An Investigation of Benzene, Toluene, Ethylbenzene, m,p-xylene; Biogenic Volatile Organic Compounds; and Carbonyl Compounds in Chiang Mai’s Atmosphere and Estimation of Their Emission Sources During the Episode Period
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Analysis
2.3. QA/QC
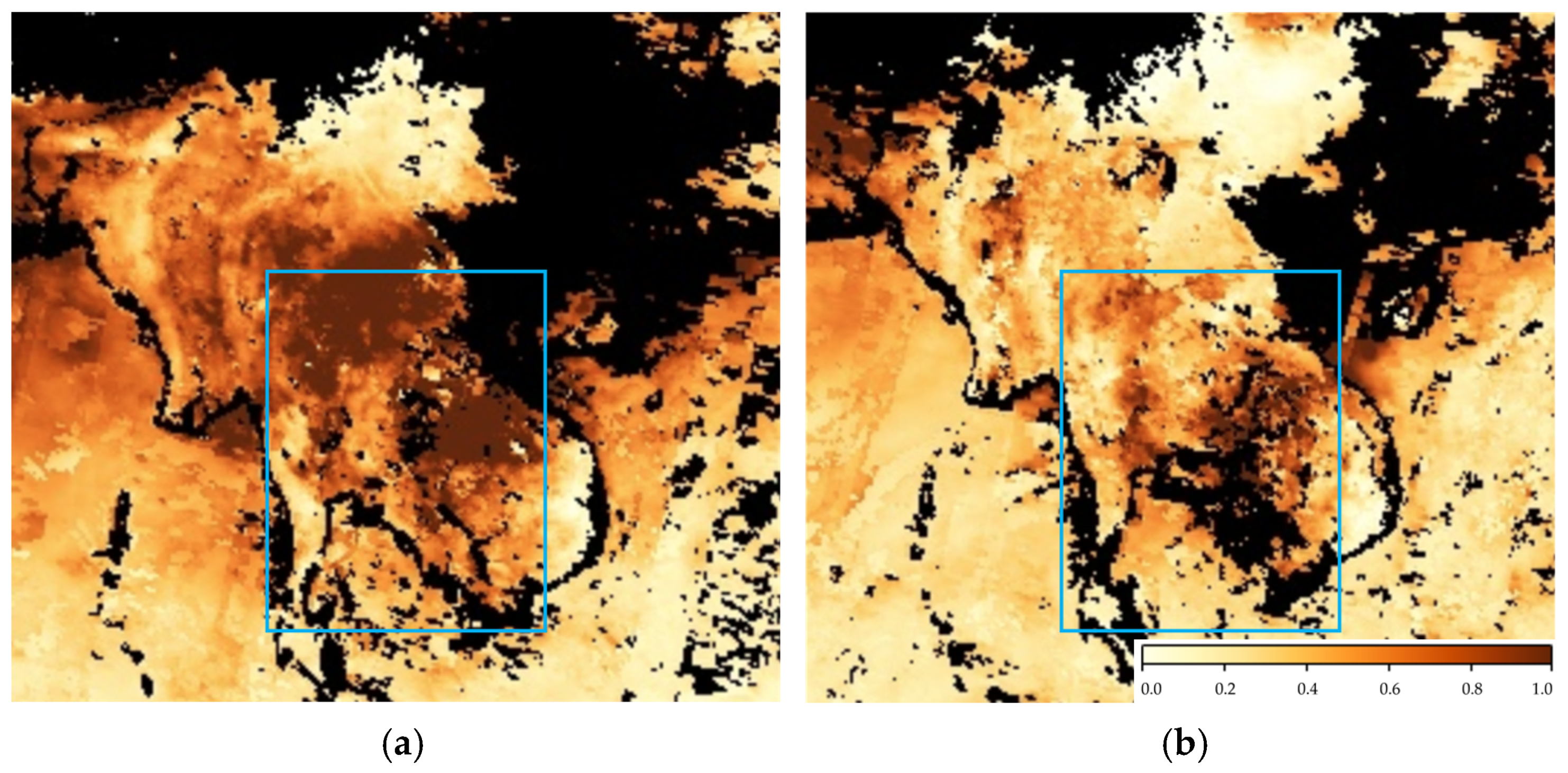
3. Results and Discussion
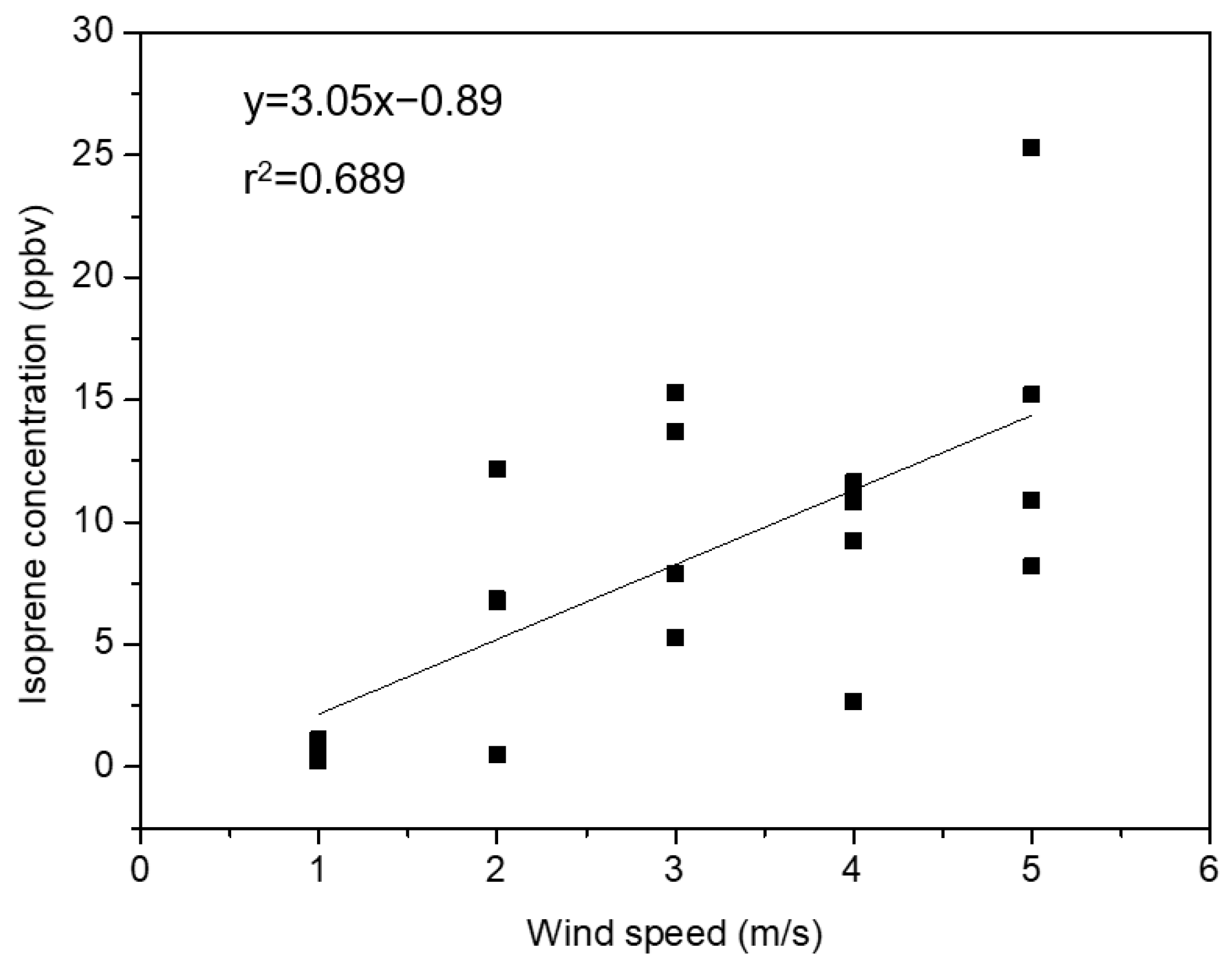
3.1. Measurement of BVOC Concentrations and Identification of Emission Sources
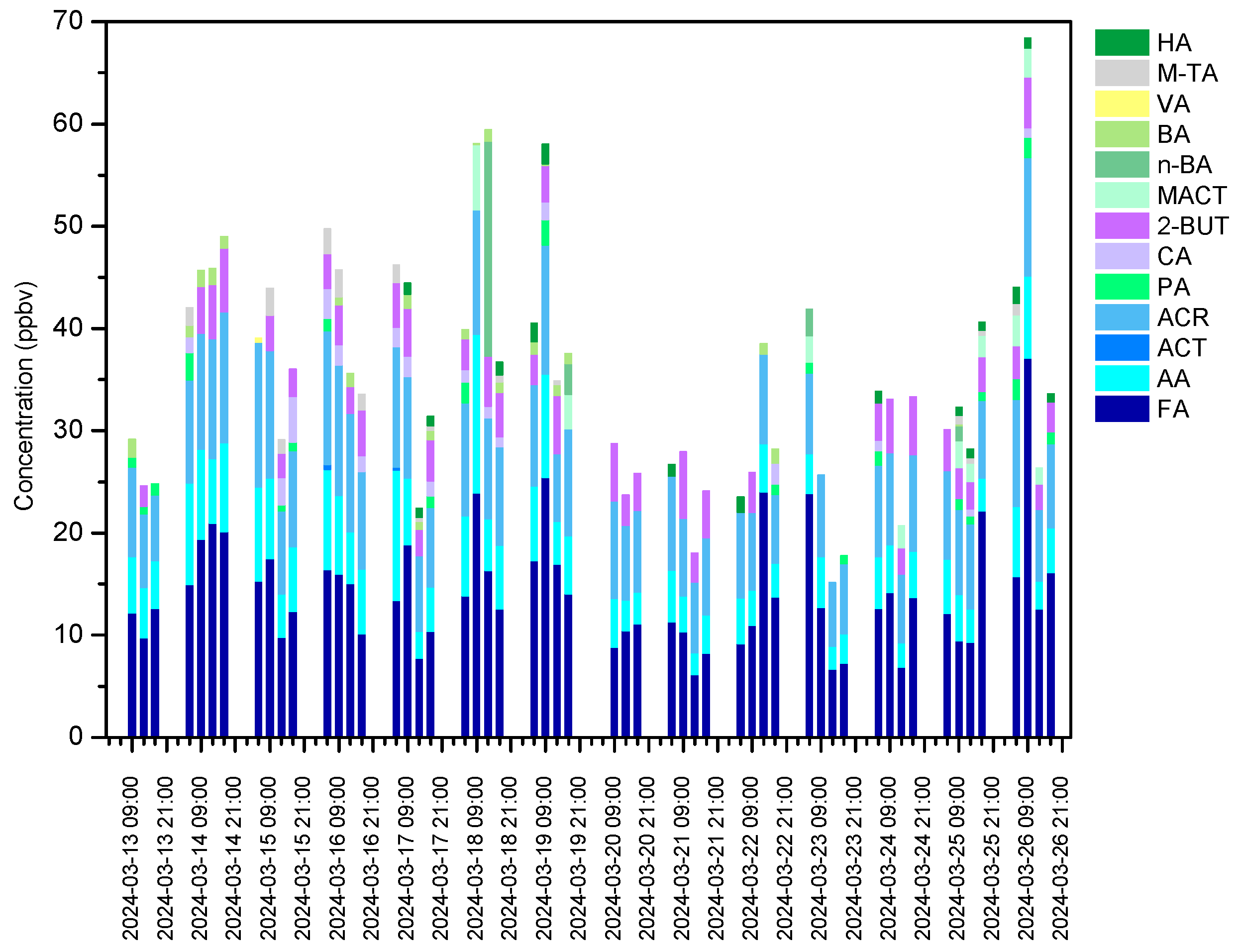
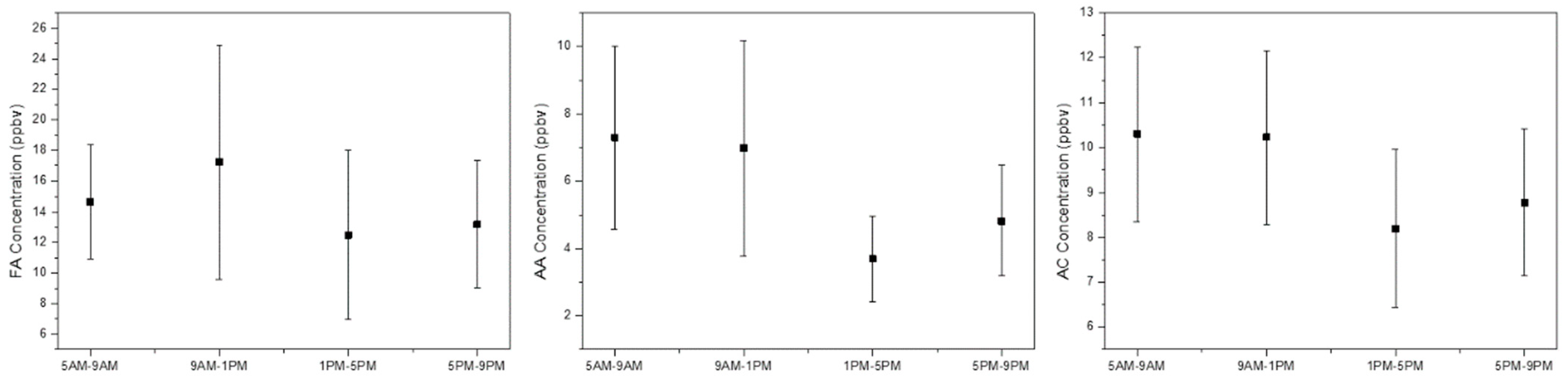
3.2. Measurement of Carbonyl Compound Concentrations and Emission Characteristics
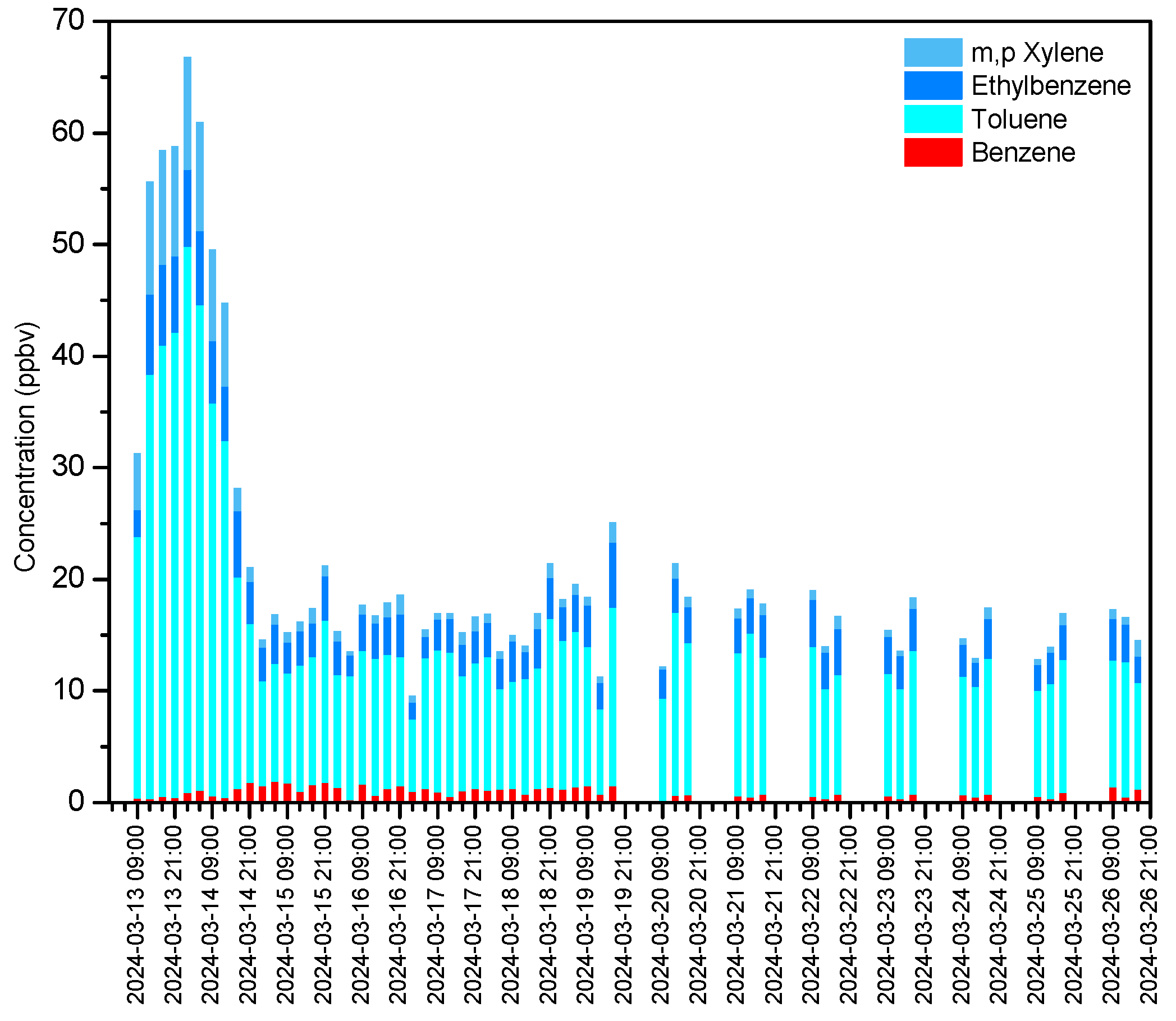
3.3. Measurement of BTEX Concentrations and Identification of Emission Sources
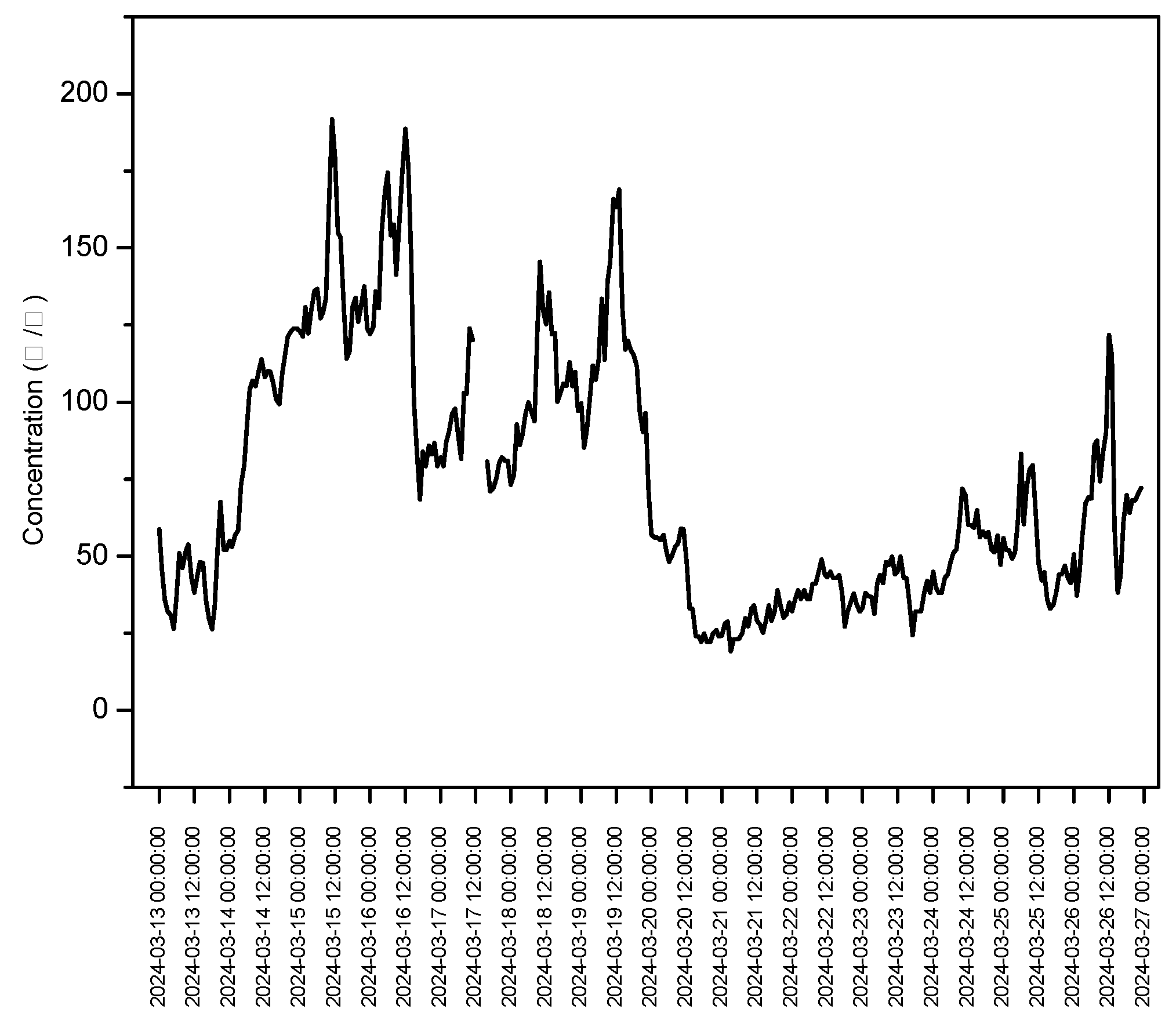
3.4. Correlation Between Gaseous Compounds and PM2.5
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amnuaylojaroen, T.; Parasin, N.; Limsakul, A. Health Risk Assessment of Exposure Near-Future PM2.5 in Northern Thailand. Air Qual. Atmos. Health 2022, 15, 1963–1979. [Google Scholar] [CrossRef]
- Chansuebsri, S.; Kolar, P.; Kraisitnitikul, P.; Kantarawilawan, N.; Yabueng, N.; Wiriya, W.; Thepnuan, D.; Chantara, S. Chemical Composition and Origins of PM2.5 in Chiang Mai (Thailand) by Integrated Source Apportionment and Potential Source Areas. Atmos. Environ. 2024, 327, 120517. [Google Scholar] [CrossRef]
- Amnuaylojaroen, T.; Macatangay, R.C.; Khodmanee, S. Modeling the Effect of VOCs from Biomass Burning Emissions on Ozone Pollution in Upper Southeast Asia. Heliyon 2019, 5, e02661. [Google Scholar] [CrossRef] [PubMed]
- Phairuang, W.; Suwattiga, P.; Chetiyanukornkul, T.; Hongtieab, S.; Limpaseni, W.; Ikemori, F.; Hata, M.; Furuuchi, M. The Influence of the Open Burning of Agricultural Biomass and Forest Fires in Thailand on the Carbonaceous Components in Size-Fractionated Particles. Environ. Pollut. 2019, 247, 238–247. [Google Scholar] [CrossRef]
- Yadav, I.C.; Linthoingambi Devi, N.; Li, J.; Syed, J.H.; Zhang, G.; Watanabe, H. Biomass Burning in Indo-China Peninsula and Its Impacts on Regional Air Quality and Global Climate Change-a Review. Environ. Pollut. 2017, 227, 414–427. [Google Scholar] [CrossRef]
