146, 445–452. [Google Scholar] [CrossRef]
- Simon, K.S.; Pipan, T.; Ohno, T.; Culver, D.C. Spatial and temporal patterns in abundance and character of dissolved organic matter in two karst aquifers. Fundam. Appl. Limnol. 2010, 177, 81–92. [Google Scholar] [CrossRef]
- He, W.; Bai, Z.; Li, Y.; Kong, X.; Liu, W.; Yang, C.; Yang, B.; Xu, F. Advances in environmental behaviors and effects of dissoved organic matter in aquatic ecosystems. Sci. China Earth Sci. 2016, 46, 341–355. [Google Scholar]
- Lechleitner, F.; Lang, S.Q.; Mcintyre, C.; Baldini, J.U.L.; Dittmar, T.; Eglinton, T.I. Source to sink characterization of dissolved organic matter in a tropical karst system. In Proceedings of the EGU General Assembly Conference, Vienna, Austria, 17–22 April 2016. [Google Scholar]
- Fooken, U.; Liebezeit, G. Distinction of marine and terrestrial origin of humic acids in North Sea surface sediments by absorption spectroscopy. Mar. Geol. 2000, 164, 173–181. [Google Scholar] [CrossRef]
- Kang, K.-H.; Shin, H.S.; Park, H. Characterization of humic substances present in landfill leachates with different landfill ages and its implications. Water Res. 2002, 36, 4023–4032. [Google Scholar] [CrossRef]
- Kowalczuk, P.; Durako, M.J.; Young, H.; Kahn, A.E.; Cooper, W.J.; Gonsior, M. Characterization of dissolved organic matter fluorescence in the South Atlantic Bight with use of PARAFAC model: Interannual variability. Mar. Chem. 2009, 113, 182–196. [Google Scholar] [CrossRef]
- Coble, P.G.; Green, S.A.; Blough, N.V.; Gagosian, R.B. Characterization of dissolved organic matter in the Black Sea by fluorescence spectroscopy. Nature 1990, 348, 432–435. [Google Scholar] [CrossRef]
- Rochelle-Newall, E.J.; Fisher, T.R. Production of chromophoric dissolved organic matter fluorescence in marine and estuarine environments: An investigation into the role of phytoplankton. Mar. Chem. 2002, 77, 7–21. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Removal of natural organic matter (NOM) and its constituents from water by adsorption—A review. Chemosphere 2017, 166, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Quiers, M.; Batiotguilhe, C.; Seidel, J.; Bicalho, C.C.; Perrette, Y.; Jourde, H. The fluorescence spectroscopy of Dissolved Organic Matter (DOM): A tool to characterize rapid infiltration flows and vulnerability in karst systems. Application to the Lez hydrosystem (Hérault, France). In Proceedings of the AGU Fall Meeting, San Francisco, CA, USA, 5–9 December 2011; pp. 411–414. [Google Scholar]
