Review: The Use of Real-Time Fluorescence Instrumentation to Monitor Ambient Primary Biological Aerosol Particles (PBAP)
Abstract
1. Introduction
1.1. PBAP Fluorescence:
1.2. Interferents—Non-Biological Compounds
2. Instrumentation and Operation
2.1. UV-APS: Ultraviolet Aerodynamic Particle Sizer
2.1.1. Laboratory-Based Studies
2.1.2. Field-Based Campaigns
2.1.3. Agricultural Campaigns
2.1.4. Indoor Campaigns
2.1.5. Ice Nucleation Studies
2.2. WIBS: The Wideband Integrated Bioaerosol Sensor
2.2.1. Laboratory Studies
2.2.2. Field Campaigns in Ambient Outdoor and Indoor Environments
2.2.3. Ice Nucleation (IN) and Rain Studies
2.2.4. Occupational Site Campaigns
2.3. BioScout
3. Overview of the Use of Real-Time Bioaerosol Detectors
4. Intercomparisons of the Real-Time Fluorescence Devices
5. Future Instrumental Developments
5.1. PA-300
5.2. WIBS-4+ and WIBS-Neo
5.3. Multiparameter Bioaerosol Spectrometer (MBS)
5.4. SIBS
6. Caveat to New Instrumental Developments
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Zhu, Y.; Hinds, W.C.; Kim, S.; Shen, S.; Sioutas, C. Study of ultrafine particles near a major highway with heavy-duty diesel traffic. Atmos. Environ. 2002, 36, 4323–4335. [Google Scholar] [CrossRef]
- Després, V.R.; Huffman, J.A.; Burrows, S.M.; Hoose, C.; Safatov, A.S.; Buryak, G.; Fröhlich-Nowoisky, J.; Elbert, W.; Andreae, M.O.; Pöschl, U. Primary biological aerosol particles in the atmosphere: A review. Tellus B 2012, 64. [Google Scholar] [CrossRef]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol health effects and exposure assessment: Progress and prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [PubMed]
- Séguin, V.; Lemauviel-Lavenant, S.; Garon, D.; Bouchart, V.; Gallard, Y.; Blanchet, B.; Diquelou, S.; Personeni, E.; Gauduchon, P.; Ourry, A. Effect of agricultural and environmental factors on the hay characteristics involved in equine respiratory disease. Agric. Ecosyst. Environ. 2010, 135, 206–215. [Google Scholar] [CrossRef]
- Sorenson, W.G. Fungal spores: Hazardous to health? Environ. Health Perspect. 1999, 107, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Peraica, M.; Radic, B.; Lucic, A.; Pavlovic, M. Toxic effects of mycotoxins in humans. Bull. World Health Organ. 1999, 77, 754–766. [Google Scholar] [PubMed]
- Pope, C.A., III; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Jerrett, M.; Burnett, R.T.; Pope, C.A., 3rd; Ito, K.; Thurston, G.; Krewski, D.; Shi, Y.; Calle, E.; Thun, M. Long-term ozone exposure and mortality. N. Engl. J. Med. 2009, 360, 1085–1095. [Google Scholar]
- Künzli, N.; Kaiser, R.; Medina, S.; Studnicka, M.; Chanel, O.; Filliger, P.; Herry, M.; Horak, F.; Puybonnieux-Texier, V.; Quénel, P. Public-health impact of outdoor and traffic-related air pollution: A European assessment. Lancet 2000, 356, 795–801. [Google Scholar] [CrossRef]
- Fenger, J. Urban air quality. Atmos. Environ. 1999, 33, 4877–4900. [Google Scholar] [CrossRef]
- Haga, D.I.; Burrows, S.M.; Iannone, R.; Wheeler, M.J.; Mason, R.H.; Chen, J.; Polishchuk, E.A.; Pöschl, U.; Bertram, A.K. Ice nucleation by fungal spores from the classes agaricomycetes, ustilaginomycetes, and eurotiomycetes, and the effect on the atmospheric transport of these spores. Atmos. Chem. Phys. 2014, 14, 8611–8630. [Google Scholar] [CrossRef]
- Rogers, D.C.; DeMott, P.J.; Kreidenweis, S.M.; Chen, Y. Measurements of ice nucleating aerosols during SUCCESS. Geophys. Res. Lett. 1998, 25, 1383–1386. [Google Scholar] [CrossRef]
- Mason, R.; Si, M.; Li, J.; Chou, C.; Dickie, R.; Toom-Sauntry, D.; Pöhlker, C.; Yakobi-Hancock, J.; Ladino, L.; Jones, K. Ice nucleating particles at a coastal marine boundary layer site: Correlations with aerosol type and meteorological conditions. Atmos. Chem. Phys. 2015, 15, 12547–12566. [Google Scholar] [CrossRef]
- Hoose, C.; Kristjánsson, J.; Burrows, S. How important is biological ice nucleation in clouds on a global scale? Environ. Res. Lett. 2010, 5, 24009. [Google Scholar] [CrossRef]
- Hummel, M.; Hoose, C.; Gallagher, M.; Healy, D.; Huffman, J.; O’Connor, D.; Pöschl, U.; Pöhlker, C.; Robinson, N.; Schnaiter, M. Regional-scale simulations of fungal spore aerosols using an emission parameterization adapted to local measurements of fluorescent biological aerosol particles. Atmos. Chem. Phys. 2015, 15, 6127–6146. [Google Scholar] [CrossRef]
- O’Gorman, C.M.; Fuller, H.T. Prevalence of culturable airborne spores of selected allergenic and pathogenic fungi in outdoor air. Atmos. Environ. 2008, 42, 4355–4368. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Sadyś, M.; Skjøth, C.A.; Healy, D.A.; Kennedy, R.; Sodeau, J.R. Atmospheric concentrations of Alternaria, Cladosporium, Ganoderma and Didymella spores monitored in Cork (Ireland) and Worcester (England) during the summer of 2010. Aerobiologia 2014, 30, 397–411. [Google Scholar] [CrossRef]
