Research on the Mechanism and Source Changes of Urban O3 Formation Under the Background of Increased Industrial Activity Levels
Abstract
1. Introduction
2. Materials and Methods
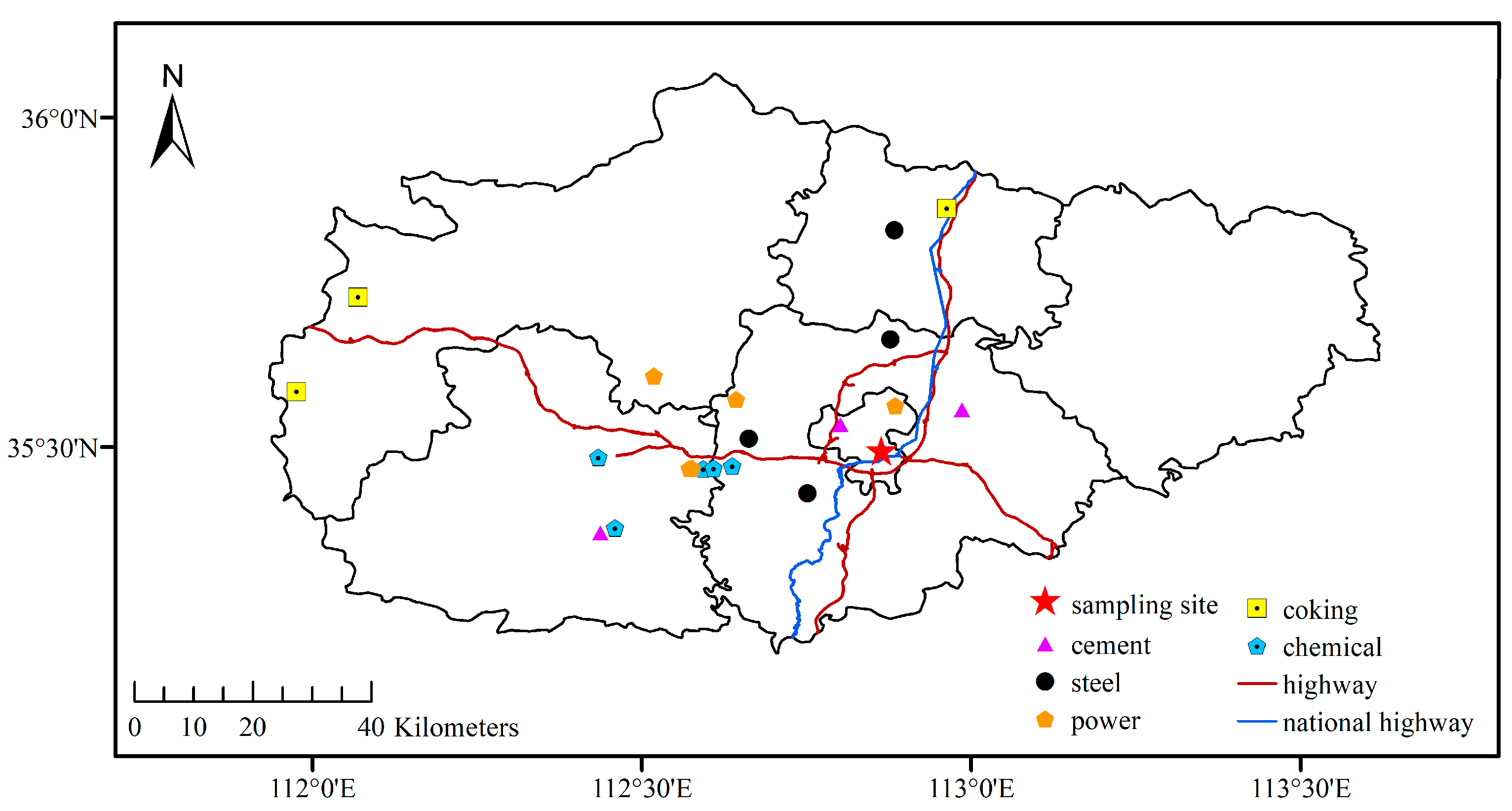
2.1. Sample Collection and Quality Control
2.2. PMF
2.3. OBM
2.3.1. O3 Production Simulation
2.3.2. RIR
3. Results and Discussion
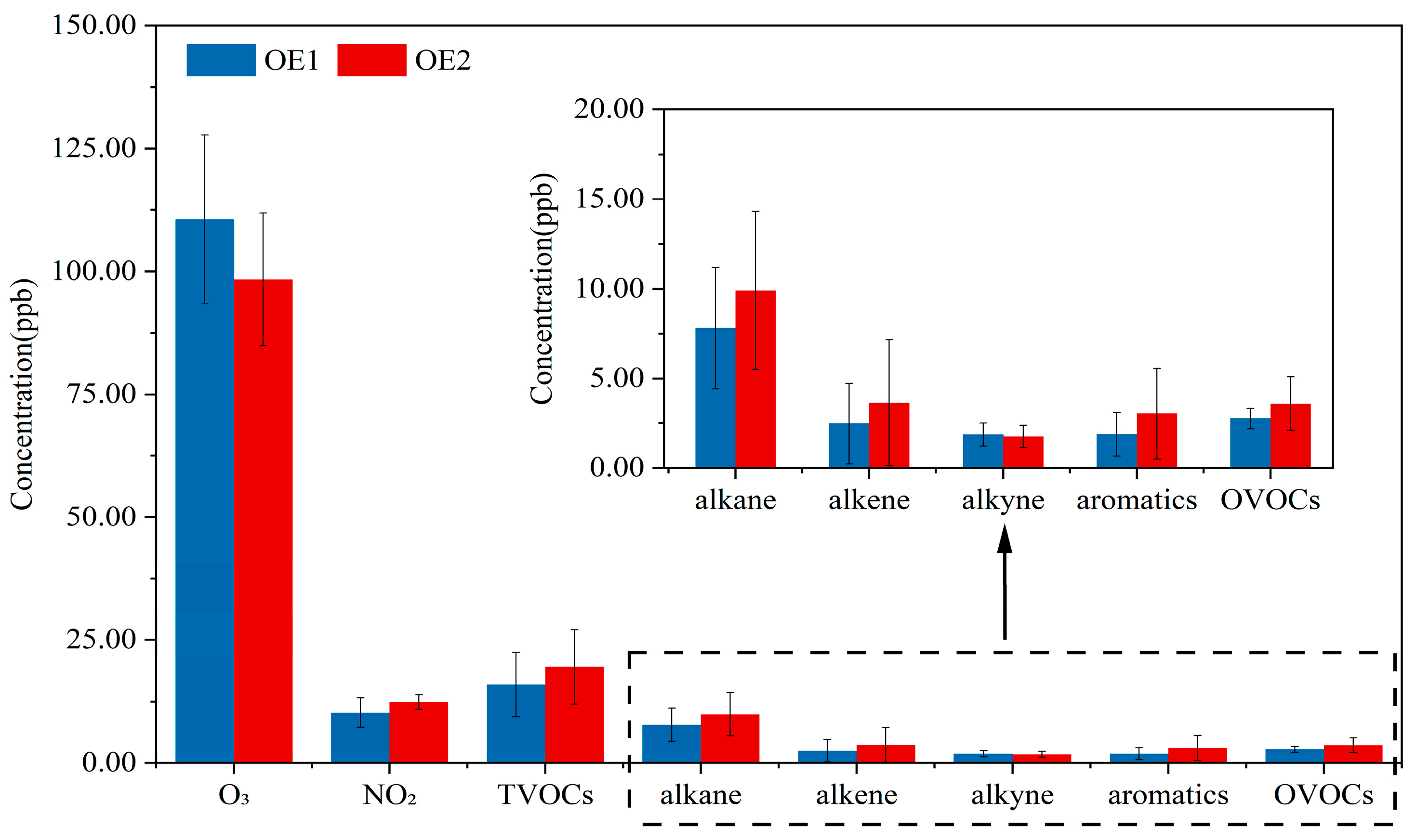
3.1. Characteristics of O3 and Its Precursors
3.2. Simulation of Atmospheric Photochemical Reaction Process
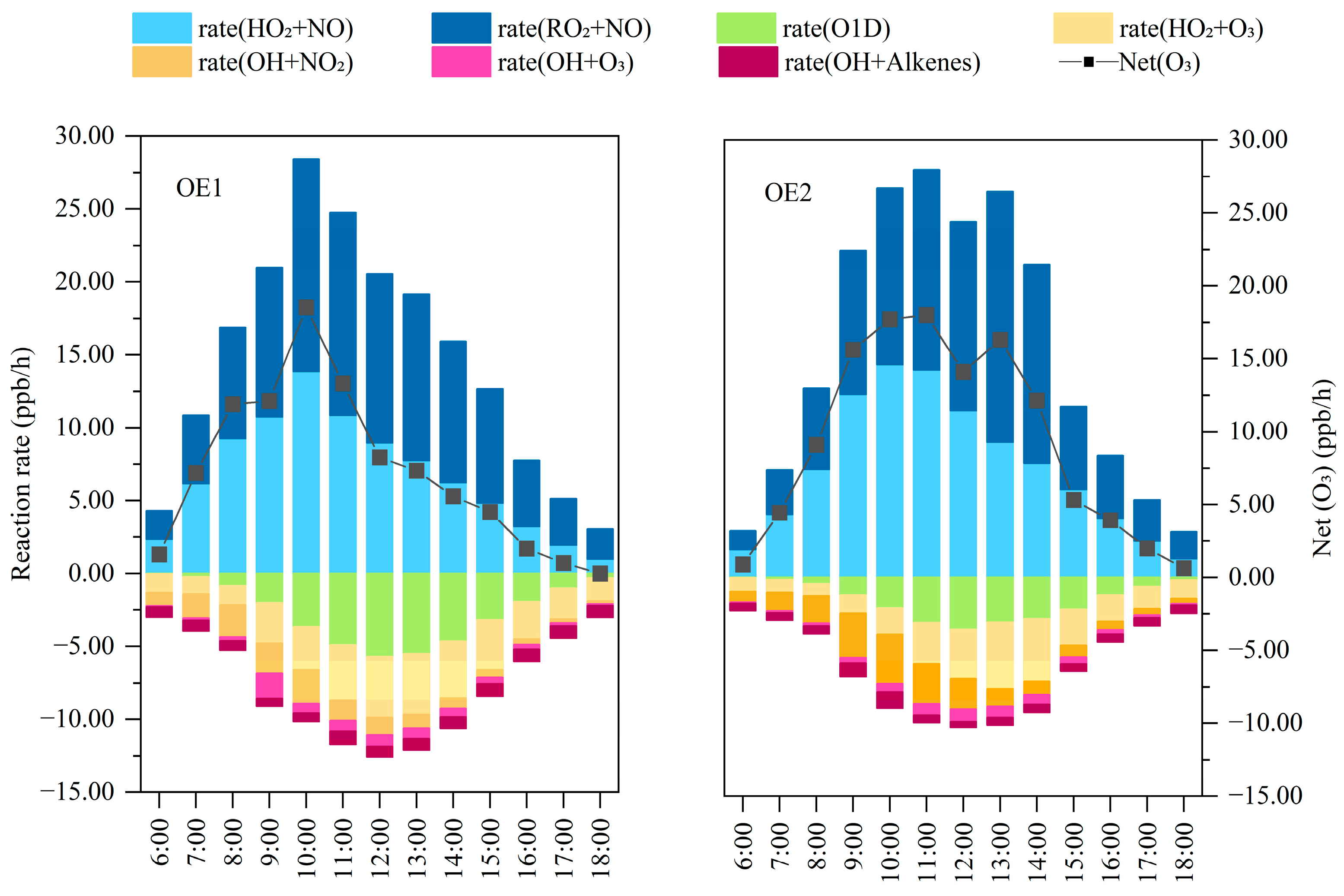
3.2.1. O3 Budget Analysis
3.2.2. Free Radical Cycle
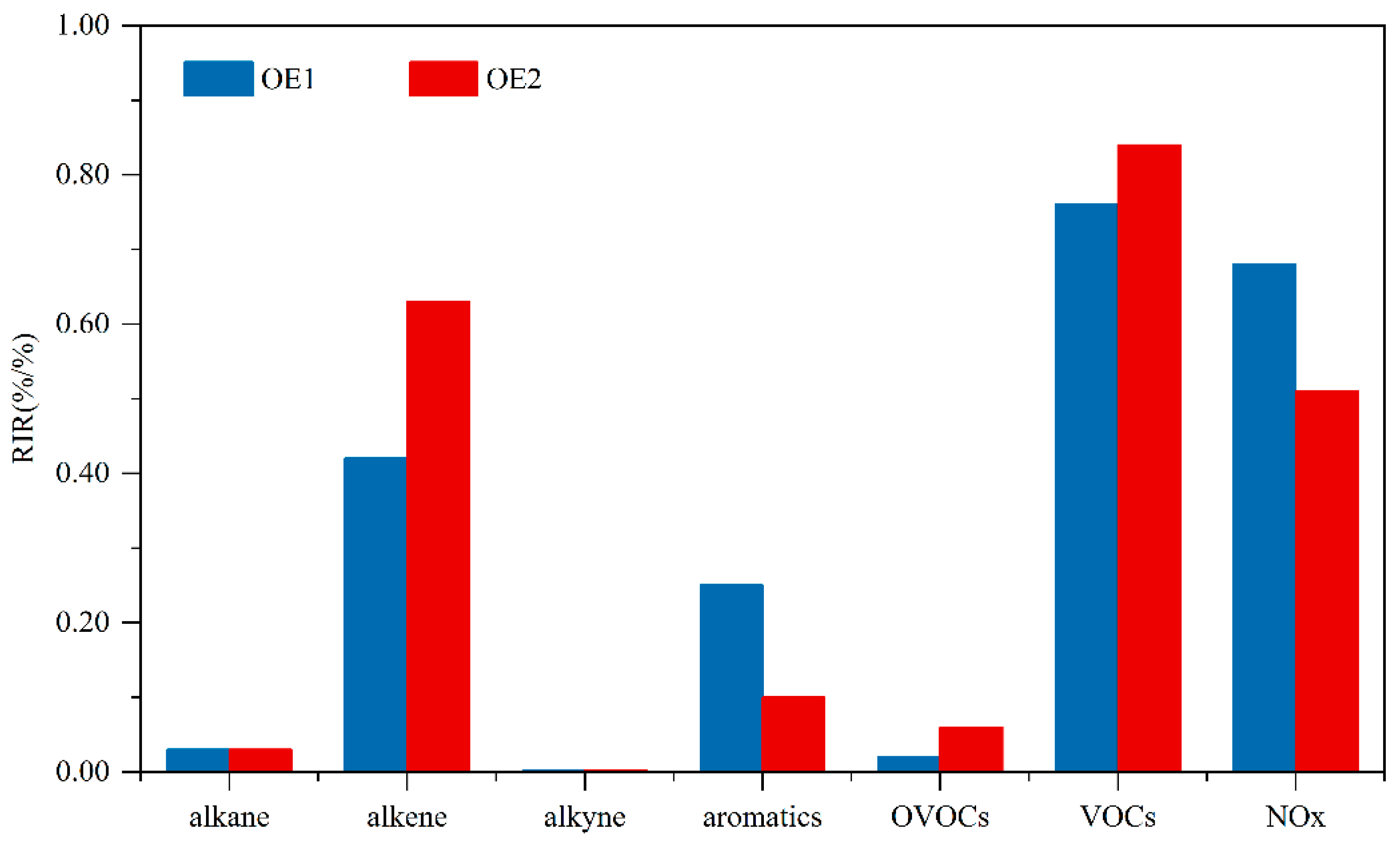
3.2.3. Sensitivity Analysis of O3 Formation
3.3. Source Apportionment of O3 and Its Precursors
3.3.1. VOCs and NOx Source Apportionment
3.3.2. O3 Source Apportionment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, X.; Hong, J.Y.; Zhang, L.; Cooper, O.R.; Schultz, M.G.; Xu, X.B.; Wang, T.; Gao, M.; Zhao, Y.H.; Zhang, Y.H. Severe Surface Ozone Pollution in China: A Global Perspective. Environ. Sci. Technol. Let. 2018, 5, 487–494. [Google Scholar]
- Zhang, Q.J.; Sun, L.N.; Wei, N.; Wu, L.; Mao, H.J. The characteristics and source analysis of VOCs emissions at roadside: Assess the impact of ethanol-gasoline implementation. Atmos. Environ. 2021, 263, 118670. [Google Scholar]