- Blondel, T.; Dudal, Y.; Boyer, D. Punctual and continuous estimation of transit time from dissolved organic matter fluorescence properties in karst aquifers, application to groundwaters of ‘Fontaine de Vaucluse’ experimental basin (SE France). Environ. Earth Sci. 2012, 65, 2299–2309. [Google Scholar] [CrossRef]
- Ishii, S.K.L.; Boyer, T.H. Behavior of Reoccurring PARAFAC Components in Fluorescent Dissolved Organic Matter in Natural and Engineered Systems: A Critical Review. Environ. Sci. Technol. 2012, 46, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Korak, J.A.; Dotson, A.D.; Summers, R.S.; Rosario-Ortiz, F.L. Critical analysis of commonly used fluorescence metrics to characterize dissolved organic matter. Water Res. 2014, 49, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Song, Y.; Du, E.; Yang, N.; Peng, J.; Liu, R. Comparison of PARAFAC components of fluorescent dissolved and particular organic matter from two urbanized rivers. Environ. Sci. Pollut. Res. 2016, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Wu, X.; Huang, H.; Fang, X.; Xu, C.; Wu, J.; Liang, X.; Shi, J. Tracking fluorescent dissolved organic matter in multistage rivers using EEM-PARAFAC analysis: Implications of the secondary tributary remediation for watershed management. Environ. Sci. Pollut. Res. Int. 2016, 23, 8756–8769. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhan, H.; Wu, Y.; Li, F.; Wang, J. Fractured-karst spring-flow protections: A case study in Jinan, China. Hydrogeol. J. 2006, 14, 1192–1205. [Google Scholar] [CrossRef]
- Xing, L.; Huang, L.; Chi, G.; Yang, L.; Li, C.; Hou, X. A dynamic study of a karst spring based on wavelet analysis and the Mann-Kendall Trend Test. Water 2018, 10, 698. [Google Scholar] [CrossRef]
- Wang, B. The Characteristics of Landforms and the Expploitation and Utilization of Landscape in the Drainage Basin of Yufu River in Shandong Province. Master’s Thesis, Shandong Normal University, Jinan, China, 2009. [Google Scholar]
- Li, B. The Influence of Wohushan Reservoir to Jinan Karst Spring Basin. Master’s Thesis, University of Jinan, Jinan, China, 2011. [Google Scholar]
- Zhang, C.; Yu, B. There are obvious differences in China’s Marine rich organic shale. Pet. Equip. 2011, 2, 33. [Google Scholar]
- Chi, G.; Xing, L.; Zhu, H.; Hou, X.; Xiang, H.; Xing, X. The study of quantitative relationship between the spring water and the dynamic change of the atmospheric precipitation in Jinan. Groundwater 2017, 39, 8–12. [Google Scholar]
- Shi, J.; Lu, G.; Jiang, S.; Liu, X.; Meng, J. Study on present condition and countermeasures of groundwater pollution in Jinan Sprig Areas. Shandong Land Resour. 2012, 28, 21–24. [Google Scholar]
- Wan, L.; Xu, H.; Yin, X.; Li, W.; Wang, Q.; Duan, X.; Gao, Z. Formation of hydrochemistry components of karst groundwater in Jinan. Hydrogeol. Eng. Geol. 2008, 35, 61–64. [Google Scholar]
