Degradation Kinetics of Common Odorants Emitted from WWTPs: A Methodological Approach for Estimating Half-Life Through Reactions with Hydroxyl Radicals
Abstract
1. Introduction
2. Materials and Methods
2.1. WWTP Description and Methodology for Sample Collection and Analysis
2.2. Odorants Selection
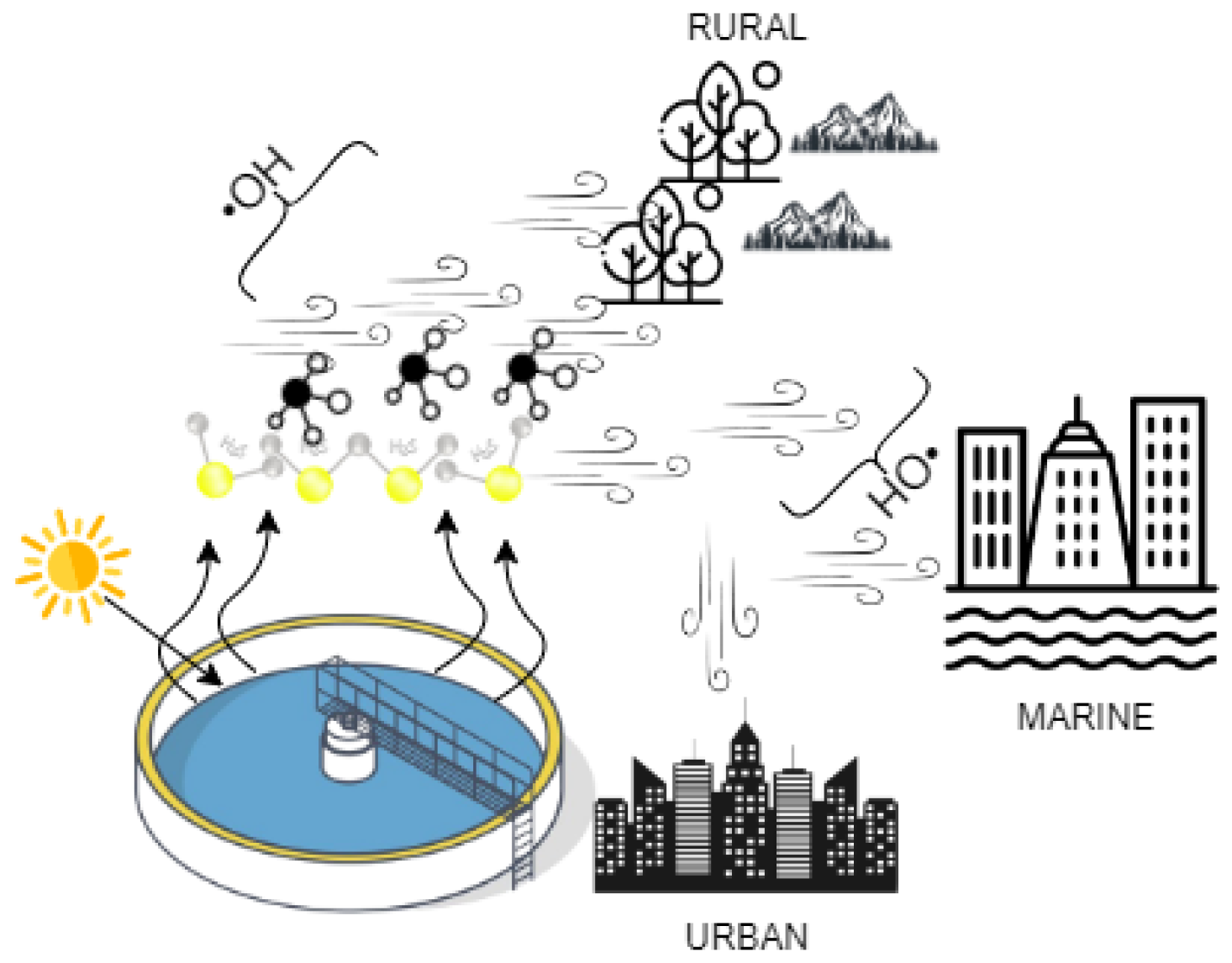
2.3. Hydroxyl Radicals Environment
2.4. Odorants Degradation Determinations
- [A] is the concentration of the odorant at time t
- [Ao] is the initial concentration of the odorant
- k′ is the pseudo-first-order rate constant
- t is the reaction time
3. Results and Discussion
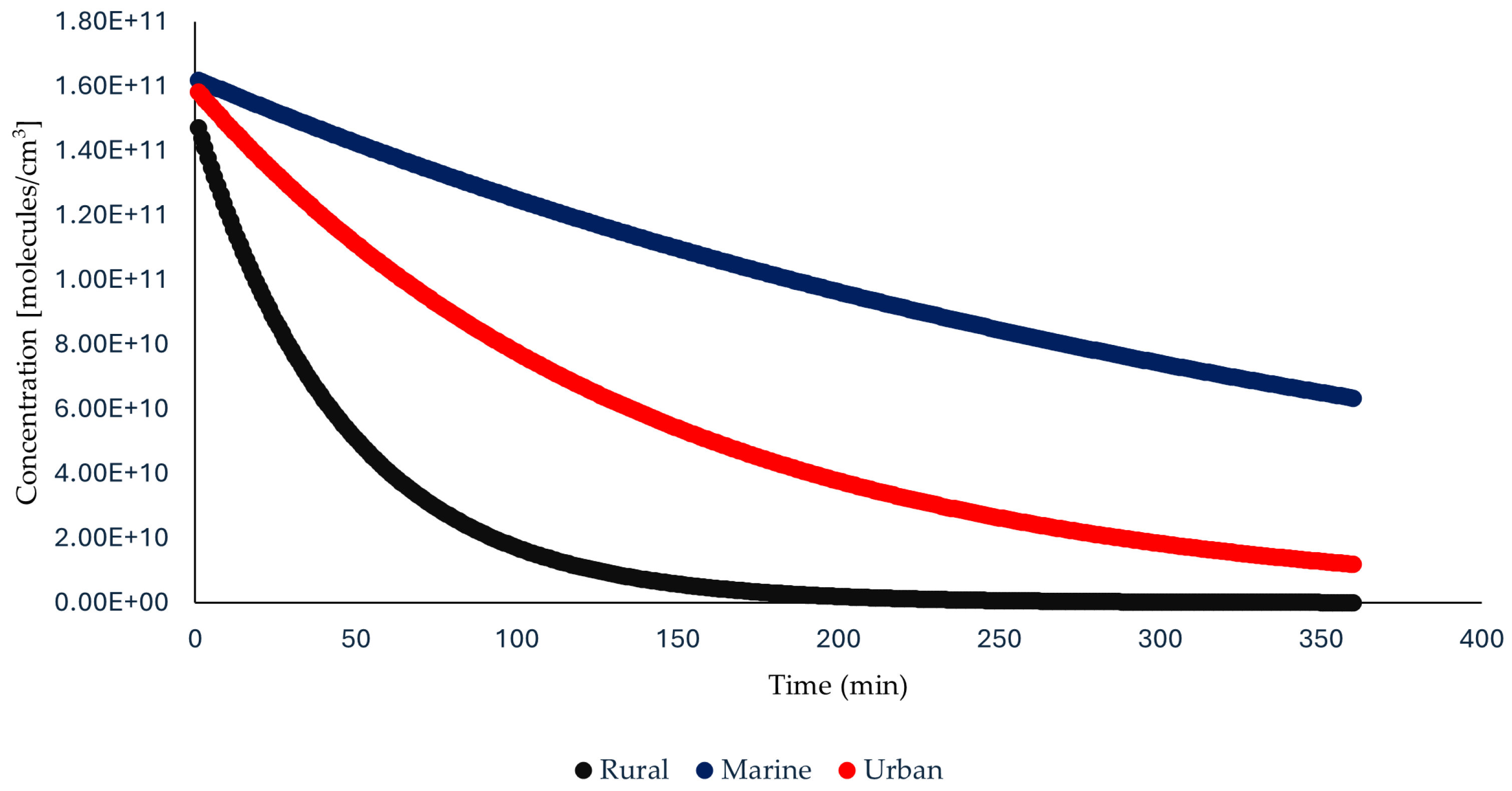
3.1. Half-Life Calculations and Persistence of Odorants
3.1.1. Short-Lived Odorants
3.1.2. Intermediate-Persistence Odorants
3.1.3. Long-Persistence Odorants
3.2. Environmental Influence on Odorant Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trabue, S.; Scoggin, K.; McConnell, L.; Maghirang, R.; Razote, E.; Hatfield, J. Identifying and tracking key odorants from cattle feedlots. Atmos. Environ. 2011, 45, 4243–4251. [Google Scholar] [CrossRef]
- Han, Z.; Qi, F.; Li, R.; Wang, H.; Sun, D. Health impact of odor from on-situ sewage sludge aerobic composting throughout different seasons and during anaerobic digestion with hydrolysis pretreatment. Chemosphere 2020, 249, 126077. [Google Scholar] [CrossRef] [PubMed]
- Blanes-Vidal, V. Air pollution from biodegradable wastes and non-specific health symptoms among residents: Direct or annoyance-mediated associations? Chemosphere 2015, 120, 371–377. [Google Scholar] [CrossRef]
- Fisher, R.M.; Le-Minh, N.; Sivret, E.C.; Alvarez-Gaitan, J.P.; Moore, S.J.; Stuetz, R.M. Distribution and sensorial relevance of volatile organic compounds emitted throughout wastewater biosolids processing. Sci. Total Environ. 2017, 599–600, 663–670. [Google Scholar] [CrossRef]
