Combination of Warming and Vegetation Composition Change Strengthens the Environmental Controls on N2O Fluxes in a Boreal Peatland
Abstract
1. Introduction
2. Methodology
2.1. Study Site
2.2. Experimental Design
2.3. Gas Measurements
2.4. Soil Water Measurements
2.5. Environmental Variables
2.6. Statistical Analysis
3. Results
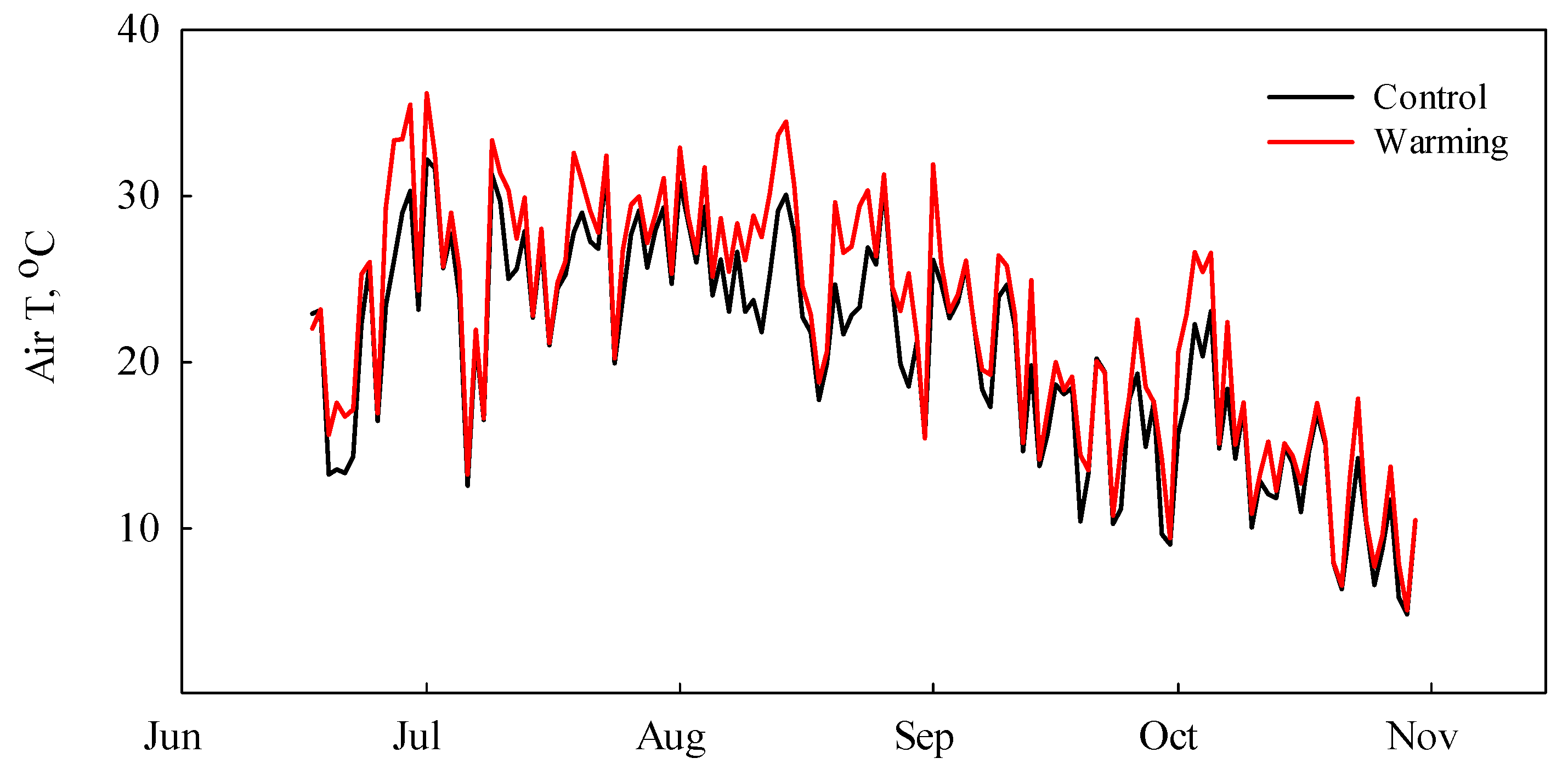
3.1. Environmental Parameters
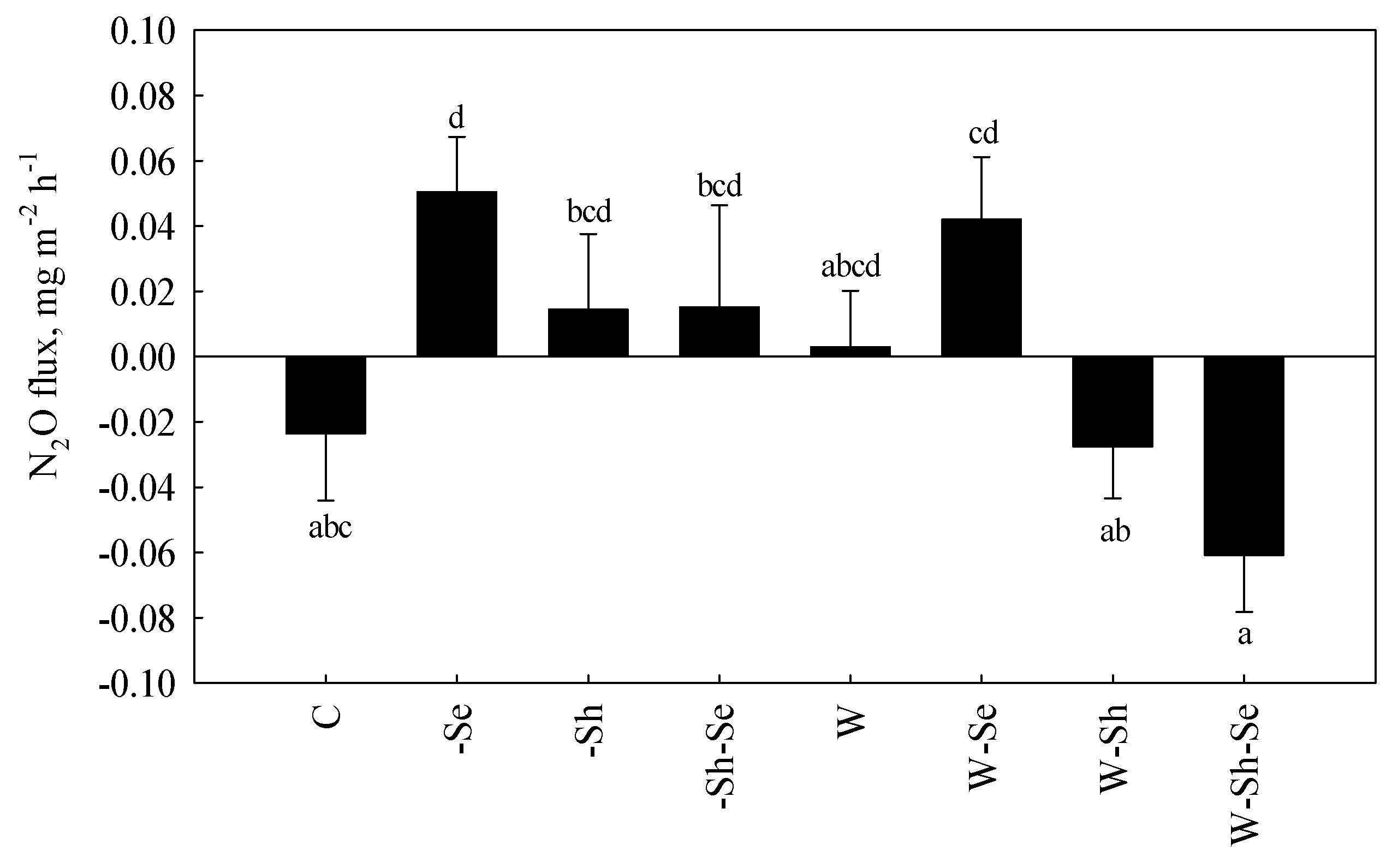
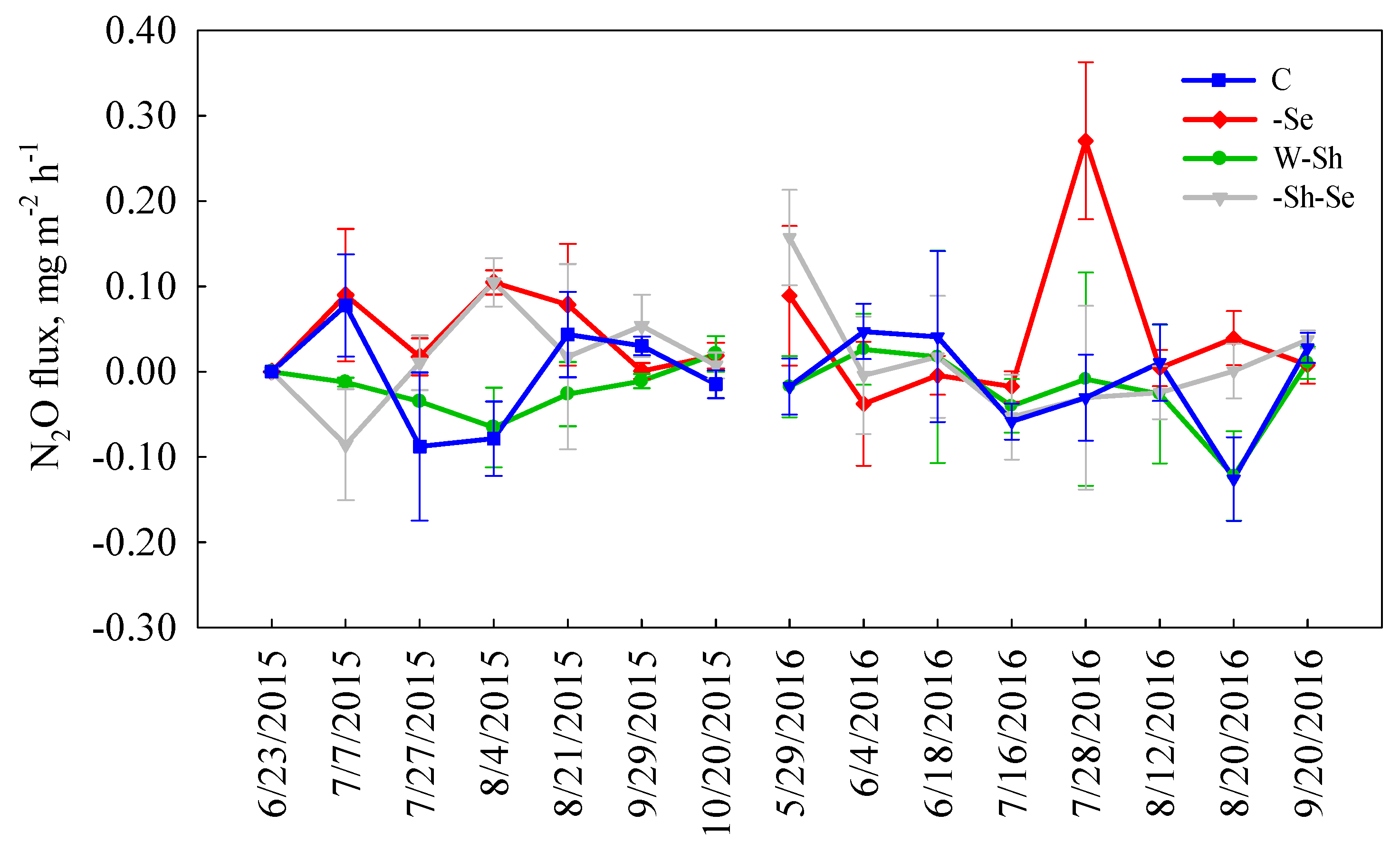
3.2. Treatment Effects on N2O Fluxes
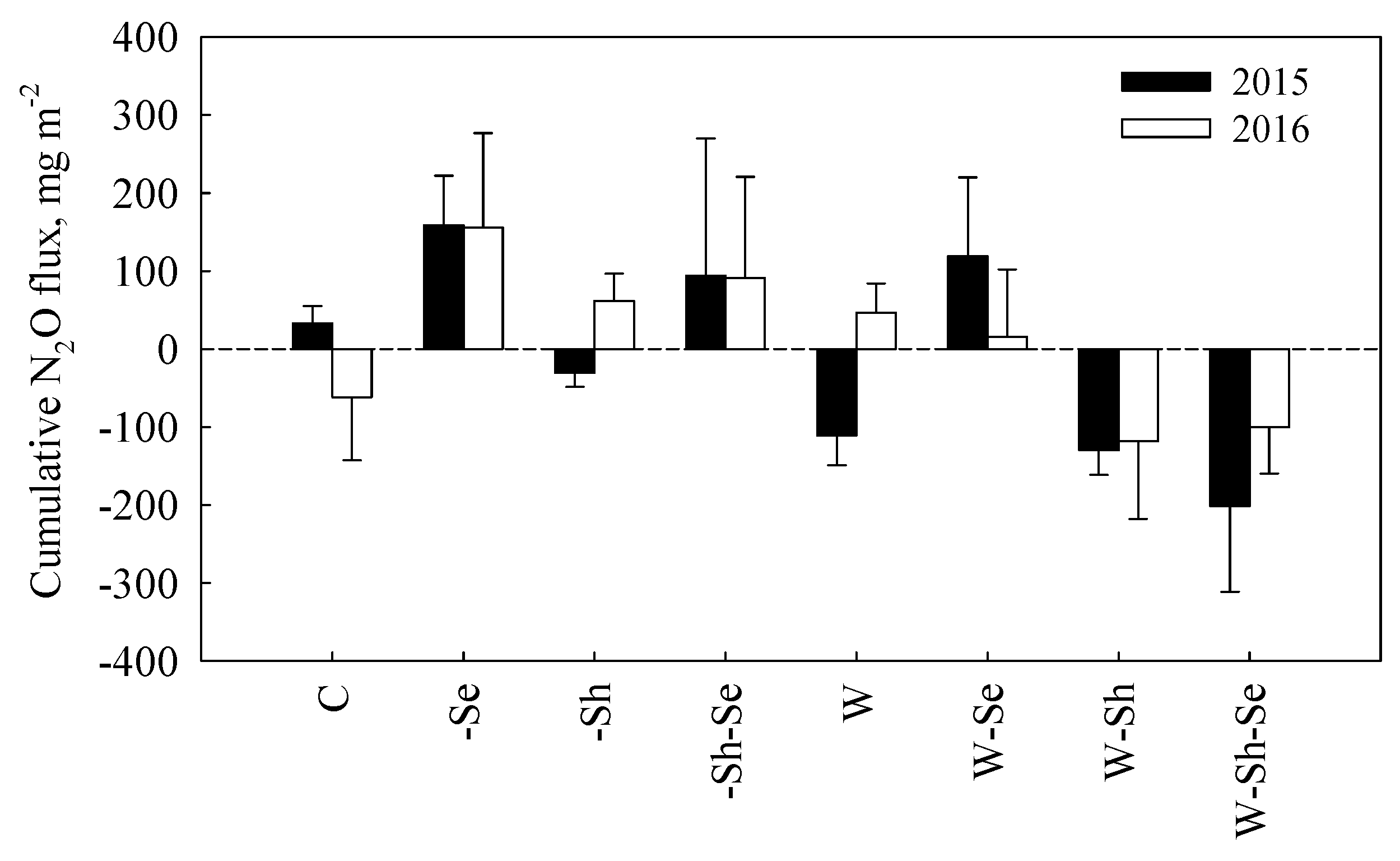
3.3. Cumulative N2O Fluxes
3.4. Relationship between N2O Fluxes and Abiotic Parameters
4. Discussion
4.1. N2O Fluxes
4.2. The Effects of Treatments on N2O Fluxes
4.3. Abiotic Controls on N2O Fluxes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tremblay, S.L.; D’Orangeville, L.; Lambert, M.C.; Houle, D. Transplanting boreal soils to a warmer region increases soil heterotrophic respiration as well as its temperature sensitivity. Soil Biol. Biochem. 2018, 116, 203–212. [Google Scholar] [CrossRef]
- Laine, A.M.; Mehtätalo, L.; Tolvanen, A.; Frolking, S.; Tuittila, E.S. Impacts of drainage, restoration and warming on boreal wetland greenhouse gas fluxes. Sci. Total Environ. 2019, 647, 169–181. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Cornelissen, J.H.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef]
- Bridgham, S.D.; Cadillo-Quiroz, H.; Keller, J.K.; Zhuang, Q. Methane emissions from wetlands: Biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Chang. Biol. 2013, 19, 1325–1346. [Google Scholar] [CrossRef]