- Ma, Y.X.; Ma, B.J.; Jiao, H.R.; Zhang, Y.F.; Xin, J.Y.; Yu, Z.A. An analysis of the effects of weather and air pollution on tropospheric ozone using a generalized additive model in Western China: Lanzhou, Gansu. Atmos. Environ. 2020, 224, 117342. [Google Scholar]
- Mcdonald, B.C.; Gouw, J.D.; Gilman, J.B.; Jathar, S.H.; Akherati, A.; Cappa, C.D.; Jimenez, J.L.; Taylor, J.L.; Hayes, P.L.; Mckeen, S.A.; et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 2018, 359, 760–764. [Google Scholar]
- Zavala, M.; Brune, W.H.; Velasco, E.; Retama, A.; Cruzalavez, L.A.; Molina, L.T. Changes in ozone production and VOC reactivity in the atmosphere of the Mexico City Metropolitan Area. Atmos. Environ. 2020, 238, 117747. [Google Scholar]
- Cohan, D.S.; Hakami, A.; Hu, Y.T.; Russell, A.G. Nonlinear Response of Ozone to Emissions: Source Apportionment and Sensitivity Analysis. Environ. Sci. Technol. 2005, 39, 6739–6748. [Google Scholar]
- Wang, T.; Xue, L.K.; Brimblecombe, P.; Lam, Y.F.; Li, L.; Zhang, L. Ozone pollution in China: A review of concentrations, meteorological influences, chemical precursors, and effects. Sci. Total Environ. 2017, 575, 1582–1596. [Google Scholar]
- Wang, X.D.; Yin, S.S.; Zhang, R.Q.; Yuan, M.H.; Ying, Q. Assessment of summertime O3 formation and the O3-NOX-VOC sensitivity in Zhengzhou, China using an observation-based model. Sci. Total Environ. 2022, 813, 152449. [Google Scholar]