- Vitko, T.G.; Cowden, S.; Suffet, I.H. Evaluation of bioscrubber and biofilter technologies treating wastewater foul air by a new approach of using odor character, odor intensity, and chemical analyses. Water Res. 2022, 220, 118691. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.M.; Swamy, V.; Ramakrishnan, N.; Chan, E.S.; Kesuma, H.P. Volatile organic compounds (VOCs) in wastewater: Recent advances in detection and quantification. Microchem. J. 2023, 195, 109537. [Google Scholar] [CrossRef]
- Zerga, A.Y.; Tahir, M.; Bouguerra, M.D.E.; Alias, H. Studying the productivity of sewage sludge (SS) components for photocatalytic CO2 transformation to CO and methane. J. Umm Al-Qura Univ. Appl. Sci. 2024. [Google Scholar] [CrossRef]
- Barczak, R.J.; Możaryn, J.; Fisher, R.M.; Stuetz, R.M. Odour concentrations prediction based on odorants concentrations from biosolid emissions. Environ. Res. 2022, 214, 113871. [Google Scholar] [CrossRef] [PubMed]
- Barczak, R.J.; Fisher, R.M.; Le-Minh, N.; Stuetz, R.M. Identification of volatile sulfur odorants emitted from ageing wastewater biosolids. Chemosphere 2022, 287 Pt 2, 132210. [Google Scholar] [CrossRef]
- Zhou, Y.; Vitko, T.G.; Suffet, I.H. A new method for evaluating nuisance of odorants by chemical and sensory analyses and the assessing of masked odors by olfactometry. Sci. Total Environ. 2023, 862, 160905. [Google Scholar] [CrossRef]
- Piccardo, M.T.; Geretto, M.; Pulliero, A.; Izzotti, A. Odor emissions: A public health concern for health risk perception. Environ. Res. 2022, 204, 112121. [Google Scholar] [CrossRef]
- Martuzzi, M.; Mitis, F.; Forastiere, F. Inequalities, inequities, environmental justice in waste management and health. Eur. J. Public Health 2010, 20, 21–26. [Google Scholar] [CrossRef]
- Bouguerra, M.D.E.; Witkowski, B.; Gierczak, T.; Barczak, R.J. Methodology of key odorants selection from wastewater treatment plants based on their kinetics with selected atmospheric oxidants. Chem. Eng. Trans. 2024, 112, 49–54. [Google Scholar]
