Design and Season Influence Nitrogen Dynamics in Two Surface Flow Constructed Wetlands Treating Nursery Irrigation Runoff
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.1.1. Constructed Wetland 1
2.1.2. Constructed Wetland 2
2.1.3. Hydraulic Loading into CW1 and CW2
2.2. Water Quality Monitoring
2.3. Statistical Analyses
3. Results
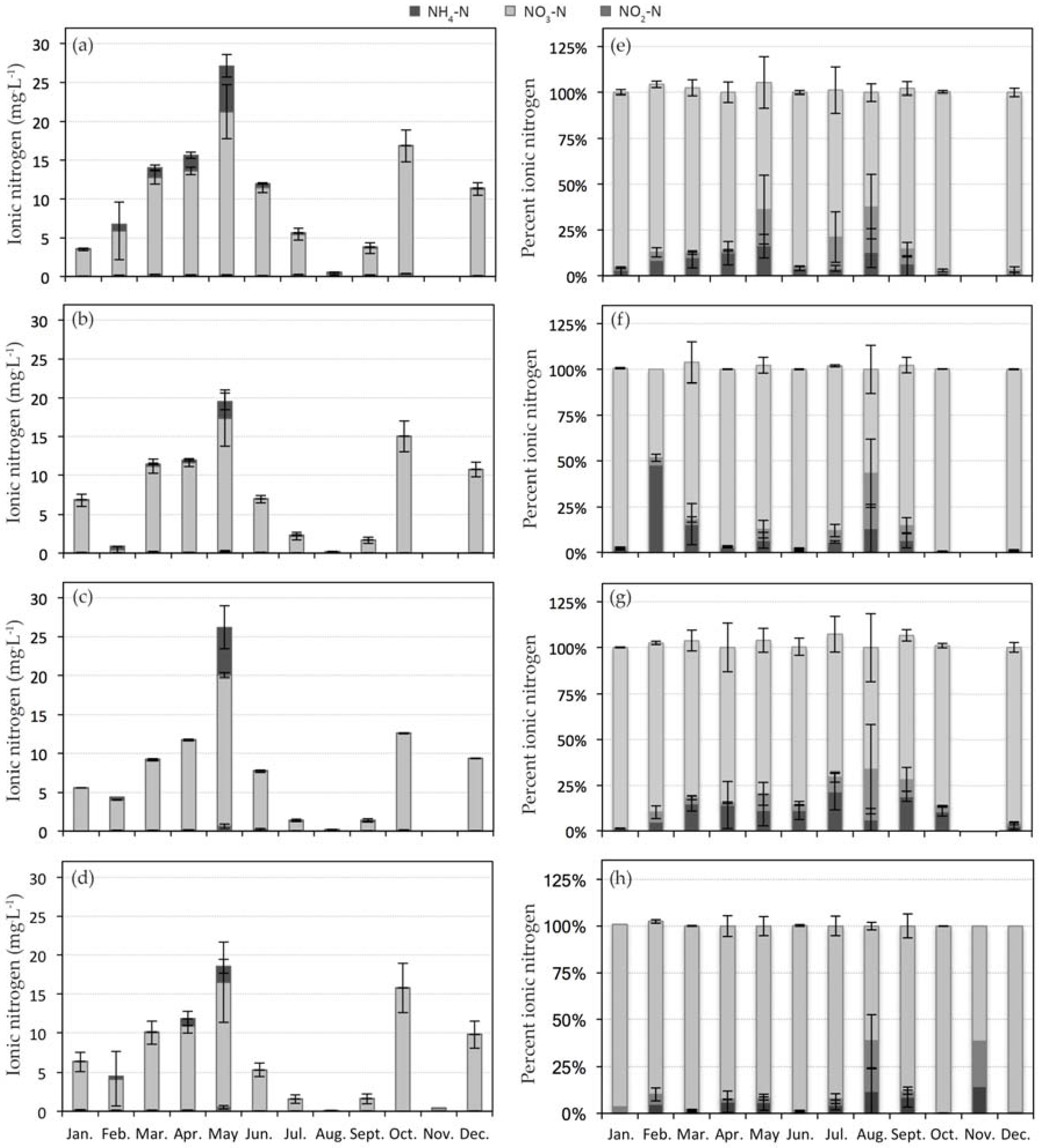
3.1. Nitrogen Dynamics in CW1
3.2. Nitrogen Dynamics in CW2
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Headley, T.R.; Dirou, J.; Huett, D.; Stovold, G.; Davison, L. Reed beds for the remediation and recycling of nursery runoff water. Australas. J. Environ. Manag. 2005, 12, 27–36. [Google Scholar] [CrossRef]
- White, S.A. Wetland technologies for nursery and greenhouse compliance with nutrient regulations. HortScience 2013, 48, 1103–1108. [Google Scholar]
- Majsztrik, J.C.; Fernandez, R.T.; Fisher, P.R.; Hitchcock, D.R.; Lea-Cox, J.; Owen, J.S., Jr.; Oki, L.R.; White, S.A. Water use and treatment in container-grown specialty crop production: A review. Water Air Soil Pollut. 2017, 228, 151. [Google Scholar] [CrossRef] [PubMed]
- Sample, D.J.; Grizzard, T.J.; Sansalone, J.; Davis, A.P.; Roseen, R.M.; Walker, J. Assessing performance of manufactured treatment devices for the removal of phosphorus from urban stormwater. J. Environ. Manag. 2012, 113, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Scholes, L.; Revitt, D.M.; Ellis, J.B. A systematic approach for the comparative assessment of stormwater pollutant removal potentials. J. Environ. Manag. 2008, 88, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Somes, N.L.G.; Fabian, J.; Wong, T.H.F. Tracking pollutant detention in constructed stormwater wetlands. Urban Water 2000, 2, 29–37. [Google Scholar] [CrossRef]
- Wittgren, H.B.; Tobiason, S. Nitrogen removal from pretreated wastewater in surface flow wetlands. Water Sci. Technol. 1995, 32, 69–78. [Google Scholar]
- Billmayer, J.J. Wastewater Technology Fact Sheet, Free Water Surface Wetlands; United States Environmental Protection Agency: Washington, DC, USA, 2000; pp. 1–8.
- García-Lledó, A.; Ruiz-Rueda, O.; Vilar-Sanz, A.; Sala, L.; Bañeras, L. Nitrogen removal efficiencies in a free water surface constructed wetland in relation to plant coverage. Ecol. Eng. 2011, 37, 678–684. [Google Scholar] [CrossRef]
