Comparison of Two Miniaturized, Rectifiable Aerosol Photometers for Personal PM2.5 Monitoring in a Dusty Occupational Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Filter Weighing
2.3. MicroPEM Methodology
2.4. UPAS Methodology
2.5. Statistical Analyses
2.6. Filter Imaging
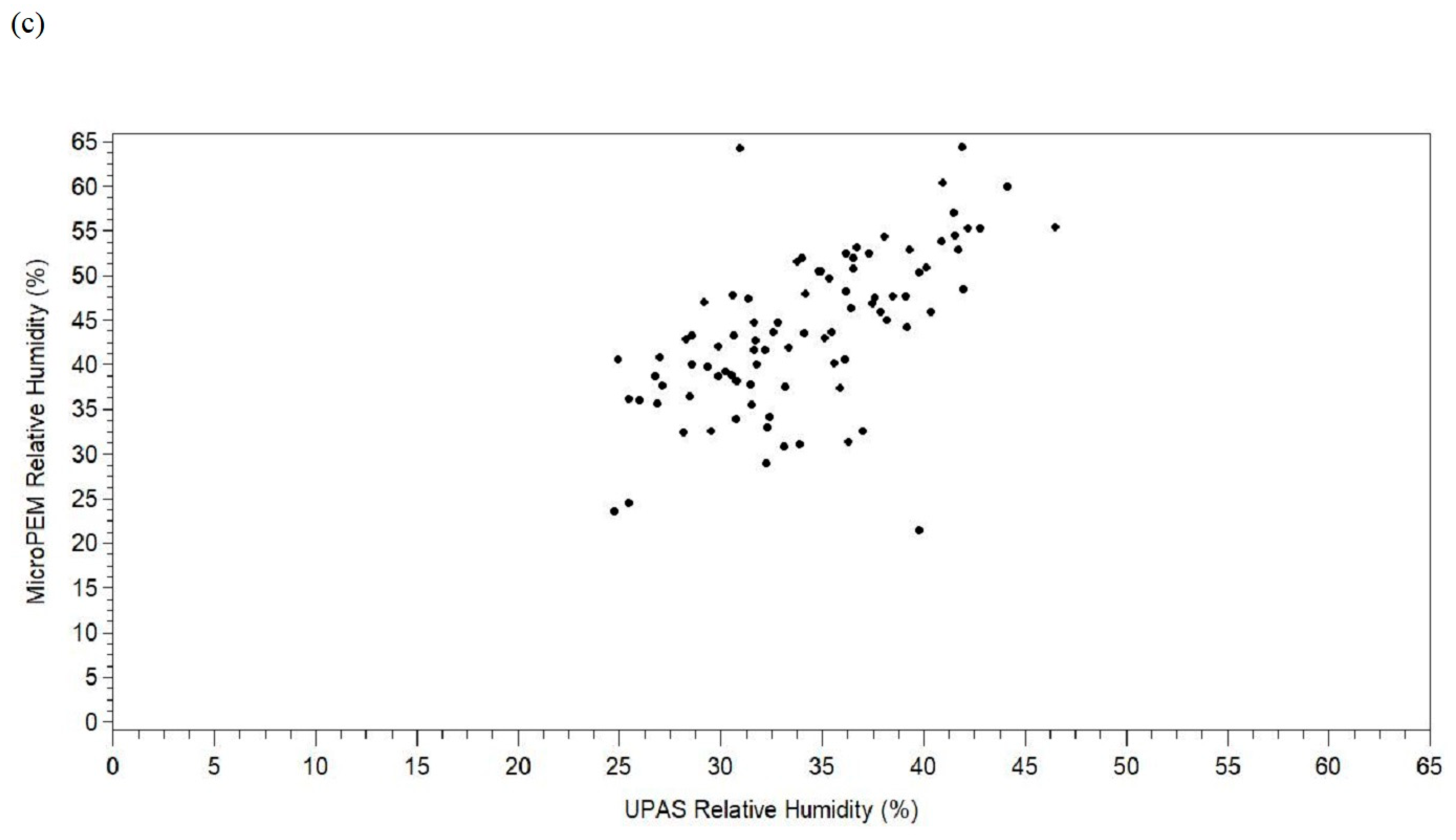
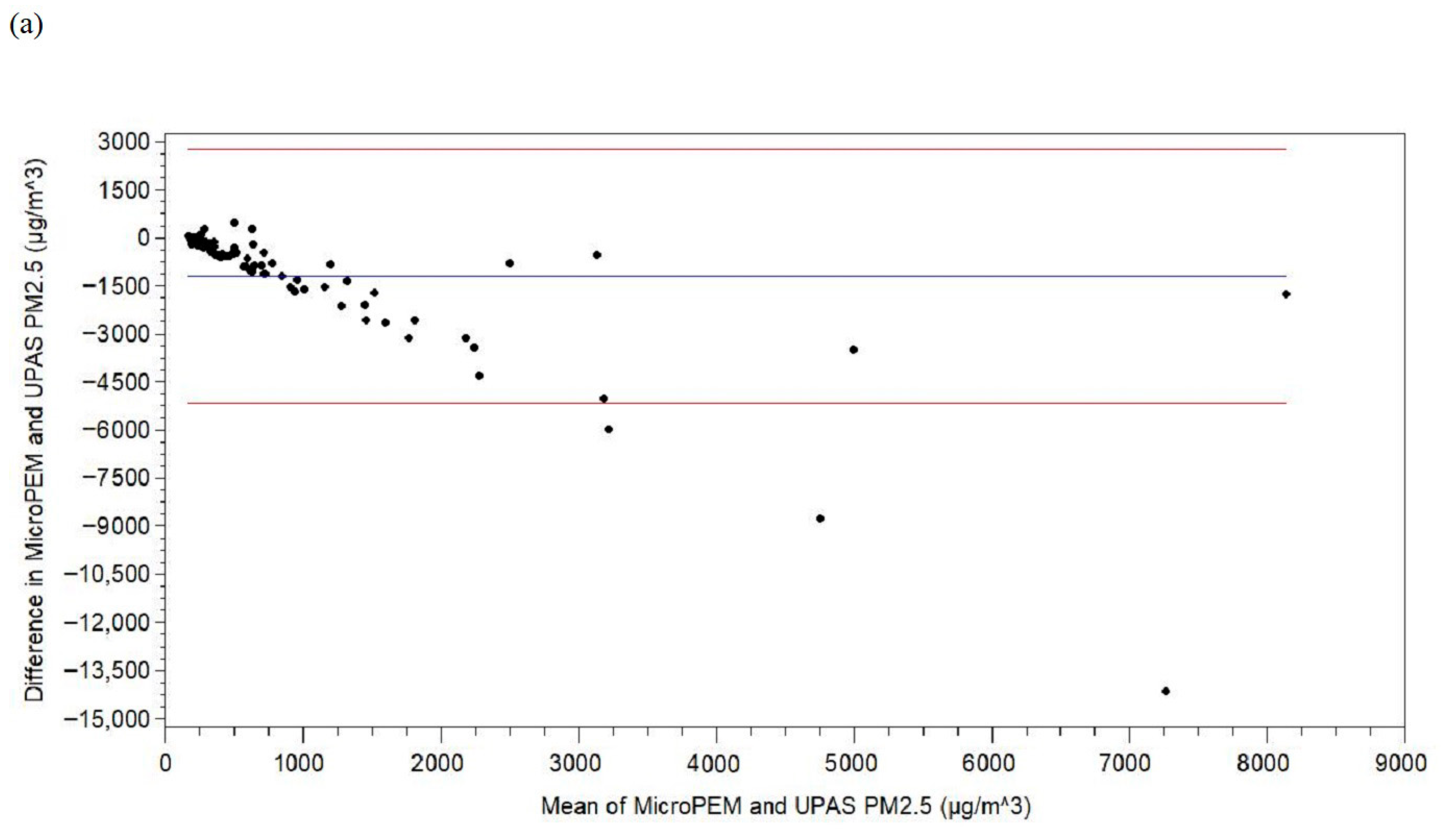
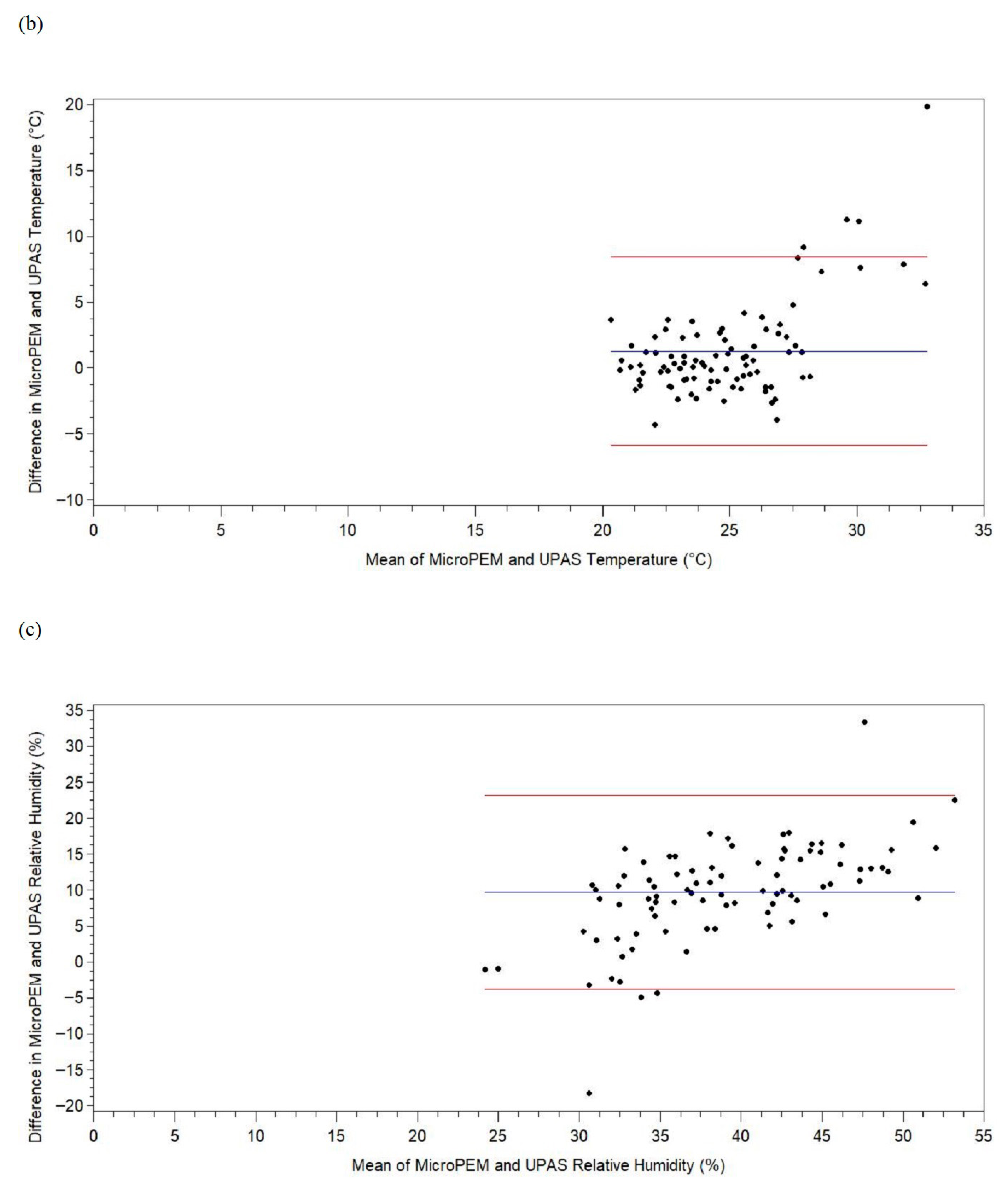
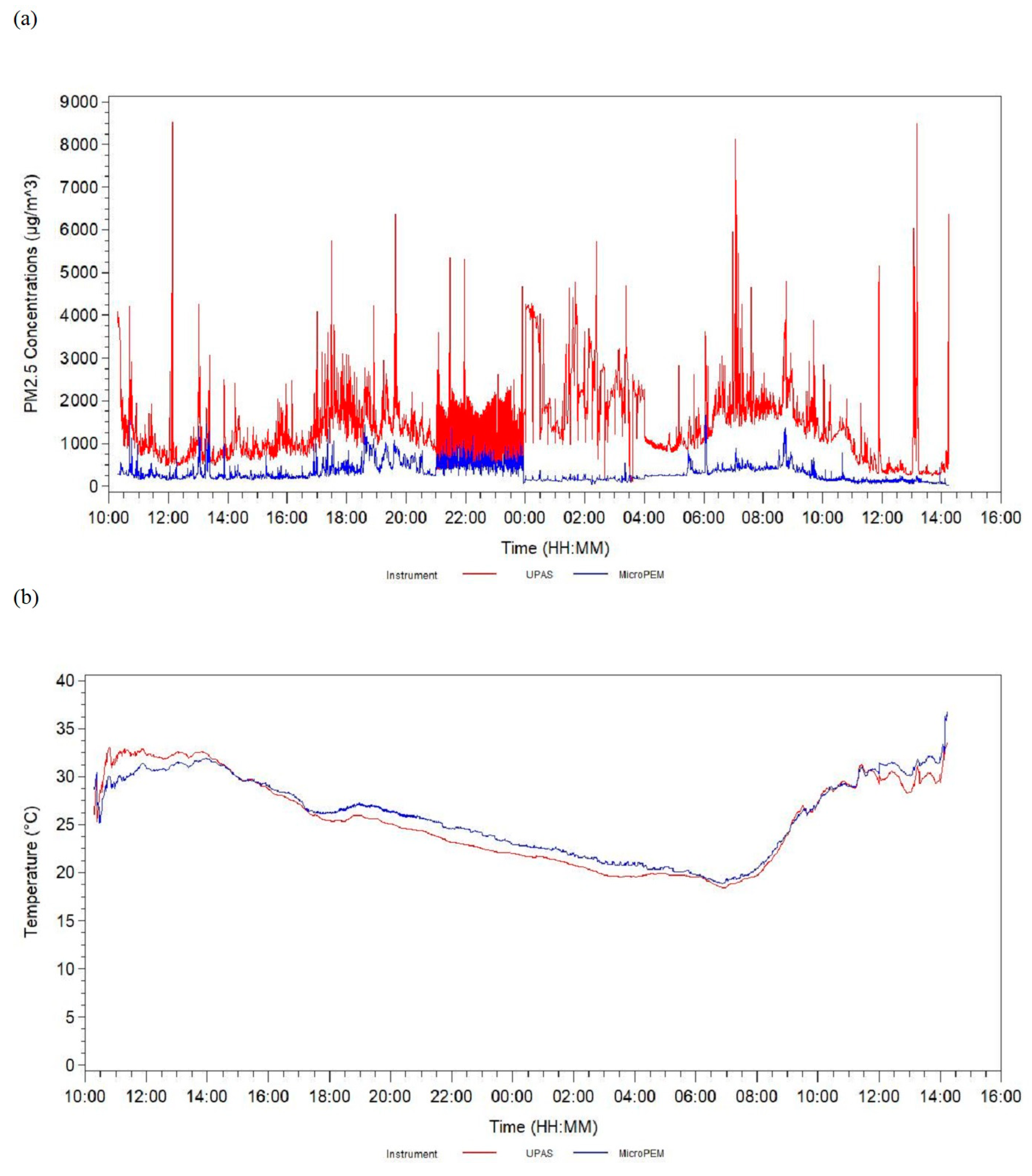
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pope, C.A., III; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef]
- Pope, C.A., III; Lefler, J.S.; Ezzati, M.; Higbee, J.D.; Marshall, J.D.; Kim, S.-Y.; Bechle, M.; Gilliat, K.S.; Vernon, S.E.; Robinson, A.L. Mortality risk and fine particulate air pollution in a large, representative cohort of US adults. Environ. Health Perspect. 2019, 127, 077007. [Google Scholar] [CrossRef]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef]
- Sang, S.; Chu, C.; Zhang, T.; Chen, H.; Yang, X. The global burden of disease attributable to ambient fine particulate matter in 204 countries and territories, 1990–2019: A systematic analysis of the Global Burden of Disease Study 2019. Ecotoxicol. Environ. Saf. 2022, 238, 113588. [Google Scholar] [CrossRef]
- Bennitt, F.B.; Wozniak, S.; Causey, K.; Spearman, S.; Okereke, C.; Garcia, V.; Hashmeh, N.; Ashbaugh, C.; Abdelkader, A.; Abdoun, M. Global, regional, and national burden of household air pollution, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2025, 405, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Bennitt, F.; Wozniak, S.; Causey, K.; Burkart, K.; Brauer, M. Estimating disease burden attributable to household air pollution: New methods within the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, S18. [Google Scholar] [CrossRef]
- Brauer, M.; Roth, G.A.; Aravkin, A.Y.; Zheng, P.; Abate, K.H.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasi, M.A.; Abbasian, M. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2162–2203. [Google Scholar] [CrossRef] [PubMed]
- Blanc, P.D.; Annesi-Maesano, I.; Balmes, J.R.; Cummings, K.J.; Fishwick, D.; Miedinger, D.; Murgia, N.; Naidoo, R.N.; Reynolds, C.J.; Sigsgaard, T. The occupational burden of nonmalignant respiratory diseases. An official American Thoracic Society and European Respiratory Society statement. Am. J. Respir. Crit. Care Med. 2019, 199, 1312–1334. [Google Scholar] [CrossRef]
