Study on the Inactivation of Pseudomonas sp and the Degradation of Trichloroethylene by Fenton-Like Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Goethite
2.2. TCE Degradation Experiments by Fenton-Like Reaction
2.3. Microbiology Experiments
2.3.1. Microbial Enrichment Culture
2.3.2. Bacterial Counting Method
2.3.3. Sterilization Experiments by Fenton-Like Reaction
2.4. The Fenton-Like and Chlorine Combined Disinfection Method
2.5. Determination Method of H2O2 Concentration
- CH2O2 = 0.025VKMnO4
- CH2O2—the concentration of H2O2, mol/L
- VKMnO4—used KMnO4 volume, mL
2.6. Characterization
3. Results and Discussions
3.1. Characterization of the Actual Growth Ring And Goethite
3.1.1. Composition Analysis of the Growth Ring of WDN
3.1.2. Morphology Analysis of the Prepared Goethite
3.1.3. Composition Analysis of the Prepared Goethite
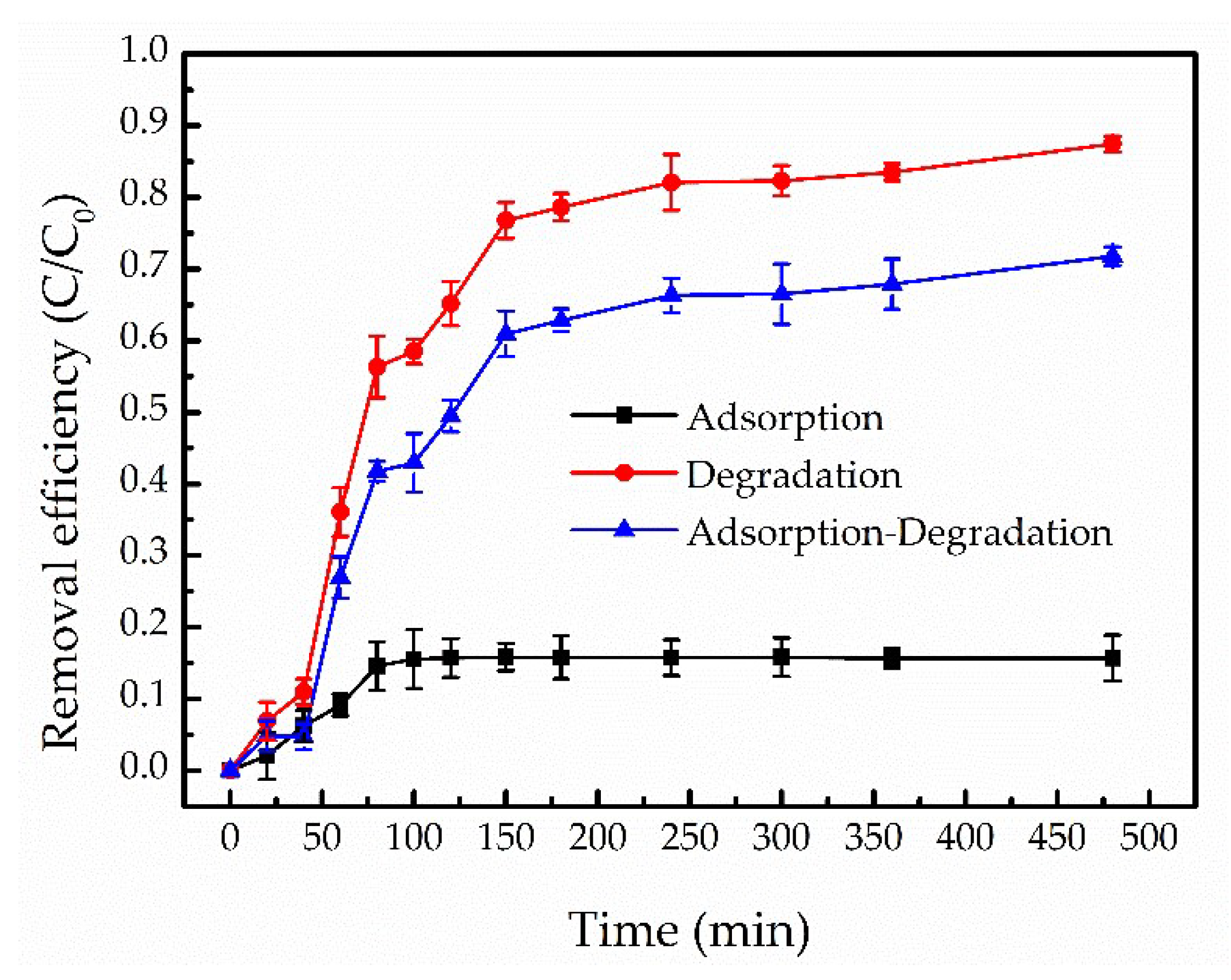
3.2. TCE Degradation by Fenton-Like Reaction
3.2.1. The Contrast of Removal Efficiency of TCE by Adsorption and Degradation
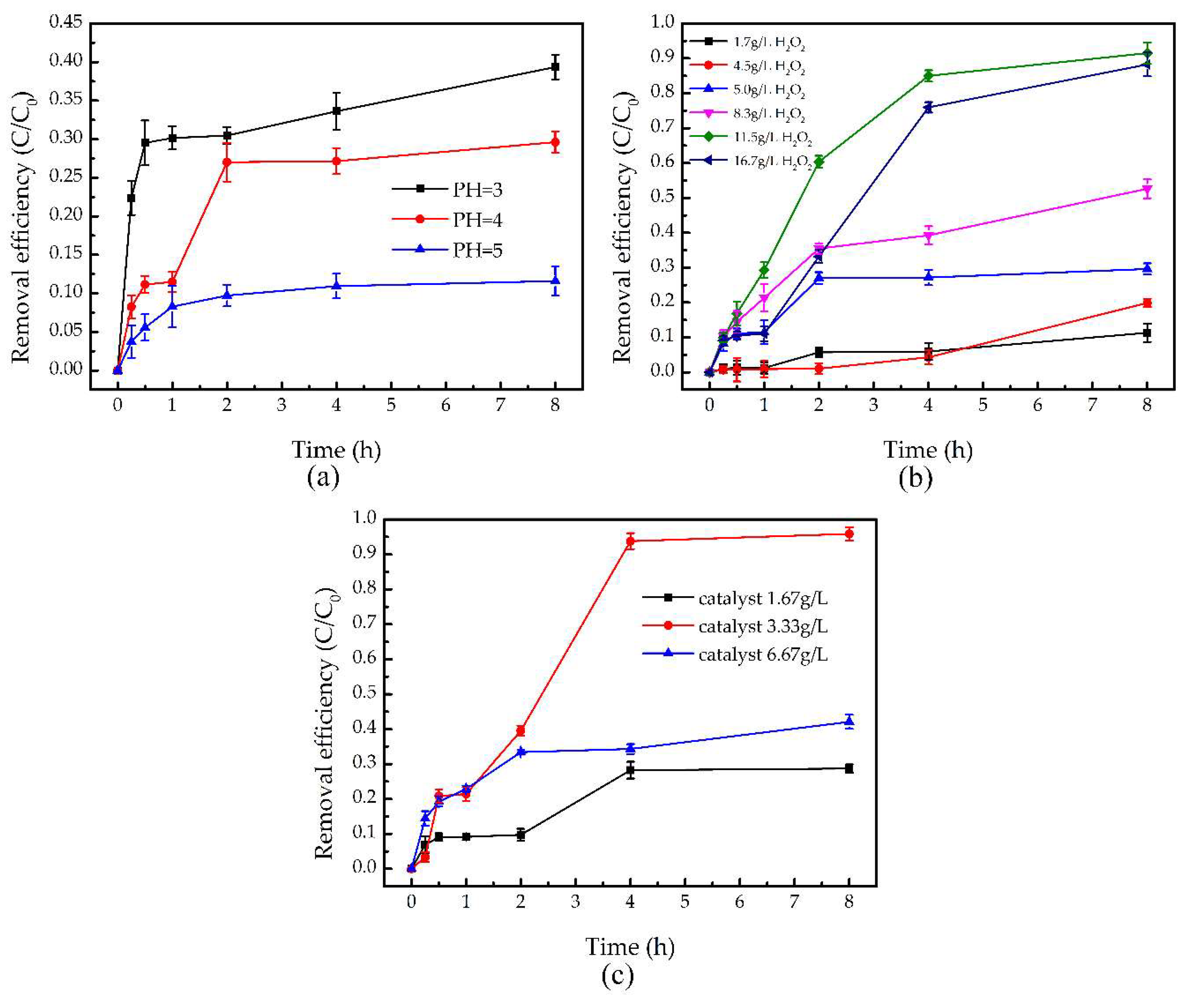
3.2.2. TCE Degradation by Fenton-Like Reaction under Different Conditions
- 1.