- Skjøth, C.A.; Sommer, J.; Stach, A.; Smith, M.; Brandt, J. The long-range transport of birch (Betula) pollen from Poland and Germany causes significant pre-season concentrations in Denmark. Clin. Exp. Allergy 2007, 37, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Galán, C.; Alcázar, P.; Cariñanos, P.; Garcia, H.; Domínguez-Vilches, E. Meteorological factors affecting daily Urticaceae pollen counts in southwest Spain. Int. J. Biometeorol. 2000, 43, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Gabey, A.; Stanley, W.; Gallagher, M.; Kaye, P.H. The fluorescence properties of aerosol larger than 0.8 μm in urban and tropical rainforest locations. Atmos. Chem. Phys. 2011, 11, 5491–5504. [Google Scholar] [CrossRef]
- O’Connor, D.; Healy, D.; Sodeau, J. A 1-month online monitoring campaign of ambient fungal spore concentrations in the harbour region of Cork, Ireland. Aerobiologia 2015, 31, 295–314. [Google Scholar] [CrossRef]
- Huffman, J.; Treutlein, B.; Pöschl, U. Fluorescent biological aerosol particle concentrations and size distributions measured with an Ultraviolet Aerodynamic Particle Sizer (UV-APS) in Central Europe. Atmos. Chem. Phys. 2010, 10, 3215–3233. [Google Scholar] [CrossRef]
- Pöhlker, C.; Huffman, J.; Poschl, U. Autofluorescence of atmospheric bioaerosols-fluorescent biomolecules and potential interferences. Atmos. Meas. Tech. 2012, 5, 37–71. [Google Scholar] [CrossRef]
- Pöhlker, C.; Huffman, J.A.; Förster, J.-D.; Pöschl, U. Autofluorescence of atmospheric bioaerosols: Spectral fingerprints and taxonomic trends of pollen. Atmos. Meas. Tech. 2013, 6, 3369–3392. [Google Scholar] [CrossRef]
- Roshchina, V.V. Fluorescing World of Plant Secreting Cells; Science Publishers: New York, NY, USA, 2008. [Google Scholar]
- Weber, G.; Teale, F. Determination of the absolute quantum yield of fluorescent solutions. Trans. Faraday Soc. 1957, 53, 646–655. [Google Scholar] [CrossRef]
- Kunit, M.; Puxbaum, H. Enzymatic determination of the cellulose content of atmospheric aerosols. Atmos. Environ. 1996, 30, 1233–1236. [Google Scholar] [CrossRef]
- Winiwarter, W.; Bauer, H.; Caseiro, A.; Puxbaum, H. Quantifying emissions of primary biological aerosol particle mass in Europe. Atmos. Environ. 2009, 43, 1403–1409. [Google Scholar] [CrossRef]
- Mel’nikova, Y.V.; Roshchina, V.; Karnaukhov, V. Microspectrofluorimetry of intact plant pollen. Biophysics 1997, 1, 243–251. [Google Scholar]
- O’Connor, D.J.; Iacopino, D.; Healy, D.A.; O’Sullivan, D.; Sodeau, J.R. The intrinsic fluorescence spectra of selected pollen and fungal spores. Atmos. Environ. 2011, 45, 6451–6458. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Healy, D.A.; Hellebust, S.; Buters, J.T.; Sodeau, J.R. Using the WIBS-4 (Waveband Integrated Bioaerosol Sensor) Technique for the On-Line Detection of Pollen Grains. Aerosol Sci. Technol. 2014, 48, 341–349. [Google Scholar] [CrossRef]
- Jabaji-Hare, S.; Perumalla, C.; Kendrick, W. Autofluorescence of vesicles, arbuscules, and intercellular hyphae of a vesicular–arbuscular fungus in leek (Allium porrum) roots. Can. J. Bot. 1984, 62, 2665–2669. [Google Scholar] [CrossRef]
- Albinsson, B.; Li, S.; Lundquist, K.; Stomberg, R. The origin of lignin fluorescence. J. Mol. Struct. 1999, 508, 19–27. [Google Scholar] [CrossRef]
- Kozioł, J.; Knobloch, E. The solvent effect on the fluorescence and light absorption of riboflavin and lumiflavin. Biochim. Biophys. Acta (BBA)-Biophys. Incl. Photosynth. 1965, 102, 289–300. [Google Scholar]
- O’Connor, D.J.; Lovera, P.; Iacopino, D.; O’Riordan, A.; Healy, D.A.; Sodeau, J.R. Using spectral analysis and fluorescence lifetimes to discriminate between grass and tree pollen for aerobiological applications. Anal. Methods 2014, 6, 1633–1639. [Google Scholar] [CrossRef]
- Pisarevskii, A.; Cherenkevich, S.; Andrianov, V. Fluorescence spectrum and quantum yield of DNA in solution. J. Appl. Spectrosc. 1966, 5, 452–454. [Google Scholar] [CrossRef]
- Morgan, J.P.; Daniels, M. Excited states of DNA and its components at room temperature—III. Spectra, polarisation and quantum yields of emissions from ApA and poly rA. Photochem. Photobiol. 1980, 31, 101–113. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Shen, B.; Gryczynski, Z.; D’Auria, S.; Gryczynski, I. Intrinsic fluorescence from DNA can be enhanced by metallic particles. Biochem. Biophys. Res. Commun. 2001, 286, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Roshchina, V.; Mel’nikova, E.; Kovaleva, L. Changes in fluorescence during development of the male gametophyte. Russ. J. Plant Physiol. 1997, 44, 36–44. [Google Scholar]
- Saxena, P. Chemistry of Alkaloids; Discovery Publishing House: New Delhi, India, 2007. [Google Scholar]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Hudson, N.; Baker, A.; Reynolds, D. Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters—A review. River Res. Appl. 2007, 23, 631–649. [Google Scholar] [CrossRef]
- Muller, C.L.; Baker, A.; Hutchinson, R.; Fairchild, I.J.; Kidd, C. Analysis of rainwater dissolved organic carbon compounds using fluorescence spectrophotometry. Atmos. Environ. 2008, 42, 8036–8045. [Google Scholar] [CrossRef]
- Bones, D.L.; Henricksen, D.K.; Mang, S.A.; Gonsior, M.; Bateman, A.P.; Nguyen, T.B.; Cooper, W.J.; Nizkorodov, S.A. Appearance of strong absorbers and fluorophores in limonene-O3 secondary organic aerosol due to NH4+-mediated chemical aging over long time scales. J. Geophys. Res. Atmos. 2010, 115. [Google Scholar] [CrossRef]
