A Single Tube Contactor for Testing Membrane Ozonation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane Contactor System
2.2. Ozonation Experiments
2.3. Wastewater Effluent and River Water
2.4. Analytical Methods
2.5. Computational Modelling
3. Results and Discussion
3.1. Ozone Concentration with Liquid Side Velocity and Membrane Size
3.2. Overall Ozone Mass Transfer Coefficient and Molar Flux through the Membrane
3.3. Removal of pCBA by Membrane Ozonation and in Presence of Additional H2O2 (Peroxone Process)
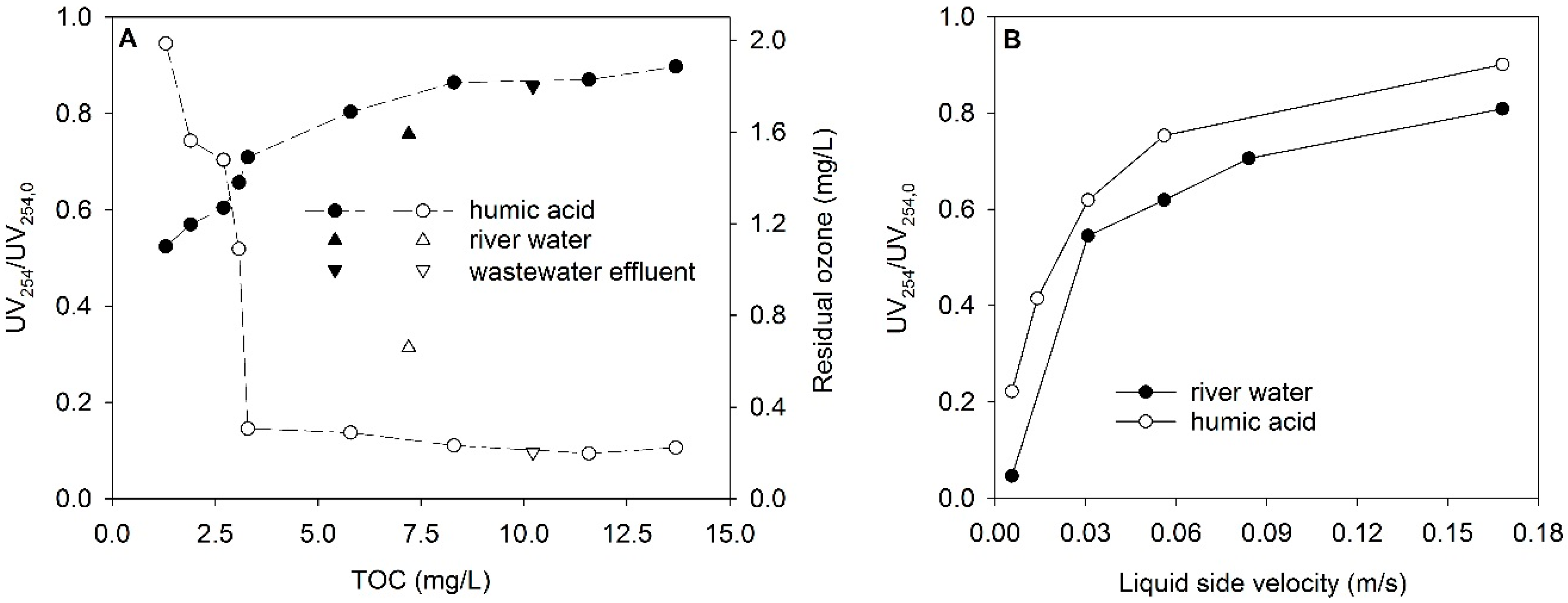
3.4. Ozonation of Dissolved Organic Matter
3.5. Membrane Longevity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Nomenclature | ||
| α | surface area of the membrane per unit volume of liquid | m−1 |
| L | length of the membrane | m |
| uL | liquid velocity | m/s |
| H | solubility of ozone in water | - |
| S | solubility of ozone in the membrane | - |
| Cg | ozone concentration in the gas phase | g/m3 |
| CL,out | ozone concentration at the outlet of the contactor | mg/L |
| KL | Overall mass transfer coefficient | m/s |
| N | Molar flux | mol/(m2 s) |
| Re | Reynolds number | - |
| Sc | Schmidt number | - |
| Acronyms | ||
| CFD | Computational fluid dynamics | |
| PDMS | Polydimethylsiloxane | |
| PES | Polyethersulfone | |
| PEI | Polyetherimide | |
| PFA | Perfluoroalkoxy alkane | |
| PTFE | Polytetrafluoroethylene | |
| PVDF | Polyvinylidene difluoride | |
| DPD | N,N-diethyl-p-phenylenediamine | |
| pCBA | para-chlorobenzoic acid | |
| ID | inner diameter | |
| OD | outer diameter | |
| TOC | total organic carbon | |
References
- Le Paulouë, J.; Langlais, B. State-of-the-art of ozonation in France. Ozone Sci. Eng. 1999, 21, 153–162. [Google Scholar] [CrossRef]