- Calheiros, C.S.C.; Rangel, A.O.S.S.; Castro, P.M.L. Constructed wetland systems vegetated with different plants applied to the treatment of tannery wastewater. Water Res. 2007, 41, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Constructed wetlands for water quality regulation. In The Wetland Book; Springer: Dordrecht, The Netherlands, 2016; pp. 1–8. [Google Scholar]
- Arheimer, B.; Wittgren, H.B. Modelling nitrogen removal in potential wetlands at the catchment scale. Ecol. Eng. 2002, 19, 63–80. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009; p. 366. [Google Scholar]
- White, S.A.; Cousins, M.M. Floating treatment wetland aided remediation of nitrogen and phosphorus from simulated stormwater runoff. Ecol. Eng. 2013, 61, 207–215. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Tanner, C.C.; Hally, V.M.; Gibbs, M.M. Nitrogen spiraling in subsurface-flow constructed wetlands: Implications for treatment response. Ecol. Eng. 2005, 25, 365–381. [Google Scholar] [CrossRef]
- Ahn, C.; Mitsch, W.J. Scaling considerations of mesocosm wetlands in simulating large created freshwater marshes. Ecol. Eng. 2002, 18, 327–342. [Google Scholar] [CrossRef]
- White, S.A.; Taylor, M.D.; Albano, J.P.; Whitwell, T.; Klaine, S.J. Phosphorus retention in lab and field-scale subsurface-flow wetlands treating plant nursery runoff. Ecol. Eng. 2011, 37, 1968–1976. [Google Scholar] [CrossRef]
- Hernandez, M.E.; Mitsch, W.J. Denitrification potential and organic matter as affected by vegetation community, wetland age, and plant introduction in created wetlands. J. Environ. Qual. 2007, 36, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Bachand, P.A.M.; Horne, A.J. Denitrification in constructed free-water surface wetlands: I. Very high nitrate removal rates in a macrocosm study. Ecol. Eng. 2000, 14, 9–15. [Google Scholar] [CrossRef]
- Maine, M.A.; Hadad, H.R.; Sánchez, G.C.; Di Luca, G.A.; Mufarrege, M.M.; Caffaratti, S.E.; Pedro, M.C. Long-term performance of two free-water surface wetlands for metallurgical effluent treatment. Ecol. Eng. 2017, 98, 372–377. [Google Scholar] [CrossRef]