- Monks, P.; Granier, C.; Fuzzi, S.; Stohl, A.; Williams, M.; Akimoto, H.; Amann, M.; Baklanov, A.; Baltensperger, U.; Bey, I. Atmospheric composition change–global and regional air quality. Atmos. Environ. 2009, 43, 5268–5350. [Google Scholar] [CrossRef]
- Gillette, D.A.; Walker, T.R. Characteristics of airborne particles produced by wind erosion of sandy soil, High Plains of west Texas. Soil Sci. 1977, 123, 97–110. [Google Scholar] [CrossRef]
- Andreae, M.; Rosenfeld, D. Aerosol–cloud–precipitation interactions. Part 1. The nature and sources of cloud-active aerosols. Earth-Sci. Rev. 2008, 89, 13–41. [Google Scholar] [CrossRef]
- Bozlee, B.J.; Misra, A.K.; Sharma, S.K.; Ingram, M. Remote Raman and fluorescence studies of mineral samples. Spectrochim. Acta Part A 2005, 61, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications; Academic Press: Waltham, MA, USA, 1999. [Google Scholar]
- Slowik, J.G.; Cross, E.S.; Han, J.-H.; Kolucki, J.; Davidovits, P.; Williams, L.R.; Onasch, T.B.; Jayne, J.T.; Kolb, C.E.; Worsnop, D.R. Measurements of morphology changes of fractal soot particles using coating and denuding experiments: Implications for optical absorption and atmospheric lifetime. Aerosol Sci. Technol. 2007, 41, 734–750. [Google Scholar] [CrossRef]
- Kumke, M.; Löhmannsröben, H.-G.; Roch, T. Fluorescence spectroscopy of polynuclear aromatic compounds in environmental monitoring. J. Fluoresc. 1995, 5, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Lewitzka, F.; Niessner, R. Application of time-resolved fluorescence spectroscopy on the analysis of PAH-coated aerosols. Aerosol Sci. Technol. 1995, 23, 454–464. [Google Scholar] [CrossRef]
- Panne, U.; Knöller, A.; Kotzick, R.; Niessner, R. On-line and in-situ detection of polycyclic aromatic hydrocarbons (PAH) on aerosols via thermodesorption and laser-induced fluorescence spectroscopy. Fresenius J. Anal. Chem. 2000, 366, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Savage, N.; Krentz, C.; Könemann, T.; Han, T.T.; Mainelis, G.; Pöhlker, C.; Huffman, J.A. Systematic Characterization and Fluorescence Threshold Strategies for the Wideband Integrated Bioaerosol Sensor (WIBS) Using Size-Resolved Biological and Interfering Particles. Atmos. Meas. Tech. 2017, 10, 4279–4302. [Google Scholar] [CrossRef]
- Hairston, P.P.; Ho, J.; Quant, F.R. Design of an instrument for real-time detection of bioaerosols using simultaneous measurement of particle aerodynamic size and intrinsic fluorescence. J. Aerosol Sci. 1997, 28, 471–482. [Google Scholar] [CrossRef]
- Huffman, J.; Sinha, B.; Garland, R.; Snee-Pollmann, A.; Gunthe, S.; Artaxo, P.; Martin, S.; Andreae, M.; Pöschl, U. Size distributions and temporal variations of biological aerosol particles in the Amazon rainforest characterized by microscopy and real-time UV-APS fluorescence techniques during AMAZE-08. Atmos. Chem. Phys. 2012, 12, 11997–12019. [Google Scholar] [CrossRef]
- Morawska, L.; Johnson, G.; Ristovski, Z.; Hargreaves, M.; Mengersen, K.; Chao, C.; Wan, M.P.; Li, Y.; Xie, X.; Katoshevski, D. Droplets expelled during human expiratory activities and their origin. In Proceedings of the International Conference on Indoor Air Quality and Climate, Copenhagen, Denmark, 17–22 August 2008. [Google Scholar]
- Brosseau, L.M.; Vesley, D.; Rice, N.; Goodell, K.; Nellis, M.; Hairston, P. Differences in detected fluorescence among several bacterial species measured with a direct-reading particle sizer and fluorescence detector. Aerosol Sci. Technol. 2000, 32, 545–558. [Google Scholar] [CrossRef]
- Kulkarni, P.; Baron, P.A.; Willeke, K. Aerosol Measurement: Principles, Techniques, and Applications; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Kanaani, H.; Hargreaves, M.; Ristovski, Z.; Morawska, L. Performance assessment of UVAPS: Influence of fungal spore age and air exposure. J. Aerosol Sci. 2007, 38, 83–96. [Google Scholar] [CrossRef]
- Agranovski, V.; Ristovski, Z.D.; Ayoko, G.A.; Morawska, L. Performance evaluation of the UVAPS in measuring biological aerosols: Fluorescence spectra from NAD (P) H coenzymes and riboflavin. Aerosol Sci. Technol. 2004, 38, 354–364. [Google Scholar] [CrossRef]
- Knibbs, L.D.; He, C.; Duchaine, C.; Morawska, L. Vacuum cleaner emissions as a source of indoor exposure to airborne particles and bacteria. Environ. Sci. Technol. 2011, 46, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Lee, J.E.; Hwang, G.B.; Lee, B.U.; Lee, S.B.; Jurng, J.S.; Bae, G.N. Electrospray-assisted ultraviolet aerodynamic particle sizer spectrometer for real-time characterization of bacterial particles. Anal. Chem. 2009, 82, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.R.; Morawska, L. The mechanism of breath aerosol formation. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Perrott, P.; Hargreaves, M. Detection of Bacteriophage in Droplets. In Human Respiratory Syncytial Virus Infection; InTech: Lexington, KY, USA, 2011. [Google Scholar]
- Agranovski, V.; Ristovski, Z.; Hargreaves, M.; Blackall, P.J.; Morawska, L. Performance evaluation of the UVAPS: Influence of physiological age of airborne bacteria and bacterial stress. J. Aerosol Sci. 2003, 34, 1711–1727. [Google Scholar] [CrossRef]
- Kaliszewski, M.; Trafny, E.A.; Lewandowski, R.; Włodarski, M.; Bombalska, A.; Kopczyński, K.; Antos-Bielska, M.; Szpakowska, M.; Młyńczak, J.; Mularczyk-Oliwa, M.; et al. A new approach to UVAPS data analysis towards detection of biological aerosol. J. Aerosol Sci. 2013, 58, 148–157. [Google Scholar] [CrossRef]
- Ratnesar-Shumate, S.; Wagner, M.L.; Kerechanin, C.; House, G.; Brinkley, K.M.; Bare, C.; Baker, N.A.; Quizon, R.; Quizon, J.; Proescher, A. Improved method for the evaluation of real-time biological aerosol detection technologies. Aerosol Sci. Technol. 2011, 45, 635–644. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, M. Liquid impinger BioSampler’s performance for size-resolved viable bioaerosol particles. J. Aerosol Sci. 2017, 106, 34–42. [Google Scholar] [CrossRef]
- Ratnesar-Shumate, S.; Pan, Y.-L.; Hill, S.C.; Kinahan, S.; Corson, E.; Eshbaugh, J.; Santarpia, J.L. Fluorescence spectra and biological activity of aerosolized bacillus spores and MS2 bacteriophage exposed to ozone at different relative humidities in a rotating drum. J. Quant. Spectrosc. Radiat. Transf. 2015, 153, 13–28. [Google Scholar] [CrossRef]
- Zou, Z.; Yao, M. Airflow resistance and bio-filtering performance of carbon nanotube filters and current facepiece respirators. J. Aerosol Sci. 2015, 79, 61–71. [Google Scholar] [CrossRef]
- Santarpia, J.L.; Pan, Y.-L.; Hill, S.C.; Baker, N.; Cottrell, B.; McKee, L.; Ratnesar-Shumate, S.; Pinnick, R.G. Changes in fluorescence spectra of bioaerosols exposed to ozone in a laboratory reaction chamber to simulate atmospheric aging. Opt. Express 2012, 20, 29867–29881. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-L.; Santarpia, J.L.; Ratnesar-Shumate, S.; Corson, E.; Eshbaugh, J.; Hill, S.C.; Williamson, C.C.; Coleman, M.; Bare, C.; Kinahan, S. Effects of ozone and relative humidity on fluorescence spectra of octapeptide bioaerosol particles. J. Quant. Spectrosc. Radiat. Transf. 2014, 133, 538–550. [Google Scholar] [CrossRef]
- Bhangar, S.; Adams, R.I.; Pasut, W.; Huffman, J.; Arens, E.A.; Taylor, J.W.; Bruns, T.D.; Nazaroff, W.W. Chamber bioaerosol study: Human emissions of size-resolved fluorescent biological aerosol particles. Indoor Air 2016, 26, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Yoon, K.Y.; Byeon, J.H.; Kim, K.; Hwang, J. Development of rapid assessment method to determine bacterial viability based on ultraviolet and visible (UV-Vis) spectroscopy analysis including application to bioaerosols. Aerosol Air Qual. Res. 2012, 12, 395–404. [Google Scholar] [CrossRef]
- Saari, S.; Putkiranta, M.; Keskinen, J. Fluorescence spectroscopy of atmospherically relevant bacterial and fungal spores and potential interferences. Atmos. Environ. 2013, 71, 202–209. [Google Scholar] [CrossRef]
- Kanaani, H.; Hargreaves, M.; Ristovski, Z.; Morawska, L. Deposition rates of fungal spores in indoor environments, factors effecting them and comparison with non-biological aerosols. Atmos. Environ. 2008, 42, 7141–7154. [Google Scholar] [CrossRef]
- Cieślik, I.; Żmija, J.; Majchrowski, A.; Pępczyńska, M.; Morawiak, P.; Włodarski, M. Synthesis and characteristics of optical properties of crystalline YAl3(BO3)4:Cr,Ce. J. Achiev. Mater. Manuf. Eng. 2011, 48, 24–28. [Google Scholar]
- Cieślik, I.; Węgłowski, R.; Żmija, J.; Kurzydłowski, K.; Płocińska, M.; Oćwieja, M. Control of optical active borates nanocrystals agglomeration. J. Achiev. Mater. Manuf. Eng. 2013, 61, 163–168. [Google Scholar]
- Agranovski, V.; Ristovski, Z.D. Real-time monitoring of viable bioaerosols: Capability of the UVAPS to predict the amount of individual microorganisms in aerosol particles. J. Aerosol Sci. 2005, 36, 665–676. [Google Scholar] [CrossRef]
- Kanaani, H.; Hargreaves, M.; Smith, J.; Ristovski, Z.; Agranovski, V.; Morawska, L. Performance of UVAPS with respect to detection of airborne fungi. J. Aerosol Sci. 2008, 39, 175–189. [Google Scholar] [CrossRef]
- Agranovski, V.; Ristovski, Z.; Hargreaves, M.; Blackall, P.J.; Morawska, L. Real-time measurement of bacterial aerosols with the UVAPS: Performance evaluation. J. Aerosol Sci. 2003, 34, 301–317. [Google Scholar] [CrossRef]
- Roshchina, V.; Karnaukhov, V. Changes in pollen autofluorescence induced by ozone. Biol. Plant. 1999, 42, 273–278. [Google Scholar] [CrossRef]
- Schumacher, C.; Pöhlker, C.; Aalto, P.; Hiltunen, V.; Petäjä, T.; Kulmala, M.; Pöschl, U.; Huffman, J. Seasonal cycles of fluorescent biological aerosol particles in boreal and semi-arid forests of Finland and Colorado. Atmos. Chem. Phys. 2013, 13, 11987–12001. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.; Zhang, X.; Xu, C.; Dong, L.; Yao, M. Bioaerosol emissions and detection of airborne antibiotic resistance genes from a wastewater treatment plant. Atmos. Environ. 2016, 124, 404–412. [Google Scholar] [CrossRef]
- Valsan, A.; Ravikrishna, R.; Biju, C.; Pöhlker, C.; Després, V.; Huffman, J.; Pöschl, U.; Gunthe, S. Fluorescent biological aerosol particle measurements at a tropical high-altitude site in southern India during the southwest monsoon season. Atmos. Chem. Phys. 2016, 16, 9805–9830. [Google Scholar] [CrossRef]