- Von Gunten, U. Oxidation processes in water treatment: Are we on track? Environ. Sci. Technol. 2018, 52, 5062–5075. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, C.; Libra, J.A.; Saupe, A. Ozonation of Water and Waste Water: A Practical Guide to Understanding Ozone and Its Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Chuang, Y.-H.; Mitch, W.A. Effect of ozonation and biological activated carbon treatment of wastewater effluents on formation of N-nitrosamines and halogenated disinfection byproducts. Environ. Sci. Technol. 2017, 51, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Reckhow, D.A. Comparison of disinfection byproduct formation from chlorine and alternative disinfectants. Water Res. 2007, 41, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Oya, M.; Hanamoto, T.; Nagashio, D. Reviewing the 20 years of operation of ozonation facilities in Hanshin Water Supply Authority with respect to water quality improvements. Ozone Sci. Eng. 2017, 39, 397–406. [Google Scholar] [CrossRef]
- Camel, V.; Bermond, A. The use of ozone and associated oxidation processes in drinking water treatment. Water Res. 1998, 32, 3208–3222. [Google Scholar] [CrossRef]
- Snyder, S.A.; Wert, E.C.; Rexing, D.J.; Zegers, R.E.; Drury, D.D. Ozone oxidation of endocrine disruptors and pharmaceuticals in surface water and wastewater. Ozone Sci. Eng. 2006, 28, 445–460. [Google Scholar] [CrossRef]
- Loeb, B.L.; Thompson, C.M.; Drago, J.; Takahara, H.; Baig, S. Worldwide ozone capacity for treatment of drinking water and wastewater: A review. Ozone Sci. Eng. 2012, 34, 64–77. [Google Scholar] [CrossRef]
- Bourgin, M.; Beck, B.; Boehler, M.; Borowska, E.; Fleiner, J.; Salhi, E.; Teichler, R.; von Gunten, U.; Siegrist, H.; McArdell, C.S. Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products. Water Res. 2018, 129, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Loeb, B.L. Forty years of advances in ozone technology. A review of ozone: Science & engineering. Ozone Sci. Eng. 2018, 40, 3–20. [Google Scholar] [CrossRef]
- Mundy, B.; Kuhnel, B.; Hunter, G.; Jarnis, R.; Funk, D.; Walker, S.; Burns, N.; Drago, J.; Nezgod, W.; Huang, J.; et al. A review of ozone systems costs for municipal applications. Report by the Municipal Committee—IOA Pan American Group. Ozone Sci. Eng. 2018, 40, 266–274. [Google Scholar] [CrossRef]