- Wu, S.; Kuschk, P.; Brix, H.; Vymazal, J.; Dong, R. Development of constructed wetlands in performance intensifications for wastewater treatment: A nitrogen and organic matter targeted review. Water Res. 2014, 57, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-G.; Fletcher, T.D.; Sun, G. Nitrogen removal in constructed wetland systems. Eng. Life Sci. 2009, 9, 11–22. [Google Scholar] [CrossRef]
- Charpentier, J.; Florentz, M.; David, G. Oxidation-reduction potential (orp) regulation: A way to optimize pollution removal and energy savings in the low load activated sludge process. Water Sci. Technol. 1987, 19, 645–655. [Google Scholar]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef] [PubMed]
- White, S.A.; Taylor, M.D.; Chandler, S.L.; Whitwell, T.; Klaine, S.J. Remediation of nitrogen and phosphorus from nursery runoff during the spring via free water surface constructed wetlands. J. Environ. Hortic. 2010, 28, 209–217. [Google Scholar]
- Taylor, M.D.; White, S.A.; Chandler, S.L.; Klaine, S.J.; Whitwell, T. Nutrient management of nursery runoff water using constructed wetland systems. HortTechnology 2006, 16, 610–614. [Google Scholar]
- Prenafeta-Boldú, F.X.; Trillas, I.; Vinas, M.; Guivernau, M.; Cáceres, R.; Marfa, O. Effectiveness of a full-scale horizontal slow sand filter for controlling phytopathogens in recirculating hydroponics: From microbial isolation to full microbiome assessment. Sci. Total Environ. 2017, 599–600, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Kadlec, R.H. The effects of deep zones on wetland nitrogen processing. Water Sci. Technol. 2007, 56, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.W. Management of freshwater marshes for wildlife. In Freshwater Wetlands: Ecological Processes and Management Potential; Good, R.E., Whingham, D.F., Simpson, R.L., Eds.; Academic Press: New York, NY, USA, 1978; pp. 267–284. [Google Scholar]
- Land, M.; Granéli, W.; Grimvall, A.; Hoffmann, C.C.; Mitsch, W.J.; Tonderski, K.S.; Verhoeven, J.T.A. How effective are created or restored freshwater wetlands for nitrogen and phosphorus removal? A systematic review. Environ. Evid. 2016, 5. [Google Scholar] [CrossRef]
- White, S.A.; Taylor, M.D.; Damrel, D.Z. Floral colonization of a free-water surface constructed wetland System in Grady County, Georgia. Castanea 2012, 77, 159–171. [Google Scholar] [CrossRef]
- USEPA. National Primary Drinking Water Regulations; Office of Ground Water and Drinking Water: Washington, DC, USA, 2009.