- Nielsen, C.S.; Michelsen, A.; Strobel, B.W.; Wulff, K.; Banyasz, I.; Elberling, B. Correlations between substrate availability, dissolved CH4, and CH4 emissions in an Arctic wetland subject to warming and plant removal. J. Geophys. Res. Biogeosci. 2017, 122, 645–660. [Google Scholar] [CrossRef]
- Tian, K.; Kong, X.; Gao, J.; Jia, Y.; Lin, H.; He, Z.; Ji, Y.; Bei, Z.; Tian, X. Local root status: A neglected bio-factor that regulates the home-field advantage of leaf litter decomposition. Plant Soil 2018, 431, 175–189. [Google Scholar] [CrossRef]
- Brummell, M.E.; Lazcano, C.; Strack, M. The effects of eriophorum vaginatum on N2O fluxes at a restored, extracted peatland. Ecol. Eng. 2017, 106, 287–295. [Google Scholar] [CrossRef]
- Hartley, I.P.; Garnett, M.H.; Sommerkorn, M.; Hopkins, D.W.; Fletcher, B.J.; Sloan, V.L.; Phoenix, G.K.; Wookey, P.A. A potential loss of carbon associated with greater plant growth in the european Arctic. Nat. Clim. Chang. 2012, 2, 875–879. [Google Scholar] [CrossRef]
- Gavazov, K.; Albrecht, R.; Buttler, A.; Dorrepaal, E.; Garnett, M.H.; Gogo, S.; Hagedorn, F.; Mills, R.T.E.; Robroek, B.J.M.; Bragazza, L. Vascular plant-mediated controls on atmospheric carbon assimilation and peat carbon decomposition under climate change. Glob. Chang. Biol. 2018, 00, 1–11. [Google Scholar] [CrossRef]
- Ward, S.E.; Ostle, N.J.; Oakley, S.; Quirk, H.; Henrys, P.A.; Bardgett, R.D. Warming effects on greenhouse gas fluxes in peatlands are modulated by vegetation composition. Ecol. Lett. 2013, 16, 1285–1293. [Google Scholar] [CrossRef]
- Pearson, T.P.; Harjunpää, L.; Laiho, R.; Laine, J.; Sarjala, T.; Silvan, K.; Silvan, N. Effects of temperature rise and water-table-level drawdown on greenhouse gas fluxes of boreal sedge fens. Boreal Environ. Res. 2015, 20, 489–505. [Google Scholar]