- Rakness, K.L.; Hunter, G.; Lew, J.; Mundy, B.; Wert, E.C. Design considerations for cost-effective ozone mass transfer in sidestream systems. Ozone Sci. Eng. 2018, 40, 159–172. [Google Scholar] [CrossRef]
- Zhou, H.; Smith, D.W. Ozone mass transfer in water and wastewater treatment: Experimental observations using a 2D laser particle dynamics analyzer. Water Res. 2000, 34, 909–921. [Google Scholar] [CrossRef]
- Basile, A.; Cassano, A.; Rastogi, N.K. Advances in Membrane Technologies for Water Treatment: Materials, Processes and Applications; Woodhead Publishing: Sawston, UK, 2015; pp. 1–342. [Google Scholar]
- Zhou, H.; Smith, D.W. Ozonation dynamics and its implication for off-gas ozone control in treating pulp mill wastewaters. Ozone Sci. Eng. 2000, 22, 31–51. [Google Scholar] [CrossRef]
- Oneby, M.A.; Bromley, C.O.; Borchardt, J.H.; Harrison, D.S. Ozone treatment of secondary effluent at U.S. municipal wastewater treatment plants. Ozone Sci. Eng. 2010, 32, 43–55. [Google Scholar] [CrossRef]
- Gabelman, A.; Hwang, S.-T. Hollow fiber membrane contactors. J. Membr. Sci. 1999, 159, 61–106. [Google Scholar] [CrossRef]
- Merle, T.; Pronk, W.; von Gunten, U. MEMBRO3X, a novel combination of a membrane contactor with advanced oxidation (O3/H2O2) for simultaneous micropollutant abatement and bromate minimization. Environ. Sci. Technol. Lett. 2017, 4, 180–185. [Google Scholar] [CrossRef]
- Van Geluwe, S.; Braeken, L.; Van der Bruggen, B. Ozone oxidation for the alleviation of membrane fouling by natural organic matter: A review. Water Res. 2011, 45, 3551–3570. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Davies, S.H.R.; Baumann, M.J.; Tarabara, V.V.; Masten, S.J. Effect of ozone dosage and hydrodynamic conditions on the permeate flux in a hybrid ozonation–ceramic ultrafiltration system treating natural waters. J. Membr. Sci. 2008, 311, 165–172. [Google Scholar] [CrossRef]