- Vymazal, J. Emergent plants used in free water surface constructed wetlands: A review. Ecol. Eng. 2013, 61, 582–592. [Google Scholar] [CrossRef]
- Bachand, P.A.M.; Horne, A.J. Denitrification in constructed free-water surface wetlands: II. Effects of vegetation and temperature. Ecol. Eng. 2000, 14, 17–32. [Google Scholar] [CrossRef]
- Hsueh, M.-L.; Yang, L.; Hsieh, L.-Y.; Lin, H.-J. Nitrogen removal along the treatment cells of a free-water surface constructed wetland in subtropical taiwan. Ecol. Eng. 2014, 73, 579–587. [Google Scholar] [CrossRef]
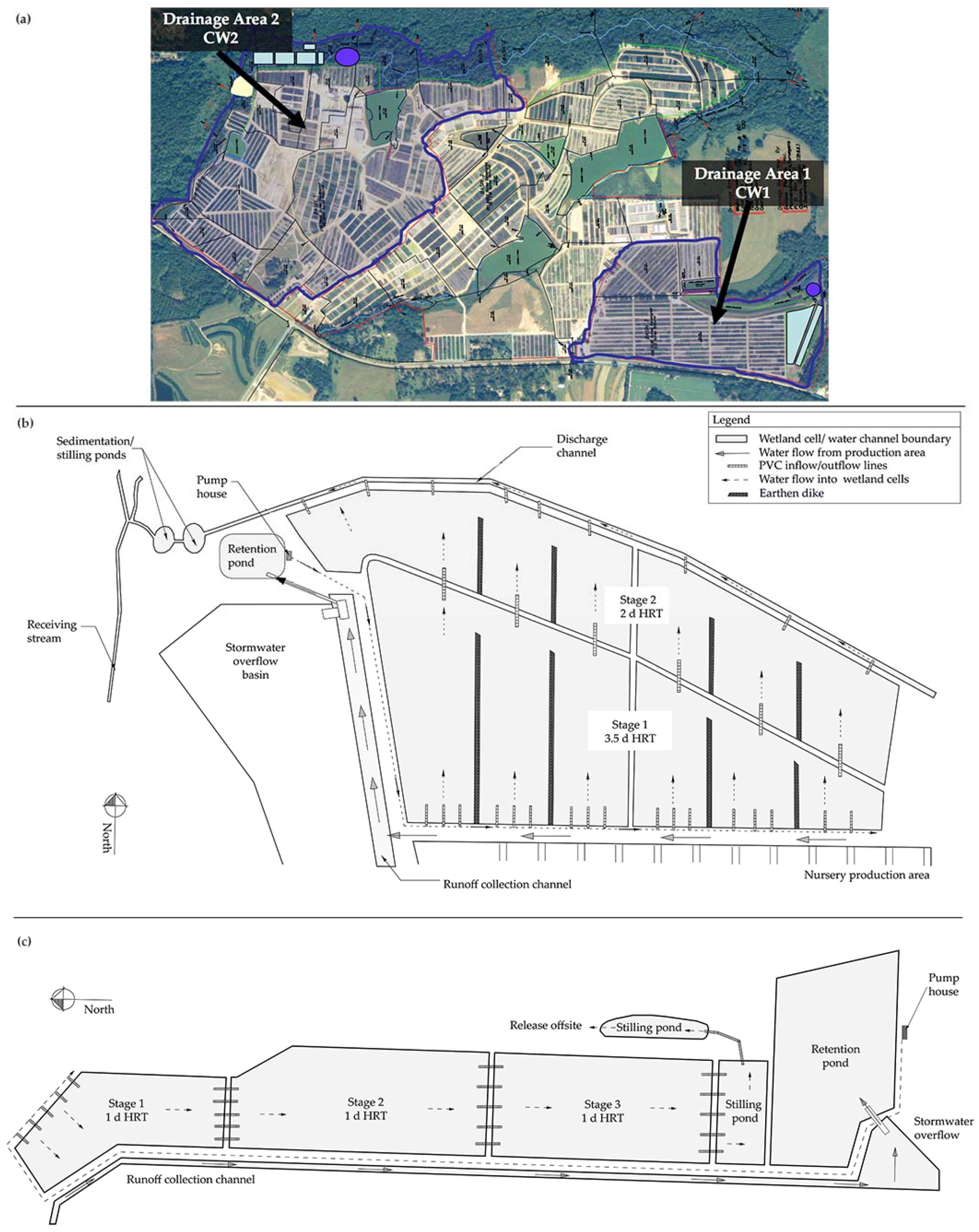
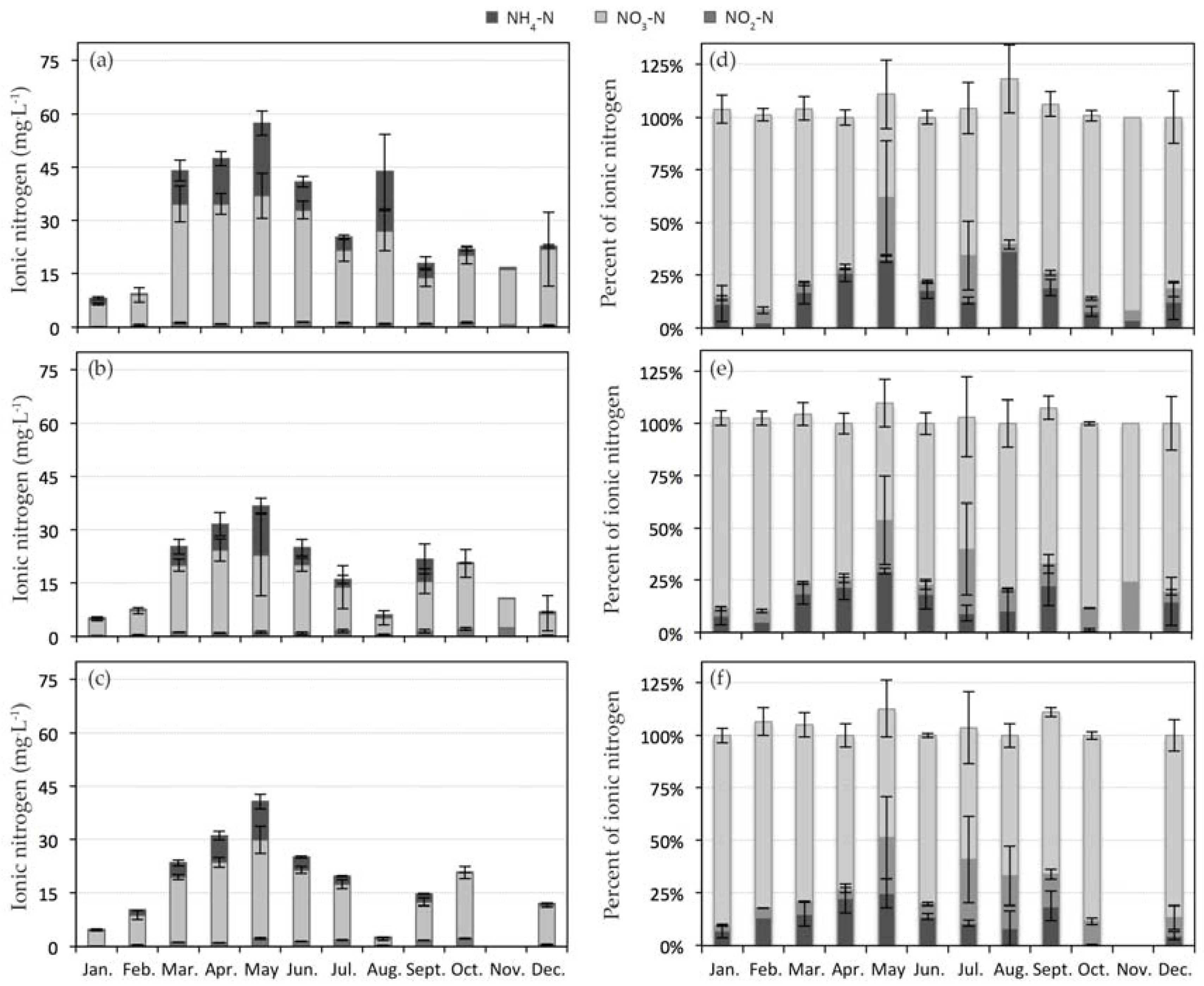
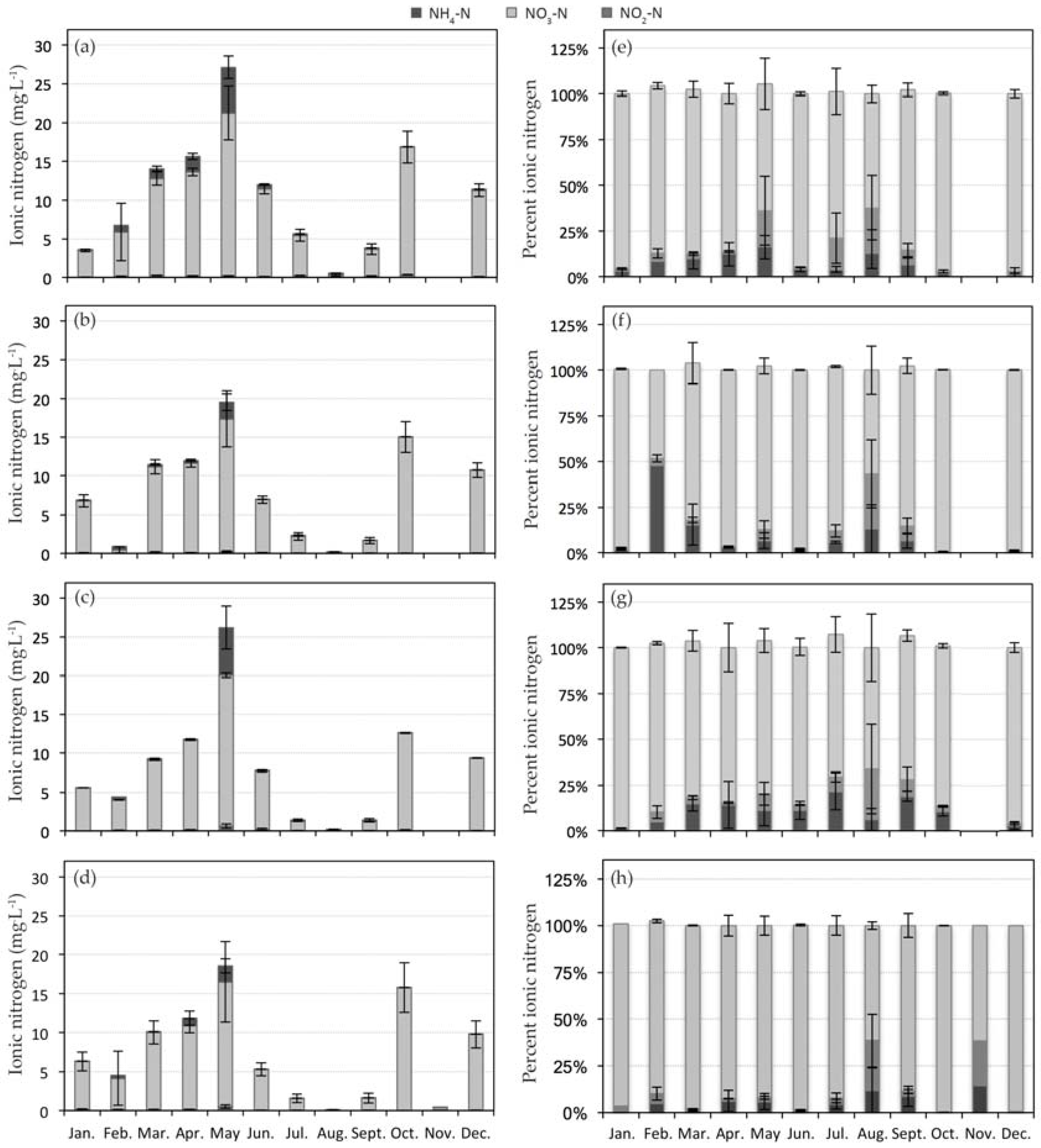
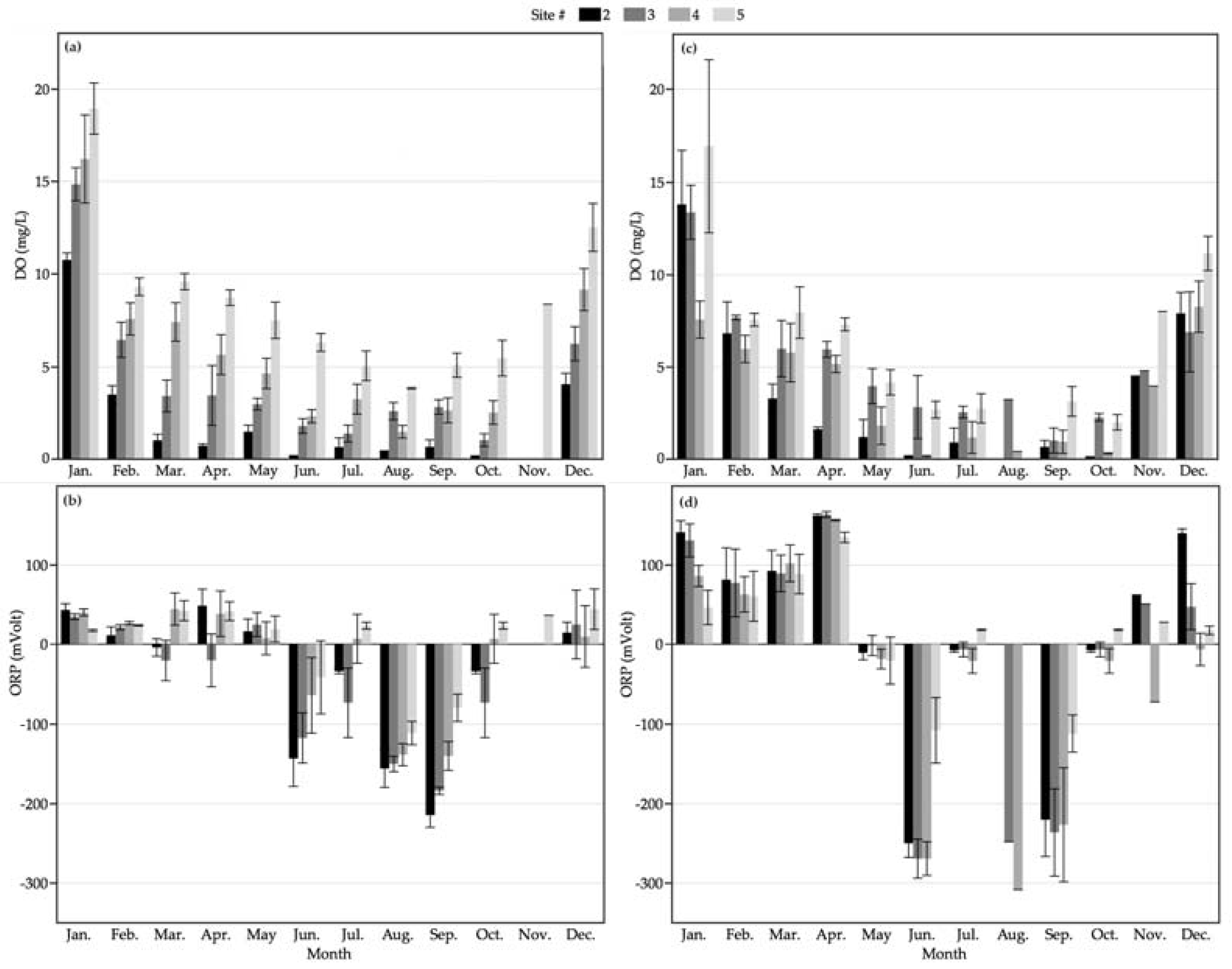