- Ling, Z.H.; Zhao, J.; Fan, S.J.; Wang, X.M. Sources of formaldehyde and their contributions to photochemical O3 formation at an urban site in the Pearl River Delta, southern China. Chemosphere 2011, 159, 2310–2319. [Google Scholar]
- Liu, H.J.; Wu, B.B.; Liu, S.H.; Shao, P.Y.; Liu, X.Y.; Zhu, C.Y.; Wang, Y.; Wu, Y.M.; Xue, Y.F.; Gao, J.J.; et al. A regional high-resolution emission inventory of primary air pollutants in 2012 for Beijing and the surrounding five provinces of North China. Atmos. Environ. 2018, 181, 20–33. [Google Scholar]
- Zou, Y.; Yan, X.L.; Flores, R.M.; Zhang, L.Y.; Yang, S.P.; Fan, L.Y.; Deng, T.; Deng, X.J.; Ye, D.Q. Source apportionment and ozone formation mechanism of VOCs considering photochemical loss in Guangzhou, China. Sci. Total Environ. 2023, 903, 166191. [Google Scholar] [PubMed]
- Li, Z.J.; Liu, T.T.; Zhang, H.; Wang, X.Q.; Zhang, J.; Qin, Z.Y.; Zhu, C.Z. Sensitivity analysis of ozone formation and source apportionment of VOCs in a typical industrial city in the Yangtze River Delta region during summer. Atmos. Pollut. Res. 2025, 16, 102424. [Google Scholar]
- Liu, Y.F.; Qiu, P.P.; Xu, K.; Li, C.L.; Yin, S.J.; Zhang, Y.J.; Ding, Y.; Zhang, C.; Wang, Z.; Zhai, R.X. Analysis of VOC emissions and O3 control strategies in the Fenhe Plain cities, China. J. Environ. Manag. 2023, 325, 116534. [Google Scholar]