- Laera, G.; Cassano, D.; Lopez, A.; Pinto, A.; Pollice, A.; Ricco, G.; Mascolo, G. Removal of organics and degradation products from industrial wastewater by a membrane bioreactor integrated with ozone or UV/H2O2 treatment. Environ. Sci. Technol. 2012, 46, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, S.K.; Sklari, S.D.; Zamboulis, D.; Zaspalis, V.T.; Zouboulis, A.I. Development of bubble-less ozonation and membrane filtration process for the treatment of contaminated water. J. Membr. Sci. 2015, 492, 40–47. [Google Scholar] [CrossRef]
- Wenten, I.G.; Julian, H.; Panjaitan, N.T. Ozonation through ceramic membrane contactor for iodide oxidation during iodine recovery from brine water. Desalination 2012, 306, 29–34. [Google Scholar] [CrossRef]
- Janknecht, P.; Picard, C.; Larbot, A.; Wilderer, P.A. Membrane ozonation in wastewater treatment. Acta Hydroch. Hydrob. 2004, 32, 33–39. [Google Scholar] [CrossRef]
- Kukuzaki, M.; Fujimoto, K.; Kai, S.; Ohe, K.; Oshima, T.; Baba, Y. Ozone mass transfer in an ozone–water contacting process with Shirasu porous glass (SPG) membranes—A comparative study of hydrophilic and hydrophobic membranes. Sep. Purif. Technol. 2010, 72, 347–356. [Google Scholar] [CrossRef]
- Stylianou, S.K.; Szymanska, K.; Katsoyiannis, I.A.; Zouboulis, A.I. Novel water treatment processes based on hybrid membrane-ozonation systems: A novel ceramic membrane contactor for bubbleless ozonation of emerging micropollutants. J. Chem. 2015, 2015, 214927. [Google Scholar] [CrossRef]
- Mosadegh-Sedghi, S.; Rodrigue, D.; Brisson, J.; Iliuta, M.C. Wetting phenomenon in membrane contactors—Causes and prevention. J. Membr. Sci. 2014, 452, 332–353. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Wang, J.; Hou, D.; Liu, H. Ozone mass transfer behaviors on physical and chemical absorption for hollow fiber membrane contactors. Water Sci. Technol. 2017, 76, 1360–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, F.R.A.D.; Borges, C.P.; Fonseca, F.V.D. Polymeric materials for membrane contactor devices applied to water treatment by ozonation. Mater. Res. 2015, 18, 1015–1022. [Google Scholar] [CrossRef]