- Effect of the initial pH
- 2.
- Effect of the H2O2 concentration
- 3.
- Effect of the catalyst dosage
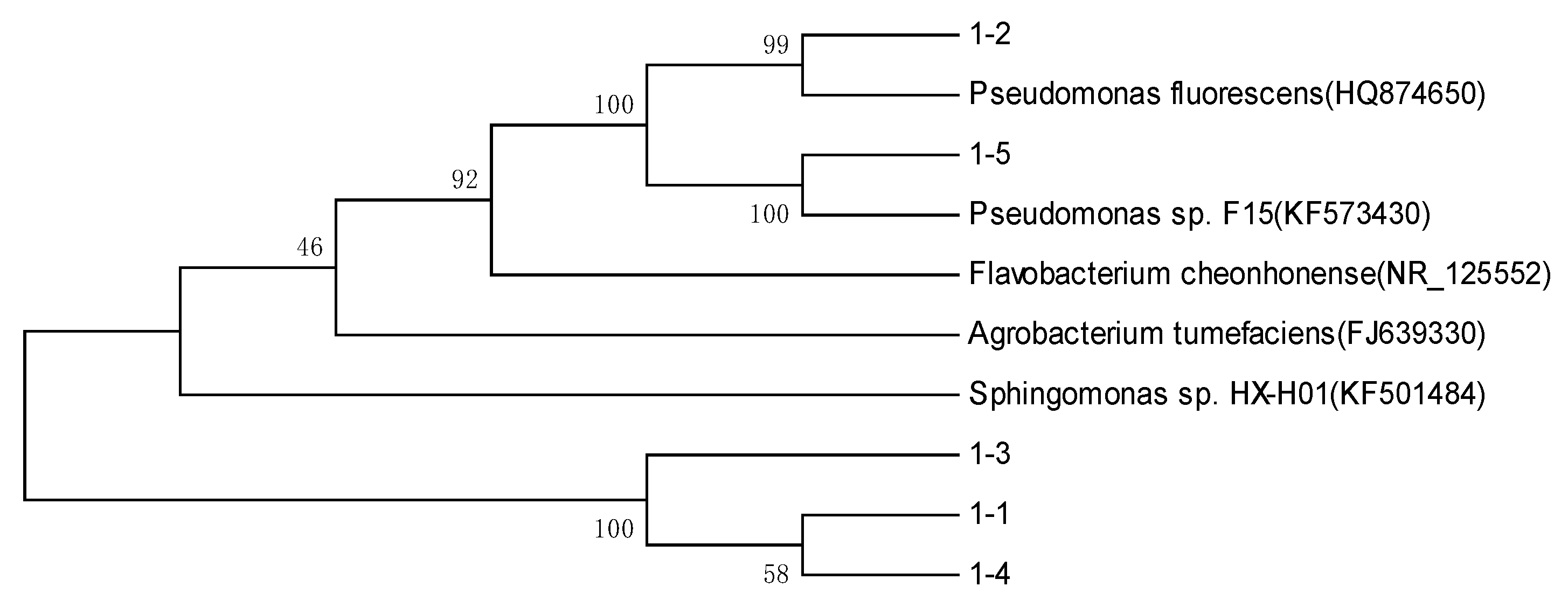