- Pasukphun, N. Environmental Health Burden of Open Burning in Northern Thailand: A Review. PSRU J. Sci. Technol. 2018, 3, 11–28. [Google Scholar]
- Narita, D.; Oanh, N.T.K.; Sato, K.; Huo, M.; Permadi, D.A.; Chi, N.N.H.; Ratanajaratroj, T.; Pawarmart, I. Pollution Characteristics and Policy Actions on Fine Particulate Matter in a Growing Asian Economy: The Case of Bangkok Metropolitan Region. Atmosphere 2019, 10, 227. [Google Scholar] [CrossRef]
- Weger, M.; Heinold, B. Air Pollution Trapping in the Dresden Basin from Gray-Zone Scale Urban Modeling. Atmos. Chem. Phys. 2023, 23, 13769–13790. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Liu, Y.; Wang, S.; Wang, L.; Li, X. Effect of Land–Atmosphere Process Parameterizations on the PM Simulation of a River Valley City with Complex Topography. Atmos. Res. 2023, 281, 106505. [Google Scholar] [CrossRef]
- Johnson, M.S.; Strawbridge, K.; Knowland, K.E.; Keller, C.; Travis, M. Long-Range Transport of Siberian Biomass Burning Emissions to North America during FIREX-AQ. Atmos. Environ. 2021, 252, 118241. [Google Scholar] [CrossRef]
- Reid, J.S.; Hyer, E.J.; Johnson, R.S.; Holben, B.N.; Yokelson, R.J.; Zhang, J.; Campbell, J.R.; Christopher, S.A.; Di Girolamo, L.; Giglio, L.; et al. Observing and Understanding the Southeast Asian Aerosol System by Remote Sensing: An Initial Review and Analysis for the Seven Southeast Asian Studies (7SEAS) Program. Atmos. Res. 2013, 122, 403–468. [Google Scholar] [CrossRef]
- Liu, M.; Chen, L.; Xie, D.; Sun, J.; He, Q.; Cai, L.; Gao, Z.; Zhang, Y. Monsoon-Driven Transport of Atmospheric Mercury to the South China Sea from the Chinese Mainland and Southeast Asia—Observation of Gaseous Elemental Mercury at a Background Station in South China. Environ. Sci. Pollut. Res. 2016, 23, 21631–21640. [Google Scholar] [CrossRef]
- Cao, J.; Situ, S.; Hao, Y.; Xie, S.; Li, L. Enhanced Summertime Ozone and SOA from Biogenic Volatile Organic Compound (BVOC) Emissions Due to Vegetation Biomass Variability during 1981-2018 in China. Atmos. Chem. Phys. 2022, 22, 2351–2364. [Google Scholar] [CrossRef]