- Cui, Q.; Song, C.; Wang, X.; Shi, F.; Yu, X.; Tan, W. Effects of warming on N2O fluxes in a boreal peatland of permafrost region, northeast China. Sci. Total Environ. 2018, 616–617, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Lohila, A.; Aurela, M.; Hatakka, J.; Pihlatie, M.; Minkkinen, K.; Penttilä, T.; Laurila, T. Responses of N2O fluxes to temperature, water table and N deposition in a northern boreal fen. Eur. J. Soil Sci. 2010, 61, 651–661. [Google Scholar] [CrossRef]
- Wang, H.; Yu, L.; Zhang, Z.; Liu, W.; Chen, L.; Cao, G.; Yue, H.; Zhou, J.; Yang, Y.; Tang, Y.; et al. Molecular mechanisms of water table lowering and nitrogen deposition in affecting greenhouse gas emissions from a tibetan alpine wetland. Glob. Chang. Biol. 2017, 23, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Song, C.; Wang, X.; Shi, F.; Wang, L.; Guo, Y. Rapid N2O fluxes at high level of nitrate nitrogen addition during freeze-thaw events in boreal peatlands of northeast China. Atmos. Environ. 2016, 135, 1–8. [Google Scholar] [CrossRef]
- Jørgensen, C.J.; Struwe, S.; Elberling, B. Temporal trends in N2O flux dynamics in a danish wetland—Effects of plant-mediated gas transport of N2O and O2 following changes in water level and soil mineral-N availability. Glob. Chang. Biol. 2012, 18, 210–222. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- He, H.; Jansson, P.-E.; Svensson, M.; Meyer, A.; Klemedtsson, L.; Kasimir, Å. Factors controlling nitrous oxide emission from a spruce forest ecosystem on drained organic soil, derived using the coupmodel. Ecol. Model. 2016, 321, 46–63. [Google Scholar] [CrossRef]
- Luan, J.; Wu, J. Gross photosynthesis explains the ‘artificial bias’ of methane fluxes by static chamber (opaque versus transparent) at the hummocks in a boreal peatland. Environ. Res. Lett. 2014, 9, 105005. [Google Scholar] [CrossRef]