3.3. The Microorganism Identification Results of Filtered Water in a Water Plant
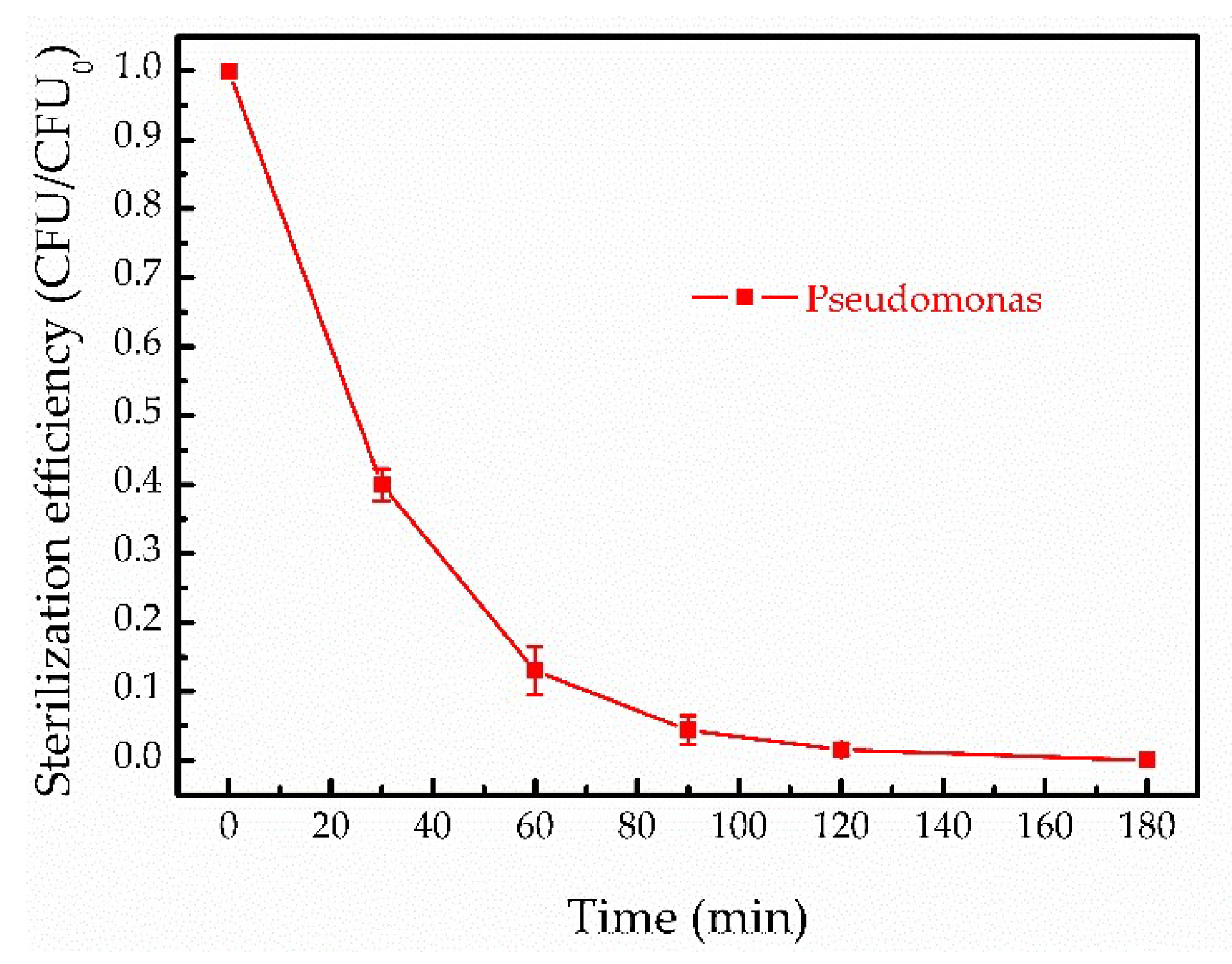
3.4. Inactivated Pseudomonas by Fenton-Like Reaction
- 1.