- Zhang, H.; Yee, L.D.; Lee, B.H.; Curtis, M.P.; Worton, D.R.; Isaacman-VanWertz, G.; Offenberg, J.H.; Lewandowski, M.; Kleindienst, T.E.; Beaver, M.R.; et al. Monoterpenes Are the Largest Source of Summertime Organic Aerosol in the Southeastern United States. Proc. Natl. Acad. Sci. USA 2018, 115, 2038–2043. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Su, G.; Li, C.; Liu, P.; Zhao, X.; Zhang, C.; Sun, X.; Mu, Y.; Wu, M.; Wang, Q.; et al. An Investigation into the Role of VOCs in SOA and Ozone Production in Beijing, China. Sci. Total Environ. 2020, 720, 137536. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, X.; Chen, D.; Gu, S.; Lu, Y.; Jiang, Q.; Wang, K.; Ou, Y.; Qian, Y.; Shao, P.; et al. Estimation of Biogenic VOC Emissions and Their Corresponding Impact on Ozone and Secondary Organic Aerosol Formation in China. Atmos. Res. 2020, 231, 104656. [Google Scholar] [CrossRef]
- Paris, A.; Gaillard, J.L.; Ledauphin, J. Impact of Biomass Combustion on Occurrence and Distribution of Aromatic Hydrocarbons in Apples. Environ. Sci. Pollut. Res. 2020, 27, 3165–3172. [Google Scholar] [CrossRef]
- Han, T.; Ma, Z.; Xu, W.; Qiao, L.; Li, Y.; He, D.; Wang, Y. Characteristics and Source Implications of Aromatic Hydrocarbons at Urban and Background Areas in Beijing, China. Sci. Total Environ. 2020, 707, 136083. [Google Scholar] [CrossRef]
- Shen, H.; Huang, Y.; Wang, R.; Zhu, D.; Li, W.; Shen, G.; Wang, B.; Zhang, Y.; Chen, Y.; Lu, Y.; et al. Global Atmospheric Emissions of Polycyclic Aromatic Hydrocarbons from 1960 to 2008 and Future Predictions. Environ. Sci. Technol. 2013, 47, 6415–6424. [Google Scholar] [CrossRef]
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; Mckay, W.A.; et al. A Global Model of Natural Volatile Organic Compound Emissions. J. Geophys. Res. 1995, 100, 8873. [Google Scholar] [CrossRef]
- Sindelarova, K.; Granier, C.; Bouarar, I.; Guenther, A.; Tilmes, S.; Stavrakou, T.; Müller, J.-F.; Kuhn, U.; Stefani, P.; Knorr, W. Global Data Set of Biogenic VOC Emissions Calculated by the MEGAN Model over the Last 30 Years. Atmos. Chem. Phys. 2014, 14, 9317–9341. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Wu, C.; Lin, G. Regional to Global Distributions, Trends, and Drivers of Biogenic Volatile Organic Compound Emission from 2001 to 2020. Atmos. Chem. Phys. 2024, 24, 3309–3328. [Google Scholar] [CrossRef]