| Site Key | CW1 | Composite Sub-Sites | CW2 | Composite Sub-Sites |
|---|---|---|---|---|
| 1.1 | Inflow ditch/runoff channel | 4 | Inflow ditch/runoff channel | 2 |
| 1.2 | Retention pond | 1 | Retention pond | 1 |
| 1.3 | Inflow to CW1 | 5 | Inflow to CW2 | 3 |
| 2 | Outflow Stage 1 | 6 | Outflow Stage 1 | 3 |
| 3 | Outflow Stage 2 | 6 | Outflow Stage 2 | 3 |
| 4 | Discharge channel | 3 | Outflow Stage 3 | 3 |
| 5 | Stilling ponds | 3 | Stilling ponds | 2 |
| Site | Concentration of Ionic Nitrogen | Percent of Ionic Nitrogen | ||||||
|---|---|---|---|---|---|---|---|---|
| NO3-N (mg/L) | NO2-N (mg/L) | NH3-N (mg/L) | ionicN (mg/L) | % NO3-N | % NO2-N | % NH3-N | ||
| CW1 | 1.1 | 24.7 ± 1.58 | 1.06 ± 0.06 | 7.97 ± 1.06 | 32.7 ± 2.47 | 78.6 ± 1.4 | 5.4 ± 1.1 | 16.7 ± 1.3 |