- Zhang, L.; Qin, X.; Liu, P.; Huang, Q.; Lan, F.; Ji, H. Estimation of carbon sink fluxes in the Pearl River basin (China) based on a water–rock–gas–organism interaction model. Environ. Earth Sci. 2015, 74, 945–952. [Google Scholar] [CrossRef]
- Calloway, D. Beer-lambert law. J. Chem. Educ. 1997, 74, 744. [Google Scholar] [CrossRef]
- Mcknight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Wolfe, A.P.; Kaushal, S.S.; Fulton, J.R.; Mcknight, D.M. Spectrofluorescence of sediment humic substances and historical changes of lacustrine organic matter provenance in response to atmospheric nutrient enrichment. Environ. Sci. Technol. 2002, 36, 3217–3223. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Pan, H.; Lei, H.; Han, Y.; Xi, B.; He, X.; Xu, Q.; Li, D.; Song, C. Analysis of spatial distribution characteristics of dissolved organic matter in trypical greenhouse soil of Northern China using three dimensional fluorescence spectra technique and parallel factor analysis model. Spectrosc. Spectr. Anal. 2014, 34, 1582–1588. [Google Scholar]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- He, X.; Xi, B.; Wei, Z.; Li, M.; Su, J.; Geng, C.; Wang, W.; Hu, C. Fluorescence characteristics of dissolved organic matter from dumped waste leachate. China Environ. Sci. 2010, 30, 752–757. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Z.; Xi, B.; Liu, J.; Yang, T.; Li, M.; Li, X.; Chen, F.; Tong, Y. Characterization of fluorescence properties of water-soluble organic compounds in multi-stage domestic refuse inoculation compost. J. Agric. Resour. Environ. 2012, 29, 79–85. [Google Scholar]
- Xie, L.; Yang, H.; Qu, X.; Zhu, Y.; Zhang, M.; Wu, F. Characerization of water extractable organic matters from the dominant plants in Lake Dianchi by multiple spectroscopy techniques. Res. Environ. Sci. 2013, 26, 72–79. [Google Scholar]
- Hao, R.; Cao, K.; Deng, Y. Characterization of dissolved organic pollutants in wastewater by three-dimensional fluorescent spectropscopy. Chin. J. Anal. Lab. 2007, 26, 41–44. [Google Scholar]
- Leenheer, J.A.; Croue, J.-P. Characterizing aquatic dissolved organic matter. Environ. Sci. Technol. 2003, 37, 18. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, S.; Xia, R.; Xu, D.; Yao, M. Dissolved organic matter (DOM) dynamics in karst aqiufer systems. Environ. Sci. 2014, 35, 1765–1772. [Google Scholar]
- Yamashita, Y.; Jaffé, R.; Maie, N.; Tanoue, E. Assessing the dynamics of dissolved organic matter (DOM) in coastal environments by excitation emission matrix fluorescence and parallel factor analysis (EEM-PARAFAC). Limnol. Oceanogr. 2008, 53, 1900–1908. [Google Scholar] [CrossRef]
