N2O Production and Reduction in Chinese Paddy Soils: Linking Microbial Functional Genes with Soil Chemical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling
2.2. Soil Chemical Properties Analysis
2.3. DNA Extraction and Quantitative PCR
2.4. Measurement of Potential N2O Production Rate and N2O Reduction Rate
2.5. Statistical Analysis
3. Results
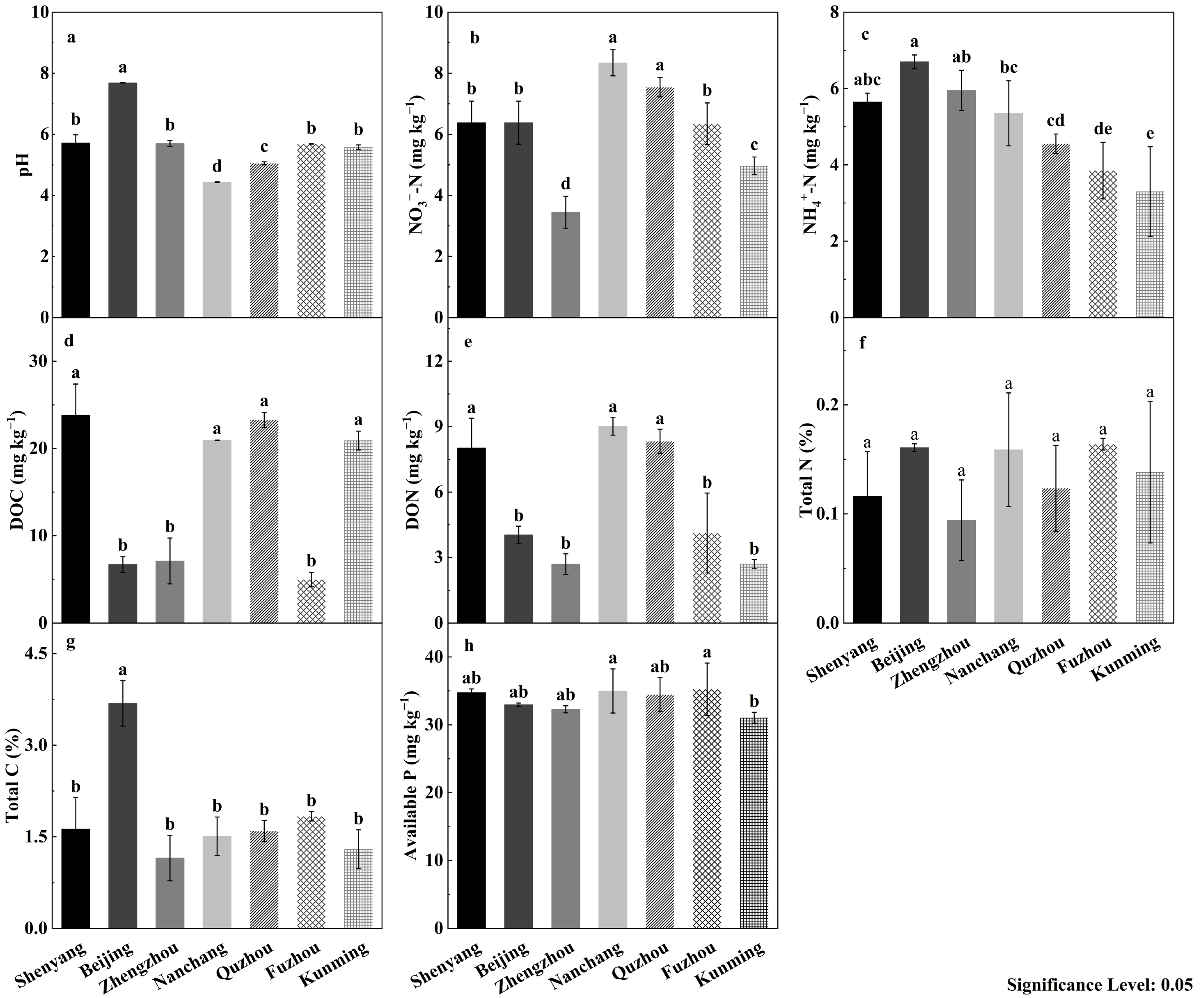
3.1. Soil Chemical Properties
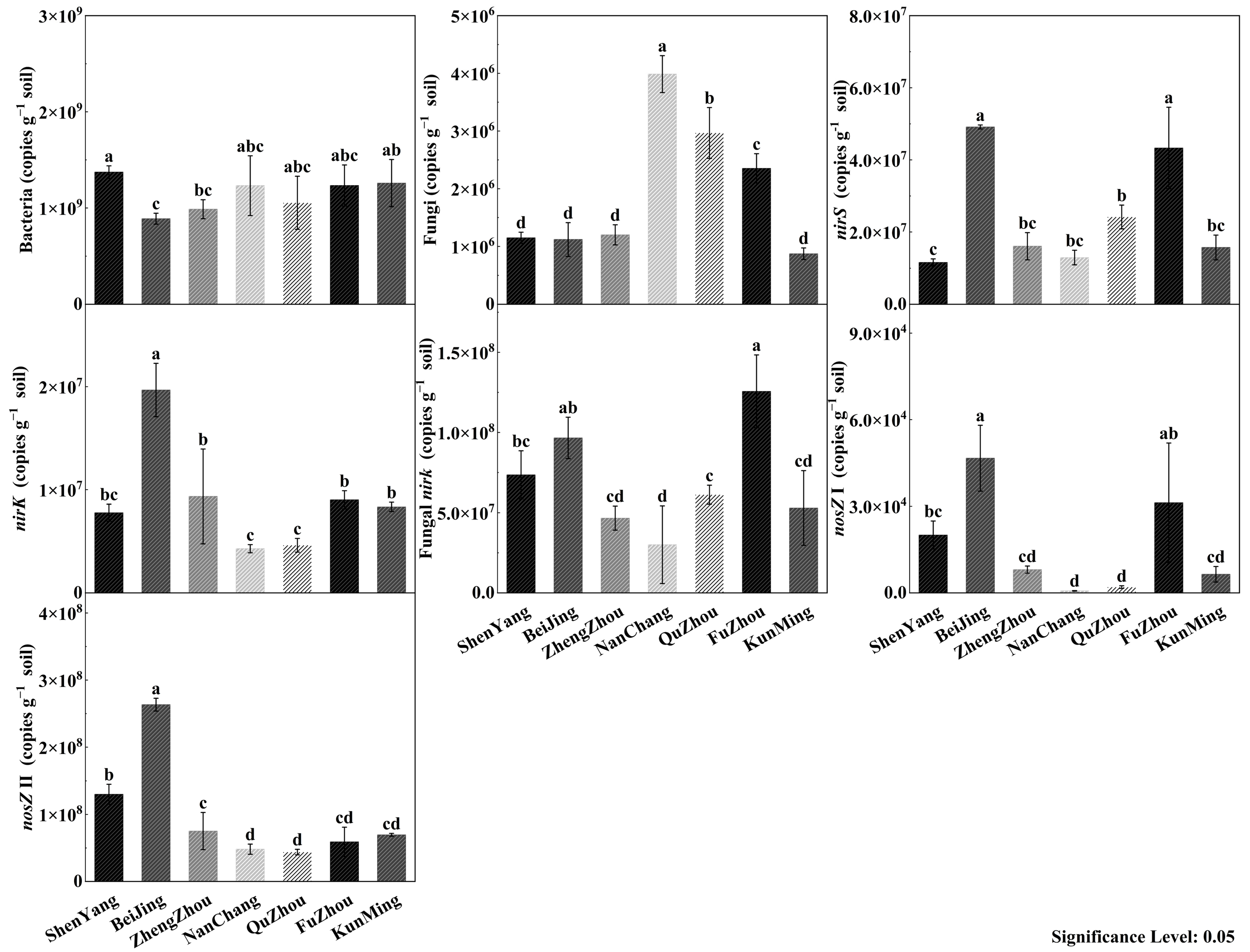
3.2. Microbial Gene Abundance
3.3. Potential N2O Production Rate and N2O Reduction Rate
3.4. Principal Component Analysis
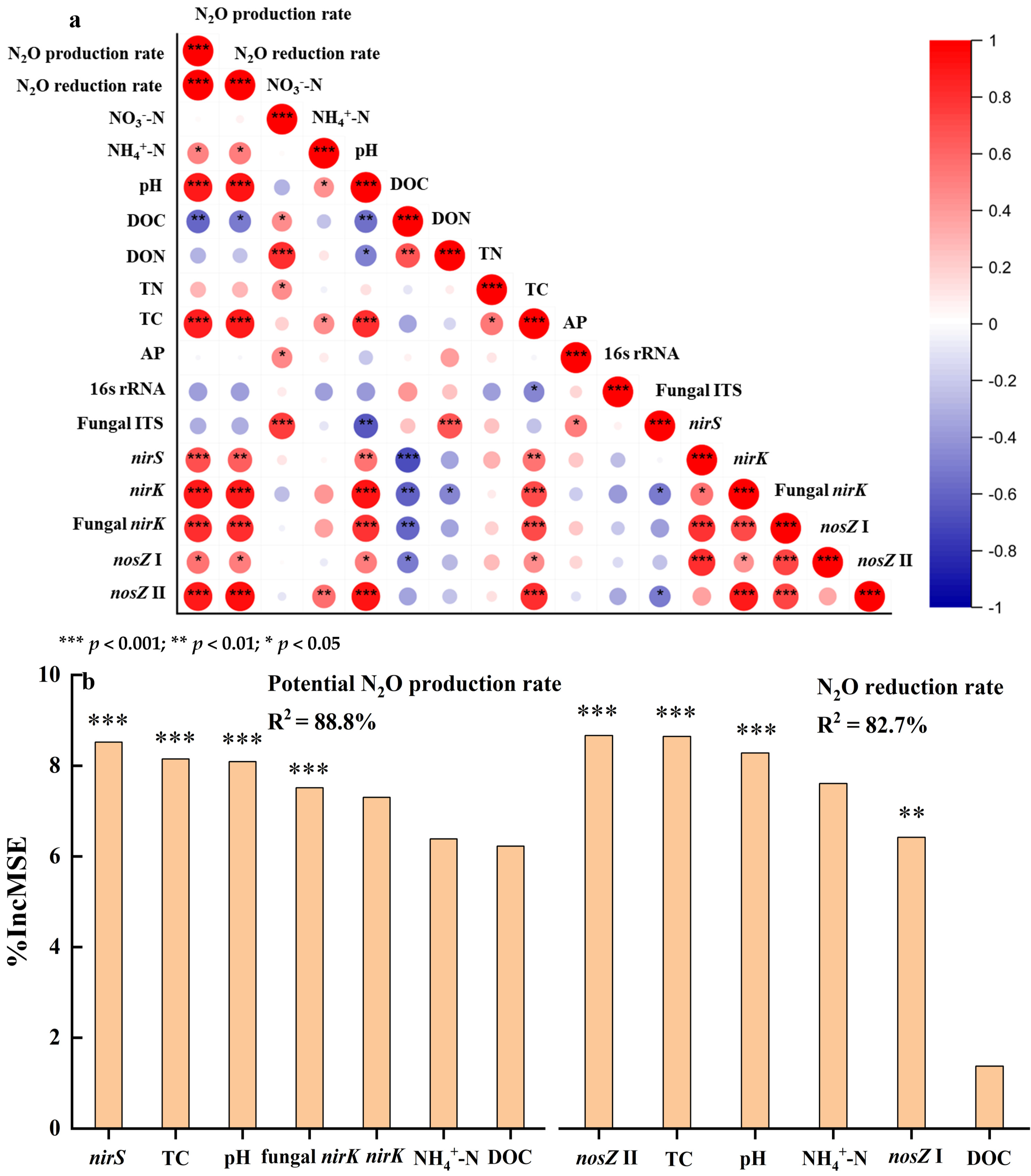
3.5. Correlation and Random Forest Analysis
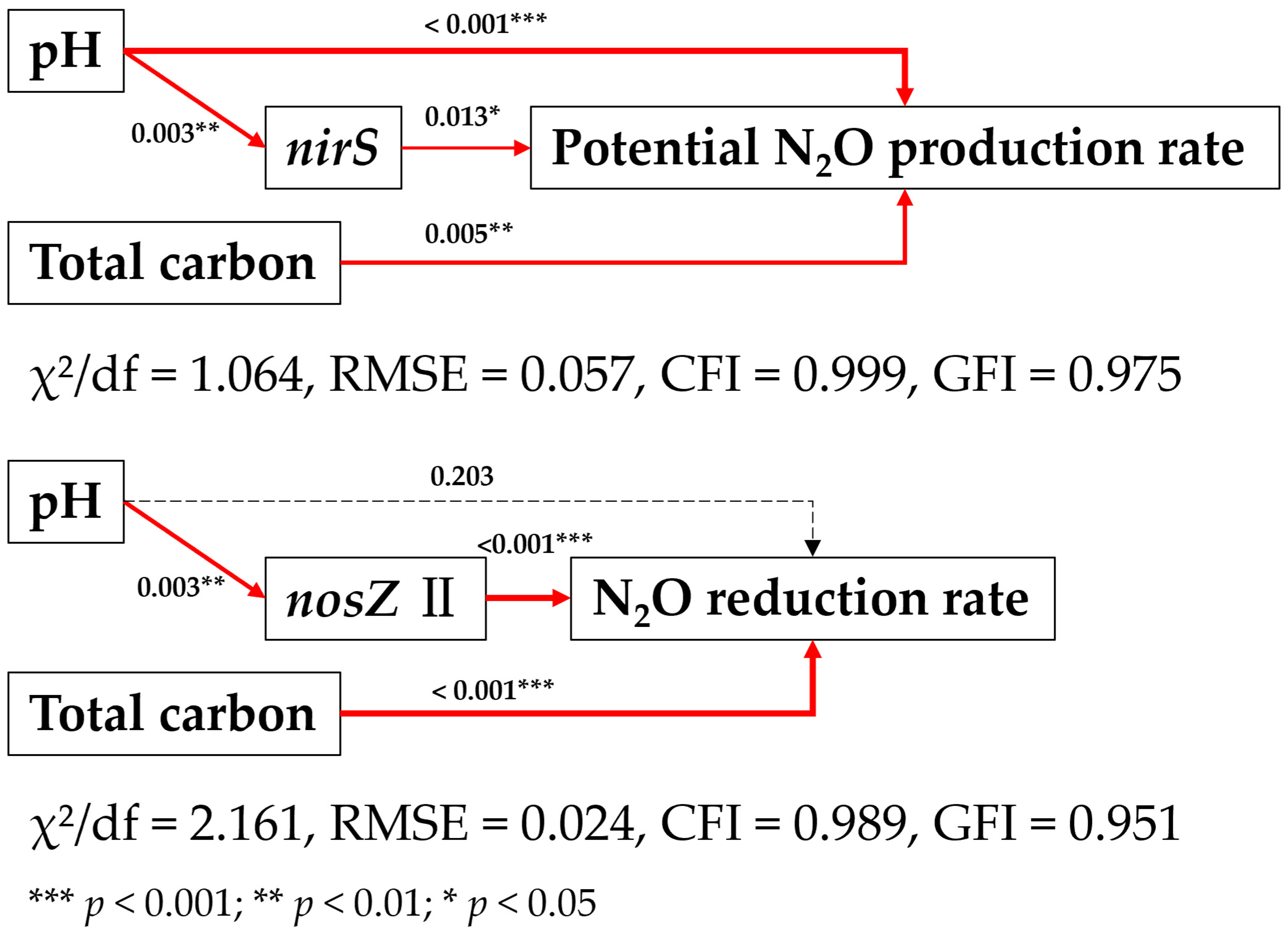
3.6. Structural Equation Modeling
4. Discussion
4.1. Biotic and Abiotic Factors Influencing Denitrification-Derived N2O Production
4.2. Biotic and Abiotic Factors Influencing N2O Reduction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of WorkingGroup I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; WGI: Dayton, OH, USA, 2021. [Google Scholar]
- Zhang, Y.; Wang, W.; Yao, H. Urea-based nitrogen fertilization in agriculture: A key source of N2O emissions and recent development in mitigating strategies. Arch. Agron. Soil Sci. 2023, 69, 663–678. [Google Scholar] [CrossRef]
- Harter, J.; Krause, H.-M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J. 2014, 8, 660–674. [Google Scholar] [CrossRef]
- Tang, Y.; Minasny, B.; McBratney, A. Partitioning denitrification pathways in N2O emissions from re-flooded dry paddy soils. Biogeochemistry 2024, 167, 1315–1333. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Huang, X.; Cheng, Y.; Cai, Z.; Zhang, J.; Müller, C. Microbial pathways account for the pH effect on soil N2O production. Eur. J. Soil Biol. 2021, 106, 103337. [Google Scholar] [CrossRef]