- Pöschl, U.; Martin, S.; Sinha, B.; Chen, Q.; Gunthe, S.; Huffman, J.; Borrmann, S.; Farmer, D.; Garland, R.; Helas, G. Rainforest aerosols as biogenic nuclei of clouds and precipitation in the Amazon. Science 2010, 329, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Hallar, A.; Chirokova, G.; McCubbin, I.; Painter, T.H.; Wiedinmyer, C.; Dodson, C. Atmospheric bioaerosols transported via dust storms in the western United States. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Huffman, J.A.; Prenni, A.; DeMott, P.; Pöhlker, C.; Mason, R.; Robinson, N.; Fröhlich-Nowoisky, J.; Tobo, Y.; Després, V.; Garcia, E.; et al. High concentrations of biological aerosol particles and ice nuclei during and after rain. Atmos. Chem. Phys. 2013, 13, 6151–6164. [Google Scholar] [CrossRef]
- Gosselin, M.I.; Rathnayake, C.M.; Crawford, I.; Pöhlker, C.; Fröhlich-Nowoisky, J.; Schmer, B.; Després, V.R.; Engling, G.; Gallagher, M.; Stone, E.; et al. Fluorescent bioaerosol particle, molecular tracer, and fungal spore concentrations during dry and rainy periods in a semi-arid forest. Atmos. Chem. Phys. 2016, 16, 15165–15184. [Google Scholar] [CrossRef]
- Wei, K.; Zou, Z.; Zheng, Y.; Li, J.; Shen, F.; Wu, C.-Y.; Wu, Y.; Hu, M.; Yao, M. Ambient bioaerosol particle dynamics observed during haze and sunny days in Beijing. Sci. Total Environ. 2016, 550, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Li, X.; Deng, J.; Da, G.; Gehin, E.; Yao, M. Time-dependent size-resolved bacterial and fungal aerosols in Beijing subway. Aerosol Air Qual. Res. 2016, 10, 1–11. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Shen, F.; Zou, Z.; Yao, M.; Wu, C.-y. Characterization of biological aerosol exposure risks from automobile air conditioning system. Environ. Sci. Technol. 2013, 47, 10660–10666. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Zheng, Y.; Li, J.; Shen, F.; Zou, Z.; Fan, H.; Li, X.; Wu, C.-y.; Yao, M. Microbial aerosol characteristics in highly polluted and near-pristine environments featuring different climatic conditions. Sci. Bull. 2015, 60, 1439–1447. [Google Scholar] [CrossRef]
- Saari, S.; Niemi, J.; Rönkkö, T.; Kuuluvainen, H.; Järvinen, A.; Pirjola, L.; Aurela, M.; Hillamo, R.; Keskinen, J. Seasonal and diurnal variations of fluorescent bioaerosol concentration and size distribution in the urban environment. Aerosol Air Qual. Res. 2015, 15, 572–581. [Google Scholar] [CrossRef]
- Manninen, H.E.; Bäck, J.; Sihto-Nissilä, S.-L.; Huffman, J.A.; Pessi, A.-M.; Hiltunen, V.; Aalto, P.P.; Hidalgo Fernández, P.J.; Hari, P.; Saarto, A.; et al. Patterns in airborne pollen and other primary biological aerosol particles (PBAP), and their contribution to aerosol mass and number in a boreal forest. Boreal Environ. Res. 2014, 19, 383–405. [Google Scholar]
- Healy, D.; Huffman, J.; O’Connor, D.; Pöhlker, C.; Pöschl, U.; Sodeau, J. Ambient measurements of biological aerosol particles near Killarney, Ireland: A comparison between real-time fluorescence and microscopy techniques. Atmos. Chem. Phys. 2014, 14, 8055–8069. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Healy, D.A.; Sodeau, J.R. The on-line detection of biological particle emissions from selected agricultural materials using the WIBS-4 (Waveband Integrated Bioaerosol Sensor) technique. Atmos. Environ. 2013, 80, 415–425. [Google Scholar] [CrossRef]
- Hameed, A.A.; Khodr, M. Suspended particulates and bioaerosols emitted from an agricultural non-point source. J. Environ. Monit. 2001, 3, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Agranovski, V.; Ristovski, Z.; Blackall, P.J.; Morawska, L. Size-selective assessment of airborne particles in swine confinement building with the UVAPS. Atmos. Environ. 2004, 38, 3893–3901. [Google Scholar] [CrossRef]
- Xu, C.; Wu, C.-Y.; Yao, M. Fluorescent Bioaerosol Particles Resulting from Human Occupancy with and without Respirators. Aerosol Air Qual. Res. 2017, 17, 198–208. [Google Scholar] [CrossRef]
- Lavoie, J.; Marchand, G.; Cloutier, Y.; Hallé, S.; Nadeau, S.; Duchaine, C.; Pichette, G. Evaluation of bioaerosol exposures during hospital bronchoscopy examinations. Environ. Sci. 2015, 17, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Bhangar, S.; Huffman, J.; Nazaroff, W. Size-resolved fluorescent biological aerosol particle concentrations and occupant emissions in a university classroom. Indoor Air 2014, 24, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.L.; Knibbs, L.D.; He, C.; Grzybowski, P.; Johnson, G.R.; Huffman, J.A.; Bell, S.C.; Wainwright, C.E.; Matte, D.L.; Dominski, F.H. Sources and dynamics of fluorescent particles in hospitals. Indoor Air 2017, 27, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Salonen, H.; Ling, X.; Crilley, L.; Jayasundara, N.; Cheung, H.C.; Hargreaves, M.; Huygens, F.; Knibbs, L.D.; Ayoko, G.A. The impact of flood and post-flood cleaning on airborne microbiological and particle contamination in residential houses. Environ. Int. 2014, 69, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Prenni, A.; Tobo, Y.; Garcia, E.; DeMott, P.; Huffman, J.; McCluskey, C.; Kreidenweis, S.; Prenni, J.; Pöhlker, C.; Pöschl, U. The impact of rain on ice nuclei populations at a forested site in Colorado. Geophys. Res. Lett. 2013, 40, 227–231. [Google Scholar] [CrossRef]