- Dons, E.; Panis, L.I.; Van Poppel, M.; Theunis, J.; Willems, H.; Torfs, R.; Wets, G. Impact of time–activity patterns on personal exposure to black carbon. Atmos. Environ. 2011, 45, 3594–3602. [Google Scholar] [CrossRef]
- Steinle, S.; Reis, S.; Sabel, C.E. Quantifying human exposure to air pollution—Moving from static monitoring to spatio-temporally resolved personal exposure assessment. Sci. Total Environ. 2013, 443, 184–193. [Google Scholar] [CrossRef]
- Cattaneo, A.; Taronna, M.; Consonni, D.; Angius, S.; Costamagna, P.; Cavallo, D.M. Personal exposure of traffic police officers to particulate matter, carbon monoxide, and benzene in the city of Milan, Italy. J. Occup. Environ. Hyg. 2010, 7, 342–351. [Google Scholar] [CrossRef]
- Maître, A.; Soulat, J.-M.; Masclet, P.; Stoklov, M.; Marquès, M.; De Gaudemaris, R. Exposure to carcinogenic air pollutants among policemen working close to traffic in an urban area. Scand. J. Work Environ. Health 2002, 28, 402–410. [Google Scholar] [CrossRef]
- Noomnual, S.; Shendell, D.G. Risk of adult street vendor exposure to traffic-related air pollution in Bangkok, Thailand. Hum. Ecol. Risk Assess. Int. J. 2017, 23, 340–349. [Google Scholar] [CrossRef]
- Hachem, M.; Saleh, N.; Paunescu, A.-C.; Momas, I.; Bensefa-Colas, L. Exposure to traffic air pollutants in taxicabs and acute adverse respiratory effects: A systematic review. Sci. Total Environ. 2019, 693, 133439. [Google Scholar] [CrossRef] [PubMed]
- Keim-Malpass, J.; Spears, C.R.; Quandt, S.A.; Arcury, T.A. Perceptions of housing conditions among migrant farmworkers and their families: Implications for health, safety and social policy. Rural Remote Health 2015, 15, 3076. [Google Scholar] [CrossRef] [PubMed]
- Caxaj, C.S.; Weiler, A.M.; Martyniuk, J. Housing conditions and health implications for migrant agricultural workers in Canada: A scoping review. Can. J. Nurs. Res. 2024, 56, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Thygerson, S.M.; Beard, J.D.; House, M.J.; Smith, R.L.; Burbidge, H.C.; Andrus, K.N.; Weber, F.X.; Chartier, R.; Johnston, J.D. Air-Quality Assessment of On-Site Brick-Kiln Worker Housing in Bhaktapur, Nepal: Chemical Speciation of Indoor and Outdoor PM2.5 Pollution. Int. J. Environ. Res. Public Health 2019, 16, 4114. [Google Scholar] [CrossRef]
