Abstract
Sand events continue to occur frequently and affect the North China region. Under unfavorable meteorological conditions, they can easily combine with haze pollution, forming sandy haze events that have a significant impact on human health. Aerosol water content (AWC) is known to have a significant impact on PM2.5, but its effect is still unclear in sandy haze. In this work, sandy haze and haze periods were observed in Zhengzhou using a series of high-time-resolution instruments. The AWC calculated by the ISORROPIA-II model reached 11 ± 5 μg m−3, accounting for 10% of the PM2.5, in the sandy haze period. Sensitivity tests show that AWC was mainly relative humidity (RH)-dependent. Additionally, elevated SO42−, TNO3, and TNH4 were crucial in the increase in AWC. The increase in Ca2+ ions in the sandy haze led to lower AWC than that in the haze periods. Specifically, (NH4)2SO4 was the major contributor to the AWC when the RH was between 30 and 46% in the sandy haze period, and NH4NO3 gradually became the main contributor with the increase in RH. In turn, AWC could enhance the formation of sulfate and nitrate, even during the sandy haze period. Therefore, the emergency control of gaseous precursors should also be implemented before the sand events.
1. Introduction
Fine particulate matter (PM2.5, aerodynamic diameter ≤ 2.5 μm), a major atmospheric pollutant, has significant adverse effects on human health [1,2], reduces visibility [3], damages ecosystems, and alters regional climates [4]. PM2.5 can absorb water from the air to form aerosol water content (AWC) on its surface, especially when relative humidity (RH) meets or exceeds the deliquescence relative humidity (DRH). AWC is an important component of PM2.5 and generally accounts for more than 50% of ambient PM2.5 mass, especially during haze events [5,6]. In addition, the AWC may affect the optical properties of PM2.5 [7], particle volumes [8], gas-particle transformations, and the formation of inorganic aerosol (nitrate, sulfate, and ammonium, SNA) and organic matter (OM) through direct participation in atmospheric complex physical and chemical mechanisms [9,10], thereby playing profound roles on air quality degradation [11].
The formation of AWC mainly depends on aerosol mass concentration, particle hygroscopicity, the aerosol phase, and RH [12]. Field surveys by Wang et al. [13] showed that simultaneously elevated RH levels and SNA resulted in an abundant AWC during haze episodes. Wu et al. [14] and Speer et al. [15] quantified that SNA was associated with 62–80% of aerosol AWC. Laboratory experiments by Ansari and Pandis [16] confirmed that inorganic salts like (NH4)2SO4 were the key factor in AWC formation. Moreover, crustal species play an important role in the AWC, in which Ca2+ can form insoluble CaSO4 with sulfate, lowering AWC concentrations [17,18]. On the other hand, AWC can affect the formation of secondary aerosol by offering a medium for heterogeneous gas/particle reactions and the partitioning of water-soluble compounds in the aerosol phase, such as the major aqueous-phase formation paths for sulfate, which are the S(IV) referred to as SO2·H2O + HSO3− + SO3− oxidation by H2O2, NO2, O3, the catalysis by transition metal ions (TMIs, e.g., Fe (III) and Mn (II)), and the heterogeneous hydrolysis reaction of NO2 and N2O5 with water to form nitrate [19,20]. In addition, AWC is a crucial factor in determining aerosol acidity. High AWC and excess NH3 have been linked to the low acidity of aerosols during severe haze in Northern China [21]. In addition, mineral dust particles play an important role in SO2 and NO2 conversion by supplying TMIs and undergoing heterogeneous reactions [22,23]. Moreover, mineral dust can modify the particle pH, and eventually affect SNA formation [17,23]. In summary, there are complex effects between AWC, particle pH, and mineral dust. Several mixing processes of sand and haze, accompanied by high concentrations of secondary and crustal species as well as high RH, have been observed in Northern China [24,25], which can supply a specific scenario for an in-depth study of their relationships.