- Pines, D.S.; Min, K.-N.; Ergas, S.J.; Reckhow, D.A. Investigation of an ozone membrane contactor system. Ozone Sci. Eng. 2005, 27, 209–217. [Google Scholar] [CrossRef]
- Shanbhag, P.V.; Sirkar, K.K. Ozone and oxygen permeation behavior of silicone capillary membranes employed in membrane ozonators. J. Appl. Polym. Sci. 1998, 69, 1263–1273. [Google Scholar] [CrossRef]
- Shanbhag, P.V.; Guha, A.K.; Sirkar, K.K. Membrane-based ozonation of organic compounds. Ind. Eng. Chem. Res. 1998, 37, 4388–4398. [Google Scholar] [CrossRef]
- Ouyang, M.; Yuan, C.; Muisener, R.J.; Boulares, A.; Koberstein, J.T. Conversion of some siloxane polymers to silicon oxide by UV/ozone photochemical processes. Chem. Mater. 2000, 12, 1591–1596. [Google Scholar] [CrossRef]
- Berry, M.; Taylor, C.; King, W.; Chew, Y.; Wenk, J. Modelling of ozone mass-transfer through non-porous membranes for water treatment. Water 2017, 9, 452. [Google Scholar] [CrossRef]
- Bader, H.; Hoigné, J. Determination of ozone in water by the indigo method. Water Res. 1981, 15, 449–456. [Google Scholar] [CrossRef]
- Bader, H.; Sturzenegger, V.; Hoigné, J. Photometric method for the determination of low concentrations of hydrogen peroxide by the peroxidase catalyzed oxidation of N,N-diethyl-p-phenylenediamine (DPD). Water Res. 1988, 22, 1109–1115. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 9963-1:1994: Water Quality—Determination of Alkalinity—Part 1: Determination of Total and Composite Alkalinity; ISO: Geneva, Switzerland, 1994. [Google Scholar]
- Atchariyawut, S.; Phattaranawik, J.; Leiknes, T.; Jiraratananon, R. Application of ozonation membrane contacting system for dye wastewater treatment. Sep. Purif. Technol. 2009, 66, 153–158. [Google Scholar] [CrossRef]