- Effect of the catalyst dosage on Pseudomonas inactivated
- 2.
- Effect of H2O2 concentration on Pseudomonas inactivated
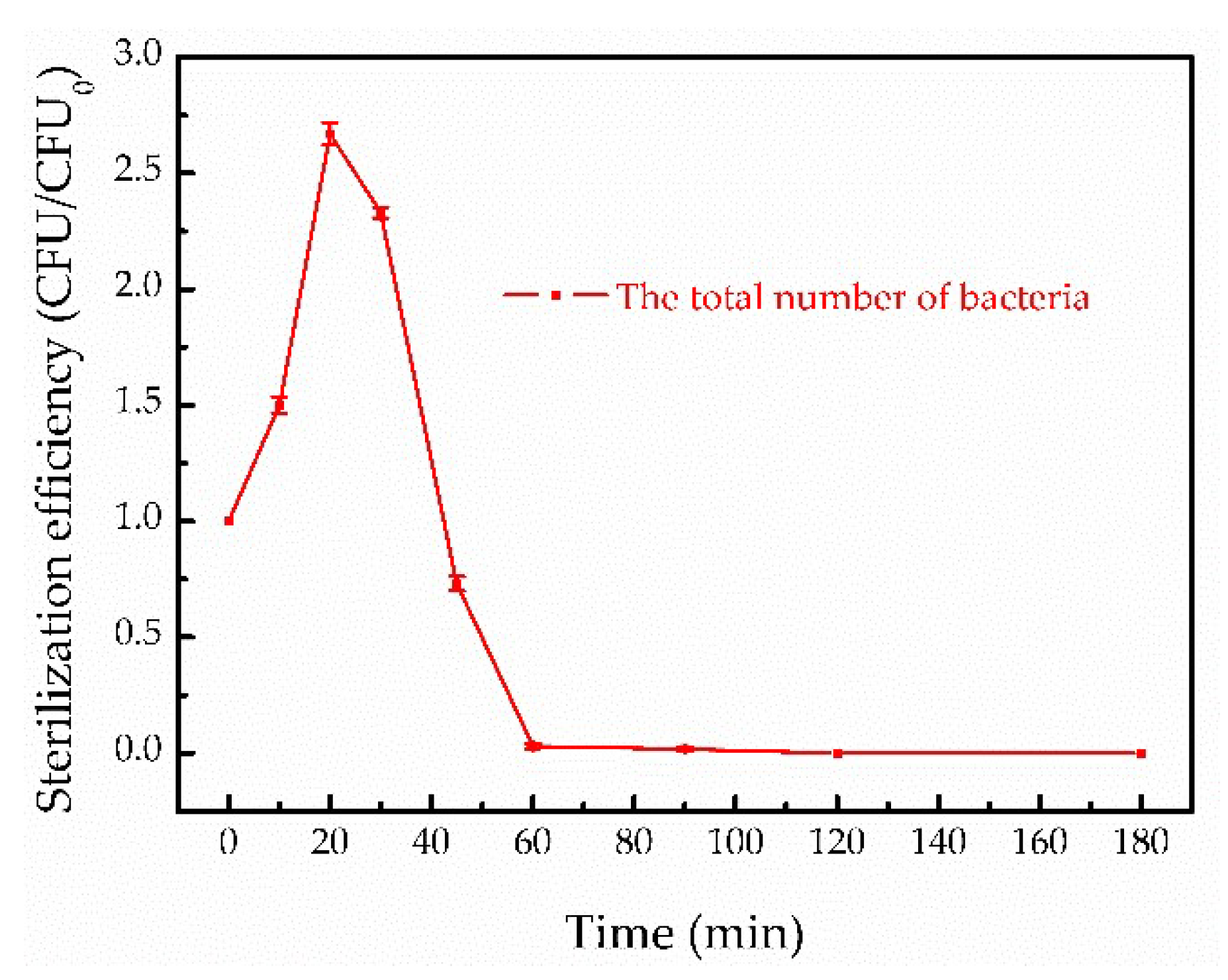
3.5. Study on Bacteria Inactivation in Filtered Water by Fenton-Like Method
3.6. Fenton-Like and Chlorine Combined Disinfection Method
3.7. The Disinfection Mechanism of Fenton-Like Reaction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- El-Chakhtoura, J.; Prest, E.; Saikaly, P.; van Loosdrecht, M.; Hammes, F.; Vrouwenvelder, H. Dynamics of bacterial communities before and after distribution in a full-scale drinking water network. Water Res. 2015, 74, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, F.; Madeira, L.M.; Juhna, T.; Block, J.C. Drinking water and biofilm disinfection by Fenton-like reaction. Water Res. 2013, 47, 5631–5638. [Google Scholar] [CrossRef] [PubMed]
- Postigo, C.; Emiliano, P.; Barceló, D.; Valero, F. Chemical characterization and relative toxicity assessment of disinfection byproduct mixtures in a large drinking water supply network. J. Hazard. Mater. 2018, 359, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Gopal, K.; Tripathy, S.S.; Bersillon, J.L.; Dubey, S.P. Chlorination byproducts, their toxicodynamics and removal from drinking water. J. Hazard. Mater. 2007, 140, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, I.J.H.G.; Boxall, D.J.B. Discolouration in potable water distribution systems: A review. Water Res. 2007, 41, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-S.; Tsai, W.-C.; Chou, C.-C. Inactivation and removal of Bacillus cereus by sanitizer and detergent. Int. J. Food Microbiol. 2002, 77, 11–18. [Google Scholar] [CrossRef]
- Wang, K.-H.; Tsai, H.-H.; Hsieh, Y.-H. The kinetics of photocatalytic degradation of trichloroethylene in gas phase over TiO2 supported on glass bead. Appl. Catal. B Environ. 1998, 17, 313–320. [Google Scholar] [CrossRef]
- Martínez Vargas, D.X.; Rivera De la Rosa, J.; Lucio-Ortiz, C.J.; Hernández-Ramirez, A.; Flores-Escamilla, G.A.; Garcia, C.D. Photocatalytic degradation of trichloroethylene in a continuous annular reactor using Cu-doped TiO2 catalysts by sol–gel synthesis. Appl. Catal. B Environ. 2015, 179, 249–261. [Google Scholar] [CrossRef]
- Meyer, C.I.; Borgna, A.; Monzón, A.; Garetto, T.F. Kinetic study of trichloroethylene combustion on exchanged zeolites catalysts. J. Hazard. Mater. 2011, 190, 903–908. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Su, Y.; Kong, J. Characterization of trichloroethylene adsorption onto waste biocover soil in the presence of landfill gas. J. Hazard. Mater. 2015, 295, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, L.; Xi, B.; Zhang, J.; Mao, X. Chemical oxidation of benzene and trichloroethylene by a combination of peroxymonosulfate and permanganate linked by in-situ generated colloidal/amorphous MnO2. Chem. Eng. J. 2017, 313, 815–825. [Google Scholar] [CrossRef]