- Kaye, P.H.; Stanley, W.R.; Foot, E.V.J. Fluid-Borne Particle Detector. Google Patents US8711353 B2, 29 April 2014. [Google Scholar]
- Technologies, D.M. Wideband Integrated Bioaerosol Sensor (WIBS-4a) Operator Manual; Droplet Measurement Technologies: Longmont, CO, USA, 2014. [Google Scholar]
- Healy, D.A.; O’Connor, D.J.; Burke, A.M.; Sodeau, J.R. A laboratory assessment of the Waveband Integrated Bioaerosol Sensor (WIBS-4) using individual samples of pollen and fungal spore material. Atmos. Environ. 2012, 60, 534–543. [Google Scholar] [CrossRef]
- Stanley, W.R.; Kaye, P.H.; Foot, V.E.; Barrington, S.J.; Gallagher, M.; Gabey, A. Continuous bioaerosol monitoring in a tropical environment using a UV fluorescence particle spectrometer. Atmos. Sci. Lett. 2011, 12, 195–199. [Google Scholar] [CrossRef]
- Gabey, A.; Gallagher, M.; Whitehead, J.; Dorsey, J.; Kaye, P.H.; Stanley, W. Measurements and comparison of primary biological aerosol above and below a tropical forest canopy using a dual channel fluorescence spectrometer. Atmos. Chem. Phys. 2010, 10, 4453–4466. [Google Scholar] [CrossRef]
- Perring, A.; Schwarz, J.; Baumgardner, D.; Hernandez, M.; Spracklen, D.; Heald, C.; Gao, R.; Kok, G.; McMeeking, G.; McQuaid, J. Airborne observations of regional variation in fluorescent aerosol across the United States. J. Geophys. Res. Atmos. 2015, 120, 1153–1170. [Google Scholar] [CrossRef]
- Robinson, N.H.; Allan, J.; Huffman, J.; Kaye, P.H.; Foot, V.; Gallagher, M. Cluster analysis of WIBS single-particle bioaerosol data. Atmos. Meas. Tech. 2013, 6, 337–347. [Google Scholar] [CrossRef]
- Healy, D.A.; O’Connor, D.J.; Sodeau, J.R. Measurement of the particle counting efficiency of the “Waveband Integrated Bioaerosol Sensor” model number 4 (WIBS-4). J. Aerosol Sci. 2012, 47, 94–99. [Google Scholar] [CrossRef]
- Toprak, E.; Schnaiter, M. Fluorescent biological aerosol particles measured with the Waveband Integrated Bioaerosol Sensor WIBS-4: Laboratory tests combined with a one year field study. Atmos. Chem. Phys. 2013, 13, 225–243. [Google Scholar] [CrossRef]
- Hernandez, M.; Perring, A.E.; McCabe, K.; Kok, G.; Granger, G.; Baumgardner, D. Chamber catalogues of optical and fluorescent signatures distinguish bioaerosol classes. Atmos. Meas. Tech. 2016, 9, 3283–3292. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, W.; Cao, Q.; Yang, L.; Chang, V.C.; Nazaroff, W.W. Influence of moisturizer and relative humidity on human emissions of fluorescent biological aerosol particles. Indoor Air 2017, 27, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.S.; Gao, R.-S.; Schwarz, J.P.; Fahey, D.W.; Perring, A.E. Fluorescence calibration method for single-particle aerosol fluorescence instruments. Atmos. Meas. Tech. 2017, 10, 1755–1768. [Google Scholar] [CrossRef]
- Gabey, A.; Vaitilingom, M.; Freney, E.; Boulon, J.; Sellegri, K.; Gallagher, M.; Crawford, I.; Robinson, N.; Stanley, W.; Kaye, P.H. Observations of fluorescent and biological aerosol at a high-altitude site in central France. Atmos. Chem. Phys. 2013, 13, 7415–7428. [Google Scholar] [CrossRef]
- Crawford, I.; Robinson, N.; Flynn, M.; Foot, V.; Gallagher, M.; Huffman, J.; Stanley, W.; Kaye, P.H. Characterisation of bioaerosol emissions from a Colorado pine forest: Results from the BEACHON-RoMBAS experiment. Atmos. Chem. Phys. 2014, 14, 8559–8578. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Zhang, M.; Kuhn, U.; Xie, Z.; Cheng, Y.; Pöschl, U.; Su, H. Ambient measurement of fluorescent aerosol particles with a WIBS in the Yangtze River Delta of China: Potential impacts of combustion-related aerosol particles. Atmos. Chem. Phys. 2016, 16, 11337–11348. [Google Scholar] [CrossRef]
- Xie, Y.; Fajardo, O.A.; Yan, W.; Zhao, B.; Jiang, J. Six-day measurement of size-resolved indoor fluorescent bioaerosols of outdoor origin in an office. Particuology 2017, 31, 161–169. [Google Scholar] [CrossRef]
- Möhler, O.; DeMott, P.; Vali, G.; Levin, Z. Microbiology and atmospheric processes: The role of biological particles in cloud physics. Biogeosciences 2007, 4, 1059–1071. [Google Scholar] [CrossRef]
- Prenni, A.J.; DeMott, P.J.; Kreidenweis, S.M.; Harrington, J.Y.; Avramov, A.; Verlinde, J.; Tjernström, M.; Long, C.N.; Olsson, P.Q. Can ice-nucleating aerosols affect Arctic seasonal climate? Bull. Am. Meteorol. Soc. 2007, 88, 541–550. [Google Scholar] [CrossRef]
- Prenni, A.J.; Petters, M.D.; Kreidenweis, S.M.; Heald, C.L.; Martin, S.T.; Artaxo, P.; Garland, R.M.; Wollny, A.G.; Pöschl, U. Relative roles of biogenic emissions and Saharan dust as ice nuclei in the Amazon basin. Nat. Geosci. 2009, 2, 402–405. [Google Scholar] [CrossRef]
- Twohy, C.H.; McMeeking, G.R.; DeMott, P.J.; McCluskey, C.S.; Hill, T.C.; Burrows, S.M.; Kulkarni, G.R.; Tanarhte, M.; Kafle, D.N.; Toohey, D.W. Abundance of fluorescent biological aerosol particles at temperatures conducive to the formation of mixed-phase and cirrus clouds. Atmos. Chem. Phys. 2016, 16, 8205–8225. [Google Scholar] [CrossRef]
