Perspectives on the Future of Ice Nucleation Research: Research Needs and Unanswered Questions Identified from Two International Workshops
Abstract
:1. Background and Motivation
2. Uncovering the Molecular Identity of Active Sites for Heterogeneous Ice Nucleation
3. The Importance of Molecular Modeling for the Understanding of Heterogeneous Ice Nucleation
4. Identifying and Quantifying Contributions of Biological Ice Nuclei from Natural and Managed Environments
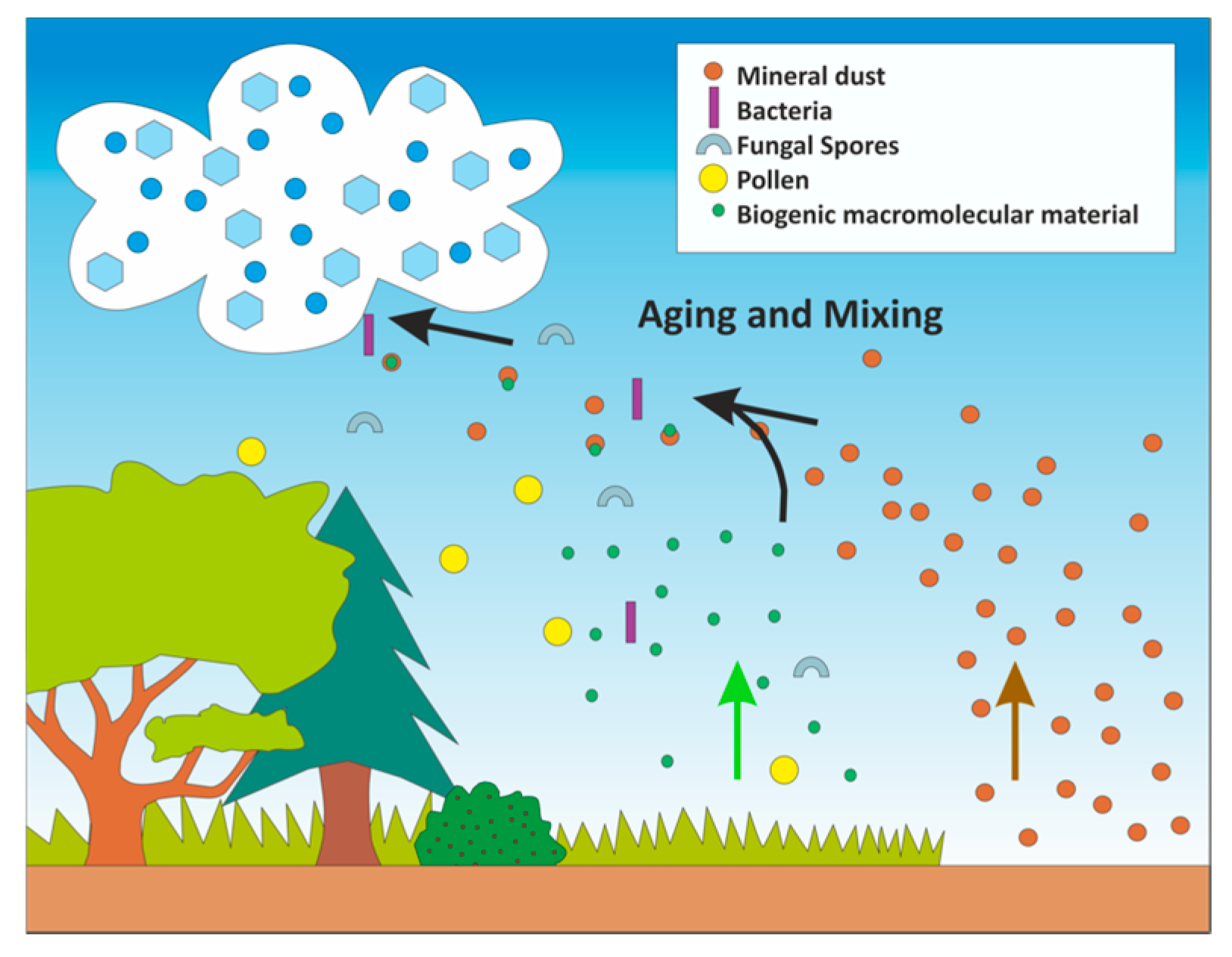
5. Examining the Role of Aging of Biological and Biogenic Ice Nuclei
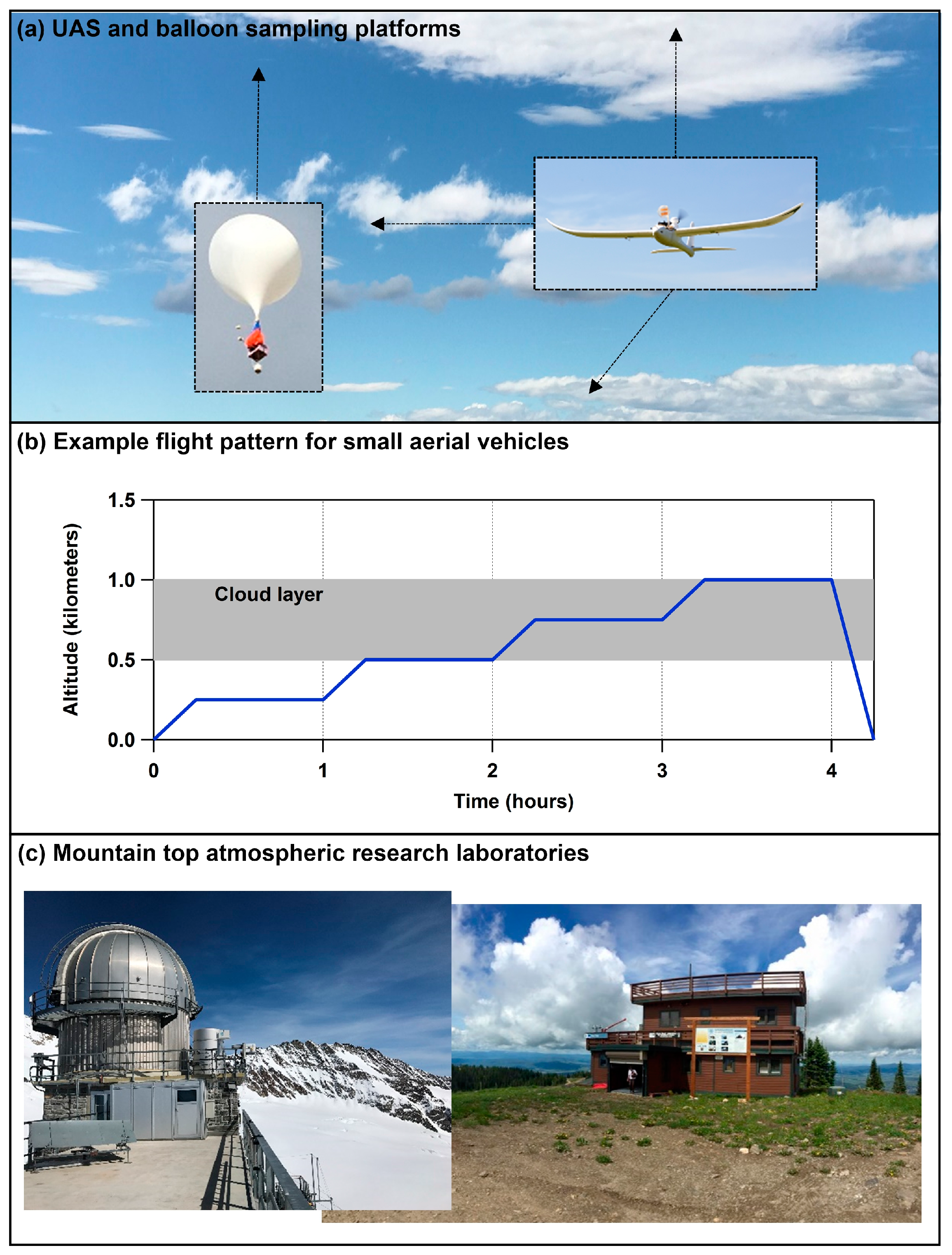
6. Conducting Targeted Sampling Campaigns in Clouds
7. Designing Lab and Field Experiments to Increase Our Understanding of the Role of Ice-Nucleating Particles in the Atmosphere
8. Conclusions: From Workshop Ideation to Action and Implementation
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pruppacher, H.R.; Klett, J.D. Microphysics of Clouds and Precipitation Kluwer; Springer: Dordrecht, The Netherlands, 2010; ISBN 978-0-306-48100-0. [Google Scholar]
- Szyrmer, W.; Zawadzki, I. Biogenic and anthropogenic sources of ice-forming nuclei: A review. Bull. Am. Meteorol. Soc. 1997, 78, 209–228. [Google Scholar] [CrossRef]
- Hoose, C.; Möhler, O. Heterogeneous ice nucleation on atmospheric aerosols: A review of results from laboratory experiments. Atmos. Chem. Phys. 2012, 12, 9817. [Google Scholar] [CrossRef]
- DeMott, P.J.; Möhler, O.; Stetzer, O.; Vali, G.; Levin, Z.; Petters, M.D.; Murakami, M.; Leisner, T.; Bundke, U.; Klein, H. Resurgence in ice nuclei measurement research. Bull. Am. Meteorol. Soc. 2011, 92, 1623–1635. [Google Scholar] [CrossRef]
- McFarquhar, G.M.; Baumgardner, D.; Heymsfield, A.J. Background and Overview. Meteorol. Monogr. 2017, 58, 5–9. [Google Scholar] [CrossRef]
- Ward, P.J.; DeMott, P.J. Preliminary experimental evaluation of Snomax (TM) snow inducer, nucleus Pseudomonas syringae, as an artificial ice for weather modification. J. Weather Modif. 1989, 21, 9–13. [Google Scholar]
- Li, J.; Lee, T.C. Bacterial ice nucleation and its potential application in the food industry. Trends Food Sci. Technol. 1995, 6, 259–265. [Google Scholar] [CrossRef]
- Kim, P.; Wong, T.S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.E.; Sands, D.C.; Bardin, M.; Jaenicke, R.; Vogel, B.; Leyronas, C.; Ariya, P.A.; Psenner, R. Microbiology and atmospheric processes: Research challenges concerning the impact of airborne micro-organisms on the atmosphere and climate. Biogeosciences 2011, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Kanji, Z.A.; Ladino, L.A.; Wex, H.; Boose, Y.; Burkert-Kohn, M.; Cziczo, D.J.; Krämer, M. Chapter 1: Overview of Ice Nucleating Particles. Meteorol. Monogr. 2017, 58, 1. [Google Scholar] [CrossRef]
- Geiger, F.M.; Tridico, A.C.; Hicks, J.M. Second harmonic generation studies of ozone depletion reactions on ice surfaces under stratospheric conditions. J. Phys. Chem. B 1999, 103, 8205–8215. [Google Scholar] [CrossRef]
- McNeill, V.F.; Loerting, T.; Geiger, F.M.; Trout, B.L.; Molina, M.J. Hydrogen chloride-induced surface disordering on ice. Proc. Natl. Acad. Sci. USA 2006, 103, 9422–9427. [Google Scholar] [CrossRef] [PubMed]
- Setyan, A.; Sauvain, J.J.; Rossi, M.J. The use of heterogeneous chemistry for the characterization of functional groups at the gas/particle interface of soot and TiO2 nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 6205–6217. [Google Scholar] [CrossRef] [PubMed]
- Wex, H.; DeMott, P.J.; Tobo, Y.; Hartmann, S.; Rösch, M.; Clauss, T.; Tomsche, L.; Niedermeier, D.; Stratmann, F. Kaolinite particles as ice nuclei: Learning from the use of different kaolinite samples and different coatings. Atmos. Chem. Phys. 2014, 14, 5529–5546. [Google Scholar] [CrossRef]
- Vali, G.; DeMott, P.J.; Möhler, O.; Whale, T.F. Technical Note: A proposal for ice nucleation terminology. Atmos. Chem. Phys. 2015, 15, 10263–10270. [Google Scholar] [CrossRef]
- Marcolli, C. Deposition nucleation viewed as homogeneous or immersion freezing in pores and cavities. Atmos. Chem. Phys. 2014, 14, 2071–2104. [Google Scholar] [CrossRef]
- Kittaka, S.; Ishimaru, S.; Kuranishi, M.; Matsuda, T.; Yamaguchi, T. Enthalpy and interfacial free energy changes of water capillary condensed in mesoporous silica, MCM-41 and SBA-15. Phys. Chem. Chem. Phys. 2006, 8, 3223–3231. [Google Scholar] [CrossRef] [PubMed]
- Persiantseva, N.M.; Popovicheva, O.B.; Shonija, N.K. Wetting and hydration of insoluble soot particles in the upper troposphere. J. Environ. Monit. 2004, 6, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Hiranuma, N.; Möhler, O.; Yamashita, K.; Tajiri, T.; Saito, A.; Kiselev, A.; Hoffmann, N.; Hoose, C.; Jantsch, E.; Koop, T. Ice nucleation by cellulose and its potential contribution to ice formation in clouds. Nat. Geosci. 2015, 8, 273. [Google Scholar] [CrossRef]
- Diehl, K.; Mitra, S.K. A laboratory study of the effects of a kerosene-burner exhaust on ice nucleation and the evaporation rate of ice crystals. Atmos. Environ. 1998, 32, 3145–3151. [Google Scholar] [CrossRef]
- Gorbunov, B.; Baklanov, A.; Kakutkina, N.; Toumi, R.; Windsor, H.L. Ice nucleation on soot particles. J. Aerosol Sci. 1998, 29, S1055–S1056. [Google Scholar] [CrossRef]
- Popovicheva, O.; Kireeva, E.; Persiantseva, N.; Khokhlova, T.; Shonija, N.; Tishkova, V.; Demirdjian, B. Effect of soot on immersion freezing of water and possible atmospheric implications. Atmos. Res. 2008, 90, 326–337. [Google Scholar] [CrossRef]
- DeMott, P.J.; Prenni, A.J.; Liu, X.; Kreidenweis, S.M.; Petters, M.D.; Twohy, C.H.; Richardson, M.S.; Eidhammer, T.; Rogers, D.C. Predicting global atmospheric ice nuclei distributions and their impacts on climate. Proc. Natl. Acad. Sci. USA 2010, 107, 11217–11222. [Google Scholar] [CrossRef] [PubMed]
- DeMott, P.J.; Hill, T.C.; McCluskey, C.S.; Prather, K.A.; Collins, D.B.; Sullivan, R.C.; Ruppel, M.J.; Mason, R.H.; Irish, V.E.; Lee, T. Sea spray aerosol as a unique source of ice nucleating particles. Proc. Natl. Acad. Sci. USA 2016, 113, 5797–5803. [Google Scholar] [CrossRef] [PubMed]
- Benson, S.W. Thermochemical Kinetics; Wiley: New York, USA, 1976; ISBN 0-471-06781-4. [Google Scholar]
- Křepelová, A.; Bartels-Rausch, T.; Brown, M.A.; Bluhm, H.; Ammann, M. Adsorption of acetic acid on ice studied by ambient-pressure XPS and partial-electron-yield NEXAFS spectroscopy at 230–240 K. J. Phys. Chem. A 2013, 117, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Orlando, F.; Waldner, A.; Bartels-Rausch, T.; Birrer, M.; Kato, S.; Lee, M.T.; Proff, C.; Huthwelker, T.; Kleibert, A.; Van Bokhoven, J. The Environmental Photochemistry of Oxide Surfaces and the Nature of Frozen Salt Solutions: A New in Situ XPS Approach. Top. Catal. 2016, 59, 591–604. [Google Scholar] [CrossRef]