- Xie, F.; Lu, Q.; de Toledo, R.A.; Shim, H. Enhanced simultaneous removal of MTBE and TCE mixture by Paracoccus sp. immobilized on waste silica gel. Int. Biodeterior. Biodegrad. 2016, 114, 222–227. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Wastewater treatment by means of advanced oxidation processes based on cavitation—A review. Chem. Eng. J. 2018, 338, 599–627. [Google Scholar] [CrossRef]
- Fernandes, A.; Makoś, P.; Boczkaj, G. Treatment of bitumen post oxidative effluents by sulfate radicals based advanced oxidation processes (S-AOPs) under alkaline pH conditions. J. Clean. Prod. 2018, 195, 374–384. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Sayed, M.; Khan, Z.U.H.; Rizwan, A.D.; Muhammad, N.; Boczkaj, G.; Murtaza, B.; Imran, M.; Khan, H.M.; et al. Solar light driven degradation of norfloxacin using as-synthesized Bi3+ and Fe2+ Co-doped ZnO with the addition of HSO5−: Toxicities and degradation pathways investigation. Chem. Eng. J. 2018, 351, 841–855. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, L.; Bao, C.; Ma, J. A facile approach to the fabrication of rattle-type magnetic carbon nanospheres for removal of methylene blue in water. Carbon 2015, 89, 378–391. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Phan, N.H.; Do, M.H.; Ngo, K.T. Magnetic Fe2MO4 (M:Fe, Mn) activated carbons: Fabrication, characterization and heterogeneous Fenton oxidation of methyl orange. J. Hazard. Mater. 2011, 185, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Santos, M.S.F.; Maldonado-Hódar, F.J.; Schaule, G.; Alves, A.; Madeira, L.M. Use of pipe deposits from water networks as novel catalysts in paraquat peroxidation. Chem. Eng. J. 2012, 210, 339–349. [Google Scholar] [CrossRef]
- Zeng, L. A method for preparing silica-containing iron(III) oxide adsorbents for arsenic removal. Water Res. 2003, 37, 4351–4358. [Google Scholar] [CrossRef]
- Wuhan University. Analytical Chemistry; Higher Education Press: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Cosultchi, A.; Rossbach, P.; Hernandez-Calderon, I. XPS analysis of petroleum well tubing adherence. Surf. Interface Anal. 2003, 35, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Fang, T. Study on Adsorption and Degradation Efficiency of PCE Based on the Growth Ring of Water Suppiy Network. Masters’ Thesis, Harbin Institute of Technology, Harbin, China, 2017. (In Chinese). [Google Scholar]