- Senesi, N. Molecular and quantitative aspects of the chemistry of fulvic acid and its interactions with metal ions and organic chemicals: Part II. The fluorescence spectroscopy approach. Anal. Chim. Acta 1990, 232, 77–106. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.; Cao, L.; Wang, C.; Yu, M.; Pi, K.; Jia, C. Characterization of DOM from soil in unsaturated zone and its implications on arsenic mobilization into groundwater. Earth Sci. 2012, 37, 605–611. [Google Scholar]
- Hering, J.G.; Morel, F.M. Humic acid complexation of calcium and copper. Environ. Sci. Technol. 1988, 22, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, G.; Drikas, M.; Hayes, R. Influence of characterised natural organic material on activated carbon adsorption: II. Effect on pore volume distribution and adsorption of 2-methylisoborneol. Water Res. 1997, 31, 1065–1073. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, J. Fluorescence characteristics of dissolved organic matter and interactionn between Cu(II) and DOM in sediments of Baiyangdian Lake, China. J. Agro-Environ. Sci. 2012, 31, 1217–1222. [Google Scholar]
- Best, M.D.; Mantai, K.E. Growth of Myriophyllum: Sediment or lake water as the source of nitrogen and phosphorus. Ecology 1978, 59, 1075–1080. [Google Scholar] [CrossRef]
- Ahmad, S.R.; Reynolds, D.M. Monitoring of water quality using fluorescence technique: Prospect of on-line process control. Water Res. 1999, 33, 2069–2074. [Google Scholar] [CrossRef]
- Ismaili, M.M.; Belin, C.; Lamotte, M.; Texier, H. Distribution and characterisation by fluorescence of the dissolved organic matter within the central Channel waters. Oceanol. Acta 1998, 21, 645–676. [Google Scholar] [CrossRef]
- Buckau, G.; Artinger, R.; Geyer, S.; Wolf, M.; Fritz, P.; Kim, J. Groundwater in-situ generation of aquatic humic and fulvic acids and the mineralization of sedimentary organic carbon. Appl. Geochem. 2000, 15, 819–832. [Google Scholar] [CrossRef]
- He, X.S.; Xi, B.D.; Wei, Z.M.; Jiang, Y.H.; Yang, Y.; An, D.; Cao, J.L.; Liu, H.L. Fluorescence excitation–emission matrix spectroscopy with regional integration analysis for characterizing composition and transformation of dissolved organic matter in landfill leachates. J. Hazard. Mater. 2011, 190, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.; Celis, R.; Hermosín, M.C.; Cornejo, J.; Zsolnay, A.; Zeller, K. Effect of organic amendments on herbicide sorption as related to the nature of the dissolved organic matter. Environ. Sci. Technol. 2000, 34, 4600–4605. [Google Scholar] [CrossRef]