- Johnston, J.D.; Hawks, M.E.; Johnston, H.B.; Johnson, L.A.; Beard, J.D. Comparison of Liquefied Petroleum Gas Cookstoves and Wood Cooking Fires on PM2.5 Trends in Brick Workers’ Homes in Nepal. Int. J. Environ. Res. Public Health 2020, 17, 5681. [Google Scholar] [CrossRef]
- Watson, J.G.; Tropp, R.J.; Kohl, S.D.; Wang, X.; Chow, J.C. Filter processing and gravimetric analysis for suspended particulate matter samples. Aerosol Sci. Eng. 2017, 1, 93–105. [Google Scholar] [CrossRef]
- Vosburgh, D.J.; Cauda, E.; O’Shaughnessy, P.T.; Sheehan, M.J.; Park, J.H.; Anderson, K. Direct-reading instruments for aerosols: A review for occupational health and safety professionals part 1: Instruments and good practices. J. Occup. Environ. Hyg. 2022, 19, 696–705. [Google Scholar] [CrossRef]
- Baron, P.A. Aerosol Photometers for respirable dust measurements. NIOSH Man. Anal. Methods 1998, 1, 15. [Google Scholar]
- Braun, K.O. Exploring aerosol-specific calibration and performance of three direct-reading photometers. J. Occup. Environ. Hyg. 2025, 22, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Anger, W.K.; Elliot, D.L.; Bodner, T.; Olson, R.; Rohlman, D.S.; Truxillo, D.M.; Kuehl, K.S.; Hammer, L.B.; Montgomery, D. Effectiveness of total worker health interventions. J. Occup. Health Psychol. 2015, 20, 226. [Google Scholar] [CrossRef]
- Volckens, J.; Quinn, C.; Leith, D.; Mehaffy, J.; Henry, C.S.; Miller-Lionberg, D. Development and evaluation of an ultrasonic personal aerosol sampler. Indoor Air 2017, 27, 409–416. [Google Scholar] [CrossRef]
- Burrowes, V.J.; Piedrahita, R.; Pillarisetti, A.; Underhill, L.J.; Fandiño-Del-Rio, M.; Johnson, M.; Kephart, J.L.; Hartinger, S.M.; Steenland, K.; Naeher, L. Comparison of next-generation portable pollution monitors to measure exposure to PM2.5 from household air pollution in Puno, Peru. Indoor Air 2020, 30, 445–458. [Google Scholar] [CrossRef]
- Cho, S.-H.; Chartier, R.T.; Mortimer, K.; Dherani, M.; Tafatatha, T. A personal particulate matter exposure monitor to support household air pollution exposure and health studies. In Proceedings of the 2016 IEEE Global Humanitarian Technology Conference (GHTC), Seattle, WA, USA, 13–16 October 2016; pp. 817–818. [Google Scholar]
- Vanker, A.; Barnett, W.; Chartier, R.; MacGinty, R.; Zar, H.J. Personal monitoring of fine particulate matter (PM2.5) exposure in mothers and young children in a South African birth cohort study—A pilot study. Atmos. Environ. 2023, 294, 119513. [Google Scholar] [CrossRef]