- Wei, X.; Miranda, P.B.; Shen, Y.R. Surface vibrational spectroscopic study of surface melting of ice. Phys. Rev. Lett. 2001, 86, 1554. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Usui, K.; Livingstone, R.A.; Fischer, S.A.; Pfaendtner, J.; Backus, E.H.; Nagata, Y.; Fröhlich-Nowoisky, J.; Schmüser, L.; Mauri, S.; Scheel, J.F.; Knopf, D.A.; Pöschl, U.; Bonn, M.; Weidner, T. Ice-nucleating bacteria control the order and dynamics of interfacial water. Sci. Adv. 2016, 2, e1501630. [Google Scholar] [CrossRef] [PubMed]
- Sanz, E.; Vega, C.; Espinosa, J.R.; Caballero-Bernal, R.; Abascal, J.L.F.; Valeriani, C. Homogeneous ice nucleation at moderate supercooling from molecular simulation. J. Am. Chem. Soc. 2013, 135, 15008–15017. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Saito, S.; Ohmine, I. Molecular dynamics simulation of the ice nucleation and growth process leading to water freezing. Nature 2002, 416, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Lupi, L.; Molinero, V. Does hydrophilicity of carbon particles improve their ice nucleation ability? J. Phys. Chem. A 2014, 118, 7330–7337. [Google Scholar] [CrossRef] [PubMed]
- Lupi, L.; Hudait, A.; Molinero, V. Heterogeneous nucleation of ice on carbon surfaces. J. Am. Chem. Soc. 2014, 136, 3156–3164. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.C.; Molinero, V. Crystallization, melting, and structure of water nanoparticles at atmospherically relevant temperatures. J. Am. Chem. Soc. 2012, 134, 6650–6659. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.B.; Molinero, V. Is it cubic? Ice crystallization from deeply supercooled water. Phys. Chem. Chem. Phys. 2011, 13, 20008–20016. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.J.; Kathmann, S.M.; Slater, B.; Michaelides, A. Molecular simulations of heterogeneous ice nucleation. I. Controlling ice nucleation through surface hydrophilicity. J. Chem. Phys. 2015, 142, 184704. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.J.; Kathmann, S.M.; Slater, B.; Michaelides, A. Molecular simulations of heterogeneous ice nucleation. II. Peeling back the layers. J. Chem. Phys. 2015, 142, 184705. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.J.; Kathmann, S.M.; Purton, J.A.; Gillan, M.J.; Michaelides, A. Non-hexagonal ice at hexagonal surfaces: The role of lattice mismatch. Phys. Chem. Chem. Phys. 2012, 14, 7944–7949. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.Y.; Patey, G.N. Heterogeneous ice nucleation induced by electric fields. J. Phys. Chem. Lett. 2011, 2, 2555–2559. [Google Scholar] [CrossRef]
- Yan, J.Y.; Patey, G.N. Molecular dynamics simulations of ice nucleation by electric fields. J. Phys. Chem. A 2012, 116, 7057–7064. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.Y.; Patey, G.N. Ice nucleation by electric surface fields of varying range and geometry. J. Chem. Phys. 2013, 139, 144501. [Google Scholar] [CrossRef] [PubMed]
- Pedevilla, P.; Cox, S.J.; Slater, B.; Michaelides, A. Can ice-like structures form on non-ice-like substrates? The example of the K-feldspar microcline. J. Phys. Chem. C 2016, 120, 6704–6713. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Cabriolu, R.; Li, T. Heterogeneous ice nucleation controlled by the coupling of surface crystallinity and surface hydrophilicity. J. Phys. Chem. C 2016, 120, 1507–1514. [Google Scholar] [CrossRef]
- Kiselev, A.; Bachmann, F.; Pedevilla, P.; Cox, S.J.; Michaelides, A.; Gerthsen, D.; Leisner, T. Active sites in heterogeneous ice nucleation—the example of K-rich feldspars. Science 2017, 355, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Abascal, J.L.F.; Sanz, E.; Garcia Fernandez, R.; Vega, C. A potential model for the study of ices and amorphous water: TIP4P/Ice. J. Chem. Phys. 2005, 122, 234511. [Google Scholar] [CrossRef] [PubMed]
- Liou, Y.C.; Tocilj, A.; Davies, P.L.; Jia, Z. Mimicry of ice structure by surface hydroxyls and water of a β-helix antifreeze protein. Nature 2000, 406, 322–324. [Google Scholar] [PubMed]
- Molinero, V.; Moore, E.B. Water modeled as an intermediate element between carbon and silicon. J. Phys. Chem. B 2008, 113, 4008–4016. [Google Scholar] [CrossRef] [PubMed]
- Bianco, V.; Franzese, G.; Dellago, C.; Coluzza, I. Role of Water in the Selection of Stable Proteins at Ambient and Extreme Thermodynamic Conditions. Phys. Rev. X 2017, 7, 21047. [Google Scholar] [CrossRef]
- Abascal, J.L.; Vega, C. A general purpose model for the condensed phases of water: TIP4P/2005. J. Chem. Phys. 2005, 123, 234505. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.; Abascal, J.L. Simulating water with rigid non-polarizable models: A general perspective. Phys. Chem. Chem. Phys. 2011, 13, 19663–19688. [Google Scholar] [CrossRef] [PubMed]
- Lederer, A.; Franke, M.; Schöpe, H.J. Heterogeneous nucleation and microstructure formation in colloidal model systems with various interactions. Eur. Phys. J. Spec. Top. 2014, 223, 389–407. [Google Scholar] [CrossRef]
- Cacciuto, A.; Auer, S.; Frenkel, D. Onset of heterogeneous crystal nucleation in colloidal suspensions. Nature 2004, 428, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Sandomirski, K.; Walta, S.; Dubbert, J.; Allahyarov, E.; Schofield, A.B.; Löwen, H.; Richtering, W.; Egelhaaf, S.U. Heterogeneous crystallization of hard and soft spheres near flat and curved walls. Eur. Phys. J. Spec. Top. 2014, 223, 439–454. [Google Scholar] [CrossRef]
- Hermes, M.; Vermolen, E.C.M.; Leunissen, M.E.; Vossen, D.L.J.; van Oostrum, P.D.J.; Dijkstra, M.; van Blaaderen, A. Nucleation of colloidal crystals on configurable seed structures. Soft Matter 2011, 7, 4623–4628. [Google Scholar] [CrossRef]
- Jungblut, S.; Dellago, C. Heterogeneous crystallization on tiny clusters. EPL Europhys. Lett. 2011, 96, 56006. [Google Scholar] [CrossRef]
- Jungblut, S.; Dellago, C. Crystallization on prestructured seeds. Phys. Rev. E 2013, 87, 12305. [Google Scholar] [CrossRef] [PubMed]
- Zielke, S.A.; Bertram, A.K.; Patey, G.N. Simulations of ice nucleation by kaolinite (001) with rigid and flexible surfaces. J. Phys. Chem. B 2015, 120, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Zielke, S.A.; Bertram, A.K.; Patey, G.N. Simulations of ice nucleation by model AgI disks and plates. J. Phys. Chem. B 2016, 120, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Viererblová, L.; Kolafa, J. A classical polarizable model for simulations of water and ice. Phys. Chem. Chem. Phys. 2011, 13, 19925–19935. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; Head-Gordon, T.; Ponder, J.W.; Ren, P.; Chodera, J.D.; Eastman, P.K.; Martinez, T.J.; Pande, V.S. Systematic improvement of a classical molecular model of water. J. Phys. Chem. B 2013, 117, 9956–9972. [Google Scholar] [CrossRef] [PubMed]