- Luan, J.; Wu, J. Long-term agricultural drainage stimulates CH4 emissions from ditches through increased substrate availability in a boreal peatland. Agric. Ecosyst. Environ. 2015, 214, 68–77. [Google Scholar] [CrossRef]
- Marion, G.M.; Henry, G.H.R.; Freckman, D.W.; Johnstone, J.; Jones, G.; Jones, M.H.; Levesque, E.; Molau, U.; Molgaard, P.; Parsons, A.N.; et al. Open-top designs for manipulating field temperature in high-latitude ecosystems. Glob. Chang. Biol. 1997, 3, 20–32. [Google Scholar] [CrossRef]
- Holland, E.A.; Robertson, G.P.; Greenberg, J.; Groffman, P.; Boone, R.D.; Gosz, J. Soil CO2, N2O, and CH4 exchange. In Standard Soil Methods for Long-Term Ecological Research; Robertson, G.P., Bledsoe, C.S., Coleman, D.C., Sollins, P., Eds.; Oxford University Press: New York, NY, USA, 1999; pp. 185–201. ISBN 0195120833. [Google Scholar]
- Drewer, J.; Lohila, A.; Aurela, M.; Laurila, T.; Minkkinen, K.; Penttilä, T.; Dinsmore, K.J.; McKenzie, R.M.; Helfter, C.; Flechard, C.; et al. Comparison of greenhouse gas fluxes and nitrogen budgets from an ombotrophic bog in scotland and a minerotrophic sedge fen in finland. Eur. J. Soil Sci. 2010, 61, 640–650. [Google Scholar] [CrossRef]
- Regina, K.; Maljanen, H.N.; Silvola, M.; Martikainen, J. Emissions of N2O and NO and net nitrogen mineralization in a boreal forested peatland treated with different nitrogen compounds. Can. J. For. Res. 1998, 28, 132–140. [Google Scholar] [CrossRef]
- Nykänen, H.V.; Huttunen, J.T.; Martikainen, P.J. Effect of experimental nitrogen load on methane and nitrous oxide fluxes on ombrotrophic boreal peatland. Plant Soil 2002, 242, 147–155. [Google Scholar] [CrossRef]