- Fisher, J.A.; Friesen, M.C.; Kim, S.; Locke, S.J.; Kefelegn, Y.; Wong, J.Y.; Albert, P.S.; Jones, R.R. Sources of variability in real-time monitoring data for fine particulate matter: Comparability of three wearable monitors in an urban setting. Environ. Sci. Technol. Lett. 2019, 6, 222–227. [Google Scholar] [CrossRef]
- Du, Y.; Wang, Q.; Sun, Q.; Zhang, T.; Li, T.; Yan, B. Assessment of PM2.5 monitoring using MicroPEM: A validation study in a city with elevated PM2.5 levels. Ecotoxicol. Environ. Saf. 2019, 171, 518–522. [Google Scholar] [CrossRef]
- Li, X.; Tryner, J.; Young, B.N.; Hernandez Ramirez, L.; Phillips, M.; WeMott, S.; Erlandson, G.; Kuiper, G.; Dean, D.; Martinez, N. Application and validation of a wearable monitor for assessing time-and location-resolved exposures to particulate matter in California’s Central Valley. Aerosol Sci. Technol. 2024, 59, 1272–1288. [Google Scholar] [CrossRef] [PubMed]
- Pillarisetti, A.; Carter, E.; Rajkumar, S.; Young, B.N.; Benka-Coker, M.L.; Peel, J.L.; Johnson, M.; Clark, M.L. Measuring personal exposure to fine particulate matter (PM2.5) among rural Honduran women: A field evaluation of the Ultrasonic Personal Aerosol Sampler (UPAS). Environ. Int. 2019, 123, 50–53. [Google Scholar] [CrossRef]
- Chartier, R.; Phillips, M.; Mosquin, P.; Elledge, M.; Bronstein, K.; Nandasena, S.; Thornburg, V.; Thornburg, J.; Rodes, C. A comparative study of human exposures to household air pollution from commonly used cookstoves in Sri Lanka. Indoor Air 2017, 27, 147–159. [Google Scholar] [CrossRef]
- Johnston, J.D.; Collingwood, S.C.; LeCheminant, J.D.; Peterson, N.E.; Reynolds, P.R.; Arroyo, J.A.; South, A.J.; Farnsworth, C.B.; Chartier, R.T.; Layton, L.N. Personal Exposure to Fine Particulate Air Pollution among Brick Workers in Nepal. Atmosphere 2023, 14, 1783. [Google Scholar] [CrossRef]
- Arku, R.E.; Birch, A.; Shupler, M.; Yusuf, S.; Hystad, P.; Brauer, M. Characterizing exposure to household air pollution within the Prospective Urban Rural Epidemiology (PURE) study. Environ. Int. 2018, 114, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, J.; Raynes-Greenow, C.; Abbott, M.; Cho, S.; Islam, S.; Tasnim, F.; Billah, S.; Alam, N. Pregnant Women’s Exposure to Household Air Pollution during a LPG Cookstove Intervention in Rural Bangladesh: The Value of Real-Time Data. Environ. Epidemiol. 2019, 3, 327–328. [Google Scholar]