Hourly data, including PM2.5, PM10, the chemical composition of PM2.5, gaseous pollutants, and meteorological parameters, were collected from January 6 to 26, 2021, using a series of online monitoring instruments. The Thermodynamic ISORROPIA-II model and Zdanovskii−Stokes−Robinson (ZSR) mixing rule were used to estimate the total AWC values of particles and AWC of major components of particles. The main purposes of this study are as follows: (1) to study the characteristics of AWC under sandy haze and haze pollution periods, and (2) to use the sensitivity tests to quantify the influence of major driving forces on AWC concentration during the observation period. To further understand the effects of AWC on secondary inorganic compound formation, we calculated the contributions of main composition forms to AWC. This work is useful to awaken awareness of the atmospheric chemistry during the sandy haze episodes and guide the measures for the emergency control of severe pollution in the future.
2. Experiment and Method
2.1. Observation Site
Field sampling was conducted from 6 to 26 January 2021, at Zhengzhou Environmental Monitoring Station (34.75° N, 113.61° E) (Figure S1), which is surrounded by busy roads, with the sampling equipment located on the roof of the monitoring station, at a height of 15 m. Moreover, this site is near a large personnel flow of the region such as the Zhengzhou Institute of Technology, residential areas, and hospitals.
2.2. Measuring Instruments
The working principle of measuring instruments, quality assurance/quality control, and measurement uncertainties can be found in the Supplementary Materials (Text S1) and previous research by Wang et al. [26]. Briefly, water-soluble inorganic ions (NH4+, SO42−, NO3−, Cl−, Na+, Mg2+, Ca2+, and K+) in PM2.5 and trace gases (NH3, HNO3, HONO, and HCl) were continuously measured by an ambient ion monitor (URG-9000D, Thermo Science, Waltham, MA, USA). The hourly concentrations of metal elements such as Fe and Mn in PM2.5 were detected by an energy-dispersive X-ray Fluorescence instrument (Xact 625, Cooper Environmental Science, Portland, OR, USA). Hourly organic carbon (OC) and elemental carbon (EC) concentrations in PM2.5 were obtained by a thermo-optical transmission method operating a semi-continuous Carbon Analyzer (Model 4, Sunset Laboratory Inc., Tigard, OR, USA). The mass concentrations of PM2.5, PM10, and gaseous pollutants (SO2, NO2, CO, and O3) were measured by a series of online analyzers (Model 1400a, 1405 42i, 43i, 48i, and 49i, Thermo Fisher Scientific, Waltham, MA, USA). Meteorological parameters including temperature (Temp), RH, wind direction (WD), and wind speed (WS) were simultaneously monitored by the city station (LUFFT WS500, Sutron Corporation, Sterling, VA, USA).
2.3. Date Analysis
2.3.1. ISORROPIA II Model
The pH values and AWCinorg of particles were calculated using the ISORROPIA-II model (http://isorropia.eas.gatech.edu, accessed on 1 January 2020), which had been implemented and reported in previous research [27]. In this study, ISORROPIA-II operates in forward mode and a “metastable” state. The calculations using total (gas and aerosol) measurements in the forward model are less affected by measurement errors. The particle water associated with the organics (AWCorg) was estimated by the organic hygroscopicity and the mass concentrations of organic matter (OC × 1.6). Detailed descriptions of pH values and AWC calculations can be found in previous studies [28,29] and Text S2 in Supplementary Materials.
2.3.2. Calculating for Aqueous-Phase Sulfate Production Rates
Refer to Cheng et al. [19] to calculate the formation rate of sulfate by liquid-phase reactions, such as S(IV) oxidation by O3, TMI, and NO2. These methods and their reaction formulations are detailed in Supplementary Materials (Tables S1–S3, Texts S3 and S4).
2.3.3. Sensitivity Test of AWC
To explore the main influencing factors of aerosol AWC and quantify the influence of inputs on AWC, sensitivity tests were conducted. In the sensitivity analysis, SO42−, TNO3 (total nitrate (gas + aerosol) represented by equivalent HNO3), TNH3 (total ammonium (gas + aerosol) represented by equivalent NH3), Ca2+, K+, Na+, Mg2+, Cl−, RH, and temperature were selected as the variables to assess how a variable affects PM2.5 AWC, and the real-time measured values of this variable and the average values of other ions in different pollution periods were input into ISORROPIA II. The relative standard deviation (RSD) of calculated aerosol AWC can reflect the influence of variable variation on aerosol water content. AWC affects these variables, but RH affects AWC. The sensitivity analysis in this work is only for PM2.5. Considering that part of the SNA may be transported in the sandy haze and haze periods [30], by comparing the correlation between nitrate, sulfate, and EC, as well as crustal material (CM) and SNA in different periods (Figure S2), we found that the high correlations between nitrate, sulfate, and EC and low correlations between SNA and CM indicated that SNA was generated locally and was less affected by sand and dust transport.