- Verma, S.K.; Kawamura, K.; Deshmukh, D.K.; Haque, M.M.; Pavuluri, C.M. Seasonal Characteristics of Biogenic Secondary Organic Aerosols Over Chichijima Island in the Western North Pacific: Impact of Biomass Burning Activity in East Asia. J. Geophys. Res. Atmos. 2021, 126, e2020JD032987. [Google Scholar] [CrossRef]
- Herman, F.; Sentian, J.; Kong, V.; Yee, W.; Ooi, M.; Gee, C.; Sharul, M.; Nad-Zir, M. Isoprene Emission under Climate Change Scenario in Southeast Asia. Preprints 2023, 2023030394. [Google Scholar] [CrossRef]
- Vella, R.; Pozzer, A.; Forrest, M.; Lelieveld, J.; Hickler, T.; Tost, H. Changes in Biogenic Volatile Organic Compound Emissions in Response to the El Niño-Southern Oscillation. Biogeosciences 2023, 20, 4391–4412. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Huang, W.; Ling, Z.; Wang, Z.; Wang, X. Carbonyl Compounds in the Atmosphere: A Review of Abundance, Source and Their Contributions to O3 and SOA Formation. Atmos. Res. 2022, 274, 106184. [Google Scholar] [CrossRef]
- Xia, H.; Huang, D.; Bao, F.; Li, M.; Zhang, Y.; Chen, C.; Zhao, J. Photochemical Aging of Beijing Urban PM2.5: Production of Oxygenated Volatile Organic Compounds. Sci. Total Environ. 2020, 743, 140751. [Google Scholar] [CrossRef]
- Shen, H.; Chen, Z.; Li, H.; Qian, X.; Qin, X.; Shi, W. Gas-Particle Partitioning of Carbonyl Compounds in the Ambient Atmosphere. Environ. Sci. Technol. 2018, 52, 10997–11006. [Google Scholar] [CrossRef]
- Pillar-Little, E.A.; Guzman, M.I. An Overview of Dynamic Heterogeneous Oxidations in the Troposphere. Environments 2018, 5, 104. [Google Scholar] [CrossRef]
- Huang, D.D.; Zhang, Q.; Cheung, H.H.Y.; Yu, L.; Zhou, S.; Anastasio, C.; Smith, J.D.; Chan, C.K. Formation and Evolution of AqSOA from Aqueous-Phase Reactions of Phenolic Carbonyls: Comparison between Ammonium Sulfate and Ammonium Nitrate Solutions. Environ. Sci. Technol. 2018, 52, 9215–9224. [Google Scholar] [CrossRef]
- Smith, J.D.; Sio, V.; Yu, L.; Zhang, Q.; Anastasio, C. Secondary Organic Aerosol Production from Aqueous Reactions of Atmospheric Phenols with an Organic Triplet Excited State. Environ. Sci. Technol. 2014, 48, 1049–1057. [Google Scholar] [CrossRef]