- Reay, D.S.; Dentener, F.; Smith, P.; Grace, J.; Feely, R.A. Global nitrogen deposition and carbon sinks. Nat. Geosci. 2008, 1, 430–437. [Google Scholar] [CrossRef]
- Liimatainen, M.; Voigt, C.; Martikainen, P.J.; Hytönen, J.; Regina, K.; Óskarsson, H.; Maljanen, M. Factors controlling nitrous oxide emissions from managed northern peat soils with low carbon to nitrogen ratio. Soil Biol. Biochem. 2018, 122, 186–195. [Google Scholar] [CrossRef]
- Silvan, N.; Tuittila, E.-S.; Kitunen, V.; Vasander, H.; Laine, J. Nitrate uptake by eriophorum vaginatum controls N2O production in a restored peatland. Soil Biol. Biochem. 2005, 37, 1519–1526. [Google Scholar] [CrossRef]
- Ward, S.E.; Ostle, N.J.; Briones, M.J.I.; Thomson, B.C. Vegetation exerts a greater control on litter decomposition than climate warming in peatlands. Ecology 2015, 96, 113–123. [Google Scholar] [CrossRef]
- Wang, X.; Siciliano, S.; Helgason, B.; Bedard-Haughn, A. Responses of a mountain peatland to increasing temperature: A microcosm study of greenhouse gas emissions and microbial community dynamics. Soil Biol. Biochem. 2017, 110, 22–33. [Google Scholar] [CrossRef]
- Zhou, Y.; Hagedorn, F.; Zhou, C.; Jiang, X.; Wang, X.; Li, M.H. Experimental warming of a mountain tundra increases soil CO2 effluxes and enhances CH4 and N2O uptake at Changbai mountain, China. Sci. Rep. 2016, 6, 21108. [Google Scholar] [CrossRef]
- Pihlatie, M.K.; Kiese, R.; Brüggemann, N.; Butterbach-Bahl, K.; Kieloaho, A.J.; Laurila, T.; Lohila, A.; Mammarella, I.; Minkkinen, K.; Penttilä, T.; et al. Greenhouse gas fluxes in a drained peatland forest during spring frost-thaw event. Biogeosciences 2010, 7, 1715–1727. [Google Scholar] [CrossRef]