- Jayarathne, T.; Stockwell, C.E.; Bhave, P.V.; Praveen, P.S.; Rathnayake, C.M.; Islam, M.R.; Panday, A.K.; Adhikari, S.; Maharjan, R.; Goetz, J.D. Nepal Ambient Monitoring and Source Testing Experiment (NAMaSTE): Emissions of particulate matter from wood and dung cooking fires, garbage and crop residue burning, brick kilns, and other sources. Atmos. Chem. Phys. Discuss. 2017, 18, 2259–2286. [Google Scholar] [CrossRef]
- Saud, B.; Paudel, G. The threat of ambient air pollution in Kathmandu, Nepal. J. Environ. Public Health 2018, 2018, 1504591. [Google Scholar] [CrossRef]
- Shakya, K.M.; Rupakheti, M.; Shahi, A.; Maskey, R.; Pradhan, B.; Panday, A.; Puppala, S.P.; Lawrence, M.; Peltier, R.E. Near-road sampling of PM 2. 5, BC, and fine-particle chemical components in Kathmandu Valley, Nepal. Atmos. Chem. Phys. 2017, 17, 6503–6516. [Google Scholar] [CrossRef]
- Islam, M.R.; Li, T.; Mahata, K.; Khanal, N.; Werden, B.; Giordano, M.R.; Praveen Puppala, S.; Dhital, N.B.; Gurung, A.; Saikawa, E. Wintertime Air Quality across the Kathmandu Valley, Nepal: Concentration, Composition, and Sources of Fine and Coarse Particulate Matter. ACS Earth Space Chem. 2022, 6, 2955–2971. [Google Scholar] [CrossRef]
- Nicolaou, L.; Rowell, E.; Gaviola, C.; Chandyo, R.K.; Sharma, A.K.; Shrestha, L.P.; Das, S.; Parker, D.L.; Thygerson, S.M.; Jacob, J. Personal exposures to air pollutants and respiratory health among brick kiln workers and household members. Environ. Res. 2025, 279, 121760. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.D.; Collingwood, S.C.; LeCheminant, J.D.; Peterson, N.E.; Reynolds, P.R.; Arroyo, J.A.; South, A.J.; Farnsworth, C.B.; Fong, G.; Cisneros, T. Respirable dust and crystalline silica concentrations among workers at a brick kiln in Bhaktapur, Nepal. J. Occup. Environ. Hyg. 2024, 21, 247–258. [Google Scholar] [CrossRef]
- Rodes, C. Standard Operating Procedure MicroPEM v 3.2 A [Gates Foundation Version] Aerosol Exposure SOP M-300: Setup, Preparation, Calibration, Deployment. Data Process. Serv. 2013, 1–46. [Google Scholar]
- Access Sensor Technologies LLC. Ultrasonic Personal Air Sampler v2.1 and v2.1 PLUS User Guide. Firmware Versions 158 and Earlier. Revision 1.6; Access Sensor Technologies LLC: Fort Collins, CO, USA, 2025; Available online: https://www.dropbox.com/scl/fi/h7gr1875ugdoqtnbp5ly0/AST_UPAS-v2.1-and-UPAS-v2.1-PLUS_UserGuide_FirmwareRev157_20250810.pdf?rlkey=gwpqi4e8aksp6k8tzy1urr6lb&e=1&dl=0 (accessed on 20 October 2025).
- Lin, L.I.K. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Measurement in medicine: The analysis of method comparison studies. J. R. Stat. Soc. Ser. D Stat. 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Cole, S.R.; Hernán, M.A. Constructing inverse probability weights for marginal structural models. Am. J. Epidemiol. 2008, 168, 656–664. [Google Scholar] [CrossRef]
- Hernán, M.A.; Hernández-Díaz, S.; Robins, J.M. A structural approach to selection bias. Epidemiology 2004, 15, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Hernán, M.Á.; Brumback, B.; Robins, J.M. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000, 11, 561–570. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Marple, V.A.; Rubow, K.L.; Turner, W.; Spengler, J.D. Low flow rate sharp cut impactors for indoor air sampling: Design and calibration. JAPCA 1987, 37, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.E.; Lewis, P.; Hofmeyr, W. Respiratory health of brickworkers in Cape Town, South Africa: Background, aims and dust exposure determinations. Scand. J. Work Environ. Health 1989, 15, 180–187. [Google Scholar] [CrossRef]
- Sanjel, S.; Khanal, S.N.; Thygerson, S.M.; Carter, W.S.; Johnston, J.D.; Joshi, S.K. Respiratory symptoms and illnesses related to the concentration of airborne particulate matter among brick kiln workers in Kathmandu valley, Nepal. Ann. Occup. Environ. Med. 2017, 29, 9. [Google Scholar] [CrossRef]
- Steve Chillrud, M.M.; Miller-Lionberg, D.; Sulemana, A.; Tryner, J.; Ross, J.; Seyram, K.; Tsotestsi, K.; Lee, A.; Jack, D.; Assante, K.P. Identification of large-PM breakthrough of PM2.5 inlets during personal filter sampling in Ghana, and testing of various multi-stage pre-cyclone impactor improvements. In Proceedings of the 2024 International Society of Exposure Science, Montreal, QC, Canada, 23 October 2024. [Google Scholar]
- Tryner, J. Choosing the Best Particulate Matter Sensor Duty Cycle for Your Measurement Application: Access Sensor Technologies. Available online: https://www.accsensors.com/blog/duty-cycling-pm-sensor (accessed on 13 September 2025).
- Alli, A.S.; Clark, S.N.; Hughes, A.; Nimo, J.; Bedford-Moses, J.; Baah, S.; Wang, J.; Vallarino, J.; Agyemang, E.; Barratt, B. Spatial-temporal patterns of ambient fine particulate matter (PM2.5) and black carbon (BC) pollution in Accra. Environ. Res. Lett. 2021, 16, 074013. [Google Scholar] [CrossRef]
- Alahmad, B.; Al-Hemoud, A.; Kang, C.-M.; Almarri, F.; Kommula, V.; Wolfson, J.M.; Bernstein, A.S.; Garshick, E.; Schwartz, J.; Koutrakis, P. A two-year assessment of particulate air pollution and sources in Kuwait. Environ. Pollut. 2021, 282, 117016. [Google Scholar] [CrossRef]
- Tryner, J. Introducing the Pre-Cyclone Impactor: Access Sensor Technologies, LLC. Available online: https://www.accsensors.com/blog/pre-cyclone-impactor (accessed on 20 August 2025).
- Daniel Miller-Lionberg, J.T.; Chillrud, S.; Mujtaba, M. Development and laboratory testing of novel multi-stage pre-cyclone impactor inserts for mitigating large-particle breakthrough during personal sampling with PM2.5 cyclones in challenging environments. In Proceedings of the 2024 International Society of Exposure Science, Montreal, QC, Canada, 23 October 2024. [Google Scholar]
- Visscher, G.; Kornet, J. Long-term tests of capacitive humidity sensors. Meas. Sci. Technol. 1994, 5, 1294. [Google Scholar] [CrossRef]
- Freitag, H.P. Calibration Procedures and Instrumental Accuracy Estimates of TAO Temperature, Relative Humidity and Radiation Measurements; Pacific Marine Environmental Laboratory: Seattle, WA, USA, 1994. [Google Scholar]
- Johnston, J.D.; Magnusson, B.M.; Eggett, D.; Mumford, K.; Collingwood, S.C.; Bernhardt, S.A. Sensor drift and predicted calibration intervals of handheld temperature and relative humidity meters under residential field-use conditions. J. Environ. Health 2014, 77, 22–29. [Google Scholar]
- Brooks, S.D.; Wise, M.E.; Cushing, M.; Tolbert, M.A. Deliquescence behavior of organic/ammonium sulfate aerosol. Geophys. Res. Lett. 2002, 29, 1917. [Google Scholar] [CrossRef]
- Freney, E.J.; Martin, S.T.; Buseck, P.R. Deliquescence and efflorescence of potassium salts relevant to biomass-burning aerosol particles. Aerosol Sci. Technol. 2009, 43, 799–807. [Google Scholar] [CrossRef]
- Biskos, G.; Malinowski, A.; Russell, L.; Buseck, P.; Martin, S. Nanosize effect on the deliquescence and the efflorescence of sodium chloride particles. Aerosol Sci. Technol. 2006, 40, 97–106. [Google Scholar] [CrossRef]
- Perkins, N.J.; Cole, S.R.; Harel, O.; Tchetgen Tchetgen, E.J.; Sun, B.; Mitchell, E.M.; Schisterman, E.F. Principled approaches to missing data in epidemiologic studies. Am. J. Epidemiol. 2018, 187, 568–575. [Google Scholar] [CrossRef]
- Harel, O.; Mitchell, E.M.; Perkins, N.J.; Cole, S.R.; Tchetgen Tchetgen, E.J.; Sun, B.; Schisterman, E.F. Multiple imputation for incomplete data in epidemiologic studies. Am. J. Epidemiol. 2018, 187, 576–584. [Google Scholar] [CrossRef]
- Mohr, D.L.; Wilson, W.J.; Freund, R.J. Statistical Methods, 4th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Carrasco, J.L.; Phillips, B.R.; Puig-Martinez, J.; King, T.S.; Chinchilli, V.M. Estimation of the concordance correlation coefficient for repeated measures using SAS and R. Comput. Methods Programs Biomed. 2013, 109, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Dawson, B.; Trapp, R.G. Basic and Clinical Biostatistics, 4th ed.; Lang Medical Books/McGraw-Hill: Columbus, OH, USA, 2004. [Google Scholar]
- King, T.S.; Chinchilli, V.M.; Carrasco, J.L. A repeated measures concordance correlation coefficient. Stat. Med. 2007, 26, 3095–3113. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.L.; King, T.S.; Chinchilli, V.M. The concordance correlation coefficient for repeated measures estimated by variance components. J. Biopharm. Stat. 2009, 19, 90–105. [Google Scholar] [CrossRef] [PubMed]




| Variable | Missing, n | Not Missing, n | AM | SD | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|---|---|---|---|
| MicroPEM PM2.5, µg/m3 | 5 | 88 | 429.48 | 885.85 | 89.29 | 145.66 | 207.06 | 361.90 | 7258.41 |
| UPAS PM2.5, µg/m3 | 3 | 90 | 1636.88 | 2251.73 | 130.21 | 420.66 | 737.74 | 1820.64 | 14,324.03 |
| PM2.5 difference b, µg/m3 | 8 | 85 | −1199.14 | 2026.12 | −14,121.76 | −1305.74 | −553.35 | 227.76 | 472.94 |
| MicroPEM temperature, °C | 2 | 91 | 25.51 | 3.97 | 19.94 | 22.91 | 24.86 | 26.77 | 42.68 |
| UPAS temperature, °C | 2 | 91 | 24.14 | 2.23 | 18.49 | 22.46 | 24.00 | 25.63 | 29.51 |
| Temperature difference b, °C | 4 | 89 | 1.28 | 3.63 | −4.27 | −0.87 | 0.39 | 2.39 | 19.86 |
| MicroPEM relative humidity, % | 2 | 91 | 43.67 | 8.71 | 21.48 | 37.84 | 43.29 | 50.48 | 64.43 |
| UPAS relative humidity, % | 2 | 91 | 34.23 | 5.00 | 24.71 | 30.62 | 33.99 | 37.88 | 46.46 |
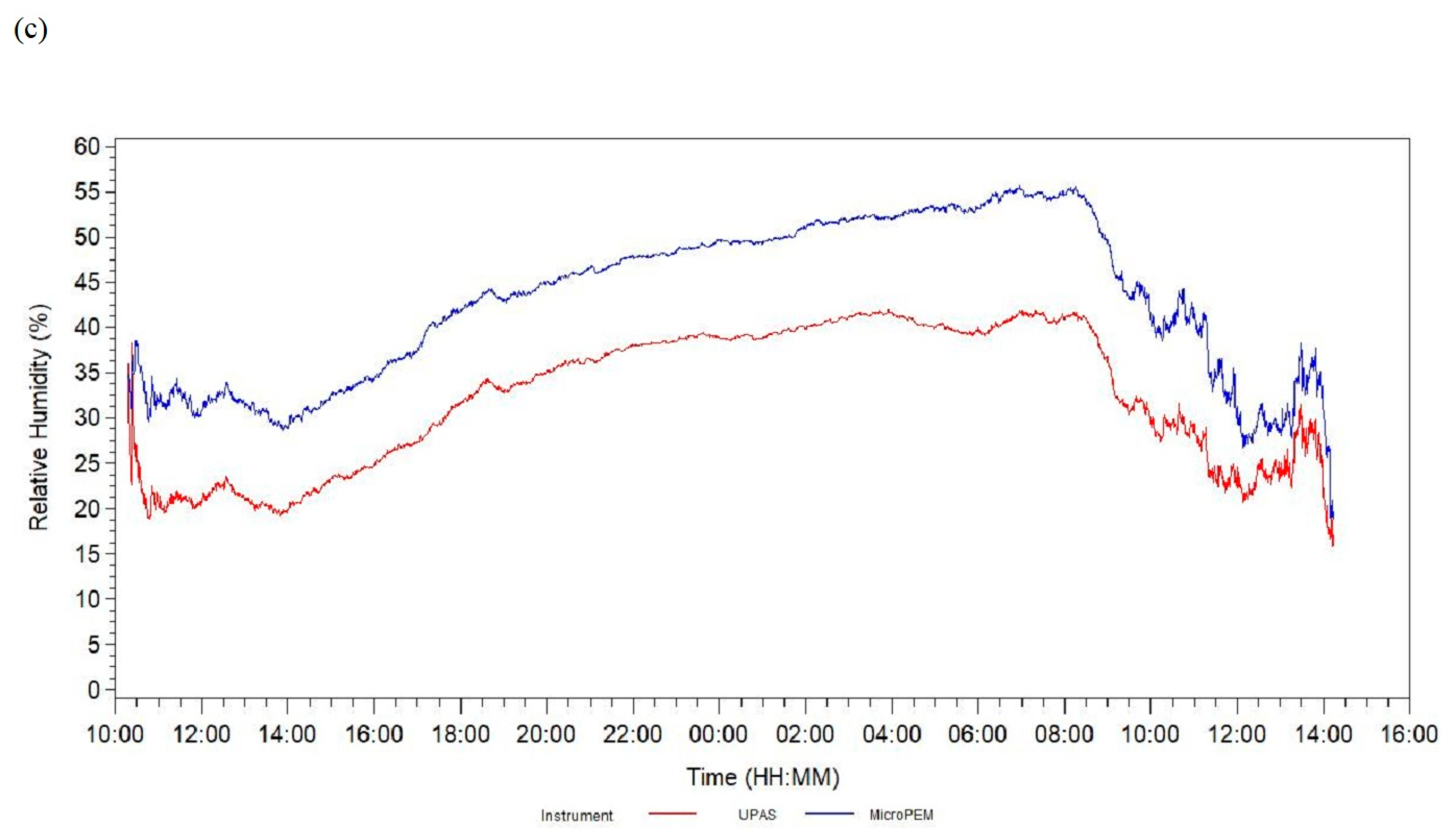
| Relative humidity difference b, % | 4 | 89 | 9.69 | 6.87 | −18.29 | 6.87 | 10.07 | 13.89 | 33.40 |
| Variable | AM b | 95% CI b | p-Value b | Median c | 95% CI c | p-Value c | PCC | SCC | CCC |
|---|---|---|---|---|---|---|---|---|---|
| PM2.5 difference d, µg/m3 | −1199.14 | −1636.16, −762.11 | <0.0001 | −553.35 | −764.76, −341.94 | <0.0001 | 0.46 | 0.34 | 0.26 |
| Temperature difference d, °C | 1.28 | 0.52, 2.05 | 0.001 | 0.39 | −0.35, 1.13 | 0.30 | 0.44 | 0.62 | 0.34 |
| Relative humidity difference d, % | 9.69 | 8.24, 11.14 | <0.0001 | 10.07 | 8.60, 11.53 | <0.0001 | 0.62 | 0.66 | 0.28 |
| Variable | Missing, n | Not Missing, n | AM | SD | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|---|---|---|---|
| MicroPEM PM2.5 a, µg/m3 | 31,498 | 207,763 | 382.14 | 1706.20 | 0.00 | 93.74 | 169.19 | 298.72 | 76,672.26 |
| UPAS PM2.5 a, µg/m3 | 91,836 | 147,425 | 1428.10 | 4734.23 | 0.00 | 275.00 | 594.08 | 1276.00 | 418,945.51 |
| PM2.5 difference a, d, µg/m3 | 113,395 | 125,866 | −1045.96 | 4147.93 | −414,927.81 | −978.91 | −390.27 | −106.33 | 37,731.66 |
| MicroPEM temperature b, °C | 213,229 | 26,032 | 25.03 | 5.51 | 12.30 | 21.00 | 24.30 | 28.60 | 66.60 |
| UPAS temperature b, °C | 224,408 | 14,853 | 24.38 | 5.26 | 9.45 | 20.33 | 23.82 | 28.10 | 43.95 |
| Temperature difference b, d, °C | 199,616 | 39,645 | 0.65 | 3.37 | −11.13 | −1.22 | 0.32 | 2.08 | 30.81 |
| MicroPEM relative humidity c, % | 213,266 | 25,995 | 44.96 | 12.38 | 8.37 | 35.53 | 44.70 | 53.63 | 99.77 |
| UPAS relative humidity c, % | 224,408 | 14,853 | 34.17 | 9.71 | 7.82 | 26.70 | 34.30 | 41.55 | 72.18 |
| Relative humidity difference c, d, % | 199,632 | 39,629 | 10.79 | 7.05 | −22.05 | 6.99 | 10.44 | 14.85 | 63.18 |
| Variable | AM d | 95% CI d | p-Value d | PCC | CCC |
|---|---|---|---|---|---|
| PM2.5 difference a, e, µg/m3 | −1205.60 | −1633.78, −777.42 | <0.0001 | 0.50 | 0.31 |
| Temperature difference b, e, °C | 3.52 | 2.72, 4.33 | <0.0001 | 0.81 | 0.80 |
| Relative humidity difference c, e, % | 9.89 | 8.58, 11.20 | <0.0001 | 0.82 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnston, J.D.; Collingwood, S.C.; LeCheminant, J.D.; Peterson, N.E.; South, A.J.; Farnsworth, C.B.; Chartier, R.T.; Thiel, M.E.; Brown, T.P.; Goss, E.S.; et al. Comparison of Two Miniaturized, Rectifiable Aerosol Photometers for Personal PM2.5 Monitoring in a Dusty Occupational Environment. Atmosphere 2025, 16, 1233. https://doi.org/10.3390/atmos16111233
Johnston JD, Collingwood SC, LeCheminant JD, Peterson NE, South AJ, Farnsworth CB, Chartier RT, Thiel ME, Brown TP, Goss ES, et al. Comparison of Two Miniaturized, Rectifiable Aerosol Photometers for Personal PM2.5 Monitoring in a Dusty Occupational Environment. Atmosphere. 2025; 16(11):1233. https://doi.org/10.3390/atmos16111233
Chicago/Turabian StyleJohnston, James D., Scott C. Collingwood, James D. LeCheminant, Neil E. Peterson, Andrew J. South, Clifton B. Farnsworth, Ryan T. Chartier, Mary E. Thiel, Tanner P. Brown, Elisabeth S. Goss, and et al. 2025. "Comparison of Two Miniaturized, Rectifiable Aerosol Photometers for Personal PM2.5 Monitoring in a Dusty Occupational Environment" Atmosphere 16, no. 11: 1233. https://doi.org/10.3390/atmos16111233
APA StyleJohnston, J. D., Collingwood, S. C., LeCheminant, J. D., Peterson, N. E., South, A. J., Farnsworth, C. B., Chartier, R. T., Thiel, M. E., Brown, T. P., Goss, E. S., Jones, P. K., Sanjel, S., Gifford, J. R., & Beard, J. D. (2025). Comparison of Two Miniaturized, Rectifiable Aerosol Photometers for Personal PM2.5 Monitoring in a Dusty Occupational Environment. Atmosphere, 16(11), 1233. https://doi.org/10.3390/atmos16111233