- Avallone, L.M. Observations for chemistry (in situ)|Resonance Fluorescence. In Encyclopedia of Atmospheric Sciences; Holton, J.R., Ed.; Academic Press: Oxford, UK, 2003; pp. 1484–1490. [Google Scholar]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Munter, R. Advanced oxidation processes—Current status and prospects. Proc. Est. Acad. Sci. Chem. 2001, 50, 59–80. [Google Scholar] [CrossRef]
- Huang, C.P.; Dong, C.; Tang, Z. Advanced chemical oxidation: Its present role and potential future in hazardous waste treatment. Waste Manag. 1993, 13, 361–377. [Google Scholar] [CrossRef]
- Holland, F.; Hofzumahaus, A.; Schäfer, J.; Kraus, A.; Pätz, H.W. Measurements of OH and HO2 radical concentrations and photolysis frequencies during BERLIOZ. J. Geophys. Res. Atmos. 2003, 108, PHO 2-1–PHO 2-23. [Google Scholar] [CrossRef]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Reid, J.P.; Sayer, R.M. Chemistry in the clouds: The role of aerosols in atmospheric chemistry. Sci. Prog. 2002, 85 Pt 3, 263–296. [Google Scholar] [CrossRef]


| Odorant | Reaction with •OH Radicals | k298/cm3 Molecule−1 s−1 |
|---|---|---|
| Ammonia | •OH + NH3 → H2O + ·NH2 | 1.83 × 10−12 |
| Trimethylamine | •OH + (CH3)3N → Products | 5.73 × 10−11 |
| Carbonyl sulfide | •OH + COS → Products | 1.93 × 10−15 |
| Methanethiol | •OH + CH3SH → Products | 3.40 × 10−11 |
| Carbonyl disulfide | •OH + CS2 → Products | 1.91 × 10−12 |
| Ethanethiol | •OH + C2H5SH → Products | 4.61 × 10−11 |
| Dimethyl sulfide | •OH + (CH3)2S → H2O + CH3SCH2 | 4.40 × 10−12 |
| Dimethyl disulfide | •OH + (CH3S) 2 → Products | 2.41 × 10−10 |
| Hydrogen sulfide | •OH + H2S → H2O + SH | 5.84 × 10−12 |
| p-xylene | •OH + 1,4-Dimethylbenzene → Products | 1.36 × 10−11 |
| Toluene | •OH + Toluene → Products | 5.70 × 10−12 |
| 1,3,5-trimethylbenzene | •OH + 1,3,5-Trimethylbenzene → Products | 5.90 × 10−11 |
| Butanol | •OH + CH3CH2CH2CH2OH → Products | 8.47 × 10−12 |
| Oxidant | Marine Site (Molecules/cm3) | Urban Site (Molecules/cm3) | Rural Site (Molecules/cm3) |
|---|---|---|---|
| •OH radical | 9.60 × 105 [14] | 2.65 × 106 [17] | 8.00 × 106 [18] |
| Odorants | T½ Rural Site (min) | T½ Marine Site (min) | T½ Urban Site (min) | T½ Class |
