Urban Health Assessment Through a Planetary Health Perspective: Methods and First Results from the Rome NBFC Experiment
Abstract
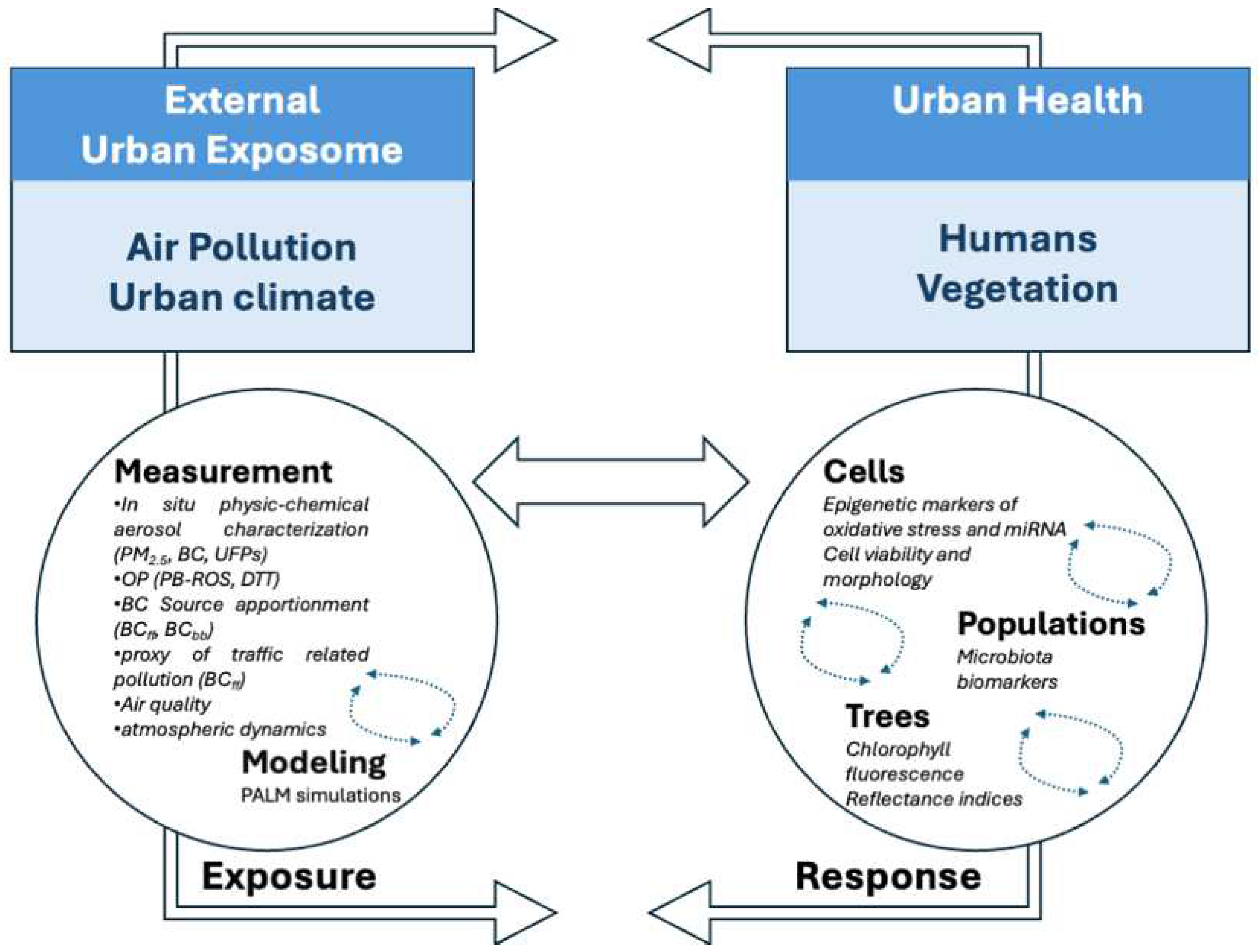
1. Introduction
2. Materials and Methods
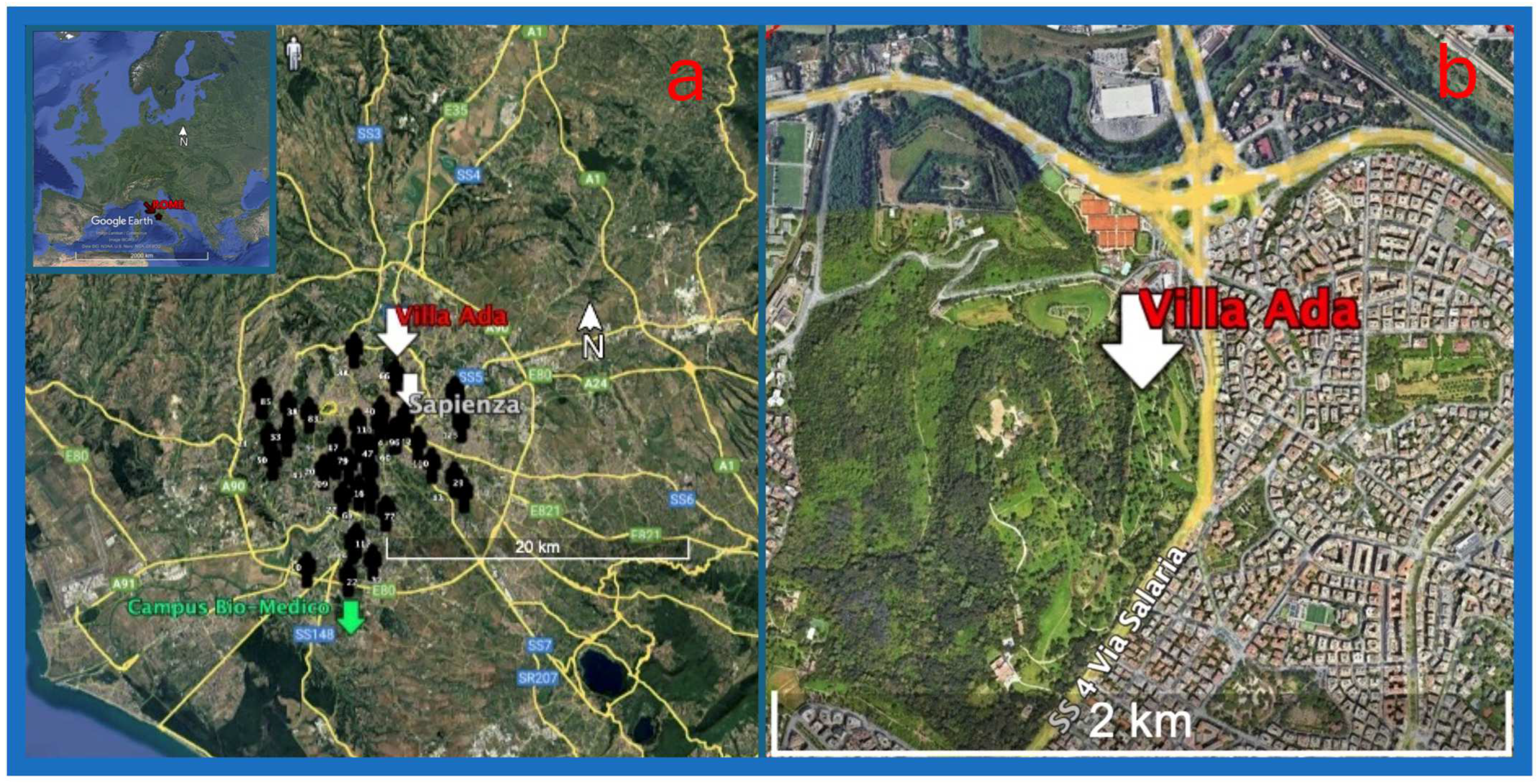
2.1. Sites
2.2. PM2.5 Modeling
2.3. Atmospheric Aerosol Measurements
2.3.1. Sampling Lines
2.3.2. Particle Number Size Distribution
2.3.3. Non-Refractory PM1 Chemical Characterization
2.3.4. Aerosol Optical Properties
2.3.5. BC and Urban Heat Island
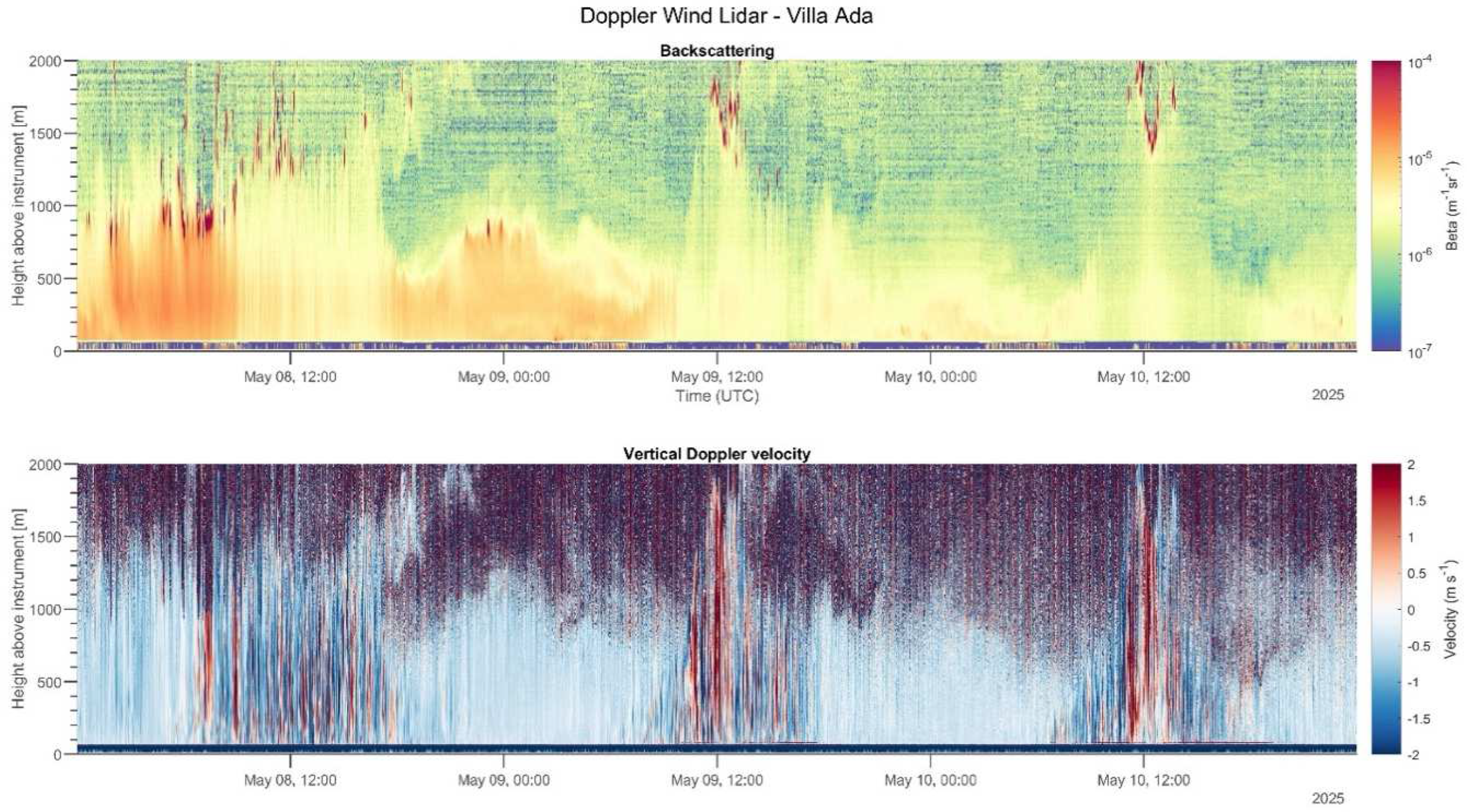