- Ji, F.; Li, C.; Zhang, J.; Deng, L. Efficient decolorization of dye pollutants with LiFe(WO4)2 as a reusable heterogeneous Fenton-like catalyst. Desalination 2011, 269, 284–290. [Google Scholar] [CrossRef]
- Zhou, L.; Shao, Y.; Liu, J.; Ye, Z.; Zhang, H.; Ma, J.; Jia, Y.; Gao, W.; Li, Y. Preparation and characterization of magnetic porous carbon microspheres for removal of methylene blue by a heterogeneous Fenton reaction. ACS Appl. Mater. Interfaces 2014, 6, 7275–7285. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J. Magnetic Nanoscaled Fe3O4/CeO2 Composite as an Efficient Fenton-Like Heterogeneous Catalyst for Degradation of 4-Chlorophenol. Environ. Sci. Technol. 2012, 46, 10145–10153. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhu, L.; Wang, N.; Tang, H.; Cao, M.; She, Y. Efficient Removal of Organic Pollutants with Magnetic Nanoscaled BiFeO3 as a Reusable Heterogeneous Fenton-Like Catalyst. Environ. Sci. Technol. 2010, 44, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Li, Y.; Ji, J.; Wong, F.-S.; Lu, X. Oxidation of 4-chlorophenol in a heterogeneous zero valent iron/H2O2 Fenton-like system: Kinetic, pathway and effect factors. Sep. Purif. Technol. 2008, 62, 551–558. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. A heterogeneous Fenton-like system with nanoparticulate zero-valent iron for removal of 4-chloro-3-methyl phenol. J. Hazard. Mater. 2011, 186, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, S.; Rosado, D.; Moreno-Andrés, J.; Cartuche, L.; Cruz, D.; Acevedo-Merino, A.; Nebot, E. Inactivation of a wild isolated Klebsiella pneumoniae by photo-chemical processes: UV-C, UV-C/H2O2 and UV-C/H2O2/Fe3+. Catal. Today 2018, 313, 94–99. [Google Scholar] [CrossRef]
- Giannakis, S.; Le, T.-T.M.; Entenza, J.M.; Pulgarin, C. Solar photo-Fenton disinfection of 11 antibiotic-resistant bacteria (ARB) and elimination of representative AR genes. Evidence that antibiotic resistance does not imply resistance to oxidative treatment. Water Res. 2018, 143, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Polo López, M.I.; Spuhler, D.; Sánchez Pérez, J.A.; Fernández Ibáñez, P.; Pulgarin, C. Solar disinfection is an augmentable, in situ-generated photo-Fenton reaction—Part 1: A review of the mechanisms and the fundamental aspects of the process. Appl. Catal. B Environ. 2016, 199, 199–223. [Google Scholar] [CrossRef]