- Rana, M.S.; Guzman, M.I. Oxidation of Phenolic Aldehydes by Ozone and Hydroxyl Radicals at the Air-Water Interface. J. Phys. Chem. A 2020, 124, 8822–8833. [Google Scholar] [CrossRef]
- Wells, K.C.; Millet, D.B.; Payne, V.H.; Deventer, M.J.; Bates, K.H.; de Gouw, J.A.; Graus, M.; Warneke, C.; Wisthaler, A.; Fuentes, J.D. Satellite Isoprene Retrievals Constrain Emissions and Atmospheric Oxidation. Nature 2020, 585, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Pimonsree, S.; Vongruang, P. Impact of Biomass Burning and Its Control on Particulate Matter over a City in Mainland Southeast Asia during a Smog Episode. Atmos. Environ. 2018, 195, 196–209. [Google Scholar] [CrossRef]
- Rattanapotanan, T.; Thongyen, T.; Bualert, S.; Choomanee, P.; Suwattiga, P.; Rungrattanaubon, T.; Utavong, T.; Phupijit, J.; Changplaiy, N. Secondary Sources of PM2.5 Based on the Vertical Distribution of Organic Carbon, Elemental Carbon, and Water-Soluble Ions in Bangkok. Environ. Adv. 2023, 11, 100337. [Google Scholar] [CrossRef]
- Vongruang, P.; Pimonsree, S. Biomass Burning Sources and Their Contributions to PM10 Concentrations over Countries in Mainland Southeast Asia during a Smog Episode. Atmos. Environ. 2020, 228, 117414. [Google Scholar] [CrossRef]
- Bran, S.H.; Macatangay, R.; Surapipith, V.; Chotamonsak, C.; Chantara, S.; Han, Z.; Li, J. Surface PM2.5 Mass Concentrations during the Dry Season over Northern Thailand: Sensitivity to Model Aerosol Chemical Schemes and the Effects on Regional Meteorology. Atmos. Res. 2022, 277, 106303. [Google Scholar] [CrossRef]
- Thepnuan, D.; Chantara, S.; Lee, C.T.; Lin, N.H.; Tsai, Y.I. Molecular Markers for Biomass Burning Associated with the Characterization of PM2.5 and Component Sources during Dry Season Haze Episodes in Upper South East Asia. Sci. Total Environ. 2019, 658, 708–722. [Google Scholar] [CrossRef]
- Bayraktar, H.; Turalioglu, F.S. A Kriging-Based Approach for Locating a Sampling Site—In the Assessment of Air Quality. Stoch. Environ. Res. Risk Assess. 2005, 19, 301–305. [Google Scholar] [CrossRef]
- NASA FIRMS. Fire Information for Resource Management System. Available online: https://firms.modaps.eosdis.nasa.gov (accessed on 20 August 2024).