- Tobo, Y.; Prenni, A.J.; DeMott, P.J.; Huffman, J.A.; McCluskey, C.S.; Tian, G.; Pöhlker, C.; Pöschl, U.; Kreidenweis, S.M. Biological aerosol particles as a key determinant of ice nuclei populations in a forest ecosystem. J. Geophys. Res. Atmos. 2013, 118, 10100–10110. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Daly, S.M.; Sodeau, J.R. On-line monitoring of airborne bioaerosols released from a composting/green waste site. Waste Manag. 2015, 42, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Crawford, I.; Lloyd, G.; Herrmann, E.; Hoyle, C.; Bower, K.; Connolly, P.; Flynn, M.; Kaye, P.; Choularton, T.; Gallagher, M. Observations of fluorescent aerosol–cloud interactions in the free troposphere at the High-Altitude Research Station Jungfraujoch. Atmos. Chem. Phys. 2016, 16, 2273–2284. [Google Scholar] [CrossRef]
- Wright, T.P.; Hader, J.D.; McMeeking, G.R.; Petters, M.D. High relative humidity as a trigger for widespread release of ice nuclei. Aerosol Sci. Technol. 2014, 48, i–v. [Google Scholar] [CrossRef]
- Yue, S.; Ren, H.; Fan, S.; Wei, L.; Zhao, J.; Bao, M.; Hou, S.; Zhan, J.; Zhao, W.; Ren, L.; et al. High Abundance of Fluorescent Biological Aerosol Particles in Winter Beijing, China. ACS Earth Space Chem. 2017, 1, 493–502. [Google Scholar] [CrossRef]
- Saari, S.; Reponen, T.; Keskinen, J. Performance of two fluorescence-based real-time bioaerosol detectors: BioScout vs. UVAPS. Aerosol Sci. Technol. 2013, 48, 371–378. [Google Scholar] [CrossRef]
- Saari, S.; Mensah-Attipoe, J.; Reponen, T.; Veijalainen, A.; Salmela, A.; Pasanen, P.; Keskinen, J. Effects of fungal species, cultivation time, growth substrate, and air exposure velocity on the fluorescence properties of airborne fungal spores. Indoor Air 2015, 25, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, Y.; Ma, Z.; Jin, Z.; Liu, X.; Wang, H.; Liu, Y.; Wang, J.; Jantunen, M.; Bi, J. Spatial and temporal trends in the mortality burden of air pollution in China: 2004–2012. Environ. Int. 2017, 98, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, D.; Bonacina, L.; Wolf, J.-P. Individual bioaerosol particle discrimination by multi-photon excited fluorescence. Opt. Express 2011, 19, 24516–24521. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, D.; Bonacina, L.; Wolf, J.-P. A flash-lamp based device for fluorescence detection and identification of individual pollen grains. Rev. Sci. Instrum. 2013, 84, 33302. [Google Scholar] [CrossRef] [PubMed]
- Crouzy, B.; Stella, M.; Konzelmann, T.; Calpini, B.; Clot, B. All-optical automatic pollen identification: Towards an operational system. Atmos. Environ. 2016, 140, 202–212. [Google Scholar] [CrossRef]
- Ruske, S.; Topping, D.O.; Foot, V.E.; Kaye, P.H.; Stanley, W.R.; Crawford, I.; Morse, A.P.; Gallagher, M.W. Evaluation of machine learning algorithms for classification of primary biological aerosol using a new UV-LIF spectrometer. Atmos. Meas. Tech. 2017, 10, 695–708. [Google Scholar] [CrossRef]
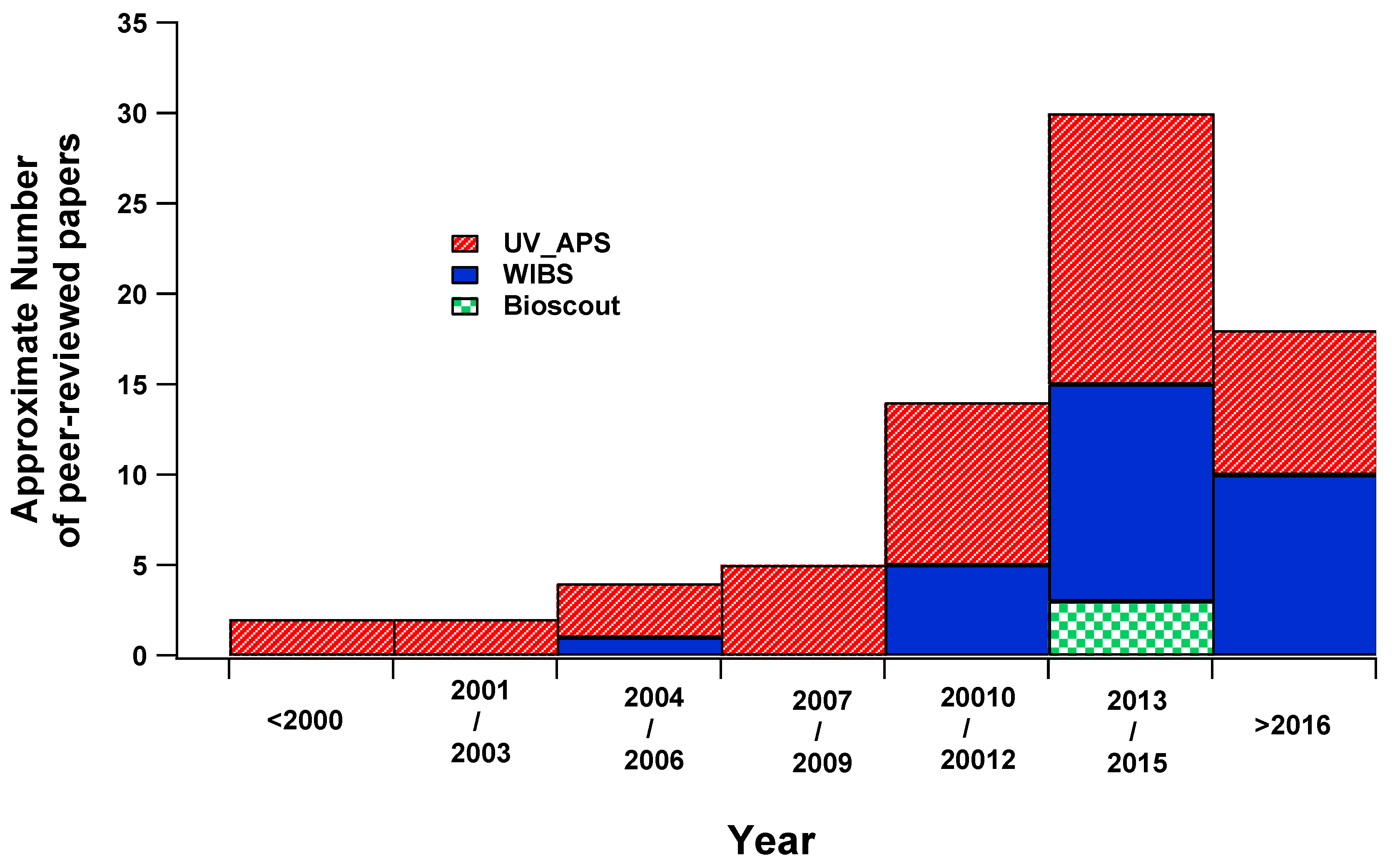
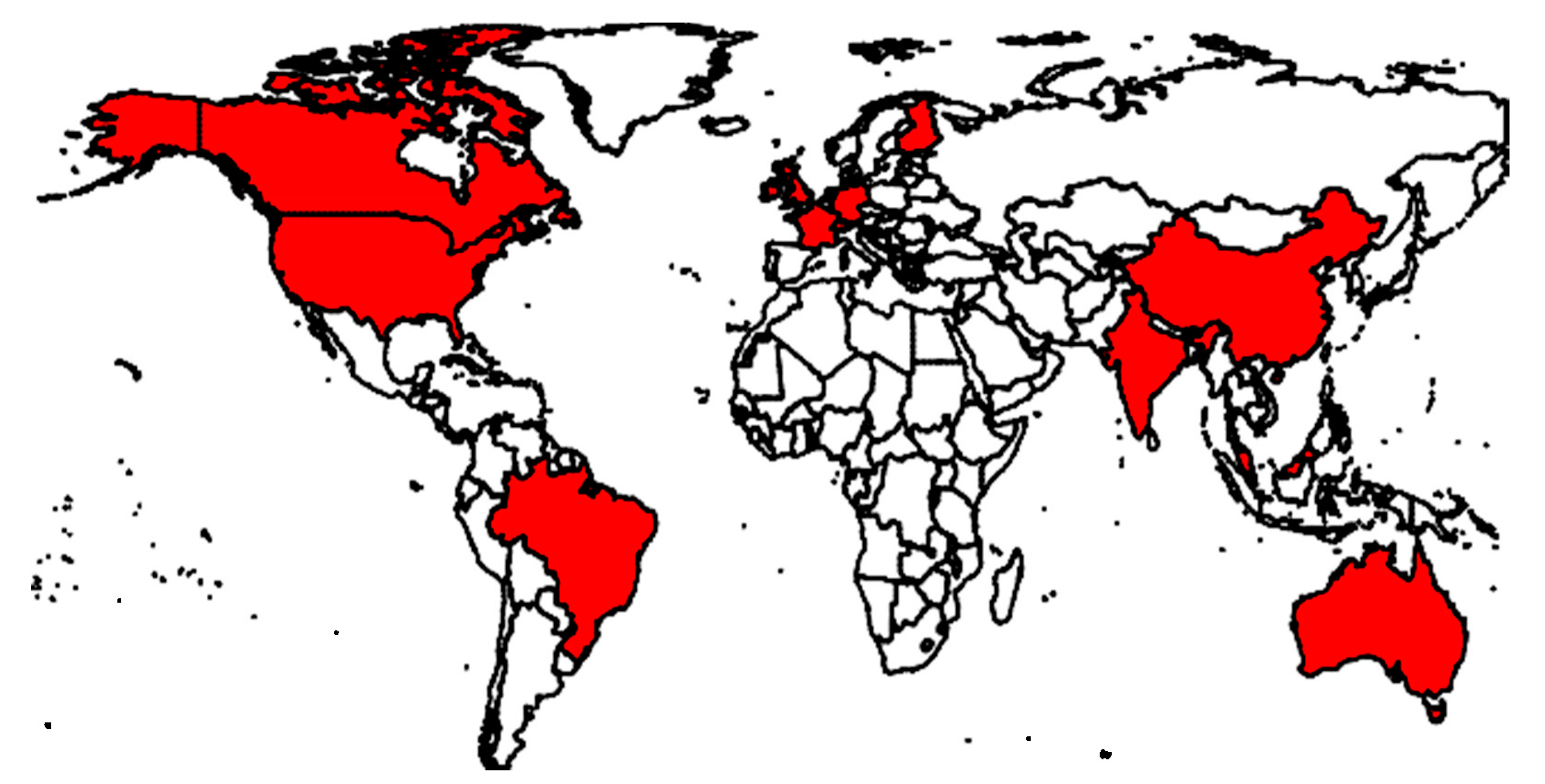
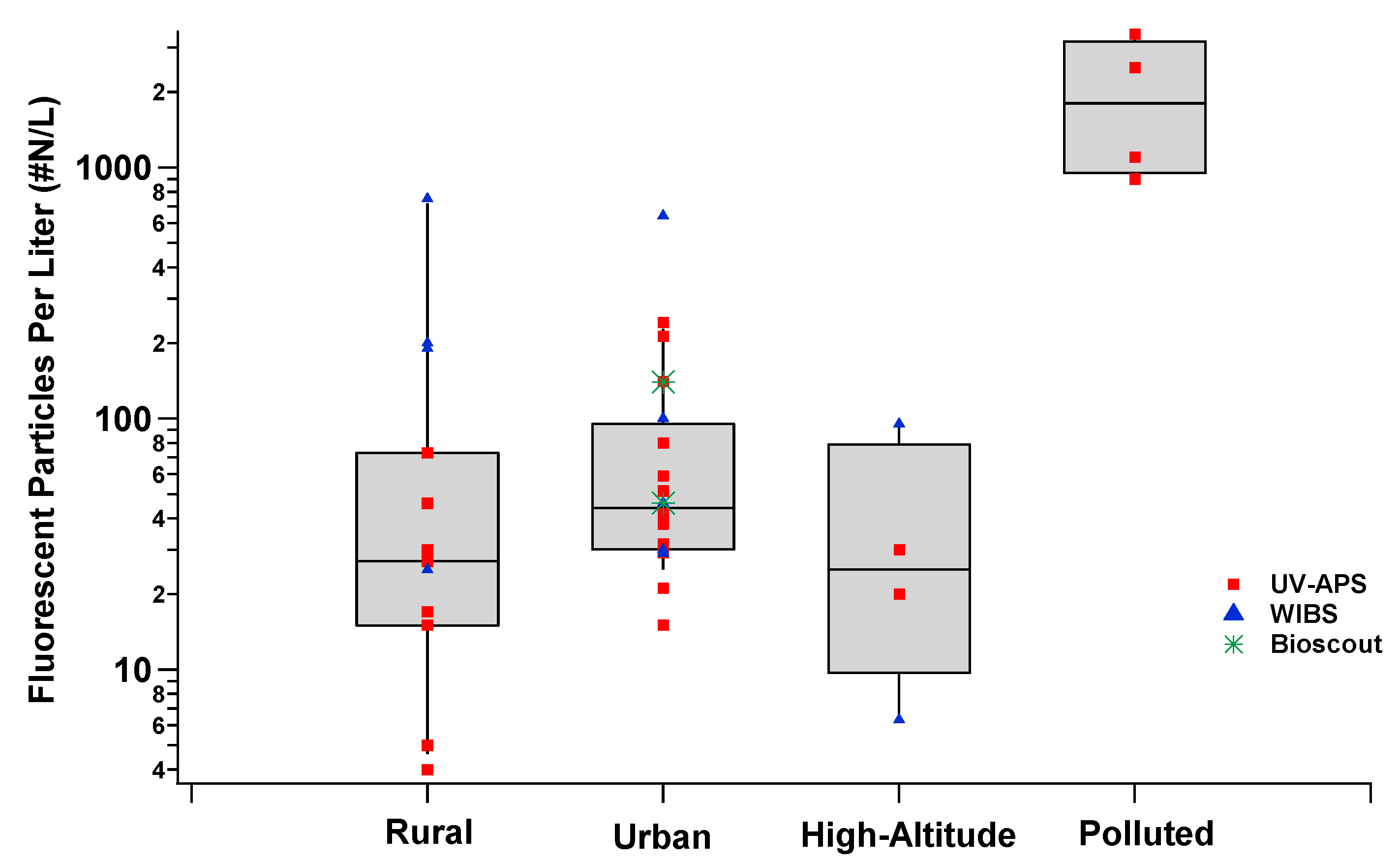
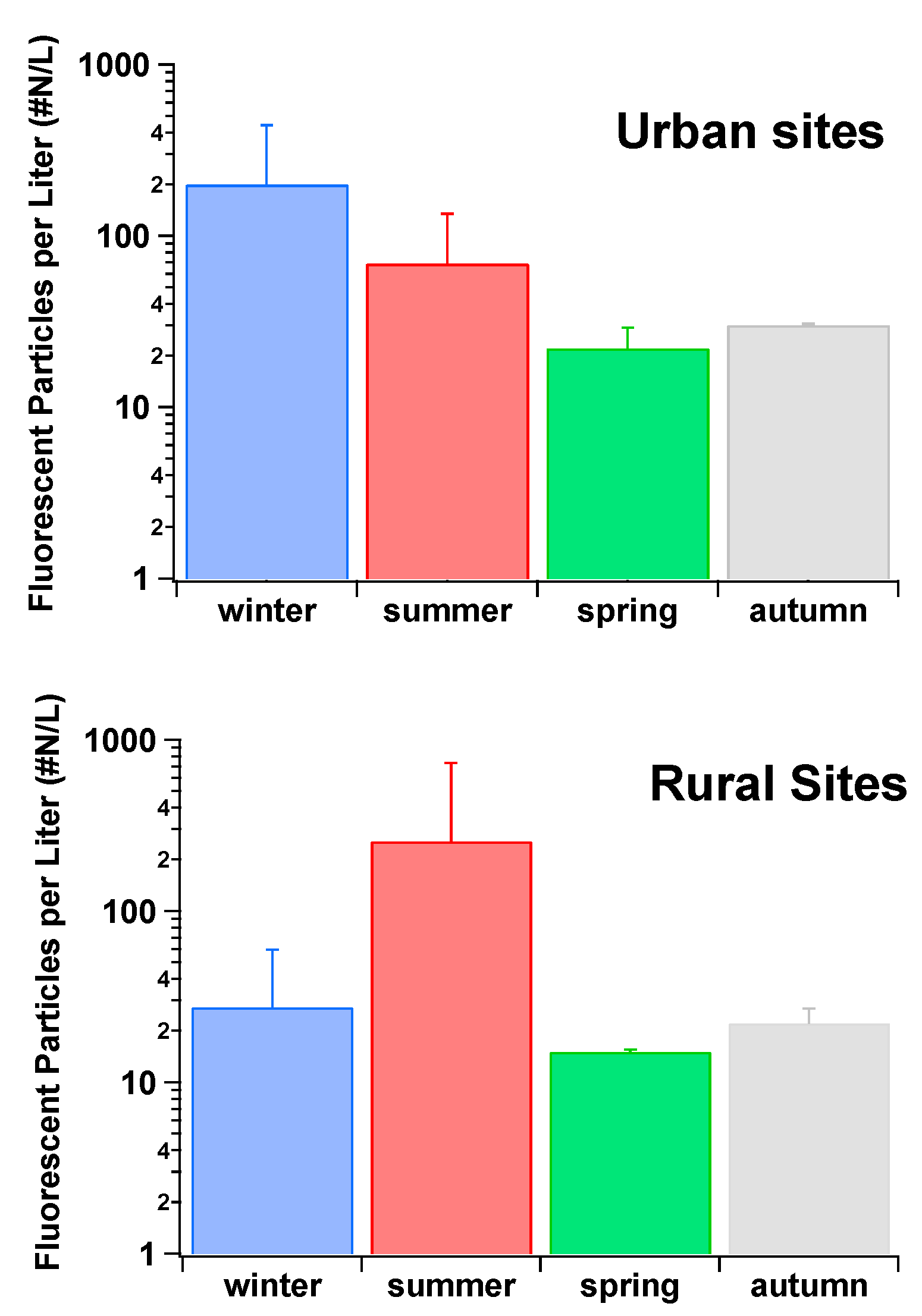
| Fluorophore | Excitation Wavelengths (nm) | Emission Wavelengths (nm) |
|---|---|---|
| Aminio acids | 260–295 | 280–360 |
| NADH and NAD(P)H | 290–295, 340–366 | 440–470 |
| Flavins | 450–488 | 520–560 |
| Cellulose | 250–350 | 350–500 |
| Chitin | 335 | 413 |
| Lignin | 240–320 | 360 |
| Melanin | 469–471 | 543–548 |
| Sporopollenin | 300–550 | 400–650 |
| Chlorophyll | 390–470 | 630–730 |
| Flavonoids | 365 | 440–610 |
| Carotenoids | 400–500 | 520–560 |
| Alkaloids | 360–380 | 410–600 |
| Nucleic acids (DNA) | 270, 320 | 280–370 and 350–470 |
| Terpenoids | 250–395 | 400–725 |
| Phenolic compounds | 300–380 | 400–500 |
| Location | Site | Length | Particle Type Analyzed | Concentration Values (Mean or Peak) | Reference |
|---|---|---|---|---|---|
| Mainz, Germany | Semi-urban | 4 months | FAPs (1–20 μm) | 30 L−1 (mean) | [23] |
| Central Amazonia, Brazil | Rainforest | ~1 month | FAPs (>1 μm) | 73 L−1 (mean) | [57,88] |
| Colorado, USA | High altitude | ~2 months | FAPs (>0.54 μm) | 30 ± 10 L−1 (mean) | [89] |
| Colorado, USA | Semi-arid forest | 10 months | FBAPs | 15 ± 24 L−1 (spring) (mean); 30 ± 30 L−1 (summer) (mean); 17 ± 31 L−1 (fall) (mean); 5.3 ± 6.3 L−1 (winter) (mean) | [85] |
| Colorado, USA | Semi-arid, forest | 35 days | FAPs (0.3–20 μm) | 30 L−1 (dry periods) (mean) | [90] |
| Colorado, USA | Forest | ~1 month | FAPs (0.5–20 μm) | ~400 L−1 (peak) | [91] |
| Beijing, China | Waste water plant | NA | FAPs | >2 μm 6.533 L−1 (peak); <2 μm 3.867 L−1 (peak) | [86] |
| Beijing, China | Urban | 2 weeks | FAPs | 500 L−1 (peak) | [92] |
| Beijing, China | Subway | ~1 month | FAPs | 2.5 × 103 L−1 | [93] |
| Multiple sites, China | Urban | March–April | FAPs (0.5–20 μm) | N/A | [94] |
| Multiple sites, China | Urban | 12 days | FAPs | 5 to 470 L−1 (range), 79 L−1 (mean) | [95] |
| Helsinki, Finland | Urban | 23 days (winter); ~60 days (summer) | FAPs (0.5–15 μm) | 10–28 L−1 (range), 15 L−1 (mean) | [96] |
| Hyytiälä, Finland | Boreal forest | 18 months | FAPs | 15 ± 24 L−1 (spring) (mean); 46 ± 48 L−1 (summer) (mean); 27 ± 32 L−1 (fall) (mean); 4 ± 46 L−1 (winter) (mean) | [85] |
| Hyytiälä, Finland | Boreal forest | 2 years | FAPs (1–20 μm) | 500 L−1 (peak) | [97] |
| Killarney, Ireland | Rural | ~1 month | FAPs (0.5–20 μm) | ~55 L−1 (peak) | [98] |
| India | High-altitude site | 11 weeks | FAPs (>1 μm) | 20 ± 20 L−1 (mean); ~520 L−1 (peak) | [87] |
| Channel | Excitation (nm) | Emission (nm) |
|---|---|---|
| A | 280 | 310–400 |
| B | 280 | 420–650 |
| C | 370 | 420–650 |
| AB | 280 | 310–400 |
| 420–650 | ||
| AC | 280 | 310–400 |
| 370 | 420–650 | |
| BC | 280 | 420–650 |
| 370 | ||
| ABC | 280 | 310–400 |
| 420–650 | ||
| 370 | 420–650 |
| Site Location | WIBS Model | Site Category | Season | EOD Range | NFL1 | NFL2 | NFL3 | NFAP | References |
|---|---|---|---|---|---|---|---|---|---|
| Manchester, England | 3 | Urban | Winter | 0.8–20 | 29 (3%) | 52 (6%) | 110 (11%) | - | [21] |
| Borneo, Malaysia | 3 | Rainforest | Summer | 0.8–20 | - | - | - | 150 | [21] |
| Puy de Dôme mountain, France | 3 | High-altitude | Summer | 0.8–20 | 12 (4.4%) | - | 95 (35.2%) | - | [120] |
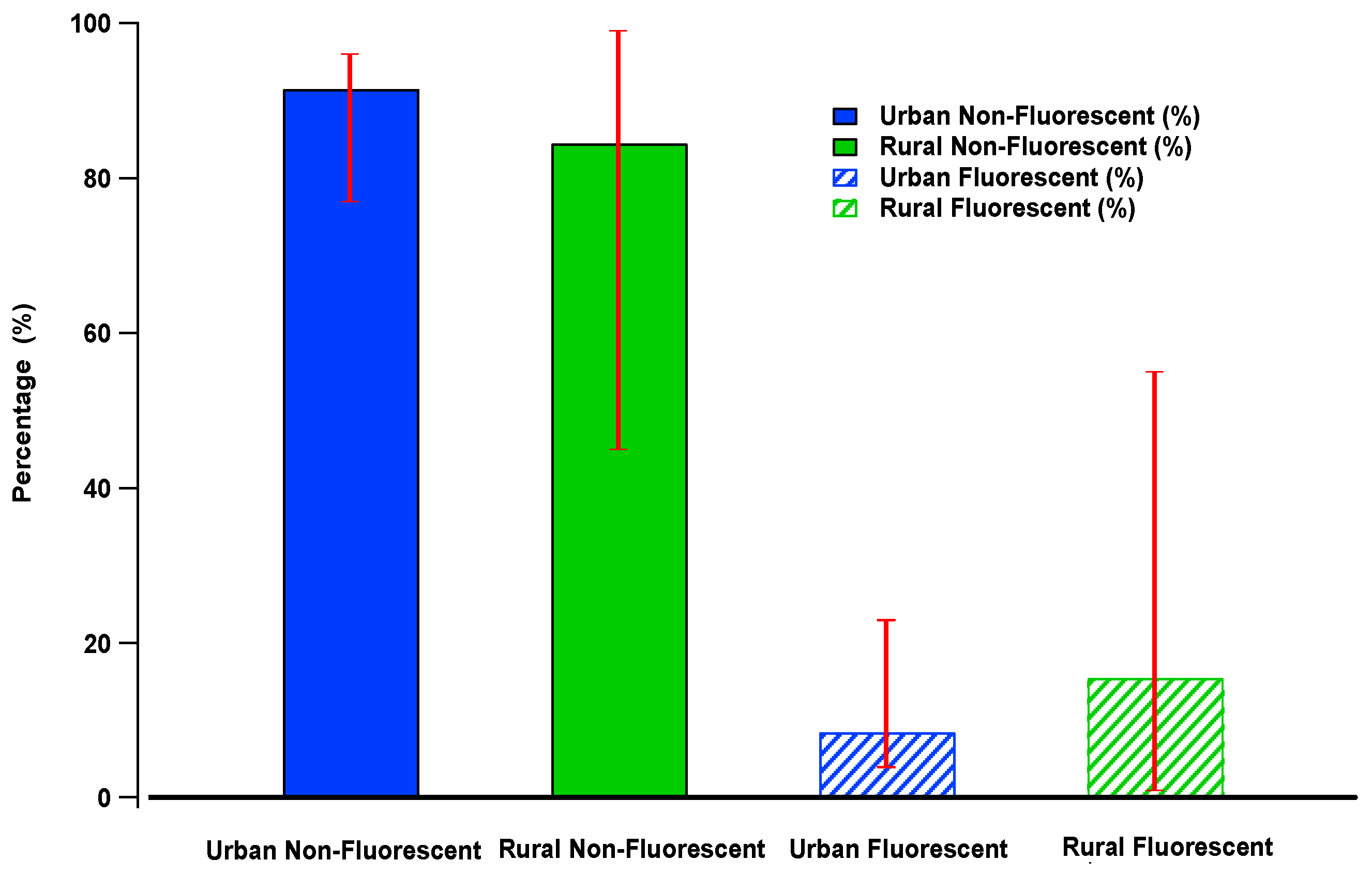
| Cork, Ireland | 4 | Coastal | Summer | 3.0–31 | ~25 | ~11 | ~2 | (~15%) | [22] |
| Killarney, Ireland | 4 | Rural | Summer | 0.5–13 | - | - | - | - | [98] |
| Karlsruhe, Germany | 4 | Semi-rural | 1-Year | 0.8–16 | - | - | - | 31 (7.3%) | [116] |
| Southern USA | 4 | High-altitude | Autumn | 1.0–10 | - | - | - | (24%) | [113] |
| Jungfrau, Switzerland | 4 | High-altitude | Winter | >0.8 | - | - | - | 6.3 ± 5.7 | [130] |
| Nanjing, China | 4a | Suburban | Autumn | 1.0–15 | 570 (4.6%) | 3350 (25.3%) | 2090 (15.6%) | - | [122] |
| Vancouver, Canada | 4a | Coastal | Autumn | 0.5–10 | - | - | - | (7.8%) | [14] |
| Colorado, USA | 3/4 | Rural Forest | 1-Year | 0.8–20 | - | - | - | (7.12%)/(4.02%) | [121] |
| North Carolina, USA | 4a | Urban | Autumn | 0.5–15 | - | - | - | (41.63%) | [131] |
| Denver, USA | 4a | High-altitude | Autumn | 0.8–12 | - | - | - | 69 (11%) | [127] |
| Nanyang, Singapore | 4a | Indoor | - | 1.0–10 | - | - | - | (~50%) | [118] |
| Beijing, China | 4a | Indoor | Spring | 0.5–10 | - | - | - | (4.37%) | [123] |
| Beijing, China | 4a | Urban | Winter | >0.8 | 155 (3.3%) | 551 (11.4%) | 79.4 (1.5%) | 642 (13.3%) | [132] |
| Device | WIBS-4/4A | UV-APS | Bio-Scout |
|---|---|---|---|
| Excitation (nm) and/or scatter source | 280/370 nm (two Xe flashlamps) | 355-nm UV laser (30 mJ) | 405 nm laser diode |
| Fluorescence detection range | 310–400 nm and 420–650 nm | 430–580 nm | >442 nm |
| Size detection range (µm) | 0.5–12 HG; 3–30 LG (WIBS-4) or 0.5–20 µm (WIBS-4A) | 0.5–20 µm | 0.5–10 µm |
| Time resolution | Millisecond | 1 s–18 h (5 min generally) | 1 s |
| Sample flow | 0.24 L/min (WIBS-4); 0.3 L/min (WIBS-4A) | 1 L/min | 2 L/min |
| Total flow | 2.4 L/min (WIBS-4); 2.5/0.3 L/min (WIBS-4A) | 5 L/min | 2 L/min |
| Parameter | PA-300 | PA-1000 | MBS | WIBS-4+ | WIBS-Neo | SIBS |
|---|---|---|---|---|---|---|
| Excitation source | 337 nm UV-laser beam | 263 nm UV-laser beam | 280 nm Xenon flashtubes | 280 and 370 nm Xenon flashtubes | 280 and 370 nm Xenon flashtubes | 280 and 370 nm Xenon flashtubes |
| Aerosol sampling flow rate | 2.0 L min−1 | 2.8 L min−1 | 0.30 L min−1 | 0.30 L min−1 | 0.30 L min−1 | 0.30 L min−1 |
| Sizing method | Optical Diameter (Do) by Mie theory | Optical Diameter (Do) by Mie theory | Optical Diameter (Do) by Mie theory | Optical Diameter (Do) by Mie theory | Optical Diameter (Do) by Mie theory | Optical Diameter (Do) by Mie theory |
| Particle size range | 1–100 µm | 0.5–100 µm | 1–20 µm | High-Gain = 0.5–12 µm Low-Gain = 3–31 µm | 0.5–50 µm | N/A |
| Fluorescence | 32 equal bins between 390–600 nm | 32 equal bins between range 290–660 nm | 300–335 nm, 340–385 nm, 390–435 nm, 440–485 nm, 490-535 nm, 540–575 nm, 580–615 nm, 620–655 nm | FL1 λex = 280 nm, λem = 310–400 nm FL2 λex = 280 nm λem = 420–650 nm FL3 λex = 370 nm λem = 420–650 nm FL4 λex = 280 nm λem = 600–750 nm FL5 λex = 370 nm λem = 600–750 nm | FL1 λex = 280 nm, λem = 310–400 nm FL2 λex = 280 nm λem = 420–650 nm FL3 λex = 370 nm λem = 420–650 nm | 16 channels between 300–720 nm |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fennelly, M.J.; Sewell, G.; Prentice, M.B.; O’Connor, D.J.; Sodeau, J.R. Review: The Use of Real-Time Fluorescence Instrumentation to Monitor Ambient Primary Biological Aerosol Particles (PBAP). Atmosphere 2018, 9, 1. https://doi.org/10.3390/atmos9010001
Fennelly MJ, Sewell G, Prentice MB, O’Connor DJ, Sodeau JR. Review: The Use of Real-Time Fluorescence Instrumentation to Monitor Ambient Primary Biological Aerosol Particles (PBAP). Atmosphere. 2018; 9(1):1. https://doi.org/10.3390/atmos9010001
Chicago/Turabian StyleFennelly, Mehael J., Gavin Sewell, Michael B. Prentice, David J. O’Connor, and John R. Sodeau. 2018. "Review: The Use of Real-Time Fluorescence Instrumentation to Monitor Ambient Primary Biological Aerosol Particles (PBAP)" Atmosphere 9, no. 1: 1. https://doi.org/10.3390/atmos9010001
APA StyleFennelly, M. J., Sewell, G., Prentice, M. B., O’Connor, D. J., & Sodeau, J. R. (2018). Review: The Use of Real-Time Fluorescence Instrumentation to Monitor Ambient Primary Biological Aerosol Particles (PBAP). Atmosphere, 9(1), 1. https://doi.org/10.3390/atmos9010001