- Men, C.; Liu, R.; Wang, Q.; Guo, L.; Shen, Z. The impact of seasonal varied human activity on characteristics and sources of heavy metals in metropolitan road dusts. Sci. Total Environ. 2018, 637, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Herold, M.B.; Giles, E.M.; Alexander, C.J.; Baggs, E.M.; Daniell, T.J. Variable response of nirK and nirS containing denitrifier communities to long-term pH manipulation and cultivation. Fems Microbiol. Lett. 2018, 365, fny035. [Google Scholar] [CrossRef]
- Wittorf, L.; Jones, C.M.; Bonilla-Rosso, G.; Hallin, S. Expression of nirK and nirS genes in two strains of Pseudomonas stutzeri harbouring both types of NO-forming nitrite reductases. Res. Microbiol. 2018, 169, 343–347. [Google Scholar] [CrossRef]
- Ming, Y.Z.; Abdullah, A.M.; Zhang, D.; Zhu, W.; Liu, H.; Cai, L.; Yu, X.; Wu, K.; Niu, M.; Zeng, Q.; et al. Insights into the evolutionary and ecological adaption strategies of nirS- and nirK-type denitrifying communities. Mol. Ecol. 2024, 33, e17507. [Google Scholar] [CrossRef]
- Wu, G.; Liang, F.; Wu, Q.; Feng, X.-G.; Shang, W.-D.; Li, H.-W.; Li, X.-X.; Che, Z.; Dong, Z.-R.; Song, H. Soil pH differently affects N2O emissions from soils amended with chemical fertilizer and manure by modifying nitrification and denitrification in wheat-maize rotation system. Biol. Fertil. Soils 2024, 60, 101–113. [Google Scholar] [CrossRef]
- Qiang, R.W.; Wang, M.; Li, Q.; Li, Y.; Sun, H.; Liang, W.; Li, C.; Zhang, J.; Liu, H. Response of Soil Nitrogen Components and nirK- and nirS-Type Denitrifying Bacterial Community Structures to Drip Irrigation Systems in the Semi-Arid Area of Northeast China. Agronomy 2024, 14, 577. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, Y.; Ciampitti, I.A.; He, P.; Xu, X.; Qiu, S.; Zhao, S. Response of soil denitrification potential and community composition of denitrifying bacterial to different rates of straw return in north-central China. Appl. Soil Ecol. 2022, 170, 104312. [Google Scholar] [CrossRef]