- Lu, B.Q.; Zhang, Z.K.; Jiang, J.K.; Meng, X.; Liu, C.; Herrmann, H.; Chen, J.M.; Xue, L.K.; Li, X. Unraveling the O3-NOx-VOCs relationships induced by anomalous ozone in industrial regions during COVID-19 in Shanghai. Atmos. Pollut. 2023, 308, 119864. [Google Scholar]
- Romero, J.; Lupascu, A.; Lowe, D.; Badia, A.; Archer, S.; Dorlin, S.; Reeves, C.E.; Butler, T. Sources of surface O3 in the UK: Tagging O3 within WRF-Chem. Atmos. Chem. Phys. 2022, 22, 13797–13815. [Google Scholar]
- Beddows, A.V.; Kitwiroon, N.; Williams, M.L.; Beevers, S.D. Emulation and Sensitivity Analysis of the Community Multiscale Air Quality Model for a UK Ozone Pollution Episode. Environ. Sci. Technol. 2017, 51, 6229–6236. [Google Scholar]
- Cheng, H.R.; Guo, H.; Ho, K.F. Erratum to: On the relationship between ozone and its precursors in the Pearl River Delta: Application of an observation-based model (OBM). Environ. Sci. Pollut. Res. 2010, 17, 1491–1492. [Google Scholar]
- Cardelino, C.A.; Chameides, W.L. An Observation-Based Model for Analyzing Ozone Precursor Relationships in the Urban Atmosphere. J. Air Waste Manag. 1995, 45, 161–180. [Google Scholar]
- Chai, W.; Wang, M.; Li, J.Y.; Tang, G.G.; Zhang, G.H.; Chen, W.T. Pollution characteristics, sources, and photochemical roles of ambient carbonyl compounds in summer of Beijing, China. Environ. Pollut. 2023, 336, 122403. [Google Scholar]