- Brukhno, A.V.; Anwar, J.; Davidchack, R.; Handel, R. Challenges in molecular simulation of homogeneous ice nucleation. J. Phys. Condens. Matter 2008, 20, 494243. [Google Scholar] [CrossRef]
- Sharp, K.A. A peek at ice binding by antifreeze proteins. Proc. Natl. Acad. Sci. USA 2011, 108, 7281–7282. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.A.; DeVries, A.L. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc. Natl. Acad. Sci. USA 1977, 74, 2589–2593. [Google Scholar] [CrossRef] [PubMed]
- Duman, J.G. Antifreeze and ice nucleator proteins in terrestrial arthropods. Annu. Rev. Physiol. 2001, 63, 327–357. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.D.; Murray, B.J.; Woodhouse, M.T.; Whale, T.F.; Baustian, K.J.; Carslaw, K.S.; Dobbie, S.; O’Sullivan, D.; Malkin, T.L. The importance of feldspar for ice nucleation by mineral dust in mixed-phase clouds. Nature 2013, 498, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef]
- Bartels-Rausch, T.; Bergeron, V.; Cartwright, J.H.; Escribano, R.; Finney, J.L.; Grothe, H.; Gutiérrez, P.J.; Haapala, J.; Kuhs, W.F.; Pettersson, J.B. Ice structures, patterns, and processes: A view across the icefields. Rev. Mod. Phys. 2012, 84, 885. [Google Scholar] [CrossRef]
- Christner, B.C.; Morris, C.E.; Foreman, C.M.; Cai, R.; Sands, D.C. Ubiquity of biological ice nucleators in snowfall. Science 2008, 319, 1214–1214. [Google Scholar] [CrossRef] [PubMed]
- Christner, B.C.; Cai, R.; Morris, C.E.; McCarter, K.S.; Foreman, C.M.; Skidmore, M.L.; Montross, S.N.; Sands, D.C. Geographic, seasonal, and precipitation chemistry influence on the abundance and activity of biological ice nucleators in rain and snow. Proc. Natl. Acad. Sci. USA 2008, 105, 18854–18859. [Google Scholar] [CrossRef] [PubMed]
- Zachariassen, K.E.; Kristiansen, E. Ice nucleation and antinucleation in nature. Cryobiology 2000, 41, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.L. Ice-binding proteins: A remarkable diversity of structures for stopping and starting ice growth. Trends Biochem. Sci. 2014, 39, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Dreischmeier, K.; Budke, C.; Wiehemeier, L.; Kottke, T.; Koop, T. Boreal pollen contain ice-nucleating as well as ice-binding “antifreeze”polysaccharides. Sci. Rep. 2017, 7, 41890. [Google Scholar] [CrossRef] [PubMed]
- Schauperl, M.; Podewitz, M.; Waldner, B.J.; Liedl, K.R. Enthalpic and Entropic Contributions to Hydrophobicity. J. Chem. Theory Comput. 2016, 12, 4600–4610. [Google Scholar] [CrossRef] [PubMed]
- Franzese, G.; Bianco, V.; Iskrov, S. Water at interface with proteins. Food Biophys. 2011, 6, 186–198. [Google Scholar] [CrossRef]
- Bianco, V.; Iskrov, S.; Franzese, G. Understanding the role of hydrogen bonds in water dynamics and protein stability. J. Biol. Phys. 2012, 38, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Franzese, G.; Bianco, V. Water at biological and inorganic interfaces. Food Biophys. 2013, 8, 153–169. [Google Scholar] [CrossRef]
- Bianco, V.; Franzese, G. Contribution of water to pressure and cold denaturation of proteins. Phys. Rev. Lett. 2015, 115, 108101. [Google Scholar] [CrossRef] [PubMed]
- Coluzza, I. A coarse-grained approach to protein design: Learning from design to understand folding. PLoS ONE 2011, 6, e20853. [Google Scholar] [CrossRef] [PubMed]
- Coluzza, I. Transferable coarse-grained potential for de novo protein folding and design. PLoS ONE 2014, 9, e112852. [Google Scholar] [CrossRef] [PubMed]
- Morawietz, T.; Singraber, A.; Dellago, C.; Behler, J. How van der Waals interactions determine the unique properties of water. Proc. Natl. Acad. Sci. USA 2016, 8368–8373. [Google Scholar] [CrossRef] [PubMed]
- Quigley, D.; Rodger, P.M.; Freeman, C.L.; Harding, J.H.; Duffy, D.M. Metadynamics simulations of calcite crystallization on self-assembled monolayers. J. Chem. Phys. 2009, 131, 94703. [Google Scholar] [CrossRef] [PubMed]
- Coluzza, I.; Frenkel, D. Virtual-Move Parallel Tempering. ChemPhysChem 2005, 6, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, P.G.; Dellago, C. 3 Trajectory-Based Rare Event Simulations. Rev. Comput. Chem. 2011, 27, 111. [Google Scholar]
- Bolhuis, P.G.; Dellago, C. Practical and conceptual path sampling issues. Eur. Phys. J. Spec. Top. 2015, 224, 2409–2427. [Google Scholar] [CrossRef]
- Baschnagel, J.; Binder, K.; Doruker, P.; Gusev, A.A.; Hahn, O.; Kremer, K.; Mattice, W.L.; Müller-Plathe, F.; Murat, M.; Paul, W. Bridging the gap between atomistic and coarse-grained models of polymers: Status and perspectives. In Viscoelasticity, Atomistic Models, Statistical Chemistry; Springer: Berlin, Germany, 2000; pp. 41–156. ISBN 978-3-540-46778-6. [Google Scholar]
- Murat, M.; Kremer, K. From many monomers to many polymers: Soft ellipsoid model for polymer melts and mixtures. J. Chem. Phys. 1998, 108, 4340–4348. [Google Scholar] [CrossRef]
- Potestio, R.; Peter, C.; Kremer, K. Computer simulations of soft matter: Linking the scales. Entropy 2014, 16, 4199–4245. [Google Scholar] [CrossRef]
- Pierleoni, C.; Capone, B.; Hansen, J.-P. A soft effective segment representation of semidilute polymer solutions. J. Chem. Phys. 2007, 127, 171102. [Google Scholar] [CrossRef] [PubMed]
- Capone, B.; Hansen, J.P.; Coluzza, I. Competing micellar and cylindrical phases in semi-dilute diblock copolymer solutions. Soft Matter 2010, 6, 6075–6078. [Google Scholar] [CrossRef]
- Coluzza, I.; Capone, B.; Hansen, J.P. Rescaling of structural length scales for “soft effective segment” representations of polymers in good solvent. Soft Matter 2011, 7, 5255–5259. [Google Scholar] [CrossRef]
- Maki, L.R.; Willoughby, K.J. Bacteria as biogenic sources of freezing nuclei. J. Appl. Meteorol. 1978, 17, 1049–1053. [Google Scholar] [CrossRef]
- Diehl, K.; Quick, C.; Matthias-Maser, S.; Mitra, S.K.; Jaenicke, R. The ice nucleating ability of pollen: Part I: Laboratory studies in deposition and condensation freezing modes. Atmos. Res. 2001, 58, 75–87. [Google Scholar] [CrossRef]
- Diehl, K.; Matthias-Maser, S.; Jaenicke, R.; Mitra, S.K. The ice nucleating ability of pollen: Part II. Laboratory studies in immersion and contact freezing modes. Atmos. Res. 2002, 61, 125–133. [Google Scholar] [CrossRef]
- O’sullivan, D.; Murray, B.J.; Malkin, T.L.; Whale, T.F.; Umo, N.S.; Atkinson, J.D.; Price, H.C.; Baustian, K.J.; Webb, M.E. Ice nucleation by fertile soil dusts: Relative importance of mineral and biogenic components. Atmos. Chem. Phys. 2014, 14, 1853–1867. [Google Scholar] [CrossRef] [Green Version]
- Schnell, R.C.; Vali, G. Biogenic ice nuclei: Part I. Terrestrial and marine sources. J. Atmos. Sci. 1976, 33, 1554–1564. [Google Scholar] [CrossRef]
- Hallett, J. Production of secondary ice particles during the riming process. Nature 1974, 249, 26–28. [Google Scholar] [CrossRef]