- Luo, X.S.; Zeng, L.; Wang, L.; Qian, H.; Hou, C.; Wen, S.; Wang, B.; Huang, Q.; Chen, W. Abundance for subgroups of denitrifiers in soil aggregates associates with denitrifying enzyme activities under different fertilization regimes. Appl. Soil Ecol. 2021, 166, 103983. [Google Scholar] [CrossRef]
- Yang, X.; Tang, S.; Ni, K.; Shi, Y.; Yi, X.; Ma, Q.; Cai, Y.; Ma, L.; Ruan, J. Long-term nitrogen addition increases denitrification potential and functional gene abundance and changes denitrifying communities in acidic tea plantation soil. Environ. Res. 2022, 216, 114679. [Google Scholar] [CrossRef]
- Tang, Q.; Moeskjær, S.; Cotton, A.; Dai, W.; Wang, X.; Yan, X.; Daniell, T.J. Organic fertilization reduces nitrous oxide emission by altering nitrogen cycling microbial guilds favouring complete denitrification at soil aggregate scale. Sci. Total Environ. 2024, 946, 174178. [Google Scholar] [CrossRef] [PubMed]
- Intrator, N.; Jayakumar, A.; Ward, B.B. Aquatic nitrous oxide reductase gene (nosZ) phylogeny and environmental distribution. Front. Microbiol. 2024, 15, 1407573. [Google Scholar] [CrossRef]
- Philippot, L.; Andert, J.; Jones, C.M.; Bru, D.; Hallin, S. Importance of denitrifiers lacking the genes encoding the nitrous oxide reductase for N2O emissions from soil. Glob. Change Biol. 2010, 17, 1497–1504. [Google Scholar] [CrossRef]
- Frostegård, Å.; Vick, S.H.W.; Lim, N.Y.N.; Bakken, L.R.; Shapleigh, J.P. Linking meta-omics to the kinetics of denitrification intermediates reveals pH-dependent causes of N2O emissions and nitrite accumulation in soil. ISME J. 2021, 16, 26–37. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.; Putz, M.; Spor, A.; Bru, D.; Breuil, M.; Hallin, S.; Philippot, L. Non-denitrifying nitrous oxide-reducing bacteria-An effective N2O sink in soil. Soil Biol. Biochem. 2016, 103, 376–379. [Google Scholar] [CrossRef]
- Meng, C.; Wang, F.; Engel, B.A.; Yang, K.; Zhang, Y. Is Cattle Manure Application with Plastic-Film Mulch a Good Choice for Potato Production? Agronomy 2019, 9, 534. [Google Scholar] [CrossRef]