- Riches, M.; Berg, T.C.; Vermeuel, M.P.; Millet, D.B.; Farmer, D.K. Wildfire Smoke Directly Changes Biogenic Volatile Organic Emissions and Photosynthesis of Ponderosa Pines. Geophys. Res. Lett. 2024, 51, e2023GL106667. [Google Scholar] [CrossRef]
- Kleist, E.; Mentel, T.F.; Andres, S.; Bohne, A.; Folkers, A.; Kiendler-Scharr, A.; Rudich, Y.; Springer, M.; Tillmann, R.; Wildt, J. Irreversible Impacts of Heat on the Emissions of Monoterpenes, Sesquiterpenes, Phenolic BVOC and Green Leaf Volatiles from Several Tree Species. Biogeosciences 2012, 9, 5111–5123. [Google Scholar] [CrossRef]
- Loreto, F.; Förster, A.; Dürr, M.; Csiky, O.; Seufert, G. On the Monoterpene Emission under Heat Stress and on the Increased Thermotolerance of Leaves of Quercus Ilex L. Fumigated with Selected Monoterpenes. Plant Cell Environ. 1998, 21, 101–107. [Google Scholar] [CrossRef]
- Lim, Y.J.; Armendariz, A.; Son, Y.S.; Kim, J.C. Seasonal Variations of Isoprene Emissions from Five Oak Tree Species in East Asia. Atmos. Environ. 2011, 45, 2202–2210. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, Y.; Calatayud, V.; Li, Z.; Feng, Z.; Loreto, F. Emissions of Isoprene and Monoterpenes from Urban Tree Species in China and Relationships with Their Driving Factors. Atmos. Environ. 2023, 314, 120096. [Google Scholar] [CrossRef]
- Niu, X.; Li, J.; Wang, Q.; Ho, S.S.H.; Sun, J.; Li, L.; Cao, J.; Ho, K.F. Characteristics of Fresh and Aged Volatile Organic Compounds from Open Burning of Crop Residues. Sci. Total Environ. 2020, 726, 138545. [Google Scholar] [CrossRef]
- Guenther, A.B.; Zimmerman, P.R.; Harley, P.C.; Monson, R.K.; Fall, R. Isoprene and Monoterpene Emission Rate Variability: Model Evaluations and Sensitivity Analyses. J. Geophys. Res. 1993, 98, 12609. [Google Scholar] [CrossRef]
- Guenther, A.B.; Monson, R.K.; Fall, R. Isoprene and Monoterpene Emission Rate Variability: Observations with Eucalyptus and Emission Rate Algorithm Development. J. Geophys. Res. 1991, 96, 10799. [Google Scholar] [CrossRef]
- Koppmann, R. Chemistry of Volatile Organic Compounds in the Atmosphere. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 267–277. [Google Scholar]
- Wolfe, G.M.; Kaiser, J.; Hanisco, T.F.; Keutsch, F.N.; De Gouw, J.A.; Gilman, J.B.; Graus, M.; Hatch, C.D.; Holloway, J.; Horowitz, L.W.; et al. Formaldehyde Production from Isoprene Oxidation across NOx Regimes. Atmos. Chem. Phys. 2016, 16, 2597–2610. [Google Scholar] [CrossRef]
- Lv, S.Y.; Liu, Q.Y.; Zhao, Y.X.; Zhang, M.Q.; Jiang, L.X.; He, S.G. Formaldehyde Generation in Photooxidation of Isoprene on Iron Oxide Nanoclusters. J. Phys. Chem. C 2019, 123, 5120–5127. [Google Scholar] [CrossRef]
- Tyndall, G.S.; Staffelbach, T.A.; Orlando, J.J.; Calvert, J.G. Rate Coefficients for the Reactions of OH Radicals with Methylglyoxal and Acetaldehyde. Int. J. Chem. Kinet. 1995, 27, 1009–1020. [Google Scholar] [CrossRef]
- Kanjanasiranont, N.; Prueksasit, T.; Morknoy, D.; Tunsaringkarn, T.; Sematong, S.; Siriwong, W.; Zapaung, K.; Rungsiyothin, A. Determination of Ambient Air Concentrations and Personal Exposure Risk Levels of Outdoor Workers to Carbonyl Compounds and BTEX in the Inner City of Bangkok, Thailand. Atmos. Pollut. Res. 2016, 7, 268–277. [Google Scholar] [CrossRef]
- Wongaree, M.; Choo-In, S. Monitoring of Benzene in an Ambient Air on the Roadside at Udon Thani of Thailand. IOP Conf. Ser. Earth Environ. Sci. 2019, 281, 012007. [Google Scholar] [CrossRef]
- Thongkum, W.; Wibuloutai, J.; Thitisutthi, S. Benzene and 1, 3 butadiene concentration and its potential health impact in Chiang Mai, Thailand. Eur. J. Sustain. Dev. 2017, 6, 2. [Google Scholar] [CrossRef][Green Version]
- Lewis, A.C.; Evans, M.J.; Hopkins, J.R.; Punjabi, S.; Read, K.A.; Purvis, R.M.; Andrews, S.J.; Moller, S.J.; Carpenter, L.J.; Lee, J.D.; et al. The Influence of Biomass Burning on the Global Distribution of Selected Non-Methane Organic Compounds. Atmos. Chem. Phys. 2013, 13, 851–867. [Google Scholar] [CrossRef]
- Yuan, B.; Liu, Y.; Shao, M.; Lu, S.; Streets, D.G. Biomass Burning Contributions to Ambient VOCs Species at a Receptor Site in the Pearl River Delta (PRD), China. Environ. Sci. Technol. 2010, 44, 4577–4582. [Google Scholar] [CrossRef]