|---|---|---|---|---|
| Ammonia | 781.67 | 4.57 * | 1.75 * | Long |
| Trimethylamine | 25.30 | 210.00 | 75.79 | Short |
| Carbonyl sulfide | 517.36 * | 129,861.00 * | 1550.00 * | Long |
| Methanethiol | 42.50 | 353.00 | 128.11 | Short |
| Carbonyl disulfide | 753.33 | 4.50 * | 986.50 | Intermediate |
| Ethanethiol | 31.36 | 261.50 | 94.80 | Short |
| Dimethyl sulfide | 326.70 | 425.00 | 989.70 | Intermediate |
| Dimethyl disulfide | 18.10 | 50.00 | 65.75 | Short |
| Hydrogen sulfide | 248.40 | 1.50 * | 750.00 | Short |
| p-xylene | 320.00 | 886.70 | 1.00 * | Intermediate |
| Toluene | 253.33 | 1.50 * | 766.67 | Intermediate |
| 1,3,5-trimethylbenzene | 25.83 | 203.33 | 73.33 | Short |
| Butanol | 170.00 | 1.00 * | 513.33 | Long |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouguerra, M.D.E.; Witkowski, B.; Gierczak, T.; Barczak, R.J. Degradation Kinetics of Common Odorants Emitted from WWTPs: A Methodological Approach for Estimating Half-Life Through Reactions with Hydroxyl Radicals. Atmosphere 2025, 16, 340. https://doi.org/10.3390/atmos16030340
Bouguerra MDE, Witkowski B, Gierczak T, Barczak RJ. Degradation Kinetics of Common Odorants Emitted from WWTPs: A Methodological Approach for Estimating Half-Life Through Reactions with Hydroxyl Radicals. Atmosphere. 2025; 16(3):340. https://doi.org/10.3390/atmos16030340
Chicago/Turabian StyleBouguerra, Marouane Dhia Eddine, Bartłomiej Witkowski, Tomasz Gierczak, and Radosław J. Barczak. 2025. "Degradation Kinetics of Common Odorants Emitted from WWTPs: A Methodological Approach for Estimating Half-Life Through Reactions with Hydroxyl Radicals" Atmosphere 16, no. 3: 340. https://doi.org/10.3390/atmos16030340
APA StyleBouguerra, M. D. E., Witkowski, B., Gierczak, T., & Barczak, R. J. (2025). Degradation Kinetics of Common Odorants Emitted from WWTPs: A Methodological Approach for Estimating Half-Life Through Reactions with Hydroxyl Radicals. Atmosphere, 16(3), 340. https://doi.org/10.3390/atmos16030340