| Analytical Element | Concentration (%) | Analytical Compound | Concentration (%) |
|---|---|---|---|
| O | 29.320 | - | - |
| Cl | 0.042 | - | - |
| Na | 0.138 | Na2O | 0.212 |
| Mg | 0.121 | MgO | 0.231 |
| Al | 0.224 | Al2O3 | 0.491 |
| Si | 1.211 | SiO2 | 3.018 |
| P | 0.060 | P2O5 | 0.163 |
| S | 0.028 | SO3 | 0.083 |
| K | 0.031 | K2O | 0.044 |
| Ca | 0.212 | CaO | 0.358 |
| Ti | 0.057 | TiO2 | 0.116 |
| Mn | 0.091 | MnO | 0.151 |
| Fe | 24.021 | Fe2O3 | 45.365 |
| Zn | 0.058 | ZnO | 0.103 |
| Sum | 55.614 | Sum | 50.335 |
| Samples | Specific Surface Area | Pore Volume | Pore Size |
|---|---|---|---|
| The growth ring | 397.06 m2/g | 0.51 cm³/g | 44.52 Å |
| Goethite | 146.85 m2/g | 0.15 cm³/g | 35.38 Å |
| No. | Sequence Length/bp | Related Bacteria Name | Related Strain Number | Similarity/% |
|---|---|---|---|---|
| 1-1 | 1403 | Flavobacterium cheonhonense | NR_125552 | 98 |
| 1-2 | 1438 | Pseudomonas fluorescens | HQ874650 | 98 |
| 1-3 | 1452 | Sphingomonas sp. HX-H01 | KF501484 | 99 |
| 1-4 | 1418 | Agrobacterium tumefaciens | FJ639330 | 99 |
| 1-5 | 1459 | Pseudomonas sp. F15 | KF573430 | 99 |
| No. | Phylum | Class | Order | Family | Genus |
|---|---|---|---|---|---|
| 1-2 | Proteobacteria | γ-proteobacteria | Pseudomonas | Pseudomonadaceae | Pseudomonas |
| 1-5 | Proteobacteria | γ-proteobacteria | Pseudomonas | Pseudomonadaceae | Pseudomonas |
| Temperature (K) | Turbidity (NTU) | pH | NH4+ (mg/L) | Cl− (mg/L) | SO42− (mg/L) | TOC (mg/L) | Total Iron (mg/L) | Total Alkalinity (CaCO3, mg/L) |
|---|---|---|---|---|---|---|---|---|
| 302 | 0.51 | 6.83 | 0.17 | 8.36 | 3.49 | 2.12 | 0.16 | 22.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, D.; He, F.; Ma, W.; Yuan, Y.; Wang, Z. Study on the Inactivation of Pseudomonas sp and the Degradation of Trichloroethylene by Fenton-Like Reaction. Water 2018, 10, 1376. https://doi.org/10.3390/w10101376
Zhong D, He F, Ma W, Yuan Y, Wang Z. Study on the Inactivation of Pseudomonas sp and the Degradation of Trichloroethylene by Fenton-Like Reaction. Water. 2018; 10(10):1376. https://doi.org/10.3390/w10101376
Chicago/Turabian StyleZhong, Dan, Fu He, Wencheng Ma, Yixing Yuan, and Ziqiang Wang. 2018. "Study on the Inactivation of Pseudomonas sp and the Degradation of Trichloroethylene by Fenton-Like Reaction" Water 10, no. 10: 1376. https://doi.org/10.3390/w10101376
APA StyleZhong, D., He, F., Ma, W., Yuan, Y., & Wang, Z. (2018). Study on the Inactivation of Pseudomonas sp and the Degradation of Trichloroethylene by Fenton-Like Reaction. Water, 10(10), 1376. https://doi.org/10.3390/w10101376