- Greenup, A.L.; Bradford, M.A., McNamara; Lee, J.A. The role of eriophorum vaginatum in CH4 flux from an ombrotrophic peatland. Plant Soil 2000, 227, 265–272. [Google Scholar] [CrossRef]
- Dodla, S.K.; Wang, J.J.; DeLaune, R.D.; Cook, R.L. Denitrification potential and its relation to organic carbon quality in three coastal wetland soils. Sci. Total Environ. 2008, 407, 471–480. [Google Scholar] [CrossRef] [PubMed]
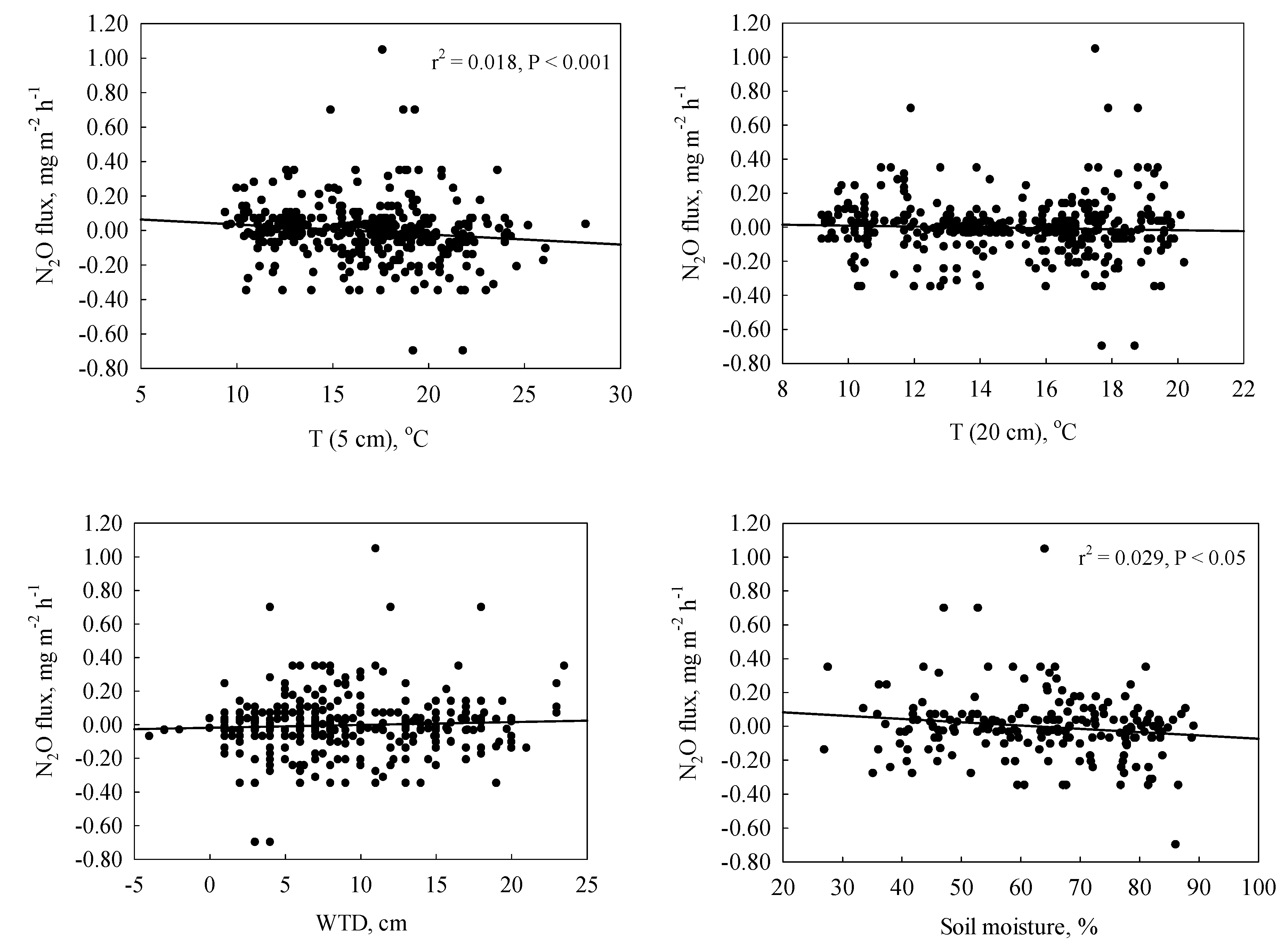
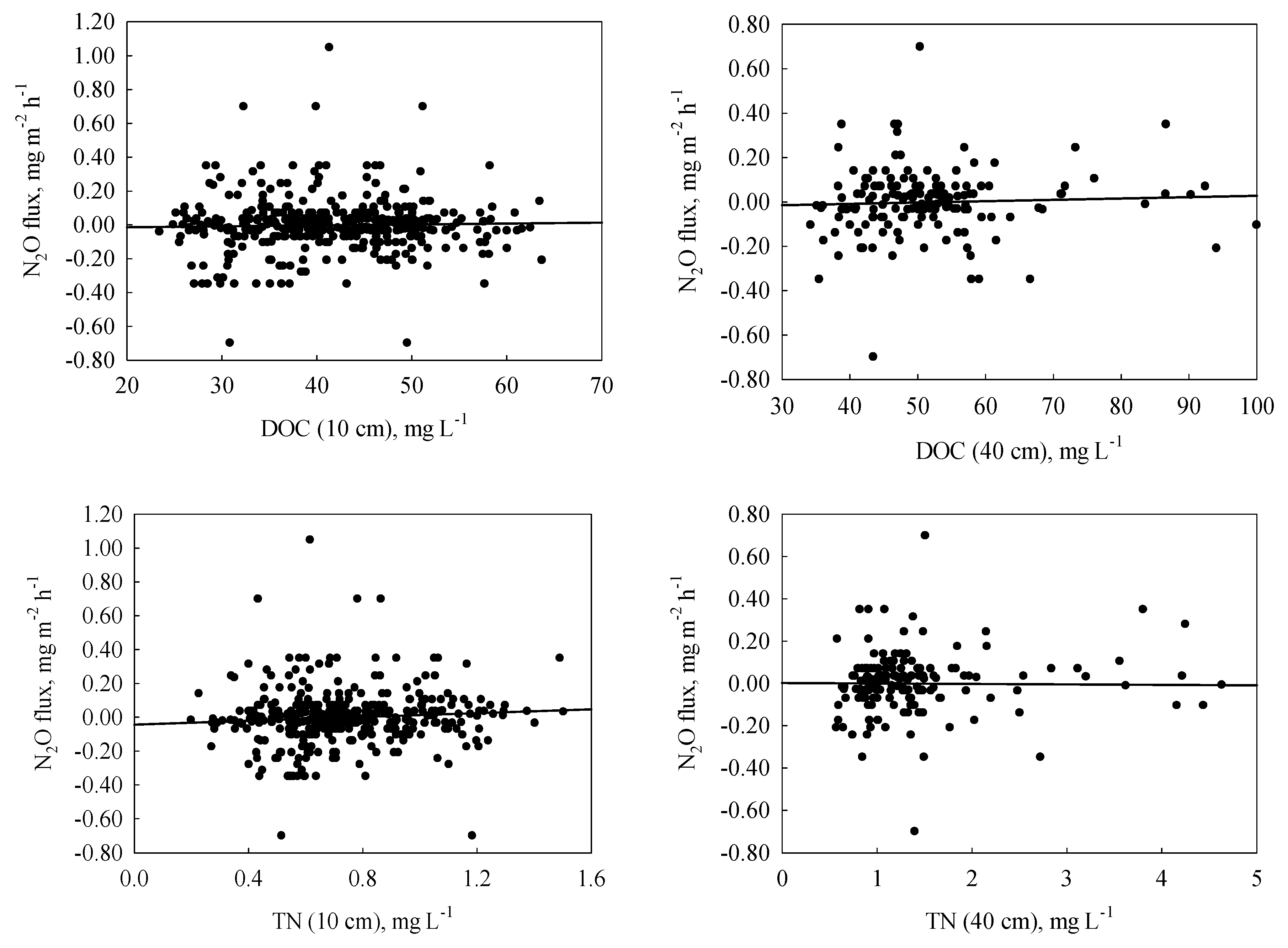
| Treatment | Soil T (0.05 m) | Soil T (0.2 m) | WTD | Soil moisture | DOC (0.1 m) | DOC (0.4 m) | TN (0.1 m) | TN (0.4 m) |
|---|---|---|---|---|---|---|---|---|
| C | 16.6 ± 0.5 a | 14.9 ± 0.4 a | 7.8 ± 0.7 a | 74.5 ± 4.5 a | 40.7 ± 1.2 ab | 53.0 ± 3.7 a | 0.8 ± 0.1 c | 1.5 ± 0.2 ab |
| -Se | 16.2 ± 0.7 a | 14.6 ± 0.4 a | 8.0 ± 0.8 a | 72.5 ± 3.3 ab | 41.3 ± 1.2 abc | 50.7 ± 1.6 a | 0.8 ± 0.1 c | 1.2 ± 0.1 a |
| -Sh | 16.9 ± 0.6 a | 15.0 ± 0.4 a | 8.3 ± 0.8 a | 71.4 ± 4.3 ab | 39.5 ± 1.1 a | 51.0 ± 4.1 a | 0.7 ± 0.1 bc | 1.5 ± 0.2 ab |
| -Sh-Se | 16.0 ± 0.5 a | 14.6 ± 0.4 a | 9.3 ± 0.7 a | 68.0 ± 3.6 ab | 44.4 ± 1.2 c | 59.3 ± 5.4 a | 0.7 ± 0.1 c | 2.1 ± 0.4 b |
| W | 17.1 ± 0.6 a | 15.5 ± 0.4 a | 7.2 ± 0.7 a | 68.6 ± 3.3 ab | 39.6 ± 0.9 a | 52.8 ± 1.2 a | 0.6 ± 0.1 a | 1.1 ± 0.1 a |
| W-Se | 16.9 ± 0.5 a | 15.1 ± 0.4 a | 8.8 ± 0.9 a | 65.7 ± 5.3 ab | 40.2 ± 1.2 a | 48.2 ± 1.4 a | 0.7 ± 0.1 abc | 1.4 ± 0.2 ab |
| W-Sh | 16.8 ± 0.5 a | 15.1 ± 0.4 a | 8.6 ± 0.7 a | 61.6 ± 4.1 b | 43.8 ± 1.2 bc | 56.7 ± 3.9 a | 0.6 ± 0.1 ab | 1.8 ± 0.3 ab |
| W-Sh-Se | 17.4 ± 0.5 a | 14.9 ± 0.4 a | 7.3 ± 0.7 a | 71.6 ± 2.5 ab | 40.8 ± 1.1 ab | 53.0 ± 3.3 a | 0.8 ± 0.1 c | 1.5 ± 0.2 ab |
| Source | Df | F | Sig.(p) |