- Qin, C.; Li, S.-L.; Wu, Y.; Bass, A.M.; Luo, W.; Ding, H.; Yue, F.-J.; Zhang, P. High sensitivity of dissolved organic carbon transport during hydrological events in a small subtropical karst catchment. Sci. Total Environ. 2024, 946, 174090. [Google Scholar] [CrossRef]
- Zhongyi, C.; He, Y.; Wang, N.; Wu, L.; Xu, J.; Shi, J. Uncovering soil amendment-induced genomic and functional divergence in soybean rhizosphere microbiomes during cadmium-contaminated soil remediation: Novel insights from field multi-omics. Environ. Pollut. 2025, 368, 125787. [Google Scholar]
- Abbas, T.; Zhang, Q.; Zou, X.; Tahir, M.; Wu, D.; Jin, S.; Di, H. Soil anammox and denitrification processes connected with N cycling genes co-supporting or contrasting under different water conditions. Environ. Int. 2020, 140, 105757. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2015, 1, e00009-15. [Google Scholar] [CrossRef]
- Chen, H.; Mothapo, N.V.; Shi, W. Fungal and bacterial N2O production regulated by soil amendments of simple and complex substrates. Soil Biol. Biochem. 2015, 84, 116–126. [Google Scholar] [CrossRef]
- Throbäck, I.N.; Enwall, K.; Jarvis, A.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. Fems Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef]
- Hallin, S.; Lindgren, P.-E. PCR detection of genes encoding nitrile reductase in denitrifying bacteria. Appl. Environ. Microbiol. 1999, 65, 1652–1657. [Google Scholar] [CrossRef]
- Wei, W.; Isobe, K.; Shiratori, Y.; Nishizawa, T.; Ohte, N.; Ise, Y.; Otsuka, S.; Senoo, K. Development of PCR primers targeting fungal nirK to study fungal denitrification in the environment. Soil Biol. Biochem. 2015, 81, 282–286. [Google Scholar] [CrossRef]
- Zhang, B.; Penton, C.R.; Yu, Z.; Xue, C.; Chen, Q.; Chen, Z.; Yan, C.; Zhang, Q.; Zhao, M.; Quensen, J.F.; et al. A new primer set for Clade I nosZ that recovers genes from a broader range of taxa. Biol. Fertil. Soils 2021, 57, 523–531. [Google Scholar] [CrossRef]