- Peckhaus, A.; Kiselev, A.; Hiron, T.; Ebert, M.; Leisner, T. A comparative study of K-rich and Na/Ca-rich feldspar ice-nucleating particles in a nanoliter droplet freezing assay. Atmos. Chem. Phys. 2016, 16, 11477–11496. [Google Scholar] [CrossRef]
- Harrison, A.D.; Whale, T.F.; Carpenter, M.A.; Holden, M.A.; Neve, L.; O’Sullivan, D.; Vergara Temprado, J.; Murray, B.J. Not all feldspars are equal: A survey of ice nucleating properties across the feldspar group of minerals. Atmos. Chem. Phys. 2016, 16, 10927–10940. [Google Scholar] [CrossRef] [Green Version]
- Sands, D.C.; Langhans, V.E.; Scharen, A.L.; De Smet, G. The association between bacteria and rain and possible resultant meteorological implications. Idojaras 1982, 86, 148–152. [Google Scholar]
- Morris, C.E.; Georgakopoulos, D.G.; Sands, D.C. Ice nucleation active bacteria and their potential role in precipitation. J. Phys. IV Fr. 2004, 121, 87–103. [Google Scholar] [CrossRef]
- Morris, C.E.; Sands, D.C.; Vinatzer, B.A.; Glaux, C.; Guilbaud, C.; Buffiere, A.; Yan, S.; Dominguez, H.; Thompson, B.M. The life history of the plant pathogen Pseudomonas syringae is linked to the water cycle. ISME J. 2008, 2, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.E.; Conen, F.; Alex Huffman, J.; Phillips, V.; Pöschl, U.; Sands, D.C. Bioprecipitation: A feedback cycle linking Earth history, ecosystem dynamics and land use through biological ice nucleators in the atmosphere. Glob. Chang. Biol. 2014, 20, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Monteil, C.L.; Bardin, M.; Morris, C.E. Features of air masses associated with the deposition of Pseudomonas syringae and Botrytis cinerea by rain and snowfall. ISME J. 2014, 8, 2290–2304. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Nowoisky, J.; Hill, T.C.; Pummer, B.G.; Yordanova, P.; Franc, G.D.; Pöschl, U. Ice nucleation activity in the widespread soil fungus Mortierella alpina. Biogeosciences 2015, 12, 1057. [Google Scholar] [CrossRef]
- Pouleur, S.; Richard, C.; Martin, J.G.; Antoun, H. Ice nucleation activity in Fusarium acuminatum and Fusarium avenaceum. Appl. Environ. Microbiol. 1992, 58, 2960–2964. [Google Scholar] [PubMed]
- Richard, C.; Martin, J.G.; Pouleur, S. Ice nucleation activity identified in some phytopathogenic Fusarium species. Phytoprotection 1996, 77, 83–92. [Google Scholar] [CrossRef]
- O’Sullivan, D.; Murray, B.J.; Ross, J.F.; Webb, M.E. The adsorption of fungal ice-nucleating proteins on mineral dusts: A terrestrial reservoir of atmospheric ice-nucleating particles. Atmos. Chem. Phys. 2016, 16, 7879–7887. [Google Scholar]
- Gurian-Sherman, D.; Lindow, S.E. Bacterial ice nucleation: Significance and molecular basis. FASEB J. 1993, 7, 1338–1343. [Google Scholar] [PubMed]
- Pietsch, R.B.; David, R.F.; Marr, L.C.; Vinatzer, B.; Schmale, D.G., III. Aerosolization of Two Strains (Ice+ and Ice–) of Pseudomonas syringae in a Collison Nebulizer at Different Temperatures. Aerosol Sci. Technol. 2015, 49, 159–166. [Google Scholar] [CrossRef]
- D’souza, N.A.; Kawarasaki, Y.; Gantz, J.D.; Lee, R.E.; Beall, B.F.N.; Shtarkman, Y.M.; Kocer, Z.A.; Rogers, S.O.; Wildschutte, H.; Bullerjahn, G.S. Diatom assemblages promote ice formation in large lakes. ISME J. 2013, 7, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Moffett, B.F. Fresh water ice nuclei. Fundam. Appl. Limnol. Hydrobiol. 2016, 188, 19–23. [Google Scholar] [CrossRef]
- Pietsch, R.B.; Vinatzer, B.A.; Schmale, D.G., III. Diversity and abundance of ice nucleating strains of Pseudomonas syringae in a freshwater lake in Virginia, USA. Front. Microbiol. 2017, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.W.; Ladino, L.A.; Alpert, P.A.; Breckels, M.N.; Brooks, I.M.; Browse, J.; Burrows, S.M.; Carslaw, K.S.; Huffman, J.A.; Judd, C.; et al. A marine biogenic source of atmospheric ice-nucleating particles. Nature 2015, 525, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.P.; Hader, J.D.; McMeeking, G.R.; Petters, M.D. High relative humidity as a trigger for widespread release of ice nuclei. Aerosol Sci. Technol. 2014, 48, 1–5. [Google Scholar] [CrossRef]
- Schnell, R.C.; Vali, G. Atmospheric ice nuclei from decomposing vegetation. Nature 1972, 236, 163–165. [Google Scholar] [CrossRef]
- Augustin, S.; Wex, H.; Niedermeier, D.; Pummer, B.; Grothe, H.; Hartmann, S.; Tomsche, L.; Clauss, T.; Voigtländer, J.; Ignatius, K. Immersion freezing of birch pollen washing water. Atmos. Chem. Phys. 2013, 13, 10989–11003. [Google Scholar] [CrossRef]
- Pummer, B.G.; Budke, C.; Augustin-Bauditz, S.; Niedermeier, D.; Felgitsch, L.; Kampf, C.J.; Huber, R.G.; Liedl, K.R.; Loerting, T.; Moschen, T.; et al. Ice nucleation by water-soluble macromolecules. Atmos. Chem. Phys. 2015, 15, 4077–4091. [Google Scholar] [CrossRef]
- Alpert, P.A.; Aller, J.Y.; Knopf, D.A. Initiation of the ice phase by marine biogenic surfaces in supersaturated gas and supercooled aqueous phases. Phys. Chem. Chem. Phys. 2011, 13, 19882–19894. [Google Scholar] [CrossRef] [PubMed]
- Janech, M.G.; Krell, A.; Mock, T.; Kang, J.S.; Raymond, J.A. Ice-Binding Proteins from Sea Ice Diatoms (Bacillariophyceae) 1. J. Phycol. 2006, 42, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Knopf, D.A.; Alpert, P.A. A water activity based model of heterogeneous ice nucleation kinetics for freezing of water and aqueous solution droplets. Faraday Discuss. 2013, 165, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Behzad, H.; Gojobori, T.; Mineta, K. Challenges and opportunities of airborne metagenomics. Genome Biol. Evol. 2015, 7, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Goulet, F. Frost heaving of forest tree seedlings: A review. New For. 1995, 9, 67–94. [Google Scholar] [CrossRef]
- Gu, L.; Hanson, P.J.; Post, W.M.; Kaiser, D.P.; Yang, B.; Nemani, R.; Pallardy, S.G.; Meyers, T. The 2007 eastern US spring freeze: Increased cold damage in a warming world? AIBS Bull. 2008, 58, 253–262. [Google Scholar] [CrossRef]
- Maqbool, A.; Shafiq, S.; Lake, L. Radiant frost tolerance in pulse crops—a review. Euphytica 2010, 172, 1–12. [Google Scholar] [CrossRef]
- Duman, J.G.; Wisniewski, M.J. The use of antifreeze proteins for frost protection in sensitive crop plants. Environ. Exp. Bot. 2014, 106, 60–69. [Google Scholar] [CrossRef]
- Neuner, G. Frost resistance in alpine woody plants. Front. Plant Sci. 2014, 5, 654. [Google Scholar] [CrossRef] [PubMed]
- Lindow, S.E. The role of bacterial ice nucleation in frost injury to plants. Annu. Rev. Phytopathol. 1983, 21, 363–384. [Google Scholar] [CrossRef]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Amato, P.; Joly, M.; Schaupp, C.; Attard, E.; Möhler, O.; Morris, C.E.; Brunet, Y.; Delort, A.M. Survival and ice nucleation activity of bacteria as aerosols in a cloud simulation chamber. Atmos. Chem. Phys. 2015, 15, 6455–6465. [Google Scholar] [CrossRef]