|---|---|---|---|
| Corrected Model | 7 | 3.207 | 0.003 |
| Intercept | 1 | 0.047 | 0.829 |
| Year | 1 | 0.004 | 0.950 |
| Date | 13 | 1.130 | 0.350 |
| Warming/ambient temperature | 1 | 0.972 | 0.327 |
| Shrub presence/absence | 1 | 2.298 | 0.133 |
| Sedge presence/absence | 1 | 6.011 | 0.016 |
| Date * Warming | 27 | 0.712 | 0.857 |
| Warming * Shrub | 1 | 0.017 | 0.897 |
| Warming * Sedge | 1 | 3.805 | 0.054 |
| Shrub * Sedge | 1 | 1.175 | 0.281 |
| Warming * Shrub * Sedge | 1 | 1.551 | 0.216 |
| Year * Warming | 2 | 1.374 | 0.254 |
| Year * Shrub | 2 | 2.484 | 0.085 |
| Year * Sedge | 2 | 1.329 | 0.266 |
| Year * Warming * Shrub * Sedge | 8 | 1.776 | 0.080 |
| Date * Warming | 12 | 0.815 | 0.635 |
| Date * Shrub | 12 | 1.006 | 0.443 |
| Date * Sedge | 12 | 2.173 | 0.013 |
| Date * Warming * Shrub | 14 | 1.735 | 0.049 |
| Date * Shrub * Sedge | 14 | 2.428 | 0.003 |
| Date * Warming * Shrub * Sedge | 14 | 1.162 | 0.304 |
| Date * Warming * Sedge | 14 | 0.369 | 0.982 |
| Treatment | Year | Soil T (0.05 m) | Soil T (0.2 m) | WTD | Soil Moisture | DOC (0.1 m) | TN (0.1 m) | DOC (0.4 m) | TN (0.4 m) |
|---|---|---|---|---|---|---|---|---|---|
| C | 2015 | −0.282 | −0.190 | 0.463 | −0.462 | 0.289 | 0.287 | n | n |
| 2016 | −0.161 | −0.265 | −0.090 | 0.304 | −0.133 | 0.217 | 0.258 | 0.457 | |
| -Se | 2015 | −0.173 | 0.142 | −0.084 | −0.328 | 0.181 | −0.061 | n | n |
| 2016 | 0.417 | 0.458 | −0.160 | 0.102 | −0.016 | −0.290 | −0.116 | 0.149 | |
| -Sh | 2015 | −0.220 | 0.029 | 0.155 | −0.375 | 0.180 | 0.244 | n | n |
| 2016 | 0.031 | 0.046 | −0.002 | 0.574 | −0.067 | −0.131 | −0.019 | −0.029 | |
| -Sh-Se | 2015 | −0.119 | −0.053 | 0.268 | 0.150 | 0.028 | 0.104 | n | n |
| 2016 | −0.316 | −0.214 | 0.109 | −0.176 | −0.201 | −0.072 | −0.159 | −0.285 | |
| W | 2015 | −0.268 | −0.236 | 0.212 | −0.344 | 0.093 | 0.031 | n | n |
| 2016 | 0.112 | 0.157 | −0.114 | 0.288 | 0.113 | −0.016 | −0.234 | −0.006 | |
| W-Se | 2015 | −0.019 | 0.424 | 0.222 | −0.442 | 0.093 | −0.270 | n | n |
| 2016 | 0.243 | 0.306 | 0.407 | 0.340 | 0.018 | 0.122 | −0.226 | 0.548 | |
| W-Sh | 2015 | −0.238 | −0.252 | −0.262 | 0.065 | −0.225 | −0.063 | n | n |
| 2016 | −0.047 | −0.091 | −0.224 | −0.543 | 0.008 | 0.436 | 0.308 | 0.256 | |
| W-Sh-Se | 2015 | −0.058 | −0.054 | −0.379 | −0.037 | 0.239 | −0.124 | n | n |
| 2016 | −0.447 | −0.473 | −0.072 | 0.551 | −0.275 | 0.008 | 0.405 | 0.280 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Wu, J.; Vogt, J.; Le, T.B.; Yuan, T. Combination of Warming and Vegetation Composition Change Strengthens the Environmental Controls on N2O Fluxes in a Boreal Peatland. Atmosphere 2018, 9, 480. https://doi.org/10.3390/atmos9120480
Gong Y, Wu J, Vogt J, Le TB, Yuan T. Combination of Warming and Vegetation Composition Change Strengthens the Environmental Controls on N2O Fluxes in a Boreal Peatland. Atmosphere. 2018; 9(12):480. https://doi.org/10.3390/atmos9120480
Chicago/Turabian StyleGong, Yu, Jianghua Wu, Judith Vogt, Thuong Ba Le, and Tao Yuan. 2018. "Combination of Warming and Vegetation Composition Change Strengthens the Environmental Controls on N2O Fluxes in a Boreal Peatland" Atmosphere 9, no. 12: 480. https://doi.org/10.3390/atmos9120480
APA StyleGong, Y., Wu, J., Vogt, J., Le, T. B., & Yuan, T. (2018). Combination of Warming and Vegetation Composition Change Strengthens the Environmental Controls on N2O Fluxes in a Boreal Peatland. Atmosphere, 9(12), 480. https://doi.org/10.3390/atmos9120480