| 1.2 | 14.0 ± 1.70 | 1.05 ± 0.13 | 4.05 ± 0.98 | 18.1 ± 2.45 | 77.4 ± 3.0 | 10.9 ± 3.0 | 12.1 ± 2.2 | |
| 1.3 | 16.1 ± 0.84 | 1.39 ± 0.07 | 3.51 ± 0.40 | 20.6 ± 1.20 | 77.5 ± 1.2 | 9.2 ± 1.0 | 13.7 ± 1.1 | |
| 2 | 10.1 ± 0.71 | 0.18 ± 0.11 | 1.15 ± 0.23 | 11.3 ± 0.88 | 86.4 ± 1.6 | 5.6 ± 1.1 | 8.3 ± 1.2 | |
| 3 | 8.07 ± 0.67 | 0.09 ± 0.01 | 0.35 ± 0.13 | 8.47 ± 0.75 | 88.8 ± 1.7 | 5.1 ± 1.1 | 6.3 ± 1.3 | |
| 4 | 7.73 ± 1.18 | 0.16 ± 0.05 | 0.80 ± 0.41 | 8.56 ± 1.50 | 87.6 ± 2.5 | 5.2 ± 1.5 | 7.5 ± 2.2 | |
| 5 | 7.52 ± 0.96 | 0.12 ± 0.03 | 0.36 ± 0.15 | 7.94 ± 1.06 | 88.9 ± 2.2 | 6.5 ± 1.6 | 4.8 ± 1.2 | |
| CW2 | 1.1 | 18.9 ± 2.84 | 0.81 ± 0.11 | 6.49 ± 1.73 | 25.0 ± 4.14 | 82.3 ± 2.8 | 6.7 ± 2.0 | 11.5 ± 2.4 |