- Joly, M.; Attard, E.; Sancelme, M.; Deguillaume, L.; Guilbaud, C.; Morris, C.E.; Amato, P.; Delort, A.M. Ice nucleation activity of bacteria isolated from cloud water. Atmos. Environ. 2013, 70, 392–400. [Google Scholar] [CrossRef]
- Quinn, P.K.; Collins, D.B.; Grassian, V.H.; Prather, K.A.; Bates, T.S. Chemistry and related properties of freshly emitted sea spray aerosol. Chem. Rev. 2015, 115, 4383–4399. [Google Scholar] [CrossRef] [PubMed]
- Ladino, L.A.; Yakobi-Hancock, J.D.; Kilthau, W.P.; Mason, R.H.; Si, M.; Li, J.; Miller, L.A.; Schiller, C.L.; Huffman, J.A.; Aller, J.Y. Addressing the ice nucleating abilities of marine aerosol: A combination of deposition mode laboratory and field measurements. Atmos. Environ. 2016, 132, 1–10. [Google Scholar] [CrossRef]
- Christner, B.C.; Mosley-Thompson, E.; Thompson, L.G.; Reeve, J.N. Isolation of bacteria and 16S rDNAs from Lake Vostok accretion ice. Environ. Microbiol. 2001, 3, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Lohmann, U.; Lesins, G. Ice nucleation of ammonia gas exposed montmorillonite mineral dust particles. Atmos. Chem. Phys. 2007, 7, 3923–3931. [Google Scholar] [CrossRef]
- Hoose, C.; Lohmann, U.; Erdin, R.; Tegen, I. The global influence of dust mineralogical composition on heterogeneous ice nucleation in mixed-phase clouds. Environ. Res. Lett. 2008, 3, 25003. [Google Scholar] [CrossRef]
- Niedermeier, D.; Hartmann, S.; Clauss, T.; Wex, H.; Kiselev, A.; Sullivan, R.C.; DeMott, P.J.; Petters, M.D.; Reitz, P.; Schneider, J. Experimental study of the role of physicochemical surface processing on the IN ability of mineral dust particles. Atmos. Chem. Phys. 2011, 11, 11131–11144. [Google Scholar] [CrossRef] [Green Version]
- Zolles, T.; Burkart, J.; Häusler, T.; Pummer, B.; Hitzenberger, R.; Grothe, H. Identification of ice nucleation active sites on feldspar dust particles. J. Phys. Chem. A 2015, 119, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- Kanji, Z.A.; Welti, A.; Chou, C.; Stetzer, O.; Lohmann, U. Laboratory studies of immersion and deposition mode ice nucleation of ozone aged mineral dust particles. Atmos. Chem. Phys. 2013, 13, 9097–9118. [Google Scholar] [CrossRef]
- Rudich, Y.; Donahue, N.M.; Mentel, T.F. Aging of organic aerosol: Bridging the gap between laboratory and field studies. Annu. Rev. Phys. Chem. 2007, 58, 321–352. [Google Scholar] [CrossRef] [PubMed]
- George, I.J.; Abbatt, J.P.D. Heterogeneous oxidation of atmospheric aerosol particles by gas-phase radicals. Nat. Chem. 2010, 2, 713. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.D.; Suter, K.; Olivarez, L. Effects of chemical aging on the ice nucleation activity of soot and polycyclic aromatic hydrocarbon aerosols. J. Phys. Chem. A 2014, 118, 10036–10047. [Google Scholar] [CrossRef] [PubMed]
- Pummer, B.G.; Bauer, H.; Bernardi, J.; Bleicher, S.; Grothe, H. Suspendable macromolecules are responsible for ice nucleation activity of birch and conifer pollen. Atmos. Chem. Phys. 2012, 12, 2541–2550. [Google Scholar] [CrossRef]
- Hartmann, S.; Augustin, S.; Clauss, T.; Wex, H.; Šantl-Temkiv, T.; Voigtländer, J.; Niedermeier, D.; Stratmann, F. Immersion freezing of ice nucleation active protein complexes. Atmos. Chem. Phys. 2013, 13, 5751–5766. [Google Scholar] [CrossRef]
- Augustin-Bauditz, S.; Wex, H.; Denjean, C.; Hartmann, S.; Schneider, J.; Schmidt, S.; Ebert, M.; Stratmann, F. Laboratory-generated mixtures of mineral dust particles with biological substances: Characterization of the particle mixing state and immersion freezing behavior. Atmos. Chem. Phys. 2016, 16, 5531–5543. [Google Scholar] [CrossRef]
- Rangel-Alvarado, R.B.; Nazarenko, Y.; Ariya, P.A. Snow-borne nanosized particles: Abundance, distribution, composition, and significance in ice nucleation processes. J. Geophys. Res. Atmos. 2015, 120. [Google Scholar] [CrossRef]
- Kajava, A.V.; Lindow, S.E. A model of the three-dimensional structure of ice nucleation proteins. J. Mol. Biol. 1993, 232, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, A.G.; Lindow, S.E. Size of bacterial ice-nucleation sites measured in situ by radiation inactivation analysis. Proc. Natl. Acad. Sci. USA 1988, 85, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Kozloff, L.M.; Turner, M.A.; Arellano, F.; Lute, M. Phosphatidylinositol, a phospholipid of ice-nucleating bacteria. J. Bacteriol. 1991, 173, 2053–2060. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, A.G.; Lindow, S.E. Phospholipid requirement for expression of ice nuclei in Pseudomonas syringae and in vitro. J. Biol. Chem. 1988, 263, 9333–9338. [Google Scholar] [PubMed]
- Sharma, V.K.; Graham, N.J. Oxidation of amino acids, peptides and proteins by ozone: A review. Ozone Sci. Eng. 2010, 32, 81–90. [Google Scholar] [CrossRef]
- Kieft, T.L.; Ruscetti, T. Characterization of biological ice nuclei from a lichen. J. Bacteriol. 1990, 172, 3519–3523. [Google Scholar] [CrossRef] [PubMed]
- Ofner, J.; Krüger, H.U.; Grothe, H.; Schmitt-Kopplin, P.; Whitmore, K.; Zetzsch, C. Physico-chemical characterization of SOA derived from catechol and guaiacol–a model substance for the aromatic fraction of atmospheric HULIS. Atmos. Chem. Phys. 2011, 11, 1–15. [Google Scholar] [CrossRef]
- Adler, G.; Koop, T.; Haspel, C.; Taraniuk, I.; Moise, T.; Koren, I.; Heiblum, R.H.; Rudich, Y. Formation of highly porous aerosol particles by atmospheric freeze-drying in ice clouds. Proc. Natl. Acad. Sci. USA 2013, 110, 20414–20419. [Google Scholar] [CrossRef] [PubMed]
- Baustian, K.J.; Wise, M.E.; Jensen, E.J.; Schill, G.P.; Freedman, M.A.; Tolbert, M.A. State transformations and ice nucleation in amorphous (semi-) solid organic aerosol. Atmos. Chem. Phys. 2013, 13, 5615. [Google Scholar] [CrossRef]
- Möhler, O.; Benz, S.; Saathoff, H.; Schnaiter, M.; Wagner, R.; Schneider, J.; Walter, S.; Ebert, V.; Wagner, S. The effect of organic coating on the heterogeneous ice nucleation efficiency of mineral dust aerosols. Environ. Res. Lett. 2008, 3, 25007. [Google Scholar] [CrossRef]
- Pöschl, U.; Martin, S.T.; Sinha, B.; Chen, Q.; Gunthe, S.S.; Huffman, J.A.; Borrmann, S.; Farmer, D.K.; Garland, R.M.; Helas, G. Rainforest aerosols as biogenic nuclei of clouds and precipitation in the Amazon. Science 2010, 329, 1513–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zobrist, B.; Marcolli, C.; Koop, T.; Luo, B.P.; Murphy, D.M.; Lohmann, U.; Zardini, A.A.; Krieger, U.K.; Corti, T.; Cziczo, D.J. Oxalic acid as a heterogeneous ice nucleus in the upper troposphere and its indirect aerosol effect. Atmos. Chem. Phys. 2006, 6, 3115–3129. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Selzle, K.; Yang, H.; Sosedova, Y.; Ammann, M.; Pöschl, U. Multiphase chemical kinetics of the nitration of aerosolized protein by ozone and nitrogen dioxide. Environ. Sci. Technol. 2012, 46, 6672–6680. [Google Scholar] [CrossRef] [PubMed]