- Nguyet, V.T.M.; Goldscheider, N. A simplified methodology for mapping groundwater vulnerability and contamination risk, and its first application in a tropical karst area, Vietnam. Hydrogeol. J. 2006, 14, 1666–1675. [Google Scholar] [CrossRef]
- Cai, W.; Xu, X.; Du, X.; Zhu, H.; Luo, G. Parallel factor analysis with EEM on dissolved organic matter in Chongqing section of Jialing River. Res. Environ. Sci. 2012, 25, 276–281. [Google Scholar]
- Guo, W.; Huang, J.; Hong, H.; Xu, J.; Deng, X. Resolving excitation emission matrix spectroscopy of estuarine CDOM with parallel factor analysis and its application in organic pollution monitoring. Environ. Sci. 2010, 31, 1419–1427. [Google Scholar]
- Tzortziou, M.; Neale, P.J.; Osburn, C.L.; Megonigal, J.P.; Maie, N.; Jaffé, R. Tidal marshes as a source of optically and chemically distinctive colored dissolved organic matter in the Chesapeake Bay. Limnol. Oceanogr. 2008, 53, 148–159. [Google Scholar] [CrossRef]
- Baker, A.; Curry, M. Fluorescence of leachates from three contrasting landfills. Water Res. 2004, 38, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tian, J.; Huang, J.; Zhang, Y.; Wang, K.; Quan, G.; Liu, P.; Yan, S. Analysis of dissolved organic matter transformation in wastewater treatment process by three dimentional fluorescence spectra technology. Environ. Pollut. Control 2017, 39, 375–383. [Google Scholar]
- Tamamura, S.; Ueno, A.; Aramaki, N.; Matsumoto, H.; Uchida, K.; Igarashi, T.; Kaneko, K. Effects of oxidative weathering on the composition of organic matter in coal and sedimentary rock. Org. Geochem. 2015, 81, 8–19. [Google Scholar] [CrossRef]
- Thayalakumaran, T.; Lenahan, M.J.; Bristow, K.L. Dissolved organic carbon in groundwater overlain by irrigated sugarcane. Groundwater 2015, 53, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Yang, J. The fluorescence properties of dissolved organic matter in Zhuozishan Mine Groundwater. Saf. Coal Mines 2014, 45, 131–137. [Google Scholar]
- Zhang, L.; Yang, H.; Tang, J.; Qin, X.; Yu, A.Y. Attenuation of arsenic in a karst subterranean stream and correlation with geochemical factors: A case study at Lihu, South China. J. Environ. Sci. 2014, 26, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
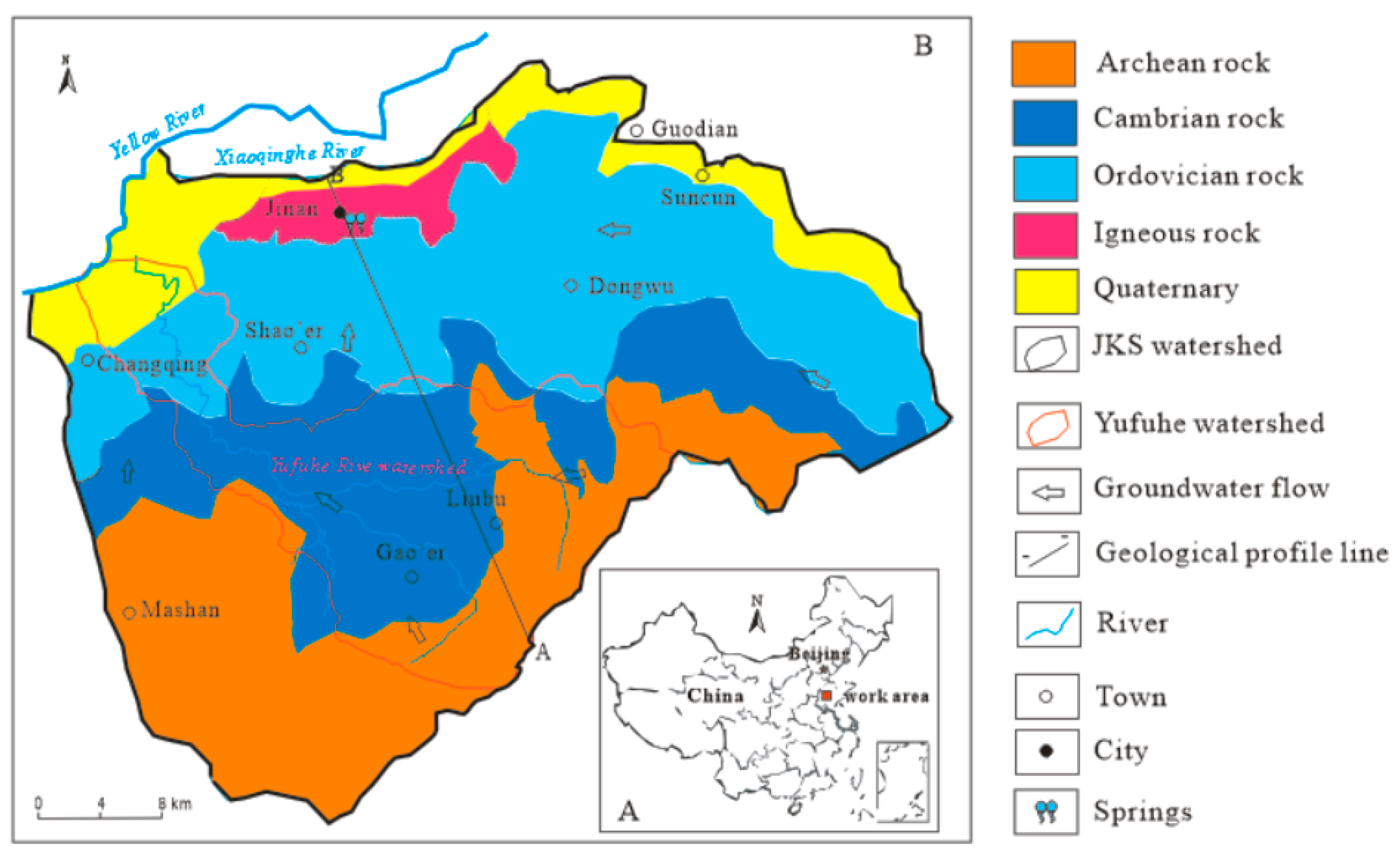

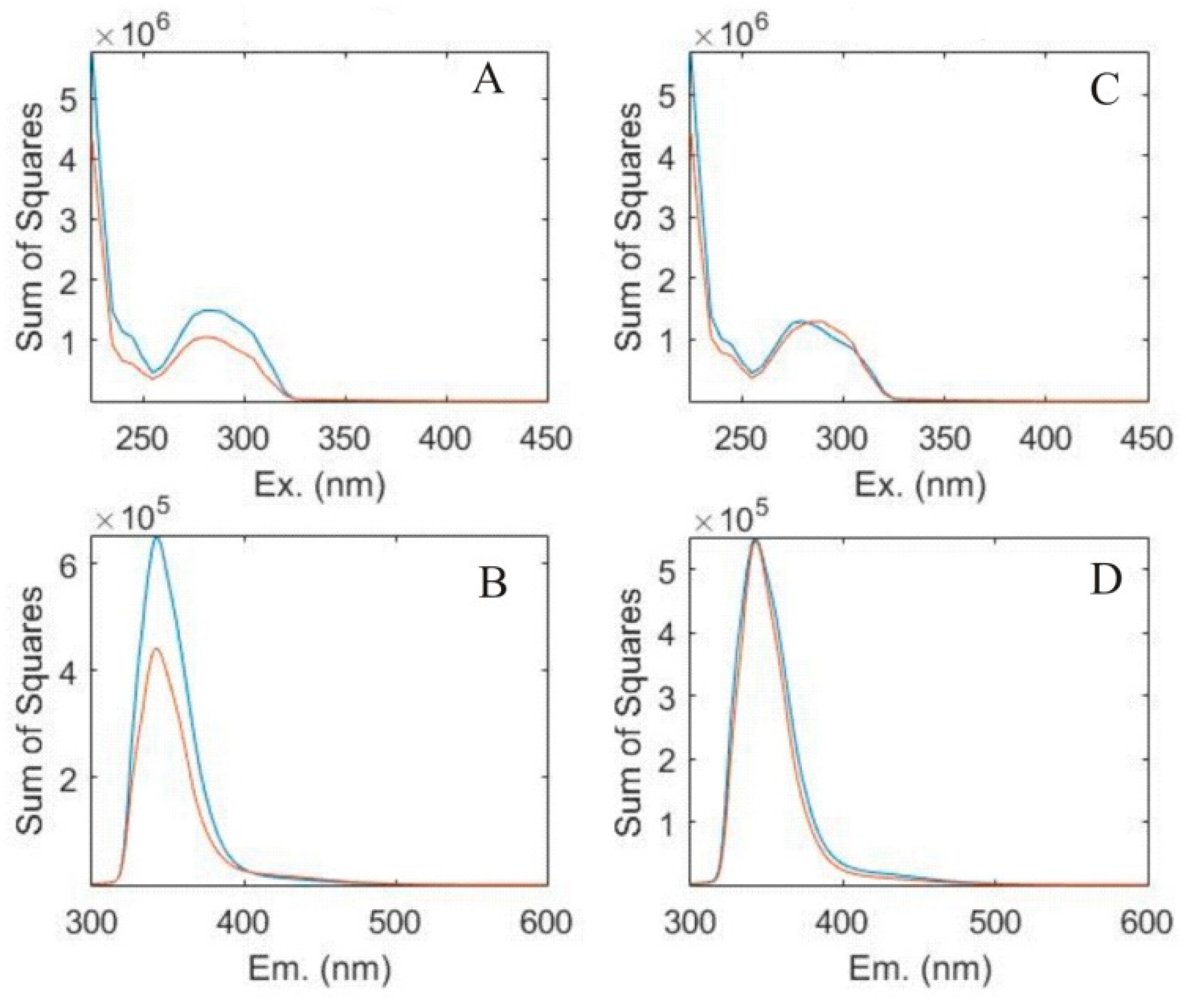
| Sample | Sampling Point | Lithology | Water Type * | Sample Site | pH | EC ** (μS/cm) | T (°C) | DO (mg/L) | TDS (mg/L) | Turbidity (NTU) | NO3– (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S01 | Hongling I | Limestone, marl, and limestone intercalated with little shale | KW | Spring | 7.45 | 566 | 15.3 | 7.15 | 245 | 0.72 | 18.32 |