| 1.2 | 10.3 ± 1.36 | 0.82 ± 0.13 | 2.50 ± 0.63 | 13.3 ± 1.97 | 80.4 ± 2.4 | 9.5 ± 2.1 | 10.5 ± 1.5 | |
| 1.3 | 10.6 ± 2.38 | 0.82 ± 0.16 | 1.46 ± 0.48 | 12.5 ± 2.65 | 80.4 ± 3.3 | 10.8 ± 3.2 | 9.1 ± 1.7 | |
| 2 | 7.28 ± 0.95 | 0.66 ± 0.09 | 1.32 ± 0.22 | 9.05 ± 1.18 | 69.8 ± 3.7 | 9.2 ± 1.4 | 22.1 ± 3.5 | |
| 3 | 5.73 ± 0.78 | 0.48 ± 0.09 | 0.84 ± 0.13 | 6.84 ± 0.94 | 64.2 ± 4.6 | 7.9 ± 1.2 | 29.2 ± 4.7 | |
| 4 | 3.94 ± 0.59 | 0.37 ± 0.08 | 0.52 ± 0.06 | 4.68 ± 0.67 | 62.1 ± 5.6 | 9.2 ± 1.7 | 30.1 ± 4.8 | |
| 5 | 3.92 ± 0.72 | 0.30 ± 0.09 | 0.38 ± 0.05 | 4.43 ± 0.78 | 66.6 ± 5.5 | 7.8 ± 1.8 | 26.9 ± 5.9 | |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, S.A. Design and Season Influence Nitrogen Dynamics in Two Surface Flow Constructed Wetlands Treating Nursery Irrigation Runoff. Water 2018, 10, 8. https://doi.org/10.3390/w10010008
White SA. Design and Season Influence Nitrogen Dynamics in Two Surface Flow Constructed Wetlands Treating Nursery Irrigation Runoff. Water. 2018; 10(1):8. https://doi.org/10.3390/w10010008
Chicago/Turabian StyleWhite, Sarah A. 2018. "Design and Season Influence Nitrogen Dynamics in Two Surface Flow Constructed Wetlands Treating Nursery Irrigation Runoff" Water 10, no. 1: 8. https://doi.org/10.3390/w10010008
APA StyleWhite, S. A. (2018). Design and Season Influence Nitrogen Dynamics in Two Surface Flow Constructed Wetlands Treating Nursery Irrigation Runoff. Water, 10(1), 8. https://doi.org/10.3390/w10010008