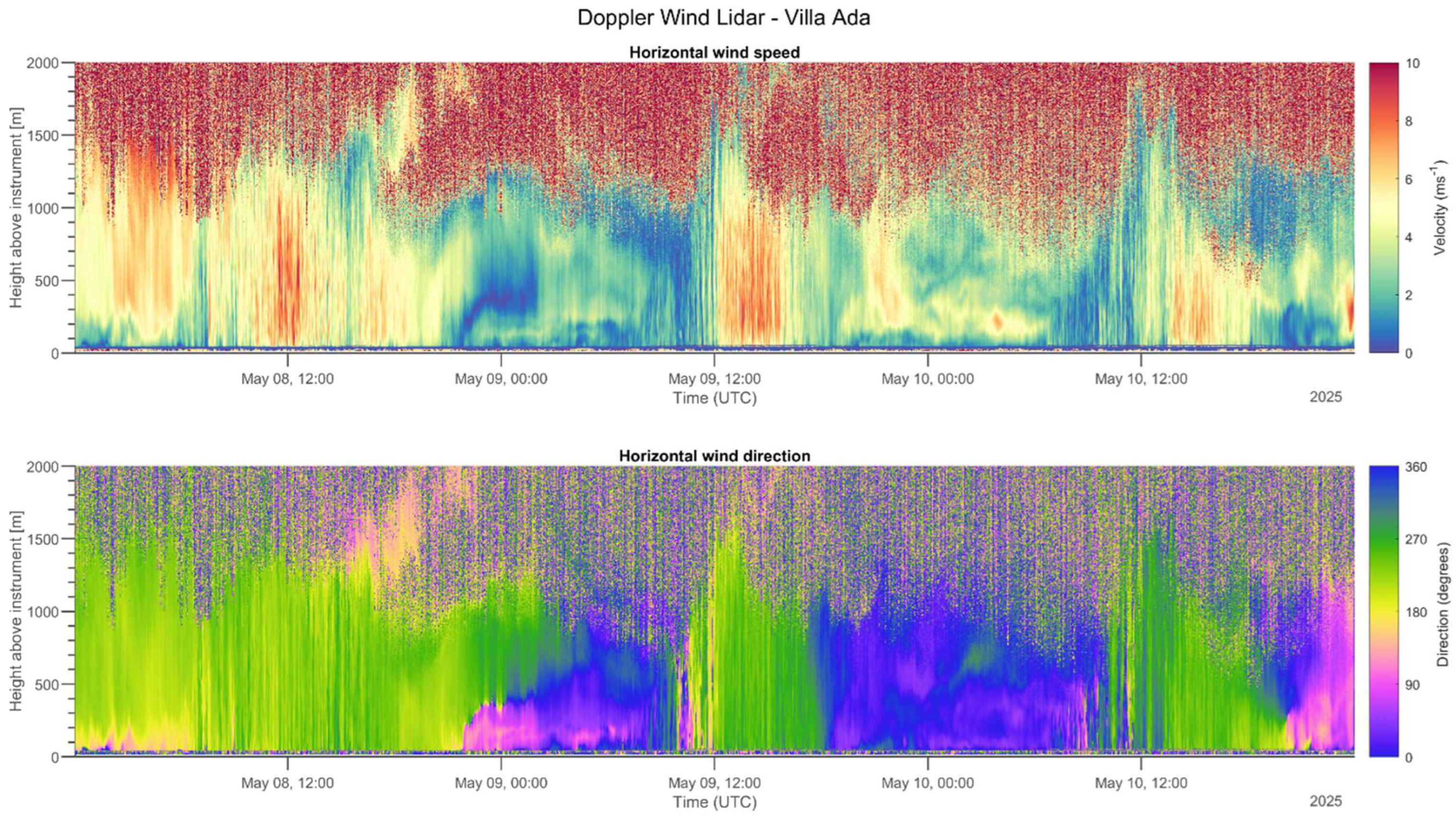
2.3.6. Aerosol Profiling
2.3.7. Insoluble and Soluble Metals
2.3.8. Bioaerosol in PM1
2.3.9. Indoor UFP
2.4. Particle-Bound Reactive Oxygen Species and Oxidative Potential
2.4.1. Particle-Bound ROS
2.4.2. Oxidative Potential
2.5. In Vitro Cell Lines
2.5.1. Cell Viability and Morphology
2.5.2. Gene Expression and miRNA
2.6. Cohorts Studies
2.6.1. The IBD/IBS Cohort for Assessing the Biodiversity of the Gut Microbiota
2.6.2. The EMERGE Cohort
2.7. Vegetation
3. Results
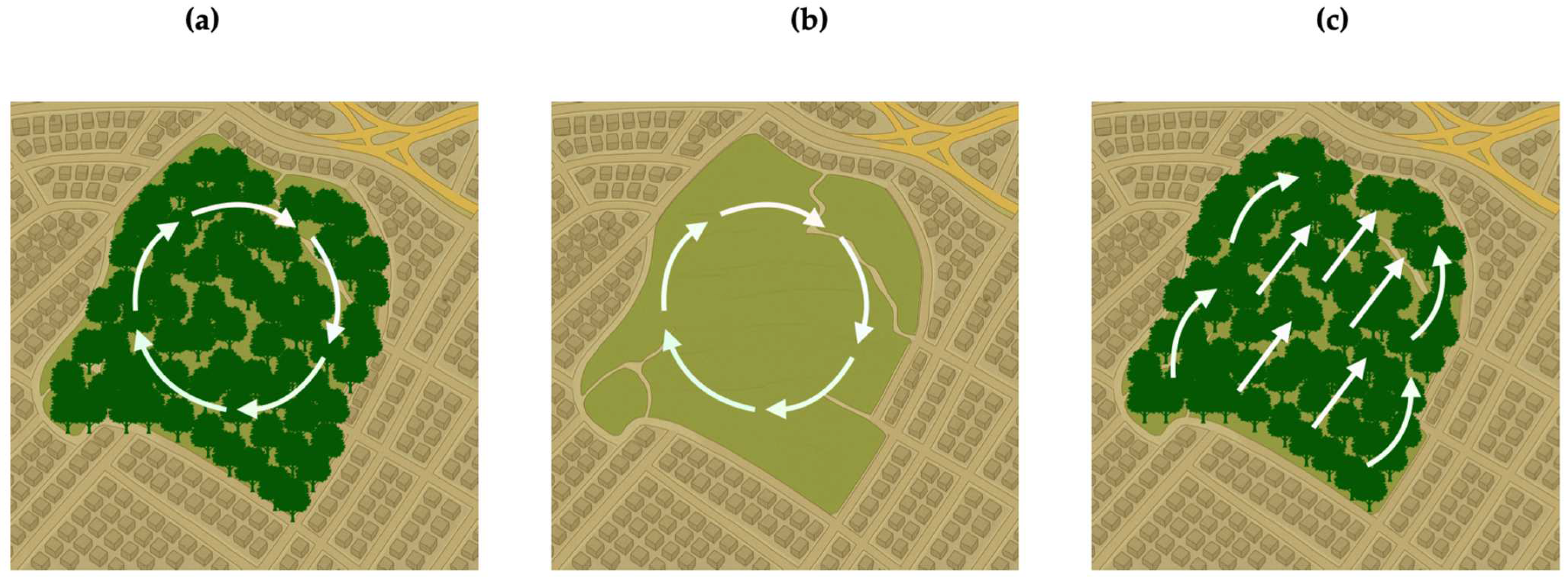
3.1. Four-Year Wind Patterns
3.2. PM2.5 Dynamics
3.2.1. Four-Year Statistics of PM2.5 and Associated Pollutants
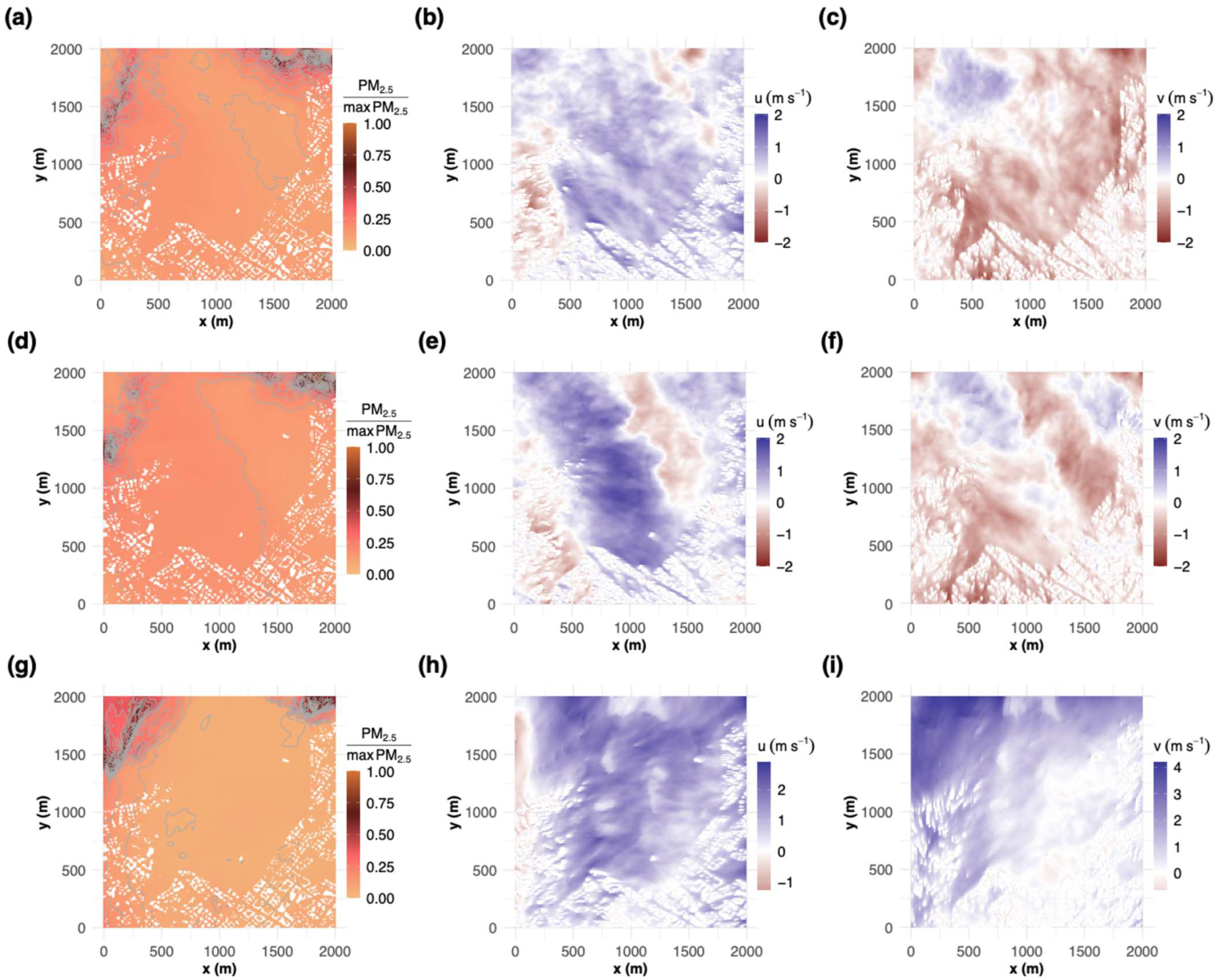
3.2.2. PM2.5 Modeling
3.3. Aerosol Profiling
3.4. Black Carbon
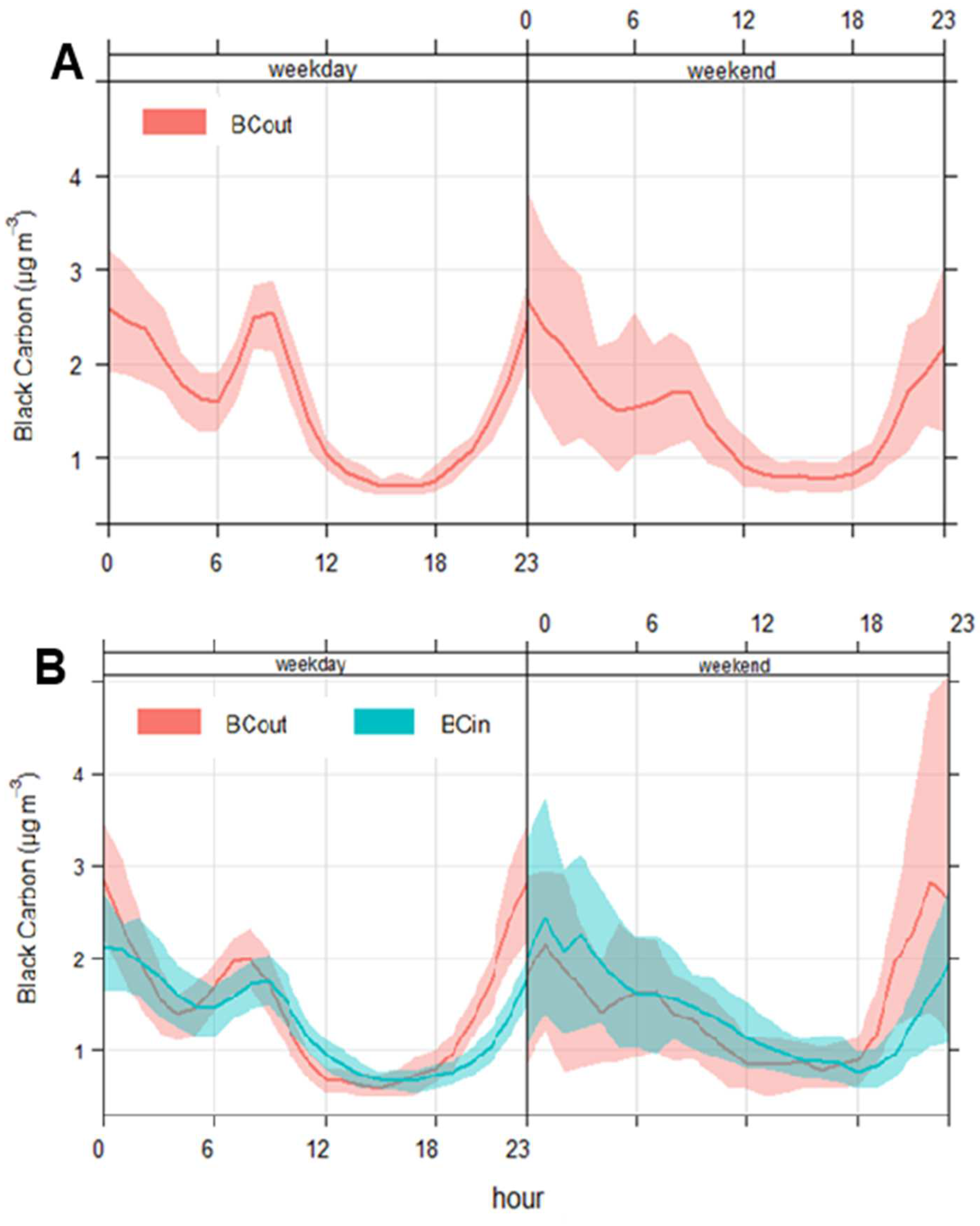
3.4.1. Four-Years BC Patterns and Source Apportionment
3.4.2. BC Indoors/Outdoors
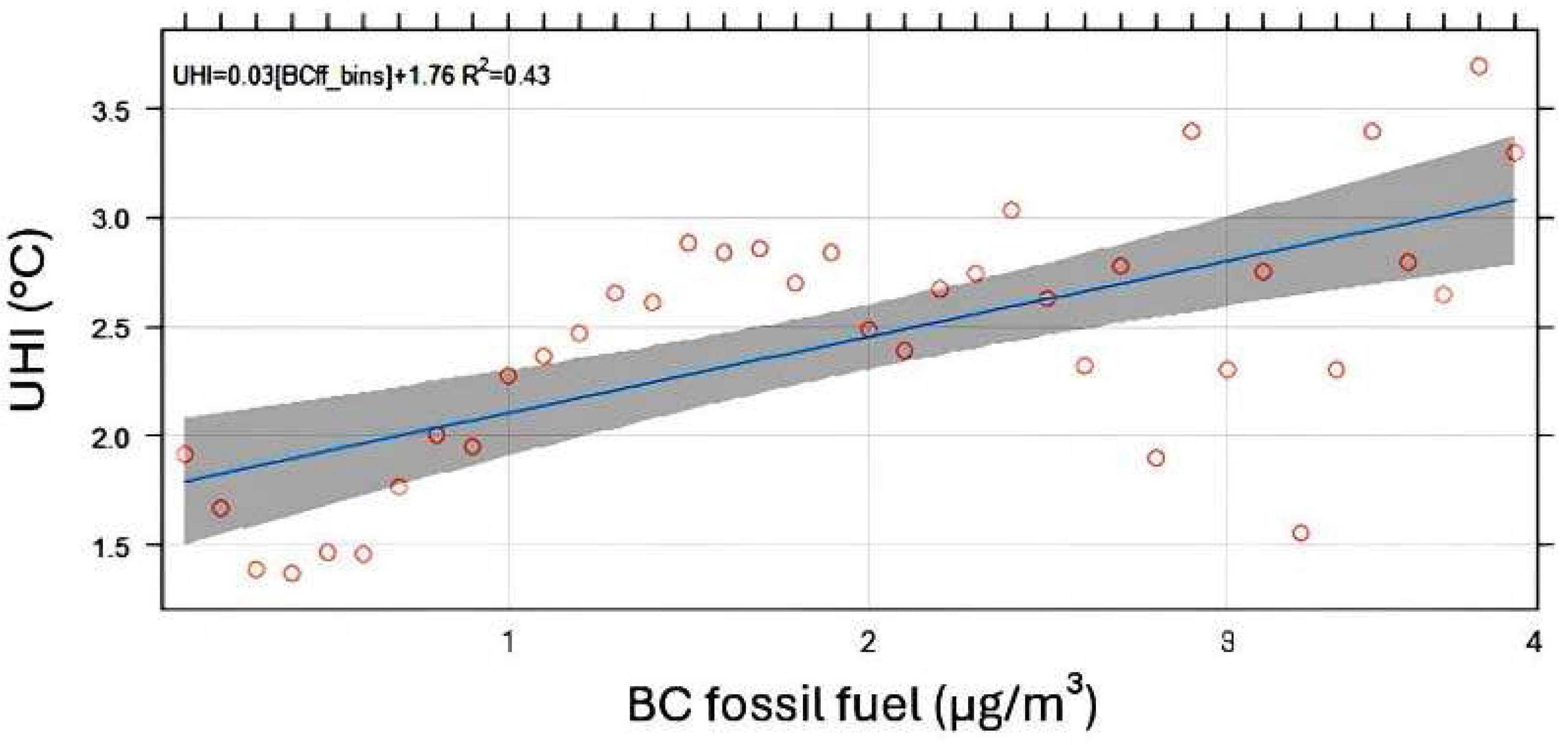
3.4.3. BC and UHI
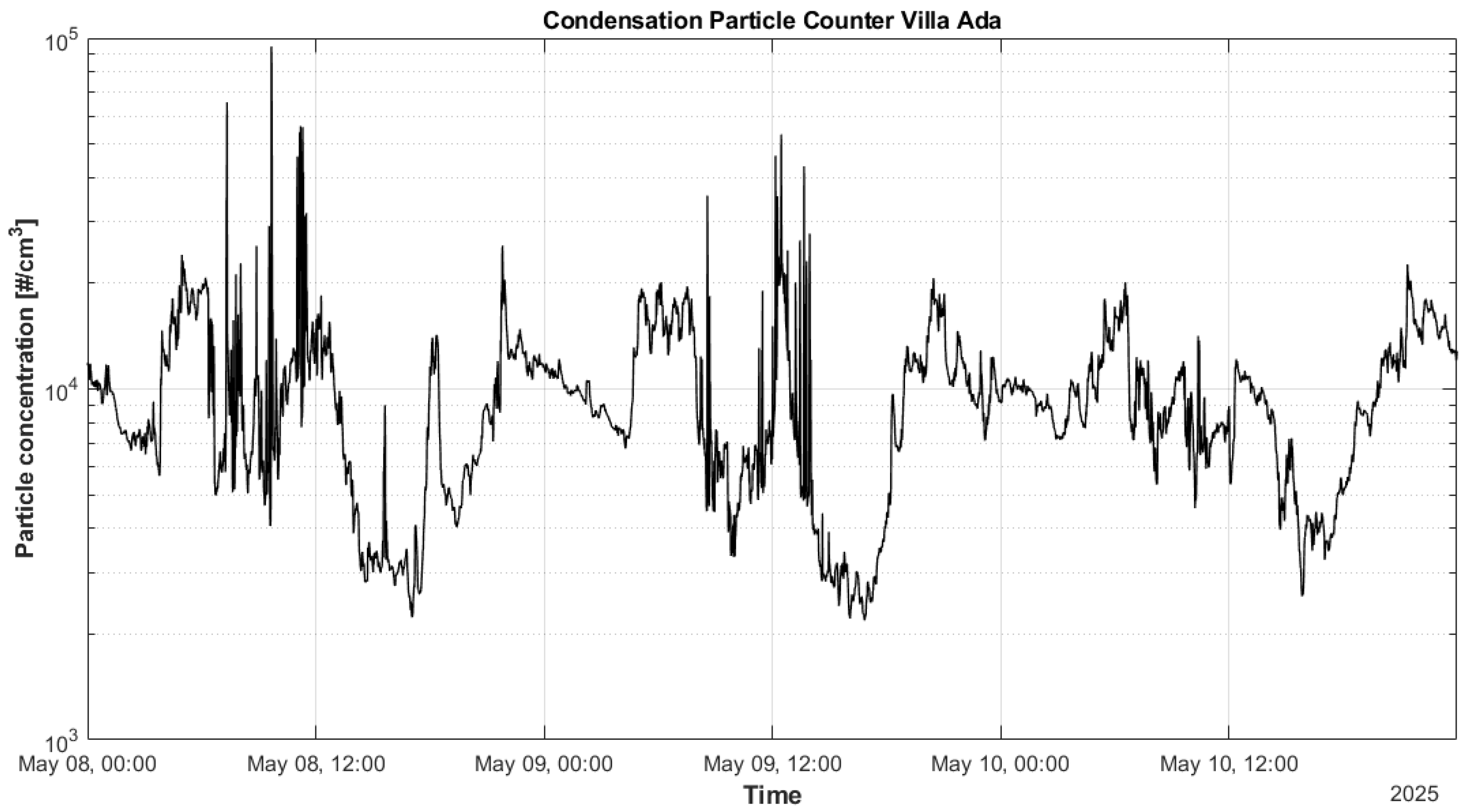
3.5. Total Particle Number Concentration
3.6. Acellular PB-ROS
3.7. Cell Viability and Morphology
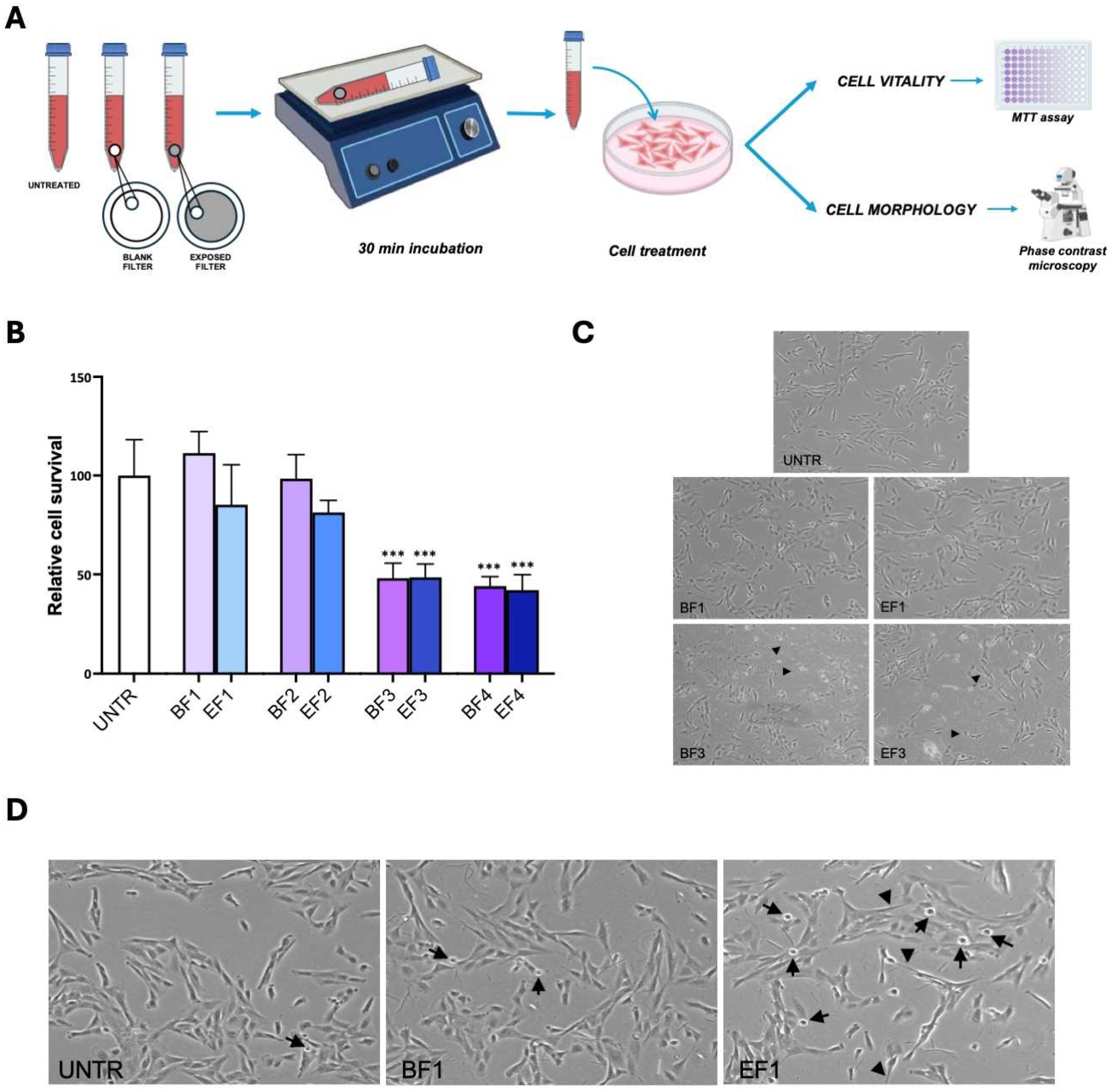
3.7.1. Extraction of Particulate Matter from Quartz Fiber Filters
3.7.2. Impact of Traffic-Related Aerosol Exposure on BEAS-2B Cell Viability
3.7.3. Assessment of Traffic-Related Aerosol Impact on Cell Morphology
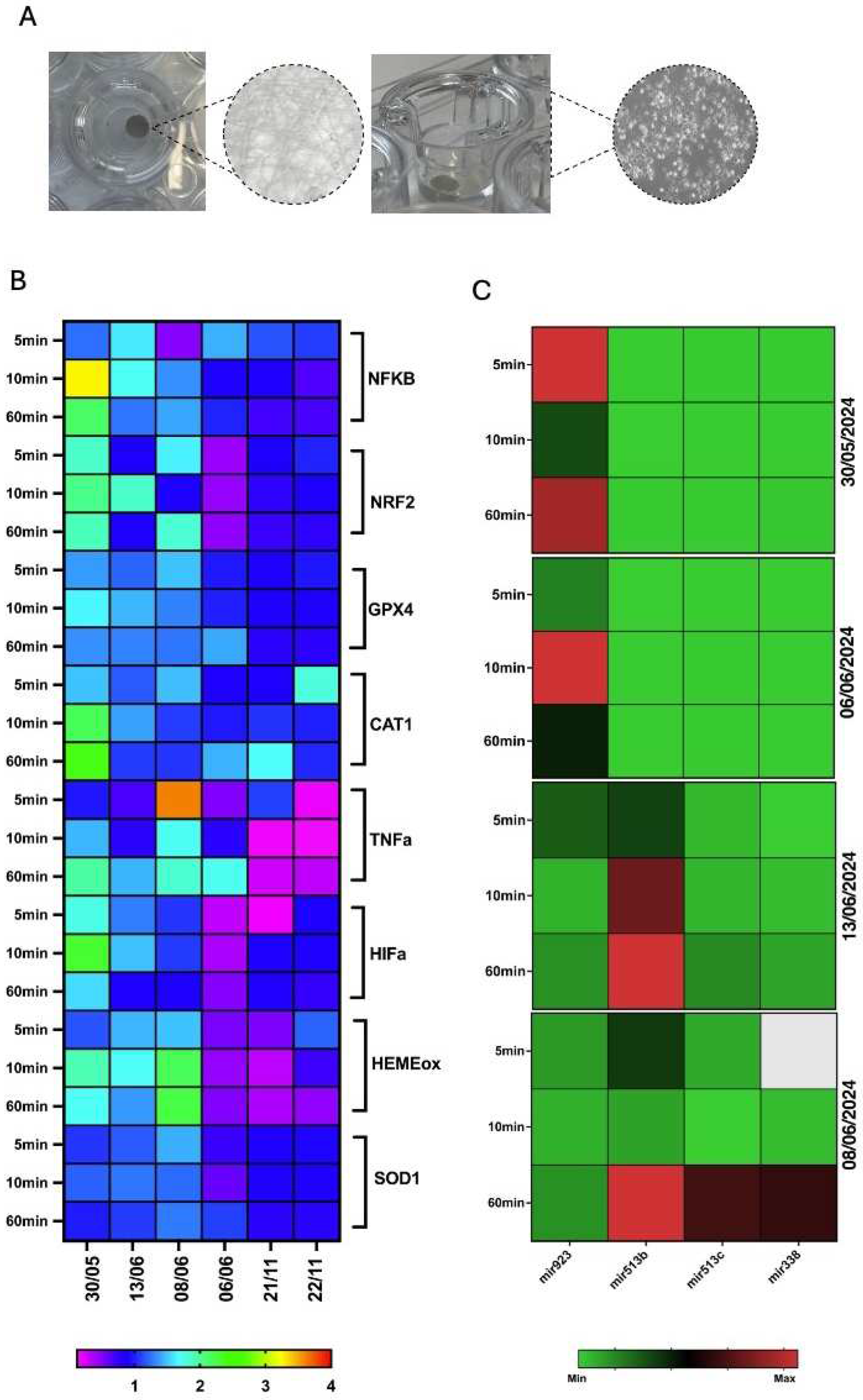
3.8. Epigenetic Markers of Oxidative Stress
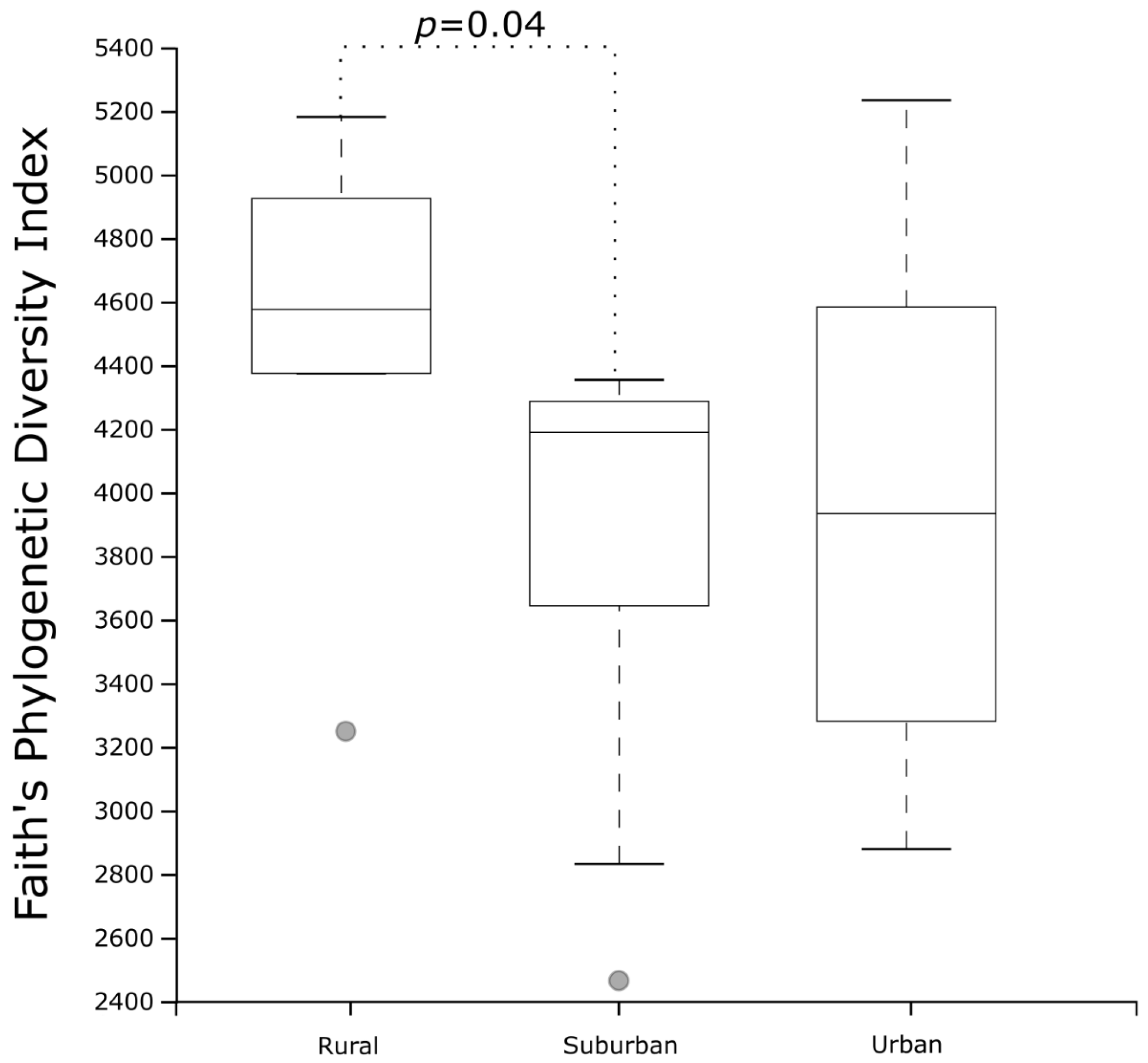
3.9. Microbiota and Urban Air Pollution Exposure
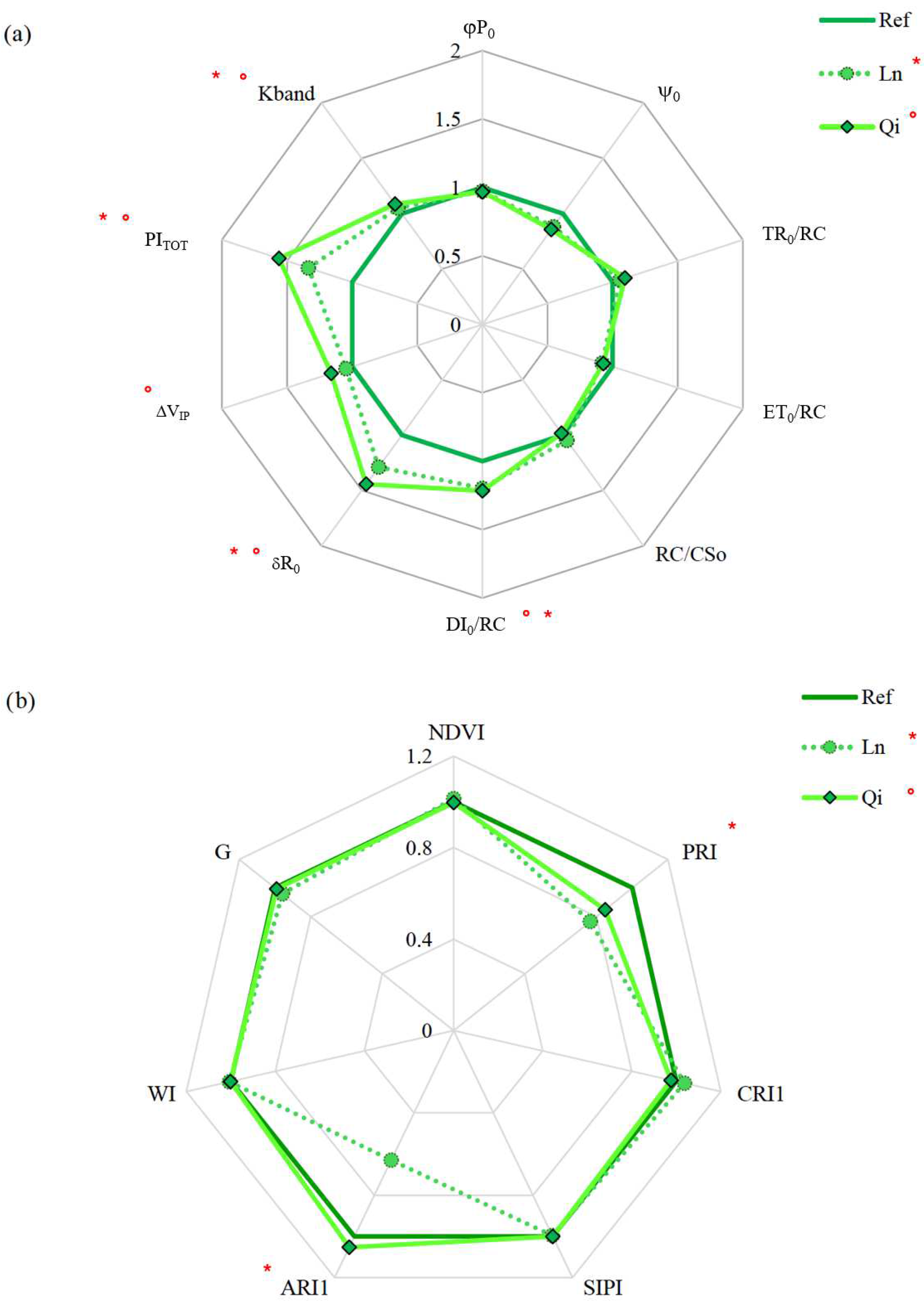
3.10. Vegetation
4. Discussion
- Defining the external urban exposome with a focus on the combination of atmospheric pollution and urban climate;
- Applying measurement and modeling tools to assess the exposome and the potential feedback;
- Investigating biological responses in humans by in vitro assays, gene and miRNA expression on cells (BEAS-2B cells), and gut microbiota diversity on human specimens (IBD/IBS cohort). Saliva biomarkers will be the focus of future work (EMERGE cohort);
- Investigating biological responses in plants, assessed by functional traits in Quercus ilex and Laurus nobilis (e.g., chlorophyll fluorescence, reflectance indices) as indicators of oxidative stress.
5. Conclusions
- Traffic-related pollution must be examined within the broader context of urban climate and ecosystem complexity.
- Exposure to urban aerosols, characterized by traffic emissions, induces a rapid and multi-phase oxidative and inflammatory response in bronchial epithelial cells, accompanied by extensive modulation of gene expression and miRNA profiles.
- Differences in gut microbiota diversity across urban, suburban, and rural settings suggest environmental influences on microbial composition.
- Functional trait alterations observed in urban trees exposed to roadside pollution point to early signs of oxidative and thermal stress.
- Through the application of measurements and modeling tools, this study highlights feedback mechanisms between vegetation and atmospheric conditions, where urban greenery not only mitigates pollution but also influences microclimate and exposure dynamics.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- HEI. State of Global Air 2024; Special Report; HEI: Boston, MA, USA, 2024. [Google Scholar]
- Lelieveld, J.; Haines, A.; Burnett, R.; Tonne, C.; Klingmüller, K.; Münzel, T.; Pozzer, A. Air Pollution Deaths Attributable to Fossil Fuels: Observational and Modelling Study. BMJ 2023, 383, e077784. [Google Scholar] [CrossRef]
- EEA Trends in EU Emissions of NH3, NMVOCs, NOx, SO2, Primary PM2.5, Primary PM10, BC and CO, Between 2005 and 2023. Available online: https://www.eea.europa.eu/en/analysis/publications/air-pollution-in-europe-2025-reporting-status-under-the-national-emission-reduction-commitments-directive/trends-in-eu-emissions-of-nh3-nmvocs-nox-so2-primary-pm2-5-primary-pm10-bc-and-co-between-2005-and-2023?activeTab=265e2bee-7de3-46e8-b6ee-76005f3f434f (accessed on 2 July 2025).
- Chen, J.; Hoek, G. Long-Term Exposure to PM and All-Cause and Cause-Specific Mortality: A Systematic Review and Meta-Analysis. Environ. Int. 2020, 143, 105974. [Google Scholar] [CrossRef]
- Gualtieri, M.; Melzi, G.; Costabile, F.; Stracquadanio, M.; La Torretta, T.; Di Iulio, G.; Petralia, E.; Rinaldi, M.; Paglione, M.; Decesari, S.; et al. On the Dose-Response Association of Fine and Ultrafine Particles in an Urban Atmosphere: Toxicological Outcomes on Bronchial Cells at Realistic Doses of Exposure at the Air Liquid Interface. Chemosphere 2024, 366, 143417. [Google Scholar] [CrossRef]
- Santoro, M.; Costabile, F.; Gualtieri, M.; Rinaldi, M.; Paglione, M.; Busetto, M.; Di Iulio, G.; Di Liberto, L.; Gherardi, M.; Pelliccioni, A.; et al. Associations between Fine Particulate Matter, Gene Expression, and Promoter Methylation in Human Bronchial Epithelial Cells Exposed within a Classroom under Air-Liquid Interface. Environ. Pollut. 2024, 358, 124471. [Google Scholar] [CrossRef] [PubMed]
- Costabile, F.; Gualtieri, M.; Rinaldi, M.; Canepari, S.; Vecchi, R.; Massimi, L.; Di Iulio, G.; Paglione, M.; Di Liberto, L.; Corsini, E.; et al. Exposure to Urban Nanoparticles at Low PM 1 Concentrations as a Source of Oxidative Stress and Inflammation. Sci. Rep. 2023, 13, 18616. [Google Scholar] [CrossRef] [PubMed]
- Weichenthal, S.; Christidis, T.; Olaniyan, T.; van Donkelaar, A.; Martin, R.; Tjepkema, M.; Burnett, R.T.; Brauer, M. Epidemiological Studies Likely Need to Consider PM2.5 Composition Even If Total Outdoor PM2.5 Mass Concentration Is the Exposure of Interest. Environ. Epidemiol. 2024, 8, e317. [Google Scholar] [CrossRef]
- Boogaard, H.; Crouse, D.L.; Tanner, E.; Mantus, E.; van Erp, A.M.; Vedal, S.; Samet, J. Assessing Adverse Health Effects of Long-Term Exposure to Low Levels of Ambient Air Pollution: The HEI Experience and What’s Next? Environ. Sci. Technol. 2024, 58, 12767–12783. [Google Scholar] [CrossRef]
- Cop28. Available online: https://www.cop28.com/en/ (accessed on 2 July 2025).
- Pöschl, U. Atmospheric Aerosols: Composition, Transformation, Climate and Health Effects. Angew. Chem. Int. Ed. 2005, 44, 7520–7540. [Google Scholar] [CrossRef]
- Rockström, J.; Gupta, J.; Qin, D.; Lade, S.J.; Abrams, J.F.; Andersen, L.S.; Armstrong McKay, D.I.; Bai, X.; Bala, G.; Bunn, S.E.; et al. Safe and Just Earth System Boundaries. Nature 2023, 619, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O.; Scholes, R.J.; Arneth, A.; Barnes, D.K.A.; Burrows, M.T.; Diamond, S.E.; Duarte, C.M.; Kiessling, W.; Leadley, P.; Managi, S.; et al. Overcoming the Coupled Climate and Biodiversity Crises and Their Societal Impacts. Science 2023, 380, eabl4881. [Google Scholar] [CrossRef]
- Cena, H.; Labra, M. Biodiversity and Planetary Health: A Call for Integrated Action. The Lancet 2024, 403, 1985–1986. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Bjørn, A.; Kılkış, Ş.; Sabag Muñoz, O.; Whiteman, G.; Hoff, H.; Seaby Andersen, L.; Rockström, J. How to Stop Cities and Companies Causing Planetary Harm. Nature 2022, 609, 463–466. [Google Scholar] [CrossRef]
- Münzel, T.; Sørensen, M.; Hahad, O.; Nieuwenhuijsen, M.; Daiber, A. The Contribution of the Exposome to the Burden of Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, R.; Schymanski, E.L.; Barabási, A.-L.; Miller, G.W. The Exposome and Health: Where Chemistry Meets Biology. Science 2020, 367, 392–396. [Google Scholar] [CrossRef]
- Hong, S.; Hui, E.C.M.; Lin, Y. Relationship between Urban Spatial Structure and Carbon Emissions: A Literature Review. Ecol. Indic. 2022, 144, 109456. [Google Scholar] [CrossRef]
- Giammona, A.; Remedia, S.; Porro, D.; Lo Dico, A.; Bertoli, G. The Biological Interplay between Air Pollutants and MiRNAs Regulation in Cancer. Front. Cell Dev. Biol. 2024, 12, 1343385. [Google Scholar] [CrossRef]
- Vriens, A.; Nawrot, T.S.; Saenen, N.D.; Provost, E.B.; Kicinski, M.; Lefebvre, W.; Vanpoucke, C.; Van Deun, J.; De Wever, O.; Vrijens, K.; et al. Recent Exposure to Ultrafine Particles in School Children Alters MIR-222 Expression in the Extracellular Fraction of Saliva. Environ. Health 2016, 15, 80. [Google Scholar] [CrossRef]
- Dennis, K.K.; Marder, E.; Balshaw, D.M.; Cui, Y.; Lynes, M.A.; Patti, G.J.; Rappaport, S.M.; Shaughnessy, D.T.; Vrijheid, M.; Barr, D.B. Biomonitoring in the Era of the Exposome. Environ. Health Perspect. 2017, 125, 502–510. [Google Scholar] [CrossRef]
- Demetriou, C.; Vineis, P. Biomarkers and Omics of Health Effects Associated with Traffic-Related Air Pollution. In Traffic-Related Air Pollution; Elsevier: Amsterdam, The Netherlands, 2020; pp. 281–309. ISBN 9780128181225. [Google Scholar]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-Omics of the Gut Microbial Ecosystem in Inflammatory Bowel Diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Hajiagha, M.N.; Taghizadeh, S.; Asgharzadeh, M.; Dao, S.; Ganbarov, K.; Köse, Ş.; Kafil, H.S. Gut Microbiota and Human Body Interactions; Its Impact on Health: A Review. Curr. Pharm. Biotechnol. 2022, 23, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Corada, K.; Woodward, H.; Alaraj, H.; Collins, C.M.; de Nazelle, A. A Systematic Review of the Leaf Traits Considered to Contribute to Removal of Airborne Particulate Matter Pollution in Urban Areas. Environ. Pollut. 2021, 269, 116104. [Google Scholar] [CrossRef]
- Fusaro, L.; Nardella, L.; Manes, F.; Sebastiani, A.; Fares, S. Supply and Demand Mismatch Analysis to Improve Regulating Ecosystem Services in Mediterranean Urban Areas: Insights from Four Italian Municipalities. Ecol. Indic. 2023, 155, 110928. [Google Scholar] [CrossRef]
- Sharma, P.; Saxena, P. Impacts and Responses of Particulate Matter Pollution on Vegetation. In Airborne Particulate Matter: Source, Chemistry and Health; Sonwani, S., Shukla, A., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 229–264. ISBN 978-981-16-5387-2. [Google Scholar]
- Popek, R.; Przybysz, A.; Gawrońska, H.; Klamkowski, K.; Gawroński, S.W. Impact of Particulate Matter Accumulation on the Photosynthetic Apparatus of Roadside Woody Plants Growing in the Urban Conditions. Ecotoxicol. Environ. Saf. 2018, 163, 56–62. [Google Scholar] [CrossRef]
- Maronga, B.; Banzhaf, S.; Burmeister, C.; Esch, T.; Forkel, R.; Fröhlich, D.; Fuka, V.; Gehrke, K.F.; Geletič, J.; Giersch, S.; et al. Overview of the PALM Model System 6.0. Geosci. Model Dev. 2020, 13, 1335–1372. [Google Scholar] [CrossRef]
- Davolio, S.; Malguzzi, P.; Drofa, O.; Mastrangelo, D.; Buzzi, A. The Piedmont Flood of November 1994: A Testbed of Forecasting Capabilities of the CNR-ISAC Meteorological Model Suite. Bull. Atmos. Sci. Technol. 2020, 1, 263–282. [Google Scholar] [CrossRef]
- Wiedensohler, A.; Wiesner, A.; Weinhold, K.; Birmili, W.; Hermann, M.; Merkel, M.; Müller, T.; Pfeifer, S.; Schmidt, A.; Tuch, T.; et al. Mobility Particle Size Spectrometers: Calibration Procedures and Measurement Uncertainties. Aerosol Sci. Technol. 2018, 52, 146–164. [Google Scholar] [CrossRef]
- Hinds, W.C. Aerosol Tecnology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1999; ISBN 0-471-19410-7. [Google Scholar]
- Ng, N.L.; Herndon, S.C.; Trimborn, A.; Canagaratna, M.R.; Croteau, P.L.; Onasch, T.B.; Sueper, D.; Worsnop, D.R.; Zhang, Q.; Sun, Y.L.; et al. An Aerosol Chemical Speciation Monitor (ACSM) for Routine Monitoring of the Composition and Mass Concentrations of Ambient Aerosol. Aerosol Sci. Technol. 2011, 45, 780–794. [Google Scholar] [CrossRef]
- Freney, E.; Zhang, Y.; Croteau, P.; Amodeo, T.; Williams, L.; Truong, F.; Petit, J.-E.; Sciare, J.; Sarda-Esteve, R.; Bonnaire, N.; et al. The Second ACTRIS Inter-Comparison (2016) for Aerosol Chemical Speciation Monitors (ACSM): Calibration Protocols and Instrument Performance Evaluations. Aerosol Sci. Technol. 2019, 53, 830–842. [Google Scholar] [CrossRef]
- Petzold, A.; Ogren, J.A.; Fiebig, M.; Laj, P.; Li, S.-M.; Baltensperger, U.; Holzer-Popp, T.; Kinne, S.; Pappalardo, G.; Sugimoto, N.; et al. Recommendations for Reporting “Black Carbon” Measurements. Atmos. Chem. Phys. 2013, 13, 8365–8379. [Google Scholar] [CrossRef]
- Sandradewi, J.; Prévôt, A.S.H.; Weingartner, E.; Schmidhauser, R.; Gysel, M.; Baltensperger, U. A Study of Wood Burning and Traffic Aerosols in an Alpine Valley Using a Multi-Wavelength Aethalometer. Atmos. Environ. 2008, 42, 101–112. [Google Scholar] [CrossRef]
- Peng, J.; Hu, M.; Guo, S.; Du, Z.; Zheng, J.; Shang, D.; Zamora, M.L.; Zeng, L.; Shao, M.; Wu, Y.S.; et al. Markedly Enhanced Absorption and Direct Radiative Forcing of Black Carbon under Polluted Urban Environments. Proc. Natl. Acad. Sci. USA 2016, 113, 4266–4271. [Google Scholar] [CrossRef]
- Wang, F.; Carmichael, G.R.; Wang, J.; Chen, B.; Huang, B.; Li, Y.; Yang, Y.; Gao, M. Circulation-Regulated Impacts of Aerosol Pollution on Urban Heat Island in Beijing. Atmos. Chem. Phys. 2022, 22, 13341–13353. [Google Scholar] [CrossRef]
- Schatz, J.; Kucharik, C.J. Urban Climate Effects on Extreme Temperatures in Madison, Wisconsin, USA. Environ. Res. Lett. 2015, 10, 094024. [Google Scholar] [CrossRef]
- Cecilia, A.; Casasanta, G.; Petenko, I.; Conidi, A.; Argentini, S. Measuring the Urban Heat Island of Rome through a Dense Weather Station Network and Remote Sensing Imperviousness Data. Urban Clim. 2023, 47, 101355. [Google Scholar] [CrossRef]
- Manninen, A.J.; Marke, T.; Tuononen, M.; O’Connor, E.J. Atmospheric Boundary Layer Classification With Doppler Lidar. J. Geophys. Res. Atmos. 2018, 123, 8172–8189. [Google Scholar] [CrossRef]
- Massimi, L.; Ristorini, M.; Simonetti, G.; Frezzini, M.A.; Astolfi, M.L.; Canepari, S. Spatial Mapping and Size Distribution of Oxidative Potential of Particulate Matter Released by Spatially Disaggregated Sources. Environ. Pollut. 2020, 266, 115271. [Google Scholar] [CrossRef]
- Canepari, S.; Pietrodangelo, A.; Perrino, C.; Astolfi, M.L.; Marzo, M.L. Enhancement of Source Traceability of Atmospheric PM by Elemental Chemical Fractionation. Atmos. Environ. 2009, 43, 4754–4765. [Google Scholar] [CrossRef]
- Heavens, D.; Chooneea, D.; Giolai, M.; Cuber, P.; Aanstad, P.; Martin, S.; Alston, M.; Misra, R.; Clark, M.D.; Leggett, R.M. How Low Can You Go? Driving down the DNA Input Requirements for Nanopore Sequencing. bioRxiv 2021. [Google Scholar] [CrossRef]
- Leggett, R.M.; Alcon-Giner, C.; Heavens, D.; Caim, S.; Brook, T.C.; Kujawska, M.; Martin, S.; Peel, N.; Acford-Palmer, H.; Hoyles, L.; et al. Rapid MinION Profiling of Preterm Microbiota and Antimicrobial-Resistant Pathogens. Nat. Microbiol. 2020, 5, 430–442. [Google Scholar] [CrossRef]
- Peel, N.; Martin, S.; Heavens, D.; Yu, D.W.; Clark, M.D.; Leggett, R.M. MARTi: A Real-Time Analysis and Visualisation Tool for Nanopore Metagenomics. bioRxiv 2025. [Google Scholar] [CrossRef]
- Orsini, D.A.; Ma, Y.; Sullivan, A.; Sierau, B.; Baumann, K.; Weber, R.J. Refinements to the Particle-into-Liquid Sampler (PILS) for Ground and Airborne Measurements of Water Soluble Aerosol Composition. Atmos. Environ. 2003, 37, 1243–1259. [Google Scholar] [CrossRef]
- Di Iulio, G.; Gualtieri, M.; Rinaldi, M.; Paglione, M.; Canepari, S.; Massimi, L.; Frezzini, M.A.; Pasqualini, F.; Sirignano, C.; Costabile, F. Association of PILS-Based and Filter-Based Particle-Bound Reactive Oxygen Species with Urban Nanoparticles, Secondary Organic Aerosols, and in-Vitro Oxidative Responses. Environ. Pollut. 2025, 385, 126874. [Google Scholar] [CrossRef]
- Sorooshian, A.; Brechtel, F.J.; Ma, Y.; Weber, R.J.; Corless, A.; Flagan, R.C.; Seinfeld, J.H. Modeling and Characterization of a Particle-into-Liquid Sampler (PILS). Aerosol Sci. Technol. 2006, 40, 396–409. [Google Scholar] [CrossRef]
- Gao, D.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J. A Method for Measuring Total Aerosol Oxidative Potential (OP) with the Dithiothreitol (DTT) Assay and Comparisons between an Urban and Roadside Site of Water-Soluble and Total OP. Atmos. Meas. Tech. 2017, 10, 2821–2835. [Google Scholar] [CrossRef]
- Giammona, A.; Gervasoni, C.; Di Iulio, G.; Sirignano, C.; Listrani, S.; Rinaldi, M.; Canepari, S.; Lo Dico, A.; Costabile, F.; Bertoli, G. Short-Term Exposure of Human Bronchial Epithelial Cell Lines to the “Oxidant” Urban Air Pollution. Environ. Toxicol. Pharmacol. 2025. under review. [Google Scholar]
- Filardo, S.; Scalese, G.; Virili, C.; Pontone, S.; Di Pietro, M.; Covelli, A.; Bedetti, G.; Marinelli, P.; Bruno, G.; Stramazzo, I.; et al. The Potential Role of Hypochlorhydria in the Development of Duodenal Dysbiosis: A Preliminary Report. Front. Cell. Infect. Microbiol. 2022, 12, 854904. [Google Scholar] [CrossRef]
- Alessio, M.; Anselmi, S.; Conforto, L.; Improta, S.; Manes, F.; Manfra, L. Radiocarbon as a Biomarker of Urban Pollution in Leaves of Evergreen Species Sampled in Rome and in Rural Areas (Lazio—Central Italy). Atmos. Environ. 2002, 36, 5405–5416. [Google Scholar] [CrossRef]
- Fusaro, L.; Salvatori, E.; Winkler, A.; Frezzini, M.A.; De Santis, E.; Sagnotti, L.; Canepari, S.; Manes, F. Urban Trees for Biomonitoring Atmospheric Particulate Matter: An Integrated Approach Combining Plant Functional Traits, Magnetic and Chemical Properties. Ecol. Indic. 2021, 126, 107707. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in Vivo Recording of Prompt and Delayed Fluorescence and 820-Nm Reflection Changes during Drying and after Rehydration of the Resurrection Plant Haberlea Rhodopensis. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Struckmeier, C.; Drewnick, F.; Fachinger, F.; Paolo Gobbi, G.; Borrmann, S. Atmospheric aerosols in Rome, Italy: Sources, dynamics and spatial variations during two seasons. Atmos. Chem. Phys. 2016, 16, 15277–15299. [Google Scholar] [CrossRef]
- EEA Air Quality Statistics. Available online: https://www.eea.europa.eu/en/analysis/maps-and-charts/air-quality-statistics-dashboards (accessed on 19 February 2025).
- Kotthaus, S.; Bravo-Aranda, J.A.; Collaud Coen, M.; Guerrero-Rascado, J.L.; Costa, M.J.; Cimini, D.; O’Connor, E.J.; Hervo, M.; Alados-Arboledas, L.; Jiménez-Portaz, M.; et al. Atmospheric Boundary Layer Height from Ground-Based Remote Sensing: A Review of Capabilities and Limitations. Atmos. Meas. Tech. 2023, 16, 433–479. [Google Scholar] [CrossRef]
- Shrestha, B.; Brotzge, J.A.; Wang, J. Observations and Impacts of Long-Range Transported Wildfire Smoke on Air Quality Across New York State During July 2021. Geophys. Res. Lett. 2022, 49, e2022GL100216. [Google Scholar] [CrossRef]
- Santinami, E.; Alberti, T.; Casasanta, G.; Costabile, F.; Di Iulio, G.; Reda, R.; Giovannelli, L. A Novel Methodological Approach Using EMD for a Multi-Scale Analysis on Black Carbon Aerosols and Urban Heat Island. Atmos. Meas. Tech. 2025. submitted. [Google Scholar]
- Xin, X.; Gong, T.; Hong, Y. Hydrogen Peroxide Initiates Oxidative Stress and Proteomic Alterations in Meningothelial Cells. Sci Rep 2022, 12, 14519. [Google Scholar] [CrossRef] [PubMed]
- Shokolenko, I.N.; Wilson, G.L.; Alexeyev, M.F. The “Fast” and the “Slow” Modes of Mitochondrial DNA Degradation. Mitochondrial DNA Part A 2016, 27, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Pollastrini, M.; Salvatori, E.; Fusaro, L.; Manes, F.; Marzuoli, R.; Gerosa, G.; Brüggemann, W.; Strasser, R.J.; Bussotti, F. Selection of Tree Species for Forests under Climate Change: Is PSI Functioning a Better Predictor for Net Photosynthesis and Growth than PSII? Tree Physiol. 2020, 40, 1561–1571. [Google Scholar] [CrossRef]
- Salvatori, E.; Fusaro, L.; Mereu, S.; Bernardini, A.; Puppi, G.; Manes, F. Different O3 Response of Sensitive and Resistant Snap Bean Genotypes (Phaseolus vulgaris L.): The Key Role of Growth Stage, Stomatal Conductance, and PSI Activity. Environ. Exp. Bot. 2013, 87, 79–91. [Google Scholar] [CrossRef]
- Joliot, P.; Johnson, G.N. Regulation of Cyclic and Linear Electron Flow in Higher Plants. Proc. Natl. Acad. Sci. USA 2011, 108, 13317–13322. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Húdoková, H.; Petrik, P.; Petek-Petrik, A.; Konôpková, A.; Leštianska, A.; Střelcová, K.; Kmeť, J.; Kurjak, D. Heat-Stress Response of Photosystem II in Five Ecologically Important Tree Species of European Temperate Forests. Biologia 2022, 77, 671–680. [Google Scholar] [CrossRef]
- Costabile, F.; Gualtieri, M.; Canepari, S.; Tranfo, G.; Consales, C.; Grollino, M.G.; Paci, E.; Petralia, E.; Pigini, D.; Simonetti, G. Evidence of Association between Aerosol Properties and In-Vitro Cellular Oxidative Response to PM1, Oxidative Potential of PM2.5, a Biomarker of RNA Oxidation, and Its Dependency on Combustion Sources. Atmos. Environ. 2019, 213, 444–455. [Google Scholar] [CrossRef]
- Costabile, F.; Alas, H.; Aufderheide, M.; Avino, P.; Amato, F.; Argentini, S.; Barnaba, F.; Berico, M.; Bernardoni, V.; Biondi, R.; et al. First Results of the “Carbonaceous Aerosol in Rome and Environs (CARE)” Experiment: Beyond Current Standards for PM10. Atmosphere 2017, 8, 249. [Google Scholar] [CrossRef]
- Altomare, A.; Del Chierico, F.; Rocchi, G.; Emerenziani, S.; Nuglio, C.; Putignani, L.; Angeletti, S.; Presti, A.L.; Ciccozzi, M.; Russo, A.; et al. Association between Dietary Habits and Fecal Microbiota Composition in Irritable Bowel Syndrome Patients: A Pilot Study. Nutrients 2021, 13, 1479. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Liu, Y.B.; Peng, Y.; Peng, J.; Ma, Q.L. Causal Effect of Air Pollution on the Risk of Cardiovascular and Metabolic Diseases and Potential Mediation by Gut Microbiota. Sci. Total Environ. 2024, 912, 169418. [Google Scholar] [CrossRef]
- Sgrigna, G.; Sæbø, A.; Gawronski, S.; Popek, R.; Calfapietra, C. Particulate Matter Deposition on Quercus Ilex Leaves in an Industrial City of Central Italy. Environ. Pollut. 2015, 197, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, H.; Tang, N.; Zhou, S.; Yu, J.; Ding, B. Spider-Web-Inspired PM0.3 Filters Based on Self-Sustained Electrostatic Nanostructured Networks. Adv. Mater. 2020, 32, e2002361. [Google Scholar] [CrossRef]
- Kim, H.; Oh, J.; Lee, H.; Jeong, S.; Ko, S.H. Next-Generation Air Filtration Nanotechnology for Improved Indoor Air Quality. Chem. Commun. 2025, 61, 1322–1341. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Wang, J.; Zhou, R.; Lu, Z.; Wang, L.; Ning, X. Polyphenylene Sulfide Fabric with Enhanced Oxidation Resistance and Hydrophobicity through Polybenzoxazine Surface Coating for Emission Control in Harsh Environment. J. Hazard. Mater. 2022, 432, 128735. [Google Scholar] [CrossRef]
- Memon, R.A.; Leung, D.Y.C. On the Heating Environment in Street Canyon. Environ. Fluid Mech. 2011, 11, 465–480. [Google Scholar] [CrossRef]
- Rai, P.K. Impacts of Particulate Matter Pollution on Plants: Implications for Environmental Biomonitoring. Ecotoxicol. Environ. Saf. 2016, 129, 120–136. [Google Scholar] [CrossRef]
- Levy, J.I.; Buonocore, J.J.; von Stackelberg, K. Evaluation of the Public Health Impacts of Traffic Congestion: A Health Risk Assessment. Environ. Health 2010, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.; Jiang, L.; Li, T.; Guo, J.; Wei, T.; Crowther, R. Environmental and Health Impacts of Banning Passenger Cars with Internal Combustion Engines: A Case Study of Leeds, UK. Transp. Res. D Transp. Environ. 2024, 134, 104343. [Google Scholar] [CrossRef]




| Pollutant | Season | Mean | SD | p25 | p75 |
|---|---|---|---|---|---|
| PM1 (μg/m3) | Spring (n = 329) | 7 | 3 | 4 | 8 |
| Summer (n = 353) | 5 | 2 | 4 | 6 | |
| Fall (n = 345) | 7 | 2 | 5 | 8 | |
| Winter (n = 326) | 8 | 4 | 5 | 11 | |
| PM2.5 (μg/m3) | Spring (n = 333) | 11 | 5 | 7 | 14 |
| Summer (n = 351) | 10 | 5 | 7 | 12 | |
| Fall (n = 348) | 11 | 4 | 8 | 13 | |
| Winter (n = 331) | 16 | 9 | 9 | 23 | |
| NO2 (μg/m3) | Spring (n = 7841) | 24 | 15 | 13 | 32 |
| Summer (n = 6259) | 19 | 11 | 11 | 26 | |
| Fall (n = 7298) | 17 | 12 | 8 | 23 | |
| Winter (n = 7265) | 26 | 14 | 15 | 34 | |
| CO (mg/m3) | Spring (n = 8331) | 0.5 | 0.2 | 0.3 | 0.6 |
| Summer (n = 6528) | 0.5 | 0.2 | 0.4 | 0.7 | |
| Fall (n = 7624) | 0.4 | 0.2 | 0.2 | 0.5 | |
| Winter (n = 7686) | 0.6 | 0.3 | 0.4 | 0.7 | |
| O3 (μg/m3) | Spring (n = 8206) | 34 | 32 | 4 | 59 |
| Summer (n = 6568) | 42 | 29 | 15 | 65 | |
| Fall (n = 7546) | 59 | 37 | 24 | 87 | |
| Winter (n = 7694) | 21 | 21 | 2 | 38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirignano, C.; Brondani, D.D.V.; Iulio, G.D.; Anselmi, C.; Argentini, S.; Bracci, A.; Calfapietra, C.; Canepari, S.; Casasanta, G.; Cattani, G.; et al. Urban Health Assessment Through a Planetary Health Perspective: Methods and First Results from the Rome NBFC Experiment. Atmosphere 2025, 16, 1144. https://doi.org/10.3390/atmos16101144
Sirignano C, Brondani DDV, Iulio GD, Anselmi C, Argentini S, Bracci A, Calfapietra C, Canepari S, Casasanta G, Cattani G, et al. Urban Health Assessment Through a Planetary Health Perspective: Methods and First Results from the Rome NBFC Experiment. Atmosphere. 2025; 16(10):1144. https://doi.org/10.3390/atmos16101144
Chicago/Turabian StyleSirignano, Carmina, Daiane De Vargas Brondani, Gianluca Di Iulio, Chiara Anselmi, Stefania Argentini, Alessandro Bracci, Carlo Calfapietra, Silvia Canepari, Giampietro Casasanta, Giorgio Cattani, and et al. 2025. "Urban Health Assessment Through a Planetary Health Perspective: Methods and First Results from the Rome NBFC Experiment" Atmosphere 16, no. 10: 1144. https://doi.org/10.3390/atmos16101144
APA StyleSirignano, C., Brondani, D. D. V., Iulio, G. D., Anselmi, C., Argentini, S., Bracci, A., Calfapietra, C., Canepari, S., Casasanta, G., Cattani, G., Ceccarelli, S., Cena, H., Landi, T. C., Coluzzi, R., De Giuseppe, R., Decesari, S., Di Cicco, A., Di Giosa, A. D., Di Liberto, L., ... Costabile, F. (2025). Urban Health Assessment Through a Planetary Health Perspective: Methods and First Results from the Rome NBFC Experiment. Atmosphere, 16(10), 1144. https://doi.org/10.3390/atmos16101144




