- Sirithian, D.; Thepanondh, S.; Sattler, M.L.; Laowagul, W. Emissions of Volatile Organic Compounds from Maize Residue Open Burning in the Northern Region of Thailand. Atmos. Environ. 2018, 176, 179–187. [Google Scholar] [CrossRef]
- Monod, A.; Monod, A.; Sive, B.C.; Avino, P.; Chen, T.; Blake, D.R.; Rowland, F.S. Monoaromatic Compounds in Ambient Air of Various Cities: A Focus on Correlations between the Xylenes and Ethylbenzene. Atmos. Environ. 2001, 35, 135–149. [Google Scholar] [CrossRef]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The Formation, Properties and Impact of Secondary Organic Aerosol: Current and Emerging Issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Zeng, L.; Shao, M.; Xie, S.; Chen, W.; Lu, S.; Wu, Y.; Cao, W. Biomass Burning Contribution to Ambient Volatile Organic Compounds (VOCs) in the Chengdu-Chongqing Region (CCR), China. Atmos. Environ. 2014, 99, 403–410. [Google Scholar] [CrossRef]
- Pun, B.K.; Seigneur, C.; Grosjean, D.; Saxena, P. Gas-Phase Formation of Water-Soluble Organic Compounds in the Atmosphere: A Retrosynthetic Analysis. J. Atmos. Chem. 2000, 35, 199–223. [Google Scholar] [CrossRef]
- Stockwell, C.E.; Veres, P.R.; Williams, J.; Yokelson, R.J. Characterization of Biomass Burning Emissions from Cooking Fires, Peat, Crop Residue, and Other Fuels with High-Resolution Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry. Atmos. Chem. Phys. 2015, 15, 845–865. [Google Scholar] [CrossRef]
- Miri, M.; Rostami Aghdam Shendi, M.; Ghaffari, H.R.; Ebrahimi Aval, H.; Ahmadi, E.; Taban, E.; Gholizadeh, A.; Yazdani Aval, M.; Mohammadi, A.; Azari, A. Investigation of Outdoor BTEX: Concentration, Variations, Sources, Spatial Distribution, and Risk Assessment. Chemosphere 2016, 163, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, D.; Kumar, K.; Singh, B.B.; Jain, V.K. Distribution of VOCs in Urban and Rural Atmospheres of Subtropical India: Temporal Variation, Source Attribution, Ratios, OFP and Risk Assessment. Sci. Total Environ. 2018, 613–614, 492–501. [Google Scholar] [CrossRef]
- Rad, H.D.; Babaei, A.A.; Goudarzi, G.; Angali, K.A.; Ramezani, Z.; Mohammadi, M.M. Levels and Sources of BTEX in Ambient Air of Ahvaz Metropolitan City. Air Qual. Atmos. Health 2014, 7, 515–524. [Google Scholar] [CrossRef]
- da Rocha, F.O.C.; Campos, V.P.; da Rocha, G.O.; Brito, A.V.S.; dos Santos Sampaio, I. Characterization of Airborne Gaseous BTEX and Aldehydes from Populated Brazilian Cities as Representative Atmospheres of Typical Urban Areas from the Southern Hemisphere. Air Qual. Atmos. Health 2023, 16, 1271–1289. [Google Scholar] [CrossRef]
- Hajizadeh, Y.; Mokhtari, M.; Faraji, M.; Mohammadi, A.; Nemati, S.; Ghanbari, R.; Abdolahnejad, A.; Fard, R.F.; Nikoonahad, A.; Jafari, N.; et al. Trends of BTEX in the Central Urban Area of Iran: A Preliminary Study of Photochemical Ozone Pollution and Health Risk Assessment. Atmos. Pollut. Res. 2018, 9, 220–229. [Google Scholar] [CrossRef]
- Wang, N.; Edtbauer, A.; Stönner, C.; Pozzer, A.; Bourtsoukidis, E.; Ernle, L.; Dienhart, D.; Hottmann, B.; Fischer, H.; Schuladen, J.; et al. Measurements of Carbonyl Compounds around the Arabian Peninsula: Overview and Model Comparison. Atmos. Chem. Phys. 2020, 20, 10807–10829. [Google Scholar] [CrossRef]
- Punsompong, P.; Chantara, S. Identification of Potential Sources of PM10 Pollution from Biomass Burning in Northern Thailand Using Statistical Analysis of Trajectories. Atmos. Pollut. Res. 2018, 9, 1038–1051. [Google Scholar] [CrossRef]


| GC/MS | HPLC | ||||
|---|---|---|---|---|---|
| Initial Temp | 20 °C (7 min hold) | Gradient | Time | A | C |
| Acetonitrile | D.I water:ACN:Tetrahydrofuran = 50:49:1 | ||||
| Oven rate 1 | 1 °C min−1 | Initial | 0 | 100 | |
| Oven Temp | 30 °C | 1 | 40 | 60 | |
| Oven rate 2 | 10 °C min−1 | 10 | 40 | 60 | |
| Oven Temp | 200 °C | 11 | 100 | 0 | |
| Oven rate 3 | 15 °C min−1 | 14 | 0 | 100 | |
| Oven Temp | 250 °C (5 min hold) | 21 | 0 | 100 | |
| Total Time | 42.33 min | Flow rate | 1 mL/min | ||
| Compound | RSD (%) | MDL (ng) | r2 of Calibration Curve |
|---|---|---|---|
| Benzene | 1.80 | 0.27 | 0.999 |
| Toluene | 4.74 | 0.51 | 0.999 |
| Ethylbenzene | 3.05 | 0.34 | 0.998 |
| m,p-xylene | 2.68 | 0.19 | 0.999 |
| Isopene | 0.63 | 0.37 | 0.998 |
| α-Pinene | 1.75 | 0.15 | 0.999 |
| Camphene | 4.88 | 0.32 | 0.999 |