- Riccobono, F.; Schobesberger, S.; Scott, C.E.; Dommen, J.; Ortega, I.K.; Rondo, L.; Almeida, J.; Amorim, A.; Bianchi, F.; Breitenlechner, M. Oxidation products of biogenic emissions contribute to nucleation of atmospheric particles. Science 2014, 344, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Ignatius, K.; Kristensen, T.B.; Järvinen, E.; Nichman, L.; Fuchs, C.; Gordon, H.; Herenz, P.; Hoyle, C.R.; Duplissy, J.; Garimella, S. Heterogeneous ice nucleation of viscous secondary organic aerosol produced from ozonolysis of α-pinene. Atmos. Chem. Phys. 2016, 16, 6495–6509. [Google Scholar] [CrossRef]
- Ellison, G.B.; Tuck, A.F.; Vaida, V. Atmospheric processing of organic aerosols. J. Geophys. Res. Atmos. 1999, 104, 11633–11641. [Google Scholar] [CrossRef]
- Baltensperger, U.; Kalberer, M.; Dommen, J.; Paulsen, D.; Alfarra, M.R.; Coe, H.; Fisseha, R.; Gascho, A.; Gysel, M.; Nyeki, S. Secondary organic aerosols from anthropogenic and biogenic precursors. Faraday Discuss. 2005, 130, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Kalberer, M.; Sax, M.; Samburova, V. Molecular size evolution of oligomers in organic aerosols collected in urban atmospheres and generated in a smog chamber. Environ. Sci. Technol. 2006, 40, 5917–5922. [Google Scholar] [CrossRef] [PubMed]
- Walser, M.L.; Park, J.; Gomez, A.L.; Russell, A.R.; Nizkorodov, S.A. Photochemical aging of secondary organic aerosol particles generated from the oxidation of d-limonene. J. Phys. Chem. A 2007, 111, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Tritscher, T.; Dommen, J.; DeCarlo, P.F.; Gysel, M.; Barmet, P.B.; Praplan, A.P.; Weingartner, E.; Prévôt, A.S.H.; Riipinen, I.; Donahue, N.M. Volatility and hygroscopicity of aging secondary organic aerosol in a smog chamber. Atmos. Chem. Phys. 2011, 11, 11477–11496. [Google Scholar] [CrossRef]
- Donahue, N.M.; Henry, K.M.; Mentel, T.F.; Kiendler-Scharr, A.; Spindler, C.; Bohn, B.; Brauers, T.; Dorn, H.P.; Fuchs, H.; Tillmann, R. Aging of biogenic secondary organic aerosol via gas-phase OH radical reactions. Proc. Natl. Acad. Sci. USA 2012, 109, 13503–13508. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Reinnig, M.C.; Naumann, K.H.; Saathoff, H.; Mentel, T.F.; Donahue, N.M.; Hoffmann, T. Formation of 3-methyl-1, 2, 3-butanetricarboxylic acid via gas phase oxidation of pinonic acid–a mass spectrometric study of SOA aging. Atmos. Chem. Phys. 2012, 12, 1483–1496. [Google Scholar] [CrossRef] [Green Version]
- Koop, T.; Bookhold, J.; Shiraiwa, M.; Pöschl, U. Glass transition and phase state of organic compounds: Dependency on molecular properties and implications for secondary organic aerosols in the atmosphere. Phys. Chem. Chem. Phys. 2011, 13, 19238–19255. [Google Scholar] [CrossRef] [PubMed]
- Cappa, C.D.; Wilson, K.R. Evolution of organic aerosol mass spectra upon heating: Implications for OA phase and partitioning behavior. Atmos. Chem. Phys. 2011, 11, 1895–1911. [Google Scholar] [CrossRef]
- Vaden, T.D.; Imre, D.; Beránek, J.; Shrivastava, M.; Zelenyuk, A. Evaporation kinetics and phase of laboratory and ambient secondary organic aerosol. Proc. Natl. Acad. Sci. USA 2011, 108, 2190–2195. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.M.; Goss, K.U.; Schwarzenbach, R.P. Sorption of a diverse set of organic vapors to urban aerosols. Environ. Sci. Technol. 2005, 39, 6638–6643. [Google Scholar] [CrossRef] [PubMed]
- Bones, D.L.; Reid, J.P.; Lienhard, D.M.; Krieger, U.K. Comparing the mechanism of water condensation and evaporation in glassy aerosol. Proc. Natl. Acad. Sci. USA 2012, 109, 11613–11618. [Google Scholar] [CrossRef] [PubMed]
- Saukko, E.; Kuuluvainen, H.; Virtanen, A. A method to resolve the phase state of aerosol particles. Atmos. Meas. Tech. 2012, 5, 259–265. [Google Scholar] [CrossRef]
- Wang, B.; Lambe, A.T.; Massoli, P.; Onasch, T.B.; Davidovits, P.; Worsnop, D.R.; Knopf, D.A. The deposition ice nucleation and immersion freezing potential of amorphous secondary organic aerosol: Pathways for ice and mixed-phase cloud formation. J. Geophys. Res. Atmos. 2012, 117. [Google Scholar] [CrossRef]
- Grayson, J.W.; Zhang, Y.; Mutzel, A.; Renbaum-Wolff, L.; Böge, O.; Kamal, S.; Herrmann, H.; Martin, S.T.; Bertram, A.K. Effect of varying experimental conditions on the viscosity of α-pinene derived secondary organic material. Atmos. Chem. Phys. 2016, 16, 6027–6040. [Google Scholar] [CrossRef]
- Szmigielski, R.; Surratt, J.D.; Gómez-González, Y.; Van der Veken, P.; Kourtchev, I.; Vermeylen, R.; Blockhuys, F.; Jaoui, M.; Kleindienst, T.E.; Lewandowski, M. 3-methyl-1, 2, 3-butanetricarboxylic acid: An atmospheric tracer for terpene secondary organic aerosol. Geophys. Res. Lett. 2007, 34, 387868. [Google Scholar] [CrossRef]
- Claeys, M.; Iinuma, Y.; Szmigielski, R.; Surratt, J.D.; Blockhuys, F.; van Alsenoy, C.; Böge, O.; Sierau, B.; Gómez-González, Y.; Vermeylen, R. Terpenylic acid and related compounds from the oxidation of α-pinene: Implications for new particle formation and growth above forests. Environ. Sci. Technol. 2009, 43, 6976–6982. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, Y.; Keywood, M.; Gnauk, T.; Herrmann, H. Diaterebic acid acetate and diaterpenylic acid acetate: Atmospheric tracers for secondary organic aerosol formation from 1, 8-cineole oxidation. Environ. Sci. Technol. 2008, 43, 280–285. [Google Scholar] [CrossRef]
- Iinuma, Y.; Engling, G.; Puxbaum, H.; Herrmann, H. A highly resolved anion-exchange chromatographic method for determination of saccharidic tracers for biomass combustion and primary bio-particles in atmospheric aerosol. Atmos. Environ. 2009, 43, 1367–1371. [Google Scholar] [CrossRef]
- Mutzel, A.; Rodigast, M.; Iinuma, Y.; Böge, O.; Herrmann, H. Monoterpene SOA–contribution of first-generation oxidation products to formation and chemical composition. Atmos. Environ. 2016, 130, 136–144. [Google Scholar] [CrossRef]
- Christoffersen, T.S.; Hjorth, J.; Horie, O.; Jensen, N.R.; Kotzias, D.; Molander, L.L.; Neeb, P.; Ruppert, L.; Winterhalter, R.; Virkkula, A. cis-Pinic acid, a possible precursor for organic aerosol formation from ozonolysis of α-pinene. Atmos. Environ. 1998, 32, 1657–1661. [Google Scholar] [CrossRef]
- Iinuma, Y.; Brüggemann, E.; Gnauk, T.; Müller, K.; Andreae, M.O.; Helas, G.; Parmar, R.; Herrmann, H. Source characterization of biomass burning particles: The combustion of selected European conifers, African hardwood, savanna grass, and German and Indonesian peat. J. Geophys. Res. Atmos. 2007, 112, 08209. [Google Scholar] [CrossRef]
- Hoffmann, D.; Tilgner, A.; Iinuma, Y.; Herrmann, H. Atmospheric stability of levoglucosan: A detailed laboratory and modeling study. Environ. Sci. Technol. 2009, 44, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Cziczo, D.J.; Ladino, L.; Boose, Y.; Kanji, Z.A.; Kupiszewski, P.; Lance, S.; Mertes, S.; Wex, H. Measurements of Ice Nucleating Particles and Ice Residuals. Meteorol. Monogr. 2017, 58, 8.1–8.13. [Google Scholar] [CrossRef]
- Field, P.R.; Lawson, R.P.; Brown, P.R.A.; Lloyd, G.; Westbrook, C.; Moisseev, D.; Miltenberger, A.; Nenes, A.; Blyth, A.; Choularton, T. Secondary Ice Production: Current State of the Science and Recommendations for the Future. Meteorol. Monogr. 2017, 58, 7.1–7.20. [Google Scholar] [CrossRef]
- Eidhammer, T.; DeMott, P.J.; Prenni, A.J.; Petters, M.D.; Twohy, C.H.; Rogers, D.C.; Stith, J.; Heymsfield, A.; Wang, Z.; Pratt, K.A. Ice initiation by aerosol particles: Measured and predicted ice nuclei concentrations versus measured ice crystal concentrations in an orographic wave cloud. J. Atmos. Sci. 2010, 67, 2417–2436. [Google Scholar] [CrossRef]
- Field, P.R.; Heymsfield, A.J.; Shipway, B.J.; DeMott, P.J.; Pratt, K.A.; Rogers, D.C.; Stith, J.; Prather, K.A. Ice in clouds experiment–layer clouds. Part II: Testing characteristics of heterogeneous ice formation in lee wave clouds. J. Atmos. Sci. 2012, 69, 1066–1079. [Google Scholar] [CrossRef]
- Heymsfield, A.; Willis, P. Cloud conditions favoring secondary ice particle production in tropical maritime convection. J. Atmos. Sci. 2014, 71, 4500–4526. [Google Scholar] [CrossRef]
- Heymsfield, A.J.; Field, P.R.; Bailey, M.; Rogers, D.; Stith, J.; Twohy, C.; Wang, Z.; Haimov, S. Ice in clouds experiment—Layer clouds. Part I: Ice growth rates derived from lenticular wave cloud penetrations. J. Atmos. Sci. 2011, 68, 2628–2654. [Google Scholar] [CrossRef]
- Lasher-Trapp, S.; Leon, D.C.; DeMott, P.J.; Villanueva-Birriel, C.M.; Johnson, A.V.; Moser, D.H.; Tully, C.S.; Wu, W. A Multisensor Investigation of Rime Splintering in Tropical Maritime Cumuli. J. Atmos. Sci. 2016, 73, 2547–2564. [Google Scholar] [CrossRef]
- Neely, R.; Blyth, A.; Bennett, L.; Dufton, D.; Cui, Z.; McQuaid, J.; Price, H.; Murray, B.; Huang, Y. Dual-Polarised Doppler X-band Radar Observations of Mixed Phased Clouds from the UK’s Ice in Clouds Experiment-Dust (ICE-D). In Proceedings of the EGU General Assembly 2016, Vienna, Austria, 17–22 April 2016; Volume 18, p. 3084. [Google Scholar]
- Pratt, K.A.; DeMott, P.J.; French, J.R.; Wang, Z.; Westphal, D.L.; Heymsfield, A.J.; Twohy, C.H.; Prenni, A.J.; Prather, K.A. In situ detection of biological particles in cloud ice-crystals. Nat. Geosci. 2009, 2, 398–401. [Google Scholar] [CrossRef]
- Prenni, A.J.; Petters, M.D.; Kreidenweis, S.M.; Heald, C.L.; Martin, S.T.; Artaxo, P.; Garland, R.M.; Wollny, A.G.; Pöschl, U. Relative roles of biogenic emissions and Saharan dust as ice nuclei in the Amazon basin. Nat. Geosci. 2009, 2, 402–405. [Google Scholar] [CrossRef]
- Meyers, M.P.; DeMott, P.J.; Cotton, W.R. New primary ice-nucleation parameterizations in an explicit cloud model. J. Appl. Meteorol. 1992, 31, 708–721. [Google Scholar] [CrossRef]
- Fan, J.; Leung, L.R.; DeMott, P.J.; Comstock, J.M.; Singh, B.; Rosenfeld, D.; Tomlinson, J.M.; White, A.; Prather, K.A.; Minnis, P. Aerosol impacts on California winter clouds and precipitation during CalWater 2011: Local pollution versus long-range transported dust. Atmos. Chem. Phys. 2014, 14, 81–101. [Google Scholar] [CrossRef]
- Thompson, G.; Eidhammer, T. A study of aerosol impacts on clouds and precipitation development in a large winter cyclone. J. Atmos. Sci. 2014, 71, 3636–3658. [Google Scholar] [CrossRef]
- Vali, G. Freezing nucleus content of hail and rain in Alberta. J. Appl. Meteorol. 1971, 10, 73–78. [Google Scholar] [CrossRef]
- Stopelli, E.; Conen, F.; Morris, C.E.; Herrmann, E.; Bukowiecki, N.; Alewell, C. Ice nucleation active particles are efficiently removed by precipitating clouds. Sci. Rep. 2015, 5, 16433. [Google Scholar] [CrossRef] [PubMed]
- Creamean, J.M.; Lee, C.; Hill, T.C.; Ault, A.P.; DeMott, P.J.; White, A.B.; Ralph, F.M.; Prather, K.A. Chemical properties of insoluble precipitation residue particles. J. Aerosol Sci. 2014, 76, 13–27. [Google Scholar] [CrossRef]
- Petters, M.D.; Wright, T.P. Revisiting ice nucleation from precipitation samples. Geophys. Res. Lett. 2015, 42, 8758–8766. [Google Scholar] [CrossRef]
- DeMott, P.J.; Cziczo, D.J.; Prenni, A.J.; Murphy, D.M.; Kreidenweis, S.M.; Thomson, D.S.; Borys, R.; Rogers, D.C. Measurements of the concentration and composition of nuclei for cirrus formation. Proc. Natl. Acad. Sci. USA 2003, 100, 14655–14660. [Google Scholar] [CrossRef] [PubMed]
- Creamean, J.M.; Suski, K.J.; Rosenfeld, D.; Cazorla, A.; DeMott, P.J.; Sullivan, R.C.; White, A.B.; Ralph, F.M.; Minnis, P.; Comstock, J.M. Dust and biological aerosols from the Sahara and Asia influence precipitation in the western US. Science 2013, 339, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Noone, K.J.; Ogren, J.A.; Heintzenberg, J.; Charlson, R.J.; Covert, D.S. Design and calibration of a counterflow virtual impactor for sampling of atmospheric fog and cloud droplets. Aerosol Sci. Technol. 1988, 8, 235–244. [Google Scholar] [CrossRef]
- Hiranuma, N.; Möhler, O.; Kulkarni, G.; Schnaiter, M.; Vogt, S.; Vochezer, P.; Järvinen, E.; Wagner, R.; Bell, D.M.; Wilson, J.; et al. Development and characterization of an ice-selecting pumped counterflow virtual impactor (IS-PCVI) to study ice crystal residuals. Atmos. Meas. Tech. 2016, 9, 3817–3836. [Google Scholar] [CrossRef]
- Mossop, S.C. Concentrations of ice crystals in clouds. Bull. Am. Meteorol. Soc. 1970, 51, 474–479. [Google Scholar] [CrossRef]
- Mossop, S.C.; Ono, A.; Wishart, E.R. Ice particles in maritime clouds near Tasmania. Q. J. R. Meteorol. Soc. 1970, 96, 487–508. [Google Scholar] [CrossRef]
- Hallett, J.; Sax, R.I.; Lamb, D.; Murty, A.S. Aircraft measurements of ice in Florida cumuli. Q. J. R. Meteorol. Soc. 1978, 104, 631–651. [Google Scholar] [CrossRef]
- Hobbs, P.V.; Rangno, A.L. Ice particle concentrations in clouds. J. Atmos. Sci. 1985, 42, 2523–2549. [Google Scholar] [CrossRef]
- Hobbs, P.V.; Rangno, A.L. Rapid development of high ice particle concentrations in small polar maritime cumuliform clouds. J. Atmos. Sci. 1990, 47, 2710–2722. [Google Scholar] [CrossRef]
- Rangno, A.L.; Hobbs, P.V. Ice particle concentrations and precipitation development in small polar maritime cumuliform clouds. Q. J. R. Meteorol. Soc. 1991, 117, 207–241. [Google Scholar] [CrossRef]
- Rosinski, J.; Morgan, G. Cloud condensation nuclei as a source of ice-forming nuclei in clouds. J. Aerosol Sci. 1991, 22, 123–133. [Google Scholar] [CrossRef]
- Santachiara, G.; Belosi, F. Does the Homogeneous Ice Nucleation Initiate in the Bulk Volume or at the Surface of Super-Cooled Water Droplets? A Review. Atmos. Clim. Sci. 2014, 4, 653. [Google Scholar] [CrossRef]
- Griggs, D.J.; Choularton, T.W. Freezing modes of riming droplets with application to ice splinter production. Q. J. R. Meteorol. Soc. 1983, 109, 243–253. [Google Scholar] [CrossRef]
- Takahashi, T.; Nagao, Y.; Kushiyama, Y. Possible high ice particle production during graupel–graupel collisions. J. Atmos. Sci. 1995, 52, 4523–4527. [Google Scholar] [CrossRef]
- Oraltay, R.G.; Hallett, J. Evaporation and melting of ice crystals: A laboratory study. Atmos. Res. 1989, 24, 169–189. [Google Scholar] [CrossRef]
- Conen, F.; Rodríguez, S.; Hülin, C.; Henne, S.; Herrmann, E.; Bukowiecki, N.; Alewell, C. Atmospheric ice nuclei at the high-altitude observatory Jungfraujoch, Switzerland. Tellus B Chem. Phys. Meteorol. 2015, 67, 25014. [Google Scholar] [CrossRef]
- Chou, C.; Stetzer, O.; Weingartner, E.; Jurányi, Z.; Kanji, Z.A.; Lohmann, U. Ice nuclei properties within a Saharan dust event at the Jungfraujoch in the Swiss Alps. Atmos. Chem. Phys. 2011, 11, 4725–4738. [Google Scholar] [CrossRef]
- Mertes, S.; Verheggen, B.; Walter, S.; Connolly, P.; Ebert, M.; Schneider, J.; Bower, K.N.; Cozic, J.; Weinbruch, S.; Baltensperger, U. Counterflow virtual impactor based collection of small ice particles in mixed-phase clouds for the physico-chemical characterization of tropospheric ice nuclei: Sampler description and first case study. Aerosol Sci. Technol. 2007, 41, 848–864. [Google Scholar] [CrossRef]
- Richardson, M.S.; DeMott, P.J.; Kreidenweis, S.M.; Cziczo, D.J.; Dunlea, E.J.; Jimenez, J.L.; Thomson, D.S.; Ashbaugh, L.L.; Borys, R.D.; Westphal, D.L. Measurements of heterogeneous ice nuclei in the western United States in springtime and their relation to aerosol characteristics. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Conen, F.; Morris, C.E.; Leifeld, J.; Yakutin, M.V.; Alewell, C. Biological residues define the ice nucleation properties of soil dust. Atmos. Chem. Phys. 2011, 11, 9643–9648. [Google Scholar] [CrossRef]
- Tobo, Y.; DeMott, P.J.; Hill, T.C.J.; Prenni, A.J.; Swoboda-Colberg, N.G.; Franc, G.D.; Kreidenweis, S.M. Organic matter matters for ice nuclei of agricultural soil origin. Atmos. Chem. Phys. 2014, 14, 8521–8531. [Google Scholar] [CrossRef]
- Murray, B.J.; O’sullivan, D.; Atkinson, J.D.; Webb, M.E. Ice nucleation by particles immersed in supercooled cloud droplets. Chem. Soc. Rev. 2012, 41, 6519–6554. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, N.H.; Squires, P. The Physics of Rainclouds: With an Introductory Chapter by P. Squires; Cambridge University Press: London, UK, 1962; Volume 3, pp. 154–196, ISBN-13: 9787100822300. [Google Scholar]
- Cooper, W.A. Ice Initiation in Natural Clouds. Meteorol. Monogr. 1986, 43, 29–32. [Google Scholar] [CrossRef]
- Niemand, M.; Möhler, O.; Vogel, B.; Vogel, H.; Hoose, C.; Connolly, P.; Klein, H.; Bingemer, H.; DeMott, P.; Skrotzki, J. A particle-surface-area-based parameterization of immersion freezing on desert dust particles. J. Atmos. Sci. 2012, 69, 3077–3092. [Google Scholar] [CrossRef]
- Marcolli, C.; Gedamke, S.; Peter, T.; Zobrist, B. Efficiency of immersion mode ice nucleation on surrogates of mineral dust. Atmos. Chem. Phys. 2007, 7, 5081–5091. [Google Scholar] [CrossRef]
- Broadley, S.L.; Murray, B.J.; Herbert, R.J.; Atkinson, J.D.; Dobbie, S.; Malkin, T.L.; Condliffe, E.; Neve, L. Immersion mode heterogeneous ice nucleation by an illite rich powder representative of atmospheric mineral dust. Atmos. Chem. Phys. 2012, 12, 287–307. [Google Scholar] [CrossRef]
- Tobo, Y.; Prenni, A.J.; DeMott, P.J.; Huffman, J.A.; McCluskey, C.S.; Tian, G.; Pöhlker, C.; Pöschl, U.; Kreidenweis, S.M. Biological aerosol particles as a key determinant of ice nuclei populations in a forest ecosystem. J. Geophys. Res. Atmos. 2013, 118. [Google Scholar] [CrossRef]
- Hiranuma, N.; Augustin-Bauditz, S.; Bingemer, H.; Budke, C.; Curtius, J.; Danielczok, A.; Diehl, K.; Dreischmeier, K.; Ebert, M.; Frank, F. A comprehensive laboratory study on the immersion freezing behavior of illite NX particles: A comparison of 17 ice nucleation measurement techniques. Atmos. Chem. Phys. 2015, 15, 2489–2518. [Google Scholar] [CrossRef]
- Wex, H.; Augustin-Bauditz, S.; Boose, Y.; Budke, C.; Curtius, J.; Diehl, K.; Dreyer, A.; Frank, F.; Hartmann, S.; Hiranuma, N. Intercomparing different devices for the investigation of ice nucleating particles using Snomax® as test substance. Atmos. Chem. Phys. 2015, 15, 1463–1485. [Google Scholar] [CrossRef]
- Grawe, S.; Augustin-Bauditz, S.; Hartmann, S.; Hellner, L.; Pettersson, J.B.; Prager, A.; Stratmann, F.; Wex, H. The immersion freezing behavior of ash particles from wood and brown coal burning. Atmos. Chem. Phys. 2016, 16, 13911–13928. [Google Scholar] [CrossRef]
- Reddington, C.L.; Carslaw, K.S.; Stier, P.; Schutgens, N.; Coe, H.; Liu, D.; Allan, J.; Browse, J.; Pringle, K.J.; Lee, L.A.; et al. The Global Aerosol Synthesis and Science Project (GASSP): Measurements and modelling to reduce uncertainty. Bull. Am. Meteorol. Soc. 2017. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Bretherton, C.; Carslaw, K.S.; Coe, H.; DeMott, P.J.; Dunlea, E.J.; Feingold, G.; Ghan, S.; Guenther, A.B.; Kahn, R. Improving our fundamental understanding of the role of aerosol− cloud interactions in the climate system. Proc. Natl. Acad. Sci. USA 2016, 113, 5781–5790. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coluzza, I.; Creamean, J.; Rossi, M.J.; Wex, H.; Alpert, P.A.; Bianco, V.; Boose, Y.; Dellago, C.; Felgitsch, L.; Fröhlich-Nowoisky, J.; et al. Perspectives on the Future of Ice Nucleation Research: Research Needs and Unanswered Questions Identified from Two International Workshops. Atmosphere 2017, 8, 138. https://doi.org/10.3390/atmos8080138
Coluzza I, Creamean J, Rossi MJ, Wex H, Alpert PA, Bianco V, Boose Y, Dellago C, Felgitsch L, Fröhlich-Nowoisky J, et al. Perspectives on the Future of Ice Nucleation Research: Research Needs and Unanswered Questions Identified from Two International Workshops. Atmosphere. 2017; 8(8):138. https://doi.org/10.3390/atmos8080138
Chicago/Turabian StyleColuzza, Ivan, Jessie Creamean, Michel J. Rossi, Heike Wex, Peter Aaron Alpert, Valentino Bianco, Yvonne Boose, Christoph Dellago, Laura Felgitsch, Janine Fröhlich-Nowoisky, and et al. 2017. "Perspectives on the Future of Ice Nucleation Research: Research Needs and Unanswered Questions Identified from Two International Workshops" Atmosphere 8, no. 8: 138. https://doi.org/10.3390/atmos8080138
APA StyleColuzza, I., Creamean, J., Rossi, M. J., Wex, H., Alpert, P. A., Bianco, V., Boose, Y., Dellago, C., Felgitsch, L., Fröhlich-Nowoisky, J., Herrmann, H., Jungblut, S., Kanji, Z. A., Menzl, G., Moffett, B., Moritz, C., Mutzel, A., Pöschl, U., Schauperl, M., ... Schmale, D. G. (2017). Perspectives on the Future of Ice Nucleation Research: Research Needs and Unanswered Questions Identified from Two International Workshops. Atmosphere, 8(8), 138. https://doi.org/10.3390/atmos8080138