- Zhang, K.; Li, L.; Huang, L.; Wang, Y.J.; Huo, Y.J.; Duan, Y.S.; Wang, Y.H.; Fu, Q.Y. The impact of volatile organic compounds on ozone formation in the suburban area of Shanghai. Atmos. Environ. 2020, 232, 117511. [Google Scholar]
- Wang, Z.Y.; Shi, Z.B.; Wang, F.; Liang, W.Q.; Shi, G.L.; Wang, W.C.; Chen, D.; Liang, D.N.; Feng, Y.C.; Russell, A.G. Implications for ozone control by understanding the survivor bias in observed ozone-volatile organic compounds system. npj Clim. Atmos. Sci. 2022, 5, 39. [Google Scholar]
- Liang, X.M.; Sun, X.B.; Xu, J.T.; Ye, D.Q. Improved emissions inventory and VOCs speciation for industrial OFP estimation in China. Sci. Total Environ. 2020, 745, 140838. [Google Scholar] [PubMed]
- Lyu, X.P.; Chen, N.; Guo, H.; Zhang, W.H.; Wang, N.; Wang, Y.; Liu, M. Ambient volatile organic compounds and their effect on ozone production in Wuhan, central China. Sci. Total Environ. 2016, 541, 200–209. [Google Scholar] [PubMed]
- Li, R.M. Source Apportionment of Surface Ozone Production Based on Field Observation; North China Electric Power University: Beijing, China, 2021. [Google Scholar]
- Jincheng Municipal Bureau of Statistics. Available online: http://tjj.jcgov.gov.cn/ (accessed on 15 February 2025).