| β-Pinene | 3.26 | 0.33 | 0.999 |
| δ-3-Carene | 1.83 | 0.46 | 0.999 |
| α-Terpinene | 2.71 | 0.42 | 0.999 |
| d-Limonene | 4.13 | 0.50 | 0.999 |
| ρ-Cymene | 3.44 | 0.48 | 0.999 |
| γ-Terpinene | 3.53 | 0.26 | 0.999 |
| Formaldehyde | 1.05 | 0.12 | 0.999 |
| Acetaldehyde | 4.25 | 0.09 | 0.997 |
| Acetone | 3.73 | 0.06 | 0.999 |
| Acrolein | 2.88 | 0.05 | 0.999 |
| Propionaldehyde | 4.32 | 0.05 | 0.999 |
| Crotonaldehyde | 4.64 | 0.05 | 0.999 |
| 2-Butanone | 3.38 | 0.06 | 0.999 |
| Methacrolein | 4.79 | 0.09 | 0.997 |
| n-Butyraldehyde | 1.29 | 0.06 | 0.997 |
| Benzaldehyde | 3.58 | 0.08 | 0.999 |
| Valeraldehyde | 4.24 | 0.09 | 0.999 |
| m-Tolualdehyde | 4.80 | 0.08 | 0.999 |
| Hexaldehyde | 3.58 | 0.12 | 0.999 |
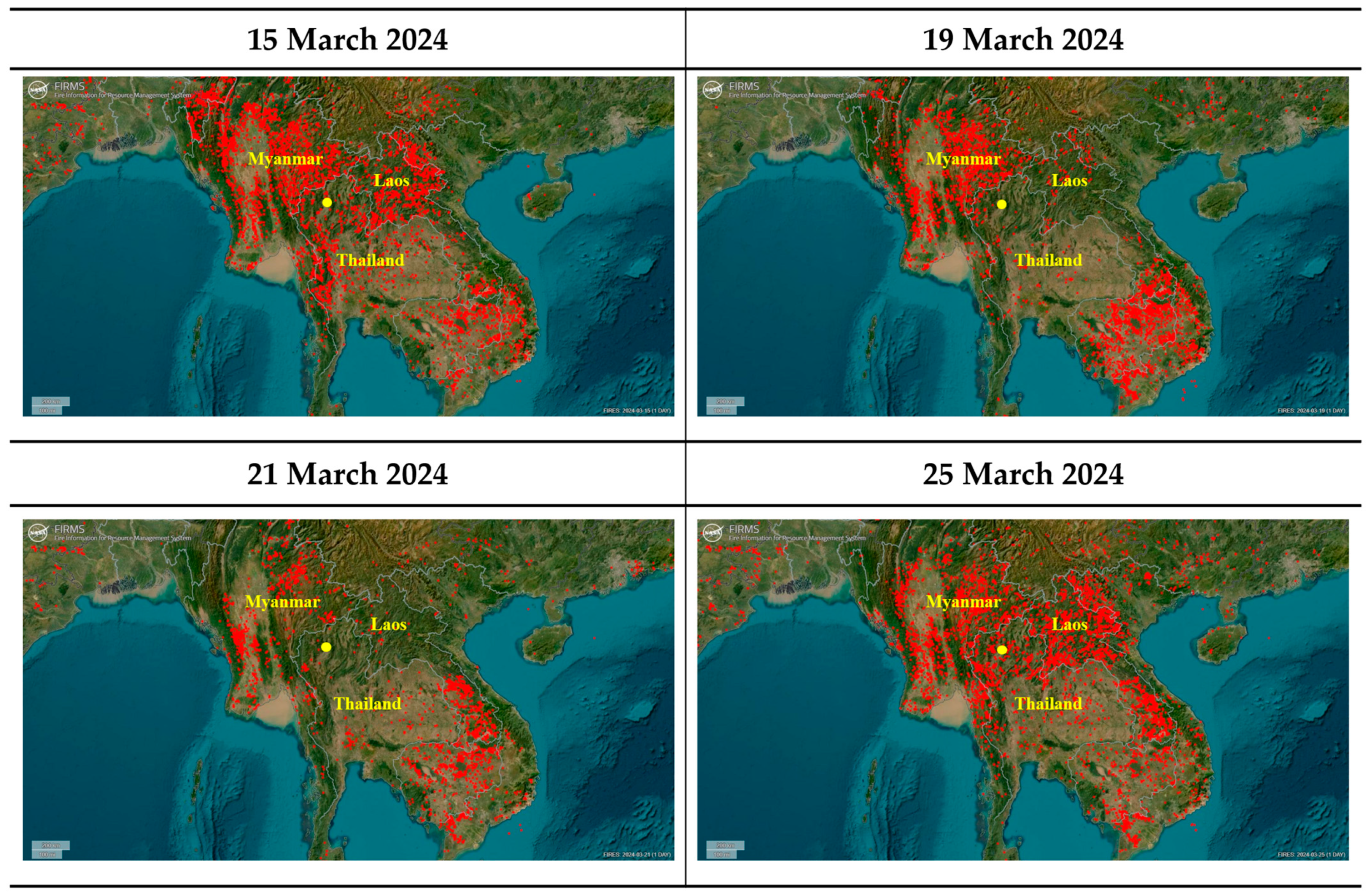
| Period | Thailand | Myanmar | Laos |
|---|---|---|---|
| 13–18 March | Serious | Serious | Serious |
| 19–20 March | Minimal | Serious | Minimal |
| 21–22 March | Minimal | Minimal | Minimal |
| 23–24 March | Moderate (Increasing) | Moderate (Increasing) | Moderate (Increasing) |
| 25–26 March | Serious | Serious | Serious |
| B | T | E | X | ISP | MTP | FA | AA | AC | PM2.5 | |
| B | 1 | 0.10 | 0.04 | 0.07 | 0.03 | 0.12 | 0.27 | 0.41 | 0.46 | 0.83 |
| T | 1 | 0.84 | 0.98 | 0.13 | 0.93 | 0.01 | 0.05 | 0.01 | 0.004 | |
| E | 1 | 0.83 | 0.04 | 0.77 | 0.10 | 0.10 | 0.10 | 0.004 | ||
| X | 1 | 0.16 | 0.97 | 0.02 | 0.06 | 0.06 | 0.0004 | |||
| ISP | 1 | 0.15 | 0.002 | 0.27 | 0.25 | 0.17 | ||||
| MTP | 1 | 0.002 | 0.03 | 0.0007 | 0.01 | |||||
| FA | 1 | 0.45 | 0.39 | 0.33 | ||||||
| AA | 1 | 0.76 | 0.71 | |||||||
| AC | 1 | 0.75 | ||||||||
| PM2.5 | 1 |
| Compound | Atmospheric Lifetime |
|---|---|
| Benzene | 9.4 days |
| Toluene | 1.9 days |
| Ethylbenzene | 1.6 days |
| m,p-Xylene | 11.8 h/19.4 h |
| Isoprene | 1 h |
| Alpha-pinene | 5.8 h |
| Formaldehyde | 4 h |
| Acetaldehyde | 6 h |
| Date | T/B | X/B | X/E | FA/AA |
|---|---|---|---|---|
| 13 March | 84.28 | 20.98 | 1.51 | 2.28 |
| 14 March | 37.45 | 7.70 | 1.08 | 2.54 |
| 15 March | 7.49 | 0.69 | 0.34 | 2.13 |
| 16 March | 10.28 | 0.80 | 0.28 | 2.16 |
| 17 March | 14.41 | 0.86 | 0.25 | 2.72 |
| 18 March | 9.48 | 0.73 | 0.25 | 1.97 |
| 19 March | 9.71 | 0.80 | 0.25 | 2.82 |
| 20 March | 25.74 | 1.63 | 0.28 | 2.77 |
| 21 March | 22.75 | 1.44 | 0.25 | 2.60 |
| 22 March | 20.58 | 1.45 | 0.21 | 4.20 |
| 23 March | 20.30 | 1.23 | 0.20 | 2.62 |
| 24 March | 17.51 | 0.99 | 0.22 | 2.98 |
| 25 March | 21.48 | 1.45 | 0.24 | 2.36 |
| 26 March | 10.87 | 0.95 | 0.30 | 4.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, D.-H.; Seo, Y.-B.; Gil, J.-S.; Lee, M.-H.; Lee, J.-S.; Lee, G.-W.; Thepnuan, D.; Choi, I.-Y.; Lee, S.-W.; Dinh, T.-V.; et al. An Investigation of Benzene, Toluene, Ethylbenzene, m,p-xylene; Biogenic Volatile Organic Compounds; and Carbonyl Compounds in Chiang Mai’s Atmosphere and Estimation of Their Emission Sources During the Episode Period. Atmosphere 2025, 16, 342. https://doi.org/10.3390/atmos16030342
Baek D-H, Seo Y-B, Gil J-S, Lee M-H, Lee J-S, Lee G-W, Thepnuan D, Choi I-Y, Lee S-W, Dinh T-V, et al. An Investigation of Benzene, Toluene, Ethylbenzene, m,p-xylene; Biogenic Volatile Organic Compounds; and Carbonyl Compounds in Chiang Mai’s Atmosphere and Estimation of Their Emission Sources During the Episode Period. Atmosphere. 2025; 16(3):342. https://doi.org/10.3390/atmos16030342
Chicago/Turabian StyleBaek, Da-Hyun, Ye-Bin Seo, Jun-Su Gil, Mee-Hye Lee, Ji-Seon Lee, Gang-Woong Lee, Duangduean Thepnuan, In-Young Choi, Sang-Woo Lee, Trieu-Vuong Dinh, and et al. 2025. "An Investigation of Benzene, Toluene, Ethylbenzene, m,p-xylene; Biogenic Volatile Organic Compounds; and Carbonyl Compounds in Chiang Mai’s Atmosphere and Estimation of Their Emission Sources During the Episode Period" Atmosphere 16, no. 3: 342. https://doi.org/10.3390/atmos16030342
APA StyleBaek, D.-H., Seo, Y.-B., Gil, J.-S., Lee, M.-H., Lee, J.-S., Lee, G.-W., Thepnuan, D., Choi, I.-Y., Lee, S.-W., Dinh, T.-V., & Kim, J.-C. (2025). An Investigation of Benzene, Toluene, Ethylbenzene, m,p-xylene; Biogenic Volatile Organic Compounds; and Carbonyl Compounds in Chiang Mai’s Atmosphere and Estimation of Their Emission Sources During the Episode Period. Atmosphere, 16(3), 342. https://doi.org/10.3390/atmos16030342