| S02 | Hongling II | Limestone, marl, and limestone intercalated with little shale | KW | Spring | 8.30 | 543 | 14.2 | 9.27 | 272 | 1.72 | 37.38 |
| S03 | Xiying | Residual sandy clay | SW | Surface river | 8.17 | 422 | 20.0 | 8.97 | 191 | 0.10 | 1.20 |
| S04 | Dashuijing | Limestone and dolomitic limestone | KW | Well 200 m in depth | 7.49 | 697 | 14.6 | 5.75 | 302 | 0.66 | 16.52 |
| S05 | Jiuqu | Residual sandy clay | SW | Surface river | 7.90 | 653 | 17.2 | 8.60 | 280 | 0.66 | 20.96 |
| S06 | Dafo | Limestone, marl, and limestone intercalated with little shale | KW | Spring | 7.35 | 693 | 15.0 | 7.73 | 290 | 1.22 | 30.75 |
| S07 | Shiyao | Gneiss | MW | Well 10 m in depth | 7.51 | 751 | 17.3 | 6.30 | 324 | 2.49 | 37.06 |
| S08 | Liubu | Residual sandy clay | SW | Surface river | 8.09 | 506 | 15.1 | 10.70 | 221 | 0.93 | 16.68 |
| S09 | Beigao’er | Limestone, marl, and limestone intercalated with little shale | KW | Spring | 7.50 | 755 | 15.5 | 8.36 | 334 | 2.19 | 50.47 |
| S10 | Menya | Residual sandy clay | SW | Surface river | 8.08 | 513 | 19.7 | 14.63 | 250 | 1.70 | 15.86 |
| S11 | Wohushan I | Residual sandy clay | SW | Reservoir | 8.68 | 1035 | 18.1 | 11.77 | 455 | 5.95 | 12.71 |
| S12 | Wohushan II | Surface water | SW | Reservoir | 8.52 | 1044 | 17.4 | 10.27 | 450 | 5.69 | 11.96 |
| S13 | Wohushan III | Residual sandy clay | SW | Reservoir | 8.29 | 1051 | 15.9 | 8.77 | 455 | 3.87 | 11.43 |
| S14 | Cuijia I | Residual sandy clay | LW | Well 6 m in depth | 7.27 | 1110 | 15.4 | 4.63 | 477 | 1.30 | 20.72 |
| S15 | Cuijia II | Limestone, marl, and limestone intercalated with little shale | KW | Spring | 7.90 | 557 | 14.4 | 9.90 | 241 | 0.46 | 18.66 |
| S16 | Zhaike | Piedmont alluvial plain | SW | Surface river | 8.58 | 971 | 19.4 | 12.16 | 418 | 3.10 | 7.69 |
| S17 | Zhai’ertou I | Limestone, marl, and limestone intercalated with little shale | KW | Well 262 m in depth | 7.64 | 671 | 17.2 | 8.60 | 288 | 1.48 | 32.00 |
| S18 | Zhai’ertou II | Piedmont alluvial plain | SW | Surface river | 8.20 | 992 | 16.3 | 10.20 | 256 | 2.56 | 7.37 |
| S19 | Eastern Kema | Piedmont alluvial plain | LW | Well 26 m in depth | 7.81 | 1280 | 18.3 | 8.95 | 550 | 0.82 | 9.08 |
| S20 | Xiangjiazhuang | Limestone, marl, and limestone intercalated with little shale | KW | Well 329 m in depth | 7.40 | 801 | 15.2 | 7.01 | 347 | 0.88 | 72.44 |
| S21 | Western Kema | Piedmont alluvial plain | SW | Surface river | 9.34 | 1647 | 19.1 | 10.80 | 701 | 4.80 | 1.08 |
| S22 | Cuimazhuang | Piedmont alluvial plain | SW | Surface river | 9.06 | 1635 | 19.2 | 10.63 | 696 | 6.57 | 4.17 |
| Components | Type | Ex/Em | |
|---|---|---|---|
| This Study | Reference | ||