- Song, Z.; Ji, Y.P.; Zhong, X.; Tang, Z.B.; Zhou, Y.Q.; Luo, H.Y.; Guo, Y.F. Determination of volatile organic pollutants in ambient airby thermal desorption-gas chromatography-mass spectrometry. Resour. Environ. Eng. 2021, 35, 914. [Google Scholar]
- Mu, S.; Zhou, C.H. Detemination of Volatile Organic Compounds (VOCs) in Car Air Using Theimal Desorption by Gas Chromatography/Mass Spectrometry (GC/MS). Environ. Sci. Manag. 2009, 34, 148–150. [Google Scholar]
- Yan, Y.L.; Niu, Y.Y.; Duan, X.L.; Yue, K.; Dong, J.Q.; Yang, C.; Hu, D.M.; Wang, Y.H.; Li, J.J.; Peng, L. Insight into carbonyl source based on improved source apportionment method: Alkene regulate secondary formation. J. Hazard. Mater. 2025, 489, 137649. [Google Scholar]
- Zhang, X.; Zhang, N.; Wei, R.; Su, W.K.; He, J.L.; Song, K.L.; Zhang, M.M.; Zhao, J.W.; Meng, K.; Zhang, Y.P. Characteristics and Source Apportionment of Ambient Volatile Organic Compounds in Shijiazhuang High-tech Industrial Development Zone in Autumn. Environ. Sci. 2024, 45, 7021–7030. [Google Scholar]
- Wang, F.; Li, L.X.; Wang, H.G.; Zhao, Z.S.; Zhang, J.P.; Zhang, D. Characteristics, Sources, and SOAP of VOCs During Winter in Jiyuan. Environ. Sci. 2022, 43, 3451–3462. [Google Scholar]
- Chen, J.; Gong, D.C.; Liao, T.; Zhou, Y.; Zhang, T.; Wang, R.W.; Wang, H.; Chen, D.H.; Wang, B.G. National Day effect and source analysis of atmospheric volatile organic compounds in the Pearl River Delta region. China Environ. Sci. 2023, 43, 3265–3280. [Google Scholar]
- United States Environmental Protection Agency. EPA Positive Matrix Factorization 5.0 Fundamentals and User Guide; United States Environmental Protection Agency: Washington, DC, USA, 2014. [Google Scholar]
- Gu, Y.X.; Li, K.; Xu, J.M.; Liao, H.; Zhou, G.Q. Observed dependence of surface ozone on increasing temperature in Shanghai, China. Atmos. Environ. 2020, 221, 117108. [Google Scholar]
- Li, W.Y.; Huang, H.Y.; Wang, Y.Z.; Zhu, Z.B.; Wang, Y.Q.; Gao, Y.S.; Peng, N.N.; Lun, X.X.; Huang, L.; Feng, R.F.; et al. Pollution Characteristics and Source Apportionment of VOCs in Urban Areas of Liaocheng in Summer. China Environ. Sci. 2023, 44, 6564–6575. [Google Scholar]
- Wang, W.J.; Fang, H.; Zhang, Y.; Ding, Y.Y.; Hua, F.; Wu, T.; Yan, Y.Z. Characterizing sources and ozone formations of summertime volatile organic compounds observed in a medium-sized city in Yangtze River Delta region. Chemosphere 2023, 328, 138609. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.J.; Liu, Y.F.; Ma, W.; Zheng, C.; Zhang, Y.J.; Wang, Z.; Cheng, Q.; Liu, X.G. Formation mechanism, precursor sensitivity and control strategies of summertime ozone on the Fenwei Plain, China. Atmos. Environ. 2023, 309, 119908. [Google Scholar]
- Song, K.X.; Liu, R.; Wang, Y.; Liu, T.; Wei, L.Y.; Wu, Y.X.; Zheng, J.Y.; Wang, B.G.; Liu, S.C. Observation-based analysis of ozone production sensitivity for two persistent ozone episodes in Guangdong, China. Atmos. Chem. Phys. 2022, 22, 8403–8416. [Google Scholar]
- Zhang, Y.N.; Xue, L.K.; Chen, T.S.; Shen, H.Q.; Li, H.; Wang, W.X. Development history of observation-based model (OBM) and its application and prospect in atmospheric chemistry studies in China. Res. Environ. Sci. 2022, 35, 621632. [Google Scholar]