- Stylianou, S.K.; Kostoglou, M.; Zouboulis, A.I. Ozone mass transfer studies in a hydrophobized ceramic membrane contactor: Experiments and analysis. Ind. Eng. Chem. Res. 2016, 55, 7587–7597. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Dingemans, M.; Dewulf, J.; Van Hecke, W.; Van Langenhove, H. Determination of ozone solubility in polymeric materials. Chem. Eng. J. 2008, 138, 172–178. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Lee, Y.; von Gunten, U. Oxidative transformation of micropollutants during municipal wastewater treatment: Comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrate VI, and ozone) and non-selective oxidants (hydroxyl radical). Water Res. 2010, 44, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Elovitz, M.S.; von Gunten, U. Hydroxyl radical/ozone ratios during ozonation processes. I. The Rct concept. Ozone Sci. Eng. 1999, 21, 239–260. [Google Scholar] [CrossRef]
- Wenk, J.; von Gunten, U.; Canonica, S. Effect of dissolved organic matter on the transformation of contaminants induced by excited triplet states and the hydroxyl radical. Environ. Sci. Technol. 2011, 45, 1334–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsoyiannis, I.A.; Canonica, S.; von Gunten, U. Efficiency and energy requirements for the transformation of organic micropollutants by ozone, O3/H2O2 and UV/H2O2. Water Res. 2011, 45, 3811–3822. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.W.; McGuire, M.J.; Koch, B.; Wolfe, R.L.; Aieta, E.M. Comparing peroxone and ozone for controlling taste and odor compounds, disinfection by-products, and microorganisms. J. Am. Water Works Assoc. 1990, 82, 181–191. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Leiknes, T.; Pronk, W. Mass transfer studies in flat-sheet membrane contactor with ozonation. J. Membr. Sci. 2005, 247, 153–167. [Google Scholar] [CrossRef]
- Stylianou, S.K.; Katsoyiannis, I.A.; Ernst, M.; Zouboulis, A.I. Impact of O3 or O3/H2O2 treatment via a membrane contacting system on the composition and characteristics of the natural organic matter of surface waters. Environ. Sci. Pollut. Res. Int. 2018, 25, 12246–12255. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, S.K.; Katsoyiannis, I.A.; Mitrakas, M.; Zouboulis, A.I. Application of a ceramic membrane contacting process for ozone and peroxone treatment of micropollutant contaminated surface water. J. Hazard. Mater. 2018, 358, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Acero, J.L.; Von Gunten, U. Characterization of oxidation processes: Ozonationn and the AOP O3/H2O2. J. Am. Water Works Assoc. 2001, 93, 90–100. [Google Scholar] [CrossRef]
- Drewes, J.E.; Jekel, M. Behavior of DOC and AOX using advanced treated wastewater for groundwater recharge. Water Res. 1998, 32, 3125–3133. [Google Scholar] [CrossRef]
- Yavich, A.A.; Lee, K.H.; Chen, K.C.; Pape, L.; Masten, S.J. Evaluation of biodegradability of NOM after ozonation. Water Res. 2004, 38, 2839–2846. [Google Scholar] [CrossRef] [PubMed]
- Elovitz, M.S.; von Gunten, U.; Kaiser, H.-P. Hydroxyl radical/ozone ratios during ozonation processes. II. The effect of temperature, pH, alkalinity, and DOM properties. Ozone Sci. Eng. 2000, 22, 123–150. [Google Scholar] [CrossRef]
- Fu, Y.J.; Qui, H.Z.; Liao, K.S.; Lue, S.J.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Effect of UV-ozone treatment on poly (dimethylsiloxane) membranes: Surface characterization and gas separation performance. Langmuir 2010, 26, 4392–4399. [Google Scholar] [CrossRef] [PubMed]
- Graubner, V.-M.; Jordan, R.; Nuyken, O.; Schnyder, B.; Lippert, T.; Kötz, R.; Wokaun, A. Photochemical modification of cross-linked poly (dimethylsiloxane) by irradiation at 172 nm. Macromolecules 2004, 37, 5936–5943. [Google Scholar] [CrossRef]
- Fujimoto, K.; Takebayashi, Y.; Inoue, H.; Ikada, Y. Ozone-induced graft polymerization onto polymer surface. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1035–1043. [Google Scholar] [CrossRef]
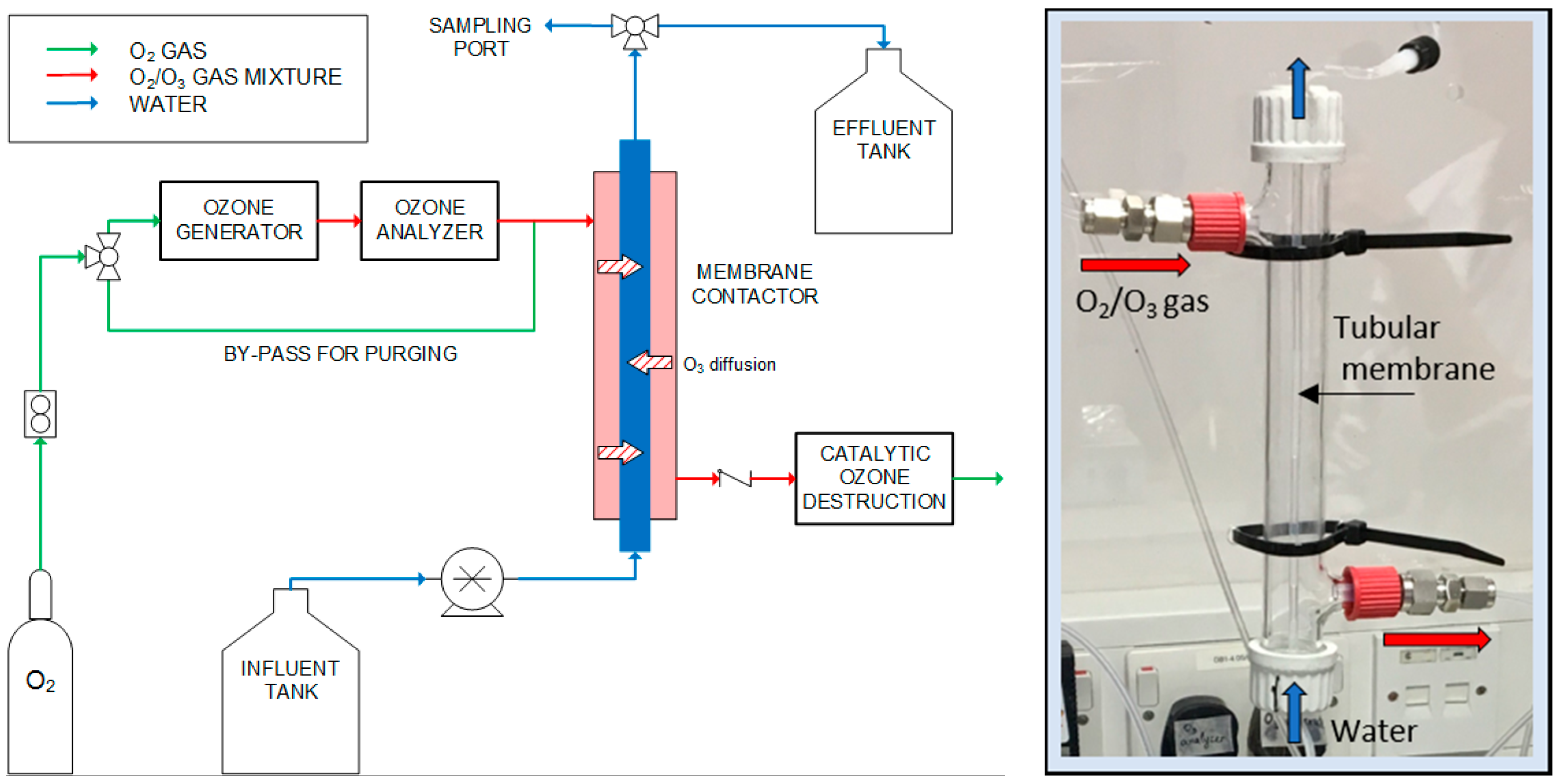
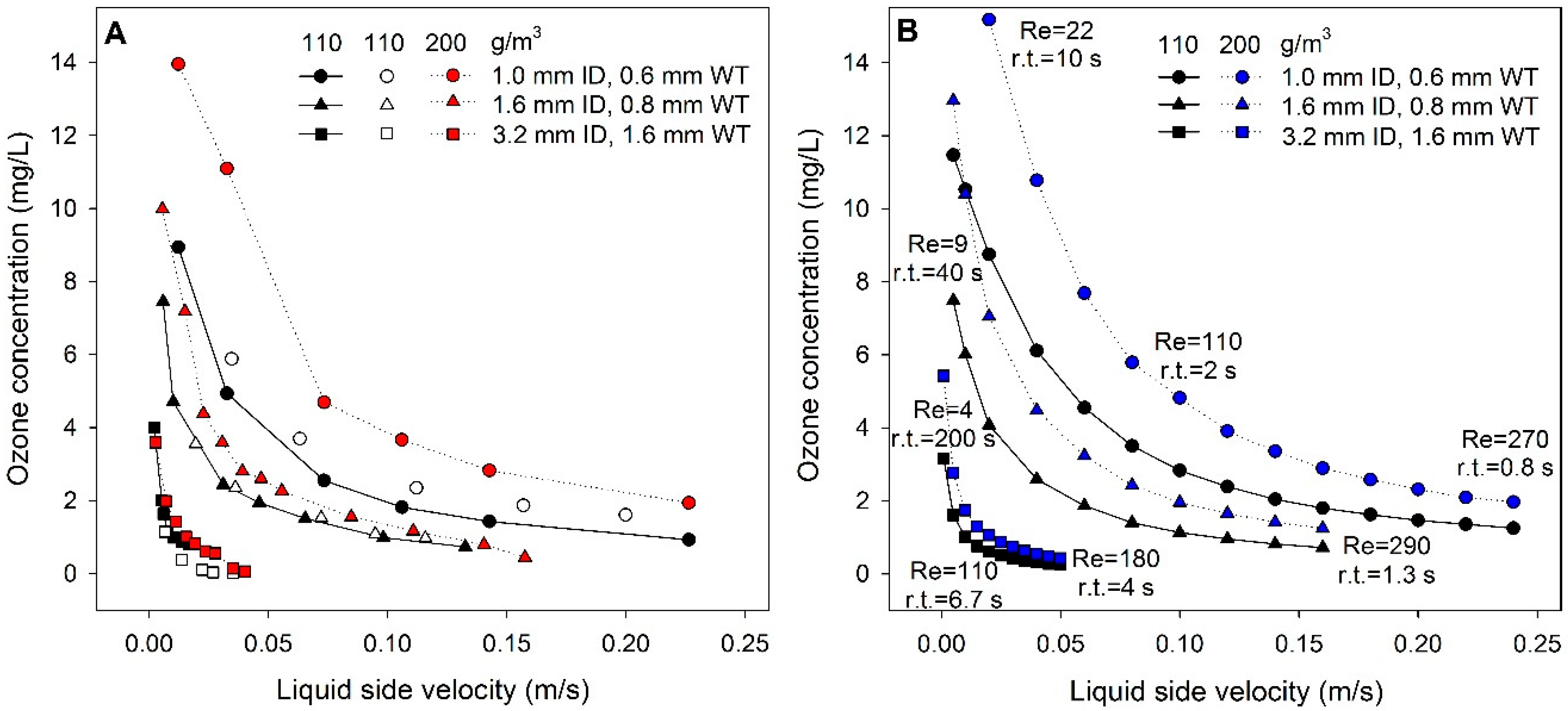
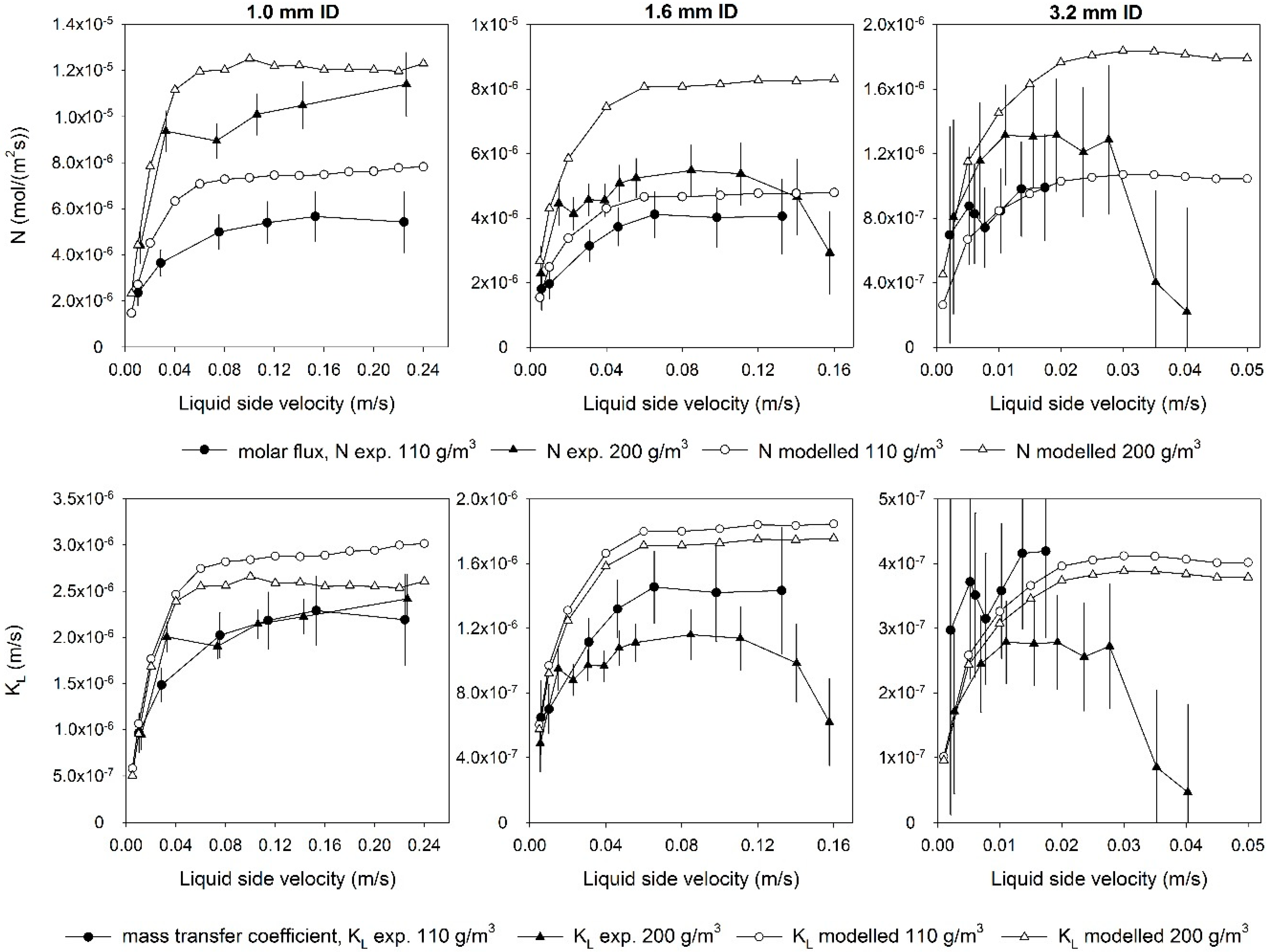
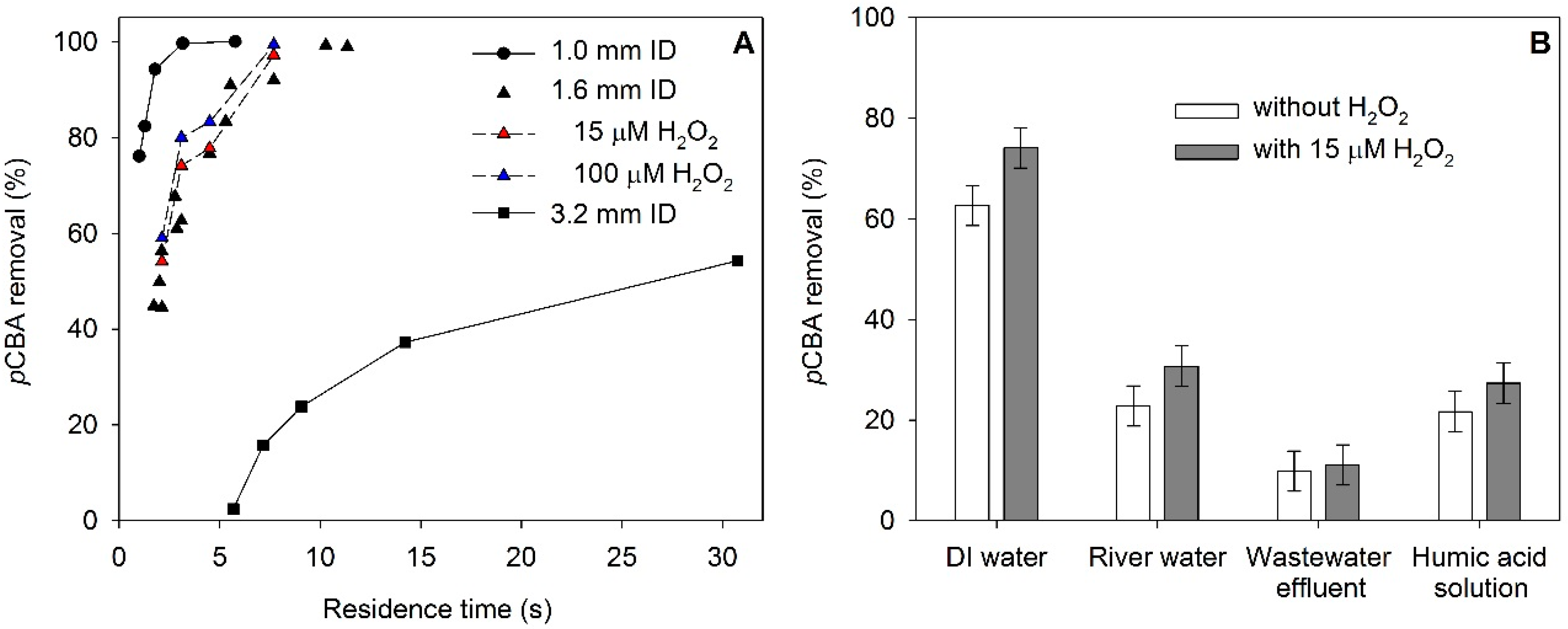
| Material | Product Code | Supplier | OD (inch) | ID (inch) | ID (mm) | Wall Thickness (mm) |
|---|---|---|---|---|---|---|