| C1 | Tyrosine of protein-like | 300(245)/345 | 275/310 [44] 275(220)/315 [45] 270–290/300–320 [46,47] |
| C2 | Tryptophan of protein-like | <225(275)/340 | 275/340 [42,44,48] 270–290/300–350 [46,47] |
| C3 | UV-fulvic acid (FA-like) | 235/414 | 240–270/370–440 [47] 235–255/410–450 [46] 235–255/410–450 [49] 230/430 [42] 260/380–460 [42] |
| Water Type | Sampling Point | Absolute Value (a.u.) | Relative Value (%) | ||||
|---|---|---|---|---|---|---|---|
| DOM Component | C1 | C2 | C3 | C1 | C2 | C3 | |
| Ex (nm) | 300(245) | <225(275) | 235 | 300(245) | <225(275) | 235 | |
| Em (nm) | 345 | 340 | 414 | 345 | 340 | 414 | |
| KW | S01 | 0.00 | 107 | 1.79 | 0.00 | 98.4 | 1.64 |
| S02 | 9.20 | 48.4 | 3.19 | 15.1 | 79.6 | 5.24 | |
| S04 | 117 | 40.3 | 2.07 | 73.4 | 25.3 | 1.30 | |
| S06 | 72.2 | 75.2 | 1.12 | 48.6 | 50.6 | 0.75 | |
| S09 | 128 | 105 | 4.32 | 53.9 | 44.3 | 1.82 | |
| S15 | 0.00 | 94.8 | 3.82 | 0.00 | 96.1 | 3.87 | |
| S17 | 3.26 | 61.8 | 3.99 | 4.72 | 89.5 | 5.77 | |
| S20 | 9.49 | 34.6 | 3.68 | 19.9 | 72.4 | 7.71 | |
| Avg. | 42.4 | 71.0 | 2.99 | 27.0 | 69.5 | 3.51 | |
| SW | S03 | 0.00 | 129 | 9.58 | 0.00 | 93.1 | 6.91 |
| S05 | 8.62 | 53.0 | 4.07 | 13.1 | 80.7 | 6.20 | |
| S08 | 2.33 | 60.0 | 4.50 | 3.50 | 89.8 | 6.75 | |
| S10 | 1.79 | 101 | 4.19 | 1.67 | 94.4 | 3.91 | |
| S11 | 2.39 | 93 | 11.7 | 2.23 | 86.9 | 10.92 | |
| S13 | 6.10 | 133 | 12.2 | 4.03 | 87.9 | 8.08 | |
| S16 | 1.39 | 123 | 13.5 | 1.00 | 89.2 | 9.79 | |
| S18 | 0.86 | 123 | 13.3 | 0.63 | 89.7 | 9.65 | |
| S21 | 11.4 | 124 | 19.7 | 7.36 | 79.9 | 12.7 | |
| S22 | 10.6 | 146 | 20.7 | 5.95 | 82.4 | 11.7 | |
| Avg. | 4.54 | 107 | 11.4 | 3.95 | 87.4 | 8.66 | |
| MW | S07 | 5.14 | 54.6 | 7.14 | 7.68 | 81.7 | 10.7 |
| LW | S14 | 3.81 | 108 | 4.99 | 3.27 | 92.4 | 4.28 |
| S19 | 6.45 | 92 | 7.14 | 6.11 | 87.1 | 6.76 | |
| Avg. | 5.13 | 100 | 6.06 | 4.69 | 89.8 | 5.52 | |
| Water Type | Sampling Point | Ex (370 nm) | FI | |
|---|---|---|---|---|
| Em (450 nm) | Em (500 nm) | |||
| KW | S01 | 54.1 | 29.2 | 1.85 |
| S02 | 102 | 47.6 | 2.15 | |
| S04 | 120 | 60.1 | 1.97 | |
| S06 | 72.7 | 36.2 | 2.01 | |
| S09 | 108 | 56.9 | 1.89 | |
| S15 | 113 | 61.4 | 1.84 | |
| S17 | 115 | 61.6 | 1.86 | |
| S20 | 122 | 62.2 | 1.97 | |
| Avg. | 101 ± 22.9 | 52.0 ± 12.1 | 1.94 ± 0.1 | |
| SW | S03 | 245 | 152 | 1.61 |
| S05 | 125 | 75.1 | 1.66 | |
| S08 | 166 | 96.4 | 1.72 | |
| S10 | 122 | 65.7 | 1.85 | |
| S11 | 261 | 152 | 1.72 | |
| S13 | 274 | 164 | 1.67 | |
| S16 | 350 | 204 | 1.72 | |
| S18 | 346 | 198 | 1.75 | |
| S21 | 472 | 286 | 1.65 | |
| S22 | 453 | 271 | 1.67 | |
| Avg. | 281 ± 118.2 | 166 ± 71.7 | 1.70 ± 0.1 | |
| MW | S07 | 197 | 106 | 1.86 |
| LW | S14 | 140 | 70.2 | 1.99 |