- Ma, W.; Feng, Z.M.; Zhan, J.L.; Liu, Y.C.; Liu, P.F.; Liu, C.T.; Ma, Q.X.; Yang, K.; Wang, Y.F.; He, H.; et al. Influence of photochemical loss of volatile organic compounds on understanding ozone formation mechanism. Atmos. Chem. Phys. 2022, 22, 4841–4851. [Google Scholar] [CrossRef]
- Zeng, P.; Lyu, X.P.; Guo, H.; Cheng, H.R.; Jiang, F.; Pan, W.Z.; Wang, Z.W.; Liang, S.W.; Hu, Y.Q. Causes of ozone pollution in summer in Wuhan, Central China. Environ. Pollut. 2018, 241, 852–861. [Google Scholar]
- Mozaffar, A.; Zhang, Y.L. Effects of VOC emission reduction on atmospheric photochemistry and ozone concentrations during high-ozone episodes. Atmos. Res. 2024, 308, 107538. [Google Scholar]
- Wang, T.T.; Tao, J.; Li, Z.; Lu, X.; Liu, Y.L.; Zhang, X.R.; Wang, B.; Zhang, D.; Yin, S.S. Characteristic, source apportionment and effect of photochemical loss of ambient VOCs in an emerging megacity of Central China. Atmos. Environ. 2024, 305, 107429. [Google Scholar]
- He, C.Q.; Zou, Y.; Lv, S.J.; Flores, R.M.; Yan, X.L.; Deng, T.; Deng, X.J. The importance of photochemical loss to source analysis and ozone formation potential: Implications from in-situ observations of volatile organic compounds (VOCs) in Guangzhou, China. Atmos. Environ. 2024, 320, 120320. [Google Scholar]
- Duan, L.Q.; Wu, Y.T.; Liu, B.S. Source apportionment of ambient VOCs during ozone pollution periods in Linfen. China Environ. Sci. 2025, 45, 78–92. [Google Scholar]
- Han, M.Z.; Huan, J.F.; Qiao, X.L.; Sun, C.; Xi, J.Y. Non-target screening of VOCs in the atmosphere of Dalian city and its potential for ozone generation. J. Dalian Univ. Technol. 2023, 63, 248–255. [Google Scholar]
- Luo, R.X.; Liu, B.S.; Liang, D.N.; Bi, X.H.; Zhang, Y.F.; Feng, Y.C. Characteristics of Ozone and Source Apportionment of the Precursor VOCs in Tianjin Suburbs in Summer. China Environ. Sci. 2021, 42, 75–87. [Google Scholar]
- Liu, Y.; Shao, M.; Fu, L.L.; Lu, S.H.; Zeng, L.M.; Tang, D.G. Source profiles of volatile organic compounds (VOCs) measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar]
- Gu, X.; Chen, K.T.; Cai, M.; Yin, Z.Y.; Liu, X.G.; Li, X.R. Study on the Fingerprint and Atmospheric Activity of Volatile Organic Compounds from Typical Industrial Emissions. Int. J. Environ. Res. Public Health 2023, 20, 3517. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, C.H.; Wang, H.L.; Zhou, M.; Lou, S.R.; Qiao, L.P.; Huang, C.; Li, L.; Su, L.Y.; Mou, Y.Y. Forming Potential of Secondary Organic Aerosols and Sources Apportionment of VOCs in Autumn of Shanghai, China. China Environ. Sci. 2013, 34, 424–433. [Google Scholar]
- Wang, K.X.; Zhang, X.; Ji, Y.Y.; Li, H.; Fan, K.; Gao, R.; He, M. Characterization of Ambient VOCs in a Coal Chemical Industry Park and Their Contribution to Complex Air Pollution. Res. Environ. Sci. 2023, 36, 294–304. [Google Scholar]
- Cao, J.Y.; Liu, C.L.; Qiu, X.H.; Peng, L. Research on high-resolution emission inventory of air pollution sources in Changzhi. Environ. Pollut. Control 2022, 44, 618–624. [Google Scholar]
- Xing, Y.R.; Niu, Y.Y.; Yan, Y.L.; Peng, L.; Dong, J.Q.; Yue, K. Research on the sources of volatile organic compounds in the summer atmosphere and the contribution to ozone generation in Changzhi, a typical industrial city. China Environ. Sci. 2024, 44, 4202–4210. [Google Scholar]
- Zheng, Z.S.; Li, K.W.; Xu, B.; Dou, J.P.; Li, L.M.; Zhang, G.T.; Li, S.J.; Geng, C.M.; Yang, W.; Azzi, M.; et al. O3–precursor relationship over multiple patterns of timescale: A case study in Zibo, Shandong Province, China. Atmos. Chem. Phys. 2023, 23, 2649–2665. [Google Scholar] [CrossRef]