| Silastic® (PDMS) | WZ-96115-22 | Cole Parmer | 1/4 | 1/8 | 3.2 | 1.6 |
| Silastic® (PDMS) | WZ-96155-00 | Cole Parmer | 1/8 | 1/16 | 1.6 | 0.8 |
| Silastic® (PDMS) | WZ-96115-08 | Cole Parmer | 1/12 | 1/25 | 1.0 | 0.6 |
| Property | Wastewater Effluent (March 2018) | River Water I (March 2018) | River Water II (July 2017) |
|---|---|---|---|
| pH | 7.9 | 7.2 | 8.2 |
| TOC (mg/L) | 10.2 | 7.2 | 4.3 |
| UV254 (cm−1) | 0.14 | 0.20 | 0.10 |
| Alkalinity (mg CaCO3/L) | 181 | 236 | n/a 1 |
| Nitrate (mg/L) | 31 | 22 | n/a |
| Property | Units | Value | Experimental Uncertainty (±) | Reference |
|---|---|---|---|---|
| α | m−1 | 4000 (1.0 mm ID) 2500 (1.6 mm ID) 1250 (3.2 mm ID) | - | calculated |
| L | m | 0.2 | - | contactor length |
| uL | m/s | 0.002–0.224 | 0.002 | calculated |
| H | - | 0.248 | - | [41] |
| S | - | 0.881 | - | [42] |
| Cg | mol/m3 | 2.1–2.5 | 0.2 | measured |
| CL,out | mol/m3 | 0.014–0.186 | 0.004 | measured (experimental) or calculated (modelled) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoumpouli, G.A.; Baker, R.; Taylor, C.M.; Chippendale, M.J.; Smithers, C.; Ho, S.S.X.; Mattia, D.; Chew, Y.M.J.; Wenk, J. A Single Tube Contactor for Testing Membrane Ozonation. Water 2018, 10, 1416. https://doi.org/10.3390/w10101416
Zoumpouli GA, Baker R, Taylor CM, Chippendale MJ, Smithers C, Ho SSX, Mattia D, Chew YMJ, Wenk J. A Single Tube Contactor for Testing Membrane Ozonation. Water. 2018; 10(10):1416. https://doi.org/10.3390/w10101416
Chicago/Turabian StyleZoumpouli, Garyfalia A., Robert Baker, Caitlin M. Taylor, Matthew J. Chippendale, Chloë Smithers, Sean S. X. Ho, Davide Mattia, Y. M. John Chew, and Jannis Wenk. 2018. "A Single Tube Contactor for Testing Membrane Ozonation" Water 10, no. 10: 1416. https://doi.org/10.3390/w10101416
APA StyleZoumpouli, G. A., Baker, R., Taylor, C. M., Chippendale, M. J., Smithers, C., Ho, S. S. X., Mattia, D., Chew, Y. M. J., & Wenk, J. (2018). A Single Tube Contactor for Testing Membrane Ozonation. Water, 10(10), 1416. https://doi.org/10.3390/w10101416