| S19 | 167 | 88.5 | 1.88 | |
| Avg. | 153 ± 13.5 | 79.3 ± 9.2 | 1.94 ± 0.1 | |
| pH | EC | T | DO | TDS | Turbidity | NO3− | C1 *** | C2 *** | C3 *** | |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | |||||||||
| EC | 0.568 ** | 1 | ||||||||
| T | 0.581 ** | 0.380 | 1 | |||||||
| DO | 0.694 ** | 0.097 | 0.569 ** | 1 | ||||||
| TDS | 0.564 ** | 0.964 ** | 0.399 | 0.096 | 1 | |||||
| Turbidity | 0.746 ** | 0.735 ** | 0.420 | 0.409 | 0.728 ** | 1 | ||||
| NO3− | −0.602 ** | −0.353 | −0.527 * | −0.417 | −0.301 | −0.311 | 1 | |||
| C1 | −0.406 | −0.142 | −0.434 * | −0.416 | −0.114 | −0.198 | 0.388 | 1 | ||
| C2 | 0.302 | 0.035 | 0.363 | 0.383 | 0.014 | 0.082 | −0.362 | −0.987 ** | 1 | |
| C3 | 0.738 ** | 0.626 ** | 0.579 ** | 0.372 | 0.574 ** | 0.700 ** | −0.321 | −0.551 ** | 0.410 | 1 |
| Unit | Absolute Value (a.u.) | Relative Value (%) | EC (μS/cm) | DO (mg/L) | TDS (mg/L) | Turbidity (NTU) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | Sum | C1 | C2 | C3 | |||||
| KW in upstream | 65.30 | 75.30 | 2.50 | 143.00 | 38.20 | 59.70 | 2.15 | 651 | 7.65 | 289 | 0.85 |
| MW in upstream | 5.14 | 54.60 | 7.14 | 67.00 | 7.68 | 81.70 | 10.67 | 751 | 6.30 | 324 | 2.49 |
| SW in upstream | 3.19 | 85.90 | 5.59 | 95.00 | 4.58 | 89.50 | 5.94 | 524 | 10.70 | 236 | 1.30 |
| RW * | 4.25 | 113.00 | 12.00 | 129.00 | 3.13 | 87.40 | 9.50 | 1043 | 10.30 | 455 | 4.91 |
| SW in middle and downstream | 6.05 | 129.00 | 16.80 | 152.00 | 3.74 | 85.30 | 10.96 | 1311 | 11.00 | 518 | 4.26 |
| LW in middle and downstream | 5.13 | 100.00 | 6.07 | 111.00 | 4.69 | 89.80 | 5.52 | 1195 | 6.79 | 514 | 1.06 |
| KW in middle and downstream | 4.25 | 64.00 | 3.83 | 72.00 | 8.20 | 86.00 | 5.78 | 676 | 8.50 | 292 | 0.94 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zhang, L.; Liu, P.; Qin, X.; Shan, X.; Yao, X. FDOM Conversion in Karst Watersheds Expressed by Three-Dimensional Fluorescence Spectroscopy. Water 2018, 10, 1427. https://doi.org/10.3390/w10101427
Liu W, Zhang L, Liu P, Qin X, Shan X, Yao X. FDOM Conversion in Karst Watersheds Expressed by Three-Dimensional Fluorescence Spectroscopy. Water. 2018; 10(10):1427. https://doi.org/10.3390/w10101427
Chicago/Turabian StyleLiu, Wen, Liankai Zhang, Pengyu Liu, Xiaoqun Qin, Xiaojing Shan, and Xin Yao. 2018. "FDOM Conversion in Karst Watersheds Expressed by Three-Dimensional Fluorescence Spectroscopy" Water 10, no. 10: 1427. https://doi.org/10.3390/w10101427
APA StyleLiu, W., Zhang, L., Liu, P., Qin, X., Shan, X., & Yao, X. (2018). FDOM Conversion in Karst Watersheds Expressed by Three-Dimensional Fluorescence Spectroscopy. Water, 10(10), 1427. https://doi.org/10.3390/w10101427