- Zhao, M.; Shen, H.Q.; Chen, T.S.; Li, J.D.; Sun, Z.Q.; Ji, Y.Y.; Xue, L.K.; Li, H.; Wang, W.X.; Shang, F.Y. Characteristics and Sensitivity Analysis of Ozone in the Representative City of the Yellow River Delta in Summer. Res. Environ. Sci. 2022, 35, 1351–1361. [Google Scholar]
- Gao, X.; Jin, B.Y.; Wu, L.; Liu, K.X.; Mao, H.J. Research progress on emission characteristics and influencing factors of VOCs from vehicle exhaust. Environ. Sci. Technol. 2023, 46, 69–78. [Google Scholar]
- Brown, S.G.; Frankel, A.; Hafner, H.R. Source apportionment of VOCs in the Los Angeles area using positive matrix factorization. Atmos. Environ. 2007, 41, 227–237. [Google Scholar]
- Niu, Y.Y.; Liu, Z.C.; Li, R.M.; Gao, Q.Z.; Deng, M.J.; Yan, Y.L.; Hu, D.M.; Wu, J.; Peng, L. Characteristics, Source Apportionment, and Environmental Impact of Volatile Organic Compounds in Summer in Yangquan. China Environ. Sci. 2020, 41, 3066–3075. [Google Scholar]
- Mo, Z.W.; Shao, M.; Lu, S.H. Compilation of a source profile database for hydrocarbon and OVOC emissions in China. Atmos. Environ. 2016, 143, 209–217. [Google Scholar] [CrossRef]
- Wang, S.H.; Yuan, B.; Wu, C.H.; Wang, C.M.; Li, T.G.; He, X.J.; Huangfu, Y.B.; Qi, J.P.; Li, X.B.; Sha, Q.E.; et al. Oxygenated volatile organic compounds (VOCs) as significant but varied contributors to VOC emissions from vehicles. Atmos. Chem. Phys. 2022, 22, 9703–9720. [Google Scholar]
- Song, C.L.; Zhang, W.M.; Pei, Y.Q.; Fan, G.L.; Xu, G.P. Comparative effects of MTBE and ethanol additions into gasoline on exhaust emissions. Atmos. Environ. 2006, 40, 1957–1970. [Google Scholar]
- Hui, L.R.; Liu, X.G.; Tan, Q.W.; Feng, M.; An, J.L.; Qu, Y.; Zhang, Y.H.; Jiang, M.Q. Characteristics, source apportionment and contribution of VOCs to ozone formation in Wuhan, Central China. Atmos. Environ. 2018, 192, 55–71. [Google Scholar]
- Niu, Y.Y.; Yan, Y.L.; Xing, Y.R.; Duan, X.L.; Yue, K.; Dong, J.Q.; Hu, D.M.; Wang, Y.H.; Peng, L. Analyzing ozone formation sensitivity in a typical industrial city in China: Implications for effective source control in the chemical transition regime. Sci. Total Environ. 2024, 919, 170559. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, D.; Yan, W.; Niu, Y.; Zhai, Y.; Tao, Q. Research on the Mechanism and Source Changes of Urban O3 Formation Under the Background of Increased Industrial Activity Levels. Atmosphere 2025, 16, 432. https://doi.org/10.3390/atmos16040432
Hu D, Yan W, Niu Y, Zhai Y, Tao Q. Research on the Mechanism and Source Changes of Urban O3 Formation Under the Background of Increased Industrial Activity Levels. Atmosphere. 2025; 16(4):432. https://doi.org/10.3390/atmos16040432
Chicago/Turabian StyleHu, Dongmei, Wen Yan, Yueyuan Niu, Yunfeng Zhai, and Qiuhong Tao. 2025. "Research on the Mechanism and Source Changes of Urban O3 Formation Under the Background of Increased Industrial Activity Levels" Atmosphere 16, no. 4: 432. https://doi.org/10.3390/atmos16040432
APA StyleHu, D., Yan, W., Niu, Y., Zhai, Y., & Tao, Q. (2025). Research on the Mechanism and Source Changes of Urban O3 Formation Under the Background of Increased Industrial Activity Levels. Atmosphere, 16(4), 432. https://doi.org/10.3390/atmos16040432