- Jones, C.M.; Graf, D.R.H.; Bru, D.; Philippot, L.; Hallin, S. The unaccounted yet abundant nitrous oxide-reducing microbial community: A potential nitrous oxide sink. ISME J. 2012, 7, 417–426. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Q.; Zhou, J.; Yuan, D.; Zhu, G. Linking abundance and community of microbial N2O-producers and N2O-reducers with enzymatic N2O production potential in a riparian zone. Sci. Total Environ. 2018, 642, 1090–1099. [Google Scholar] [CrossRef]
- Machefert, S.E.; Dise, N.B. Hydrological controls on denitrification in riparian ecosystems. Hydrol. Earth Syst. Sci. 2004, 8, 686–694. [Google Scholar] [CrossRef][Green Version]
- Foltz, M.E.; Kent, A.D.; Koloutsou-Vakakis, S.; Zilles, J.L. Influence of rye cover cropping on denitrification potential and year-round field N2O emissions. Sci. Total Environ. 2021, 765, 144295. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, L.; Huang, G.; Rasul, F.; Awan, M.I.; Cui, H.; Liu, K.; Yu, X.; Tang, H.; Wang, S.; et al. nirS and nosZII bacterial denitrifiers as well as fungal denitrifiers are coupled with N2O emissions in long-term fertilized soils. Sci. Total Environ. 2023, 897, 165426. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Francis, C.A. Francis, Spatiotemporal Characterization of San Francisco Bay Denitrifying Communities: A Comparison of nirK and nirS Diversity and Abundance. Microb. Ecol. 2017, 73, 271–284. [Google Scholar] [CrossRef]
- Song, H.; Che, Z.; Jin, W.; Meng, Y.; Wang, J.; Cao, W.; Dong, Z. Changes in denitrifier communities and denitrification rates in an acidifying soil induced by excessive N fertilization. Arch. Agron. Soil Sci. 2019, 66, 1203–1217. [Google Scholar] [CrossRef]
- Hu, X.; Gu, H.; Liu, J.; Liu, Z.; Li, L.; Du, S.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; et al. Biogeographic distribution patterns and assembly processes of nirS-type and nirK-type denitrifiers across the black soil zone in Northeast China. Soil Sci. Soc. Am. J. 2021, 86, 1383–1396. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, N.; Li, Y.; Xu, S.; Liu, Y.; Miao, S.; Ding, W. Extreme Rainfall Amplified the Stimulatory Effects of Soil Carbon Availability on N2O Emissions. Glob. Change Biol. 2025, 31, e70164. [Google Scholar] [CrossRef]
- Highton, M.P.; Bakken, L.R.; Dörsch, P.; Wakelin, S.; de Klein, C.A.; Molstad, L.; Morales, S.E. Soil N2O emission potential falls along a denitrification phenotype gradient linked to differences in microbiome, rainfall and carbon availability. Soil Biol. Biochem. 2020, 150, 108004. [Google Scholar] [CrossRef]
- Wei, Z.; Well, R.; Ma, X.; Lewicka-Szczebak, D.; Rohe, L.; Zhang, G.; Li, C.; Ma, J.; Bol, R.; Xu, H.; et al. Organic fertilizer amendment decreased N2O/(N2O+N2) ratio by enhancing the mutualism between bacterial and fungal denitrifiers in high nitrogen loading arable soils. Soil Biol. Biochem. 2024, 198, 109550. [Google Scholar] [CrossRef]
- Hallin, S.; Philippot, L.; Löffler, F.E.; Sanford, R.A.; Jones, C.M. Genomics and Ecology of Novel N2O-Reducing Microorganisms. Trends Microbiol. 2018, 26, 43–55. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, H.-W.; Deng, M.; Yang, P.; Ye, G. Microorganisms carrying nosZ I and nosZ II share similar ecological niches in a subtropical coastal wetland. Sci. Total Environ. 2023, 870, 162008. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhou, H.; Wu, M.; Shan, J.; Yan, X. Investigating drivers of N2 loss and N2O reducers in paddy soils across China. Sci. Total Environ. 2024, 954, 176287. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Liu, C.; Yang, M.; Zhong, J.; Wang, W.; Li, Z.; Li, K. Stronger link of nosZ I than nosZ II to the higher total N2O consumption in anoxic paddy surface soils. Geoderma 2022, 425, 116035. [Google Scholar] [CrossRef]
- Shaaban, M.; Hu, R.; Wu, Y.; Song, L.; Xu, P. Soil pH management for mitigating N2O emissions through nosZ (Clade I and II) gene abundance in rice paddy system. Environ. Res. 2023, 225, 115542. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yin, Y.; He, G.; Cha, G.; Ayala-Del-Río, H.L.; González, G.; Konstantinidis, K.T.; Löffler, E.F. pH selects for distinct N2O-reducing microbiomes in tropical soil microcosms. ISME Commun. 2024, 4, ycae070. [Google Scholar] [CrossRef]
- Qian, X.; Chen, H.; Li, Q.; Wang, F. Converse Responses of Biochar Application on N2O Emissions in Soils at Different pH Values in a Subtropical Citrus Orchard. Agronomy 2024, 14, 1831. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Pold, G.; Liu, X.-J.A.; Frey, S.D.; Melillo, J.M.; DeAngelis, K.M. Microbial diversity drives carbon use efficiency in a model soil. Nat. Commun. 2020, 11, 3684. [Google Scholar] [CrossRef]
- Su, F.; Li, Y.; Qian, J.; Li, T.; Wang, Y. Impact of freeze-thaw cycles and influent C/N ratios on N2O emissions in subsurface wastewater infiltration systems. J. Environ. Chem. Eng. 2024, 12, 114293. [Google Scholar] [CrossRef]



| Gene | Primer | Sequence 5′ to 3′ | Thermal Cycling Conditions |
|---|---|---|---|
| 16s rRNA | 515F/806R | GTGYCAGCMGCCGCGGTAA/ GGACTACNVGGGTWTCTAAT | 95 °C for 1 min, 1 cycle; 95 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s, 40 cycles; 75 °C for 10 min. [24] |
| Fungal ITS | 5.8S-F/ITS1f-R | CGCTGCGTTCTTCATCG/ TCCGTAGGTGAACCTGCGG | 95 °C for 1 min, cycle; 98 °C for 10 s, 53 °C for 30 s, 72 °C for 30 s, 40 cycles; 75 °C for 10 min. [25] |
| nirS | nirS-cd3aF/ nirS-R3cd | GASTTCGGRTGSGTCTTGA/ ATCATGGTSCTGCCGCG | 95 °C for 1 min, 1 cycle; 98 °C for 10 s, 53 °C for 30 s, 72 °C for 30 s, 40 cycles; 75 °C for 10 min. [26] |
| nirK | nirK-FlaCu/ nirK-R3Cu-GCb | ATCATGGTSCTGCCGCG/ GCCTCGATCAGRTTGTGGTT | 94 °C for 3 min, 1 cycle; 94 °C for 30 s, 57 °C for 1 min, 73 °C for 1 min, 35 cycles; 75 °C for 10 min. [27] |