3. Results and Discussion
3.1. Sandy Haze and Haze Events
Figure 1 illustrates the temporal variation in particle mass concentration, gas pollutants, and meteorological factors from January 6 to 26, 2021. The observation period is divided into three scenes based on pollutant concentration under varying meteorological conditions with their average values listed in Table S4. During the sandy haze period (January 11–16), PM10 and Ca2+ concentrations rose sharply from 71 μg/m³ in the clean period to 274 μg/m³ and from 1.4 to 3.9 μg/m³, respectively, driven by high WS likely due to sandstorms originating from Northwest China (Figure S3). After that, an extended haze event developed over the January 16–26 period (labeled haze in Figure 1), with an average PM2.5 concentration of 111 ± 63 μg/m³. During the sandy haze period, the average concentrations of gaseous pollutants (SO2, NO2, and CO) in the sandy haze periods were about twice as high as those in the clean period. In addition, the Temp and RH slightly increased in the sandy haze period. These results indicate that atmospheric pollutants accumulated during the sandy haze period accompanied by sandstorm effects.
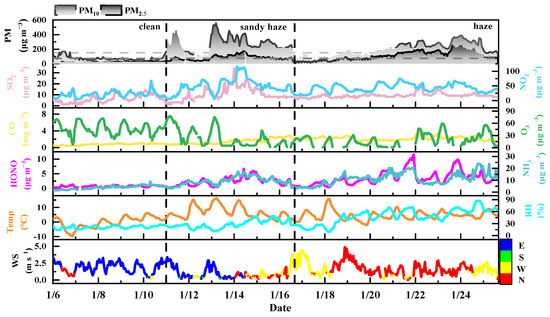
Figure 1.
Temporal variations of observed concentrations of PM10, PM2.5, gaseous pollutants (e.g., SO2, NO2, CO, and O3), and meteorological parameters (e.g., WS, WD, Temp, and RH) from 6 to 26 January 2021.
3.2. Role of Aerosol Water Content on PM2.5
Figure 2a shows the 2D kernel density graphs between the PM2.5 and the ratio of AWC/PM2.5 under different pollution levels. The ratio of AWC/PM2.5 increased as PM2.5 levels increased. Ratios were three times higher during the most polluted periods (0.67, PM2.5 > 225 μg m−3) than those during clean and moderately polluted conditions (ratios of 0.2 when PM2.5 < 75 μg m−3). This is consistent with previous studies that enhanced PM2.5 mass concentration was accompanied by the persistent growth of AWC [31,32,33]. The mass fractions of the PM2.5 components and calculated AWC are grouped by the different pollution periods, as displayed in Figure 2b. SNA dominated the PM2.5 mass concentration and accounted for 38 and 50% of the sandy haze and haze periods, respectively. In addition, the proportion of CM in the sandy haze period (29 ± 10 μg m−3) was more than four times that in the haze period (7 ± 3 μg m−3), accounting for 27 and 3% in PM2.5 mass concentration, respectively.
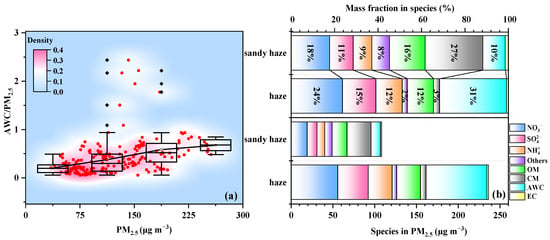
Figure 2.
(a) Aerosol water content/PM2.5 mass concentrations ratio (AWC/PM2.5) under different fine particle (PM2.5) concentration levels. Red dots represent the distribution density, while black dