Traffic-Related Particulate Matter and Cardiometabolic Syndrome: A Review
Abstract
:1. Introduction
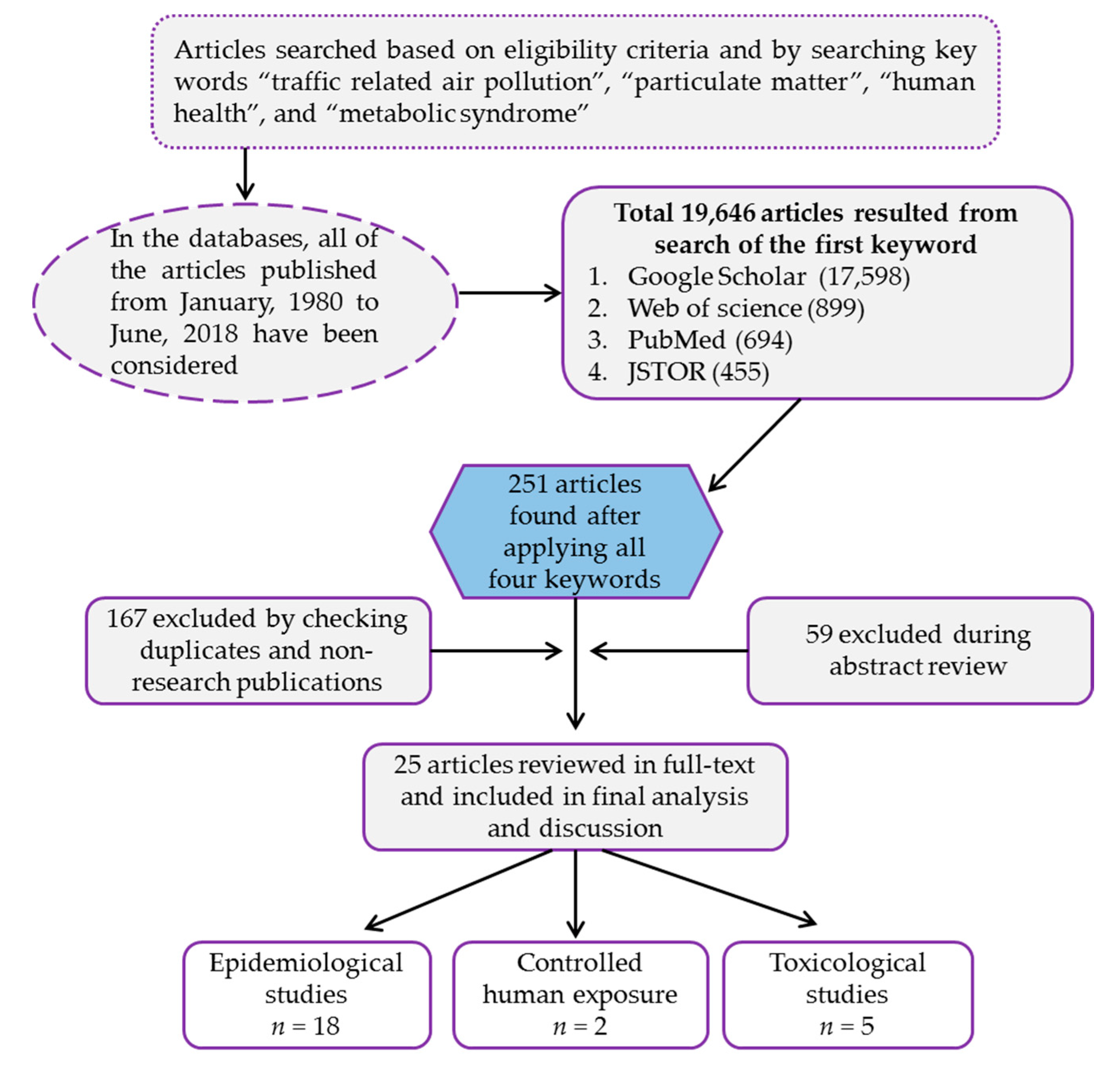
2. Methodology
3. Results
3.1. Search Results of Databases
3.2. Current Understanding of Cardiometabolic Syndrome Induced by Traffic-Related PM Exposure
3.2.1. Exposure Metrics
3.2.2. Measured Health Endpoints
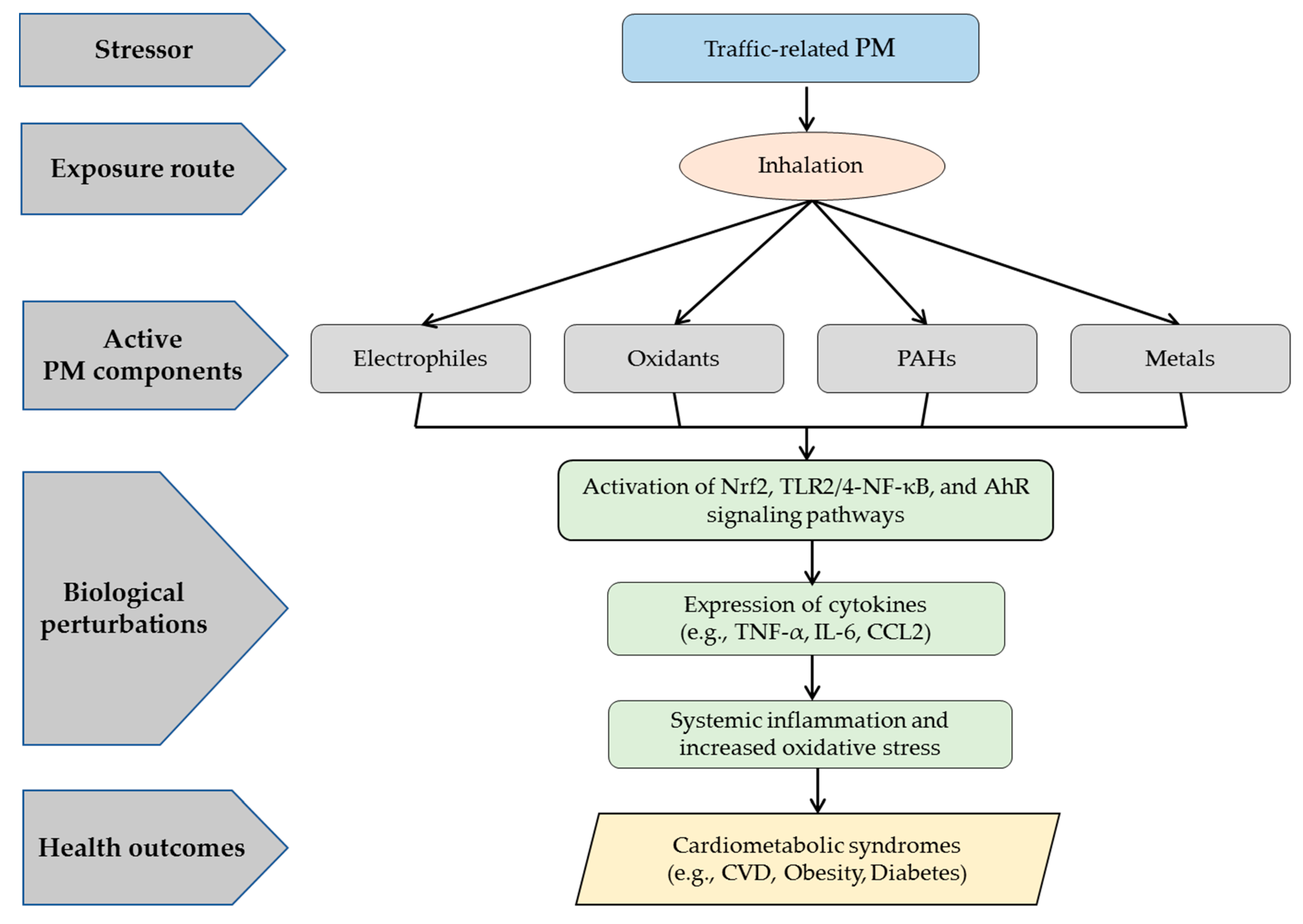
3.2.3. Active Components within Traffic-Related PM
3.2.4. Potential Underlying Mechanisms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brook, R.D.; Xu, X.H.; Bard, R.L.; Dvonch, J.T.; Morishita, M.; Kaciroti, N.; Sun, Q.H.; Harkema, J.; Rajagopalan, S. Reduced metabolic insulin sensitivity following sub-acute exposures to low levels of ambient fine particulate matter air pollution. Sci. Total Environ. 2013, 448, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.X.; Xu, X.H.; Chu, M.; Guo, Y.; Wang, J.H. Air particulate matter and cardiovascular disease: The epidemiological, biomedical and clinical evidence. J. Thoracic Dis. 2016, 8, E8–E19. [Google Scholar]
- Wei, Y.J.; Zhang, J.F.; Li, Z.G.; Gow, A.; Chung, K.F.; Hu, M.; Sun, Z.S.; Zeng, L.M.; Zhu, T.; Jia, G.; et al. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: Findings from a natural experiment in Beijing. FASEB J. 2016, 30, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak-Lasocka, J.; Lasocki, J.; Siekmeier, R.; Chlopek, Z.; Pokorski, M. Impact of traffic-related air pollution on health. In Environment Exposure to Pollutants; Springer: Cham, Switzerland, 2015; Volume 834, pp. 21–29. [Google Scholar]
- Libalova, H.; Rossner, P.; Vrbova, K.; Brzicova, T.; Sikorova, J.; Vojtisek-Lom, M.; Beranek, V.; Klema, J.; Ciganek, M.; Neca, J.; et al. Transcriptional response to organic compounds from diverse gasoline and biogasoline fuel emissions in human lung cells. Toxicol. In Vitro 2018, 48, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Batterman, S. Air pollution and health risks due to vehicle traffic. Sci. Total Environ. 2013, 450, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhu, Y.F.; Mu, L.N.; Zhang, Z.F.; Liu, S.J. Pulmonary diseases induced by ambient ultrafine and engineered nanoparticles in twenty-first century. Natl. Sci. Rev. 2016, 3, 416–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, B.A.; Brook, R.; Pope, C.A. Air pollution and cardiovascular disease. Curr. Prob. Cardiol. 2015, 40, 207–238. [Google Scholar] [CrossRef] [PubMed]
- Hoet, P.H.M.; Brüske-Hohlfeld, I.; Salata, O.V. Nanoparticles—Known and unknown health risks. J. Nanobiotechnol. 2004, 2, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO (World Health Organization). Ambient (Outdoor) Air Quality and Health; Fact Sheet; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Karagulian, F.; Belis, C.A.; Dora, C.F.C.; Prüss-Ustün, A.M.; Bonjour, S.; Adair-Rohani, H.; Amann, M. Contributions to cities’ ambient particulate matter (PM): A systematic review of local source contributions at global level. Atmos. Environ. 2015, 120, 475–483. [Google Scholar] [CrossRef]
- Karavalakis, G.; Short, D.; Vu, D.; Russell, R.; Hajbabaei, M.; Asa-Awuku, A.; Durbin, T.D. Evaluating the effects of aromatics content in gasoline on gaseous and particulate matter emissions from SI-PFI and SIDI vehicles. Environ. Sci. Technol. 2015, 49, 7021–7031. [Google Scholar] [CrossRef] [PubMed]
- Bisig, C.; Roth, M.; Müller, L.; Comte, P.; Heeb, N.; Mayer, A.; Czerwinski, J.; Petri-Fink, A.; Rothen-Rutishauser, B. Hazard identification of exhausts from gasoline-ethanol fuel blends using a multi-cellular human lung model. Environ. Res. 2016, 151, 789–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, M.; Usemann, J.; Bisig, C.; Comte, P.; Czerwinski, J.; Mayer, A.C.R.; Beier, K.; Rothen-Rutishauser, B.; Latzin, P.; Müller, L. Effects of gasoline and ethanol-gasoline exhaust exposure on human bronchial epithelial and natural killer cells in vitro. Toxicol. In Vitro 2017, 45, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Roth, P.; Durbin, T.D.; Johnson, K.C.; Cocker, D.R.; Asa-Awuku, A.; Brezny, R.; Geller, M.; Karavalakis, G. Gasoline particulate filters as an effective tool to reduce particulate and polycyclic aromatic hydrocarbon emissions from gasoline direct injection (GDI) vehicles: A case study with two GDI vehicles. Environ. Sci. Technol. 2018, 52, 3275–3284. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, Y.; Zhang, S.J.; Hu, J.N.; Zhang, K.M.; Li, Z.H.; He, L.Q.; Hao, J.M. Characterizing particulate polycyclic aromatic hydrocarbon emissions from diesel vehicles using a portable emissions measurement system. Sci. Rep. 2017, 7, 10058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverman, D.T.; Samanic, C.M.; Lubin, J.H.; Blair, A.E.; Stewart, P.A.; Vermeulen, R.; Coble, J.B.; Rothman, N.; Schleiff, P.L.; Travis, W.D.; et al. The diesel exhaust in miners study: A nested case-control study of lung cancer and diesel exhaust. J. Natl. Cancer Inst. 2012, 104, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Libalova, H.; Rossner, P.; Vrbova, K.; Brzicova, T.; Sikorova, J.; Vojtisek-Lom, M.; Beranek, V.; Klema, J.; Ciganek, M.; Neca, J.; et al. Comparative analysis of toxic responses of organic extracts from diesel and selected alternative fuels engine emissions in human lung BEAS-2B cells. Int. J. Mol. Sci. 2016, 17, 1833. [Google Scholar] [CrossRef] [PubMed]
- Storey, J.M.; Lewis, S.; Szybist, J.; Thomas, J.; Barone, T.; Eibl, M.; Nafziger, E.; Kaul, B. Novel characterization of GDI engine exhaust for gasoline and mid-level gasoline-alcohol blends. SAE Int. J. Fuels Lubr. 2014, 7, 571–579. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Vinzents, P.; Petersen, J.H.; Kleinjans, J.C.S.; Plas, G.; Kirsch-Volders, M.; Dostal, M.; Rossner, P.; Beskid, O.; Sram, R.J.; et al. Cytogenetic effects in children and mothers exposed to air pollution assessed by the frequency of micronuclei and fluorescence in situ hybridization (FISH): A family pilot study in the czech republic. Mutat. Res. 2006, 608, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Memon, A.N.; Sheikh, S.A.; Rouq, F.A.; Usmani, A.M.; Hassan, A.; Arain, S.A. Effect of environmental air pollution on type 2 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 123–128. [Google Scholar] [PubMed]
- Eze, I.C.; Schaffner, E.; Foraster, M.; Imboden, M.; von Eckardstein, A.; Gerbase, M.W.; Rothe, T.; Rochat, T.; Kunzli, N.; Schindler, C.; et al. Long-term exposure to ambient air pollution and metabolic syndrome in adults. PLoS ONE 2015, 10, e0130337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, K.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- World Bank. The Cost of Air Pollution: Strengthening the Economic Case for Action; World Bank: Washington, DC, USA, 2016. [Google Scholar]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Economic burden of cardiovascular disease in type 2 diabetes: A systematic review. Value Health 2018, 21, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Riediker, M. Cardiovascular effects of fine particulate matter components in highway patrol officers. Inhal. Toxicol. 2007, 19, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Alexeeff, S.E.; Roy, A.; Shan, J.; Liu, X.; Messier, K.; Apte, J.S.; Portier, C.; Sidney, S.; Van Den Eeden, S.K. High-resolution mapping of traffic related air pollution with google street view cars and incidence of cardiovascular events within neighborhoods in Oakland, CA. Environ. Health 2018, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Riediker, M.; Franc, Y.; Bochud, M.; Meier, R.; Rousson, V. Exposure to fine particulate matter leads to rapid heart rate variability changes. Front. Environ. Sci. 2018, 6, 2. [Google Scholar] [CrossRef]
- Mazidi, M.; Speakman, J.R. Impact of obesity and ozone on the association between particulate air pollution and cardiovascular disease and stroke mortality among US adults. J. Am. Heart Assoc. 2018, 7, e008006. [Google Scholar] [CrossRef] [PubMed]
- Peretz, A.; Sullivan, J.H.; Leotta, D.F.; Trenga, C.A.; Sands, F.N.; Allen, J.; Carlsten, C.; Wilkinson, C.W.; Gill, E.A.; Kaufman, J.D. Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environ. Health Perspect. 2008, 116, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.W.; Deng, F.R.; Niu, J.; Huang, Q.S.; Liu, Y.C.; Guo, X.B. The relationship between traffic-related air pollutants and cardiac autonomic function in a panel of healthy adults: A further analysis with existing data. Inhal. Toxicol. 2011, 23, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.Q.; Koehoorn, M.; Davies, H.W.; Demers, P.A.; Tamburic, L.; Brauer, M. Long-term exposure to traffic-related air pollution and the risk of coronary heart disease hospitalization and mortality. Environ. Health Perspect. 2011, 119, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Wang, S.; Dou, C.; Zhang, X.; Yu, Y.; Zheng, Y.; Avula, U.; Hoxha, M.; Díaz, A.; McCracken, J.; et al. Air pollution exposure and telomere length in highly exposed subjects in Beijing, China: A repeated-measure study. Environ. Int. 2012, 48, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steenhof, M.; Janssen, N.A.H.; Strak, M.; Hoek, G.; Gosens, I.; Mudway, I.S.; Kelly, F.J.; Harrison, R.M.; Pieters, R.H.H.; Cassee, F.R.; et al. Air pollution exposure affects circulating white blood cell counts in healthy subjects: The role of particle composition, oxidative potential and gaseous pollutants—The raptes project. Inhal. Toxicol. 2014, 26, 141–165. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.G.; Allen, K.; Yang, H.Y.; Nan, B.; Morishita, M.; Mukherjee, B.; Dvonch, J.T.; Spino, C.; Fink, G.D.; Rajagopalan, S.; et al. Cardiovascular depression in rats exposed to inhaled particulate matter and ozone: Effects of diet-induced metabolic syndrome. Environ. Health Perspect. 2014, 122, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Burnett, R.T.; Kwong, J.C.; Villeneuve, P.J.; Goldberg, M.S.; Brook, R.D.; van Donkelaar, A.; Jerrett, M.; Martin, R.V.; Brook, J.R.; et al. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ. Health Perspect. 2013, 121, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Dijkema, M.B.A.; Mallant, S.F.; Gehring, U.; van den Hurk, K.; Alssema, M.; van Strien, R.T.; Fischer, P.H.; Nijpels, G.; Stehouwer, C.D.A.; Hoek, G. Long-term exposure to traffic-related air pollution and type 2 diabetes prevalence in a cross-sectional screening-study in the Netherlands. Environ. Health 2011, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Ruckerl, R.; Hampel, R.; Breitner, S.; Cyrys, J.; Kraus, U.; Carter, J.; Dailey, L.; Devlin, R.B.; Diaz-Sanchez, D.; Koenig, W.; et al. Associations between ambient air pollution and blood markers of inflammation and coagulation/fibrinolysis in susceptible populations. Environ. Int. 2014, 70, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Sun, H.; Xiao, L.; Zhou, Y.; Yin, W.; Xu, T.; Cheng, J.; Chen, W.; Yuan, J. Combined effect of urinary monohydroxylated polycyclic aromatic hydrocarbons and impaired lung function on diabetes. Environ. Res. 2016, 148, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Ponticiello, B.G.; Capozzella, A.; Di Giorgio, V.; Casale, T.; Giubilati, R.; Tomei, G.; Tomei, F.; Rosati, M.V.; Sancini, A. Overweight and urban pollution: Preliminary results. Sci. Total Environ. 2015, 518–519, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Kan, H.D.; Kearney, G.D.; Xu, X.H. Associations between exposure to polycyclic aromatic hydrocarbons and glucose homeostasis as well as metabolic syndrome in nondiabetic adults. Sci. Total Environ. 2015, 505, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Qian, Z.M.; Vaughn, M.G.; Nelson, E.J.; Dharmage, S.C.; Heinrich, J.; Lin, S.; Lawrence, W.R.; Ma, H.; Chen, D.H.; et al. Is prehypertension more strongly associated with long-term ambient air pollution exposure than hypertension? Findings from the 33 communities Chinese health study. Environ. Pollut. 2017, 229, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Wang, D.D.; Rizzo, A.M.; Gachette, D.; Delnord, M.; Parambi, R.; Kang, C.M.; Brugge, D. Association of PNC, BC, and PM2.5 measured at a central monitoring site with blood pressure in a predominantly near highway population. Int. J. Environ. Res. Public Health 2015, 12, 2765–2780. [Google Scholar] [CrossRef] [PubMed]
- Thiering, E.; Cyrys, J.; Kratzsch, J.; Meisinger, C.; Hoffmann, B.; Berdel, D.; Von Berg, A.; Koletzko, S.; Bauer, C.P.; Heinrich, J. Long-term exposure to traffic-related air pollution and insulin resistance in children: Results from the giniplus and lisaplus birth cohorts. Diabetologia 2013, 56, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Cosselman, K.E.; Krishnan, R.M.; Oron, A.P.; Jansen, K.; Peretz, A.; Sullivan, J.H.; Larson, T.V.; Kaufman, J.D. Blood pressure response to controlled diesel exhaust exposure in human subjects. Hypertension 2012, 59, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Krempel, P.G.; André, P.A.; Freitas, J.R. Traffic-related air pollution effect on fast glycemia of aged obese type 2 diabetic mice. J. Clin. Exp. Cardiol. 2013, 4, 255. [Google Scholar]
- Brocato, J.; Sun, H.; Shamy, M.; Kluz, T.; Alghamdi, M.A.; Khoder, M.I.; Chen, L.C.; Costa, M. Particulate matter from Saudi Arabia induces genes involved in inflammation, metabolic syndrome and atherosclerosis. J. Toxicol. Environ. Health A 2014, 77, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.X.; Zhu, Y.F.; Chang, H.F.; Liang, Y. Nanoceria restrains PM2.5-induced metabolic disorder and hypothalamus inflammation by inhibition of astrocytes activation related NF-κB pathway in Nrf2 deficient mice. Free Radic. Biol. Med. 2016, 99, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.K.; Chen, M.J.; Zhong, M.H.; Hu, Z.Y.; Qiu, L.L.; Rajagopalan, S.; Fossett, N.G.; Chen, L.C.; Ying, Z.K. Exposure to concentrated ambient PM2.5 shortens lifespan and induces inflammation-associated signaling and oxidative stress in drosophila. Toxicol. Sci. 2017, 156, 199–207. [Google Scholar] [PubMed]
- Araujo, J.A.; Nel, A.E. Particulate matter and atherosclerosis: Role of particle size, composition and oxidative stress. Part. Fibre Toxicol. 2009, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Franck, U.; Odeh, S.; Wiedensohler, A.; Wehner, B.; Herbarth, O. The effect of particle size on cardiovascular disorders—The smaller the worse. Sci. Total Environ. 2011, 409, 4217–4221. [Google Scholar] [CrossRef] [PubMed]
- Karottki, D.G.; Bekö, G.; Clausen, G.; Madsen, A.M.; Andersen, Z.J.; Massling, A.; Ketzel, M.; Ellermann, T.; Lund, R.; Sigsgaard, T.; et al. Cardiovascular and lung function in relation to outdoor and indoor exposure to fine and ultrafine particulate matter in middle-aged subjects. Environ. Int. 2014, 73, 372–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Yu, Y.; Yin, D.; Qin, D.; He, J.; Dong, L. Spatial patterns and temporal variations of six criteria air pollutants during 2015 to 2017 in the city clusters of Sichuan basin, China. Sci. Total Environ. 2018, 624, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Agrawal, M. Air pollutant levels are 12 times higher than guidelines in Varanasi, India. Sources and transfer. Environ. Chem. Lett. 2018, 16, 1009–1016. [Google Scholar] [CrossRef]
- Khaefi, M.; Geravandi, S.; Hassani, G.; Yari, A.R.; Soltani, F.; Dobaradaran, S.; Moogahi, S.; Mohammadi, M.J.; Mahboubi, M.; Alavi, N.; et al. Association of particulate matter impact on prevalence of chronic obstructive pulmonary disease in Ahvaz, southwest Iran during 2009–2013. Aerosol Air Qual. Res. 2017, 17, 230–237. [Google Scholar] [CrossRef]
- Oakes, M.; Baxter, L.; Long, T.C. Evaluating the application of multipollutant exposure metrics in air pollution health studies. Environ. Int. 2014, 69, 90–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, W.Q.; Allen, R.W.; Brauer, M.; Davies, H.W.; Mancini, G.B.J.; Lear, S. Long-term exposure to traffic-related air pollution and progression of carotid artery atherosclerosis: A prospective cohort study. BMJ Open 2014, 4, e004743. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variabilitystandards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Park, S.K.; Auchincloss, A.H.; O’Neill, M.S.; Prineas, R.; Correa, J.C.; Keeler, J.; Barr, R.G.; Kaufman, J.D.; Diez Roux, A.V. Particulate air pollution, metabolic syndrome, and heart rate variability: The multi-ethnic study of atherosclerosis (MESA). Environ. Health Perspect. 2010, 118, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Connes, P.; Martin, C.; Barthelemy, J.-C.; Monchanin, G.; Atchou, G.; Forsuh, A.; Massarelli, R.; Wouassi, D.; Thiriet, P.; Pichot, V. Nocturnal autonomic nervous system activity impairment in sickle cell trait carriers. Clin. Physiol. Funct. Imaging 2006, 26, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Jennings, B.J.; Ozanne, S.E.; Hales, C.N. Nutrition, oxidative damage, telomere shortening, and cellular senescence: Individual or connected agents of aging? Mol. Genet. Metab. 2000, 71, 32–42. [Google Scholar] [CrossRef] [PubMed]
- IDF Diabetes Atlas Group. Update of mortality attributable to diabetes for the IDF Diabetes Atlas: Estimates for the year 2013. Diabetes Res. Clin. Pract. 2015, 109, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus—Present and future perspectives. Nat. Rev. Endocrinol. 2011, 8, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Eze, I.C.; Hemkens, L.G.; Bucher, H.C.; Hoffmann, B.; Schindler, C.; Künzli, N.; Schikowski, T.; Probst-Hensch, N.M. Association between ambient air pollution and diabetes mellitus in Europe and North America: Systematic review and meta-analysis. Environ. Health Perspect. 2015, 123, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sun, Q. Fine particulate matter air pollution and atherosclerosis: Mechanistic insights. BBA Gen. Subj. 2016, 1860, 2863–2868. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.L.; Owens, E.O.; Dutton, S.J.; Luben, T.J. Systematic review of the effects of black carbon on cardiovascular disease among individuals with pre-existing disease. Int. J. Public Health 2013, 58, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wilker, E.H.; Dorans, K.S.; Rice, M.B.; Schwartz, J.; Coull, B.A.; Koutrakis, P.; Gold, D.R.; Keaney, J.F.; Lin, H.; et al. Short-term exposure to air pollution and biomarkers of oxidative stress: The framingham heart study. J. Am. Heart Assoc. 2016, 5, e002742. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; O’Neill, M.S.; Vokonas, P.S.; Sparrow, D.; Spiro, A., III; Tucker, K.L.; Suh, H.; Hu, H.; Schwartz, J. Traffic-related particles are associated with elevated homocysteine. Am. J. Resp. Crit. Care 2008, 178, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Vivanco-Hidalgo, R.M.; Wellenius, G.A.; Basagaña, X.; Cirach, M.; González, A.G.; Ceballos, P.; Zabalza, A.; Jiménez-Conde, J.; Soriano-Tarraga, C.; Giralt-Steinhauer, E.; et al. Short-term exposure to traffic-related air pollution and ischemic stroke onset in Barcelona, Spain. Environ. Res. 2018, 162, 160–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wichmann, H.E. Diesel exhaust particles. Inhal. Toxicol. 2007, 19, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Cascio, W.E.; Ghio, A.J.; Wild, P.; Danuser, B.; Riediker, M. Associations of short-term particle and noise exposures with markers of cardiovascular and respiratory health among highway maintenance workers. Environ. Health Perspect. 2014, 122, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Van Eeden, S.F.; Tan, W.C.; Suwa, T.; Mukae, H.; Terashima, T.; Fujii, T.; Qui, D.; Vincent, R.; Hogg, J.C. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM10). Am. J. Resp. Crit. Care 2001, 164, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Fossati, S.; Baccarelli, A.; Zanobetti, A.; Hoxha, M.; Vokonas, P.S.; Wright, R.O.; Schwartz, J. Ambient particulate air pollution and microRNAS in elderly men. Epidemiology 2014, 25, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Cherng, T.W.; Campen, M.J.; Knuckles, T.L.; Bosc, L.G.; Kanagy, N.L. Impairment of coronary endothelial cell ETB receptor function after short-term inhalation exposure to whole diesel emissions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R640–R647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rückerl, R.; Greven, S.; Ljungman, P.; Aalto, P.; Antoniades, C.; Bellander, T.; Berglind, N.; Chrysohoou, C.; Forastiere, F.; Jacquemin, B.; et al. Air pollution and inflammation (interleukin-6, c-reactive protein, fibrinogen) in myocardial infarction survivors. Environ. Health Perspect. 2007, 115, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.B.; Ravnskjaer, L.; Loft, S.; Andersen, K.K.; Brauner, E.V.; Baastrup, R.; Yao, C.; Ketzel, M.; Becker, T.; Brandt, J.; et al. Long-term exposure to fine particulate matter and incidence of diabetes in the Danish Nurse Cohort. Environ. Int. 2016, 91, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Jessica, M.L.; Puett, R.; Rajagopalan, S.; Brook, R.D. Ambient air pollution: An emerging risk factor for diabetes mellitus. Curr. Diabetes Rep. 2015, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Brook, R.D. Air pollution and type 2 diabetes mechanistic insights. Diabetes 2012, 61, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Delfino, R.J.; Gillen, D.L.; Tjoa, T.; Staimer, N.; Polidori, A.; Arhami, M.; Sioutas, C.; Longhurst, J. Electrocardiographic ST-segment depression and exposure to traffic-related aerosols in elderly subjects with coronary artery disease. Environ. Health Perspect. 2011, 119, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Mauderly, J.L.; Chow, J.C. Health effects of organic aerosols. Inhal. Toxicol. 2008, 20, 257–288. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yamaguchi, K.; Lee, S.H.; Tithof, P.K.; Sayler, G.S.; Yoon, J.H.; Baek, S.J. Evaluation of polycyclic aromatic hydrocarbons in the activation of early growth response-1 and Peroxisome Proliferator Activated Receptors. Toxicol. Sci. 2005, 85, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P. The biology of peroxisome proliferator-activated receptors: Relationship with lipid metabolism and insulin sensitivity. Diabetes 2004, 53, S43–S50. [Google Scholar] [CrossRef] [PubMed]
- Lamb, R.E.; Goldstein, B.J. Modulating an oxidative-inflammatory cascade: Potential new treatment strategy for improving glucose metabolism, insulin resistance, and vascular function. Int. J. Clin. Pract. 2008, 62, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Everett, C.J.; King, D.E.; Player, M.S.; Matheson, E.M.; Post, R.E.; Mainous, A.G., III. Association of urinary polycyclic aromatic hydrocarbons and serum c-reactive protein. Environ. Res. 2010, 110, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sandau, C.D.; Romanoff, L.C.; Caudill, S.P.; Sjodin, A.; Needham, L.L.; Patterson, D.G., Jr. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ. Res. 2008, 107, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zotter, P.; Bruns, E.A.; Stefenelli, G.; Bhattu, D.; Brown, S.; Bertrand, A.; Marchand, N.; Lamkaddam, H.; Slowik, J.G.; et al. Particle-bound reactive oxygen species (Pb-ROS) emissions and formation pathways in residential wood smoke under different combustion and aging conditions. Atmos. Chem. Phys. 2018, 18, 6985–7000. [Google Scholar] [CrossRef]
- Venkatachari, P.; Hopke, P.K.; Grover, B.D.; Eatough, D.J. Measurement of particle-bound reactive oxygen species in rubidoux aerosols. J. Atmos. Chem. 2005, 50, 49–58. [Google Scholar] [CrossRef]
- Venkatachari, P.; Hopke, P.K. Development and laboratory testing of an automated monitor for the measurement of atmospheric particle-bound reactive oxygen species (ROS). Aerosol Sci. Technol. 2008, 42, 629–635. [Google Scholar] [CrossRef]
- Brook, R.D.; Urch, B.; Dvonch, J.T.; Bard, R.L.; Speck, M.; Keeler, G.; Morishita, M.; Marsik, F.J.; Kamal, A.S.; Kaciroti, N.; et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 2009, 54, 659. [Google Scholar] [CrossRef] [PubMed]
- Langrish, J.P.; Mills, N.L.; Chan, J.K.K.; Leseman, D.L.A.C.; Aitken, R.J.; Fokkens, P.H.B.; Cassee, F.R.; Li, J.; Donaldson, K.; Newby, D.E.; et al. Beneficial cardiovascular effects of reducing exposure to particulate air pollution with a simple facemask. Part. Fibre Toxicol. 2009, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rosa, S.; Arcidiacono, B.; Chiefari, E.; Brunetti, A.; Indolfi, C.; Foti, D.P. Type 2 diabetes mellitus and cardiovascular disease: Genetic and epigenetic links. Front. Endocrinol. 2018, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Leshinsky-Silver, E.; Cheng, S.; Grow, M.A.; Schwartz, S.; Scharf, L.; Lev, D.; Boaz, M.; Brunner, D.; Zimlichman, R. Candidate gene polymorphism in cardiovascular disease: The BIP cohort. Isr. Med. Assoc. J. 2006, 2, 103–105. [Google Scholar]
- Altshuler, D.; Hirschhorn, J.N.; Klannemark, M.; Lindgren, C.M.; Vohl, M.; Nemesh, J.; Lane, C.R.; Schaffner, S.F.; Bolk, S.; Brewer, C. The common PPARγ Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat. Genet. 2000, 26, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Bao, W.; Rong, S.; Fang, M.; Wang, D.; Yao, P.; Hu, F.B.; Liu, L. Hemochromatosis gene (HFE) polymorphisms and risk of type 2 diabetes mellitus: A meta-analysis. Am. J. Epidemiol. 2012, 176, 461–472. [Google Scholar] [CrossRef] [PubMed]
- De Majo, F.; Calore, M. Chromatin remodelling and epigenetic state regulation by non-coding RNAs in the diseased heart. Non-Coding RNA Res. 2018, 3, 20–28. [Google Scholar] [CrossRef]
- Pandey, A.K.; Agarwal, P.; Kaur, K.; Datta, M. Micrornas in diabetes: Tiny players in big disease. Cell. Physiol. Biochem. 2009, 23, 221–232. [Google Scholar] [CrossRef] [PubMed]
| Health Outcomes | Reference | Location | Sample Size |
|---|---|---|---|
| Cardiovascular disease (CVD)-related outcomes: Cardiac rhythm inflammation coagulation, changes in heart rate variability, stroke, cardiac autonomic function coronary diseases, telomere length, and changes in white blood cells, neutrophils, monocytes, and inflammatory responses | Riediker et al., 2007 [29] | NC, USA | 9 |
| Alexeeff et al., 2018 [30] | CA, USA | 41,869 | |
| Riediker et al., 2018 [31] | Western Switzerland | 18 | |
| Mazidi & Speakman, 2018 [32] | USA | 26,349 (NHANES) 1 1,283,722 (BRFSS) 2 | |
| Peretz et al., 2008 [33] | Seattle, WA, USA | 27 | |
| Wu et al., 2011 [34] | Beijing, China | 14 | |
| Gan et al., 2011 [35] | Vancouver, Canada | 452,735 | |
| Hou et al., 2012 [36] | Beijing, China | 120 | |
| Steenhof et al., 2014 [37] | Netherlands | 31 | |
| Wagner et al., 2014 [38] | MI, USA | 64 | |
| Diabetes | Chen et al., 2013 [39] | ON, Canada | 62,012 |
| Dijkema et al., 2011 [40] | Westfriesland, Netherlands | 8018 | |
| Ruckerl et al., 2014 [41] | Augsburg, Germany | 274 | |
| Hou et al., 2016 [42] | Wuhan Zhuhai, China | 2730 | |
| Obesity | Ponticiello et al., 2015 [43] | Rome, Italy | 150 |
| Metabolic syndrome: insulin resistance, β-cell dysfunction, hypertension, blood pressure, hyperglycemia, atherosclerosis, systemic inflammation, and dysregulated insulin signaling | Hu et al., 2015 [44] | USA | 1878 (NHANES) 1 |
| Yang et al., 2017 [45] | Liaoning, China | 24,845 | |
| Chung et al., 2015 [46] | Boston, MA, USA | 220 | |
| Thiering et al., 2013 [47] | Munich and Wesel, Germany | 397 | |
| Brook et al., 2013 [1] | MI, USA | 25 | |
| Cosselman et al., 2012 [48] | Seattle, WA, USA | 45 | |
| Matsuda et al., 2013 [49] | Brazil | 66 | |
| Brocato et al., 2014 [50] | Jeddah, Saudi Arabia | 9 | |
| Xu et al., 2016 [51] | Beijing, China | N/A 3 | |
| Wang et al., 2017 [52] | MD, USA | 400 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, C.M.S.; Jiang, H.; Chen, J.Y.; Lin, Y.-H. Traffic-Related Particulate Matter and Cardiometabolic Syndrome: A Review. Atmosphere 2018, 9, 336. https://doi.org/10.3390/atmos9090336
Ahmed CMS, Jiang H, Chen JY, Lin Y-H. Traffic-Related Particulate Matter and Cardiometabolic Syndrome: A Review. Atmosphere. 2018; 9(9):336. https://doi.org/10.3390/atmos9090336
Chicago/Turabian StyleAhmed, C. M. Sabbir, Huanhuan Jiang, Jin Y. Chen, and Ying-Hsuan Lin. 2018. "Traffic-Related Particulate Matter and Cardiometabolic Syndrome: A Review" Atmosphere 9, no. 9: 336. https://doi.org/10.3390/atmos9090336
APA StyleAhmed, C. M. S., Jiang, H., Chen, J. Y., & Lin, Y.-H. (2018). Traffic-Related Particulate Matter and Cardiometabolic Syndrome: A Review. Atmosphere, 9(9), 336. https://doi.org/10.3390/atmos9090336