| Fungal nirK | nirKfF/ nirKfR | TACGGGCTCATGtaygtnsarcc/ AGGAATCCCACAscnccyttntc | 95 °C for 5 min, 1 cycle; 95 °C for 30 s, 61.5 °C for 30 s, 72 °C for 1 min, 35 cycle; 72 °C for 10 min. [28] |
| nosZ I | nosZ I F/ nosZ I R | GGCAARCTVTCDCCVAC/ AVCGGTCYTTVGAGAAYTT | 95 °C for 2 min, 1 cycle; 95 °C for 45 s, 53 °C for 45 s, 72 °C for 1 min, 35 cycles; 72 °C for 10 min. [29] |
| nosZ II | nosZ- II -F/ nosZ- II -R | CTIGGICCIYTKCAYAC/ GCIGARCARAAITCBGTRC | 95 °C for 5 min, 1 cycle; 95 °C for 30 s, 54 °C for 60 s, 72 °C for 1 min, 35 cycles; 72 °C for 10 min. [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, C.; Jiang, A.; Gao, Y.; Yu, X.; Zhou, Y.; Chen, R.; Shen, W.; Yang, K.; Wang, W.; Qi, D.; et al. N2O Production and Reduction in Chinese Paddy Soils: Linking Microbial Functional Genes with Soil Chemical Properties. Atmosphere 2025, 16, 788. https://doi.org/10.3390/atmos16070788
Meng C, Jiang A, Gao Y, Yu X, Zhou Y, Chen R, Shen W, Yang K, Wang W, Qi D, et al. N2O Production and Reduction in Chinese Paddy Soils: Linking Microbial Functional Genes with Soil Chemical Properties. Atmosphere. 2025; 16(7):788. https://doi.org/10.3390/atmos16070788
Chicago/Turabian StyleMeng, Chaobiao, Aoqi Jiang, Yumeng Gao, Xiangyun Yu, Yujie Zhou, Ruiquan Chen, Weijian Shen, Kaijing Yang, Weihan Wang, Dongliang Qi, and et al. 2025. "N2O Production and Reduction in Chinese Paddy Soils: Linking Microbial Functional Genes with Soil Chemical Properties" Atmosphere 16, no. 7: 788. https://doi.org/10.3390/atmos16070788
APA StyleMeng, C., Jiang, A., Gao, Y., Yu, X., Zhou, Y., Chen, R., Shen, W., Yang, K., Wang, W., Qi, D., Xu, C., & Duan, Y. (2025). N2O Production and Reduction in Chinese Paddy Soils: Linking Microbial Functional Genes with Soil Chemical Properties. Atmosphere, 16(7), 788. https://doi.org/10.3390/atmos16070788





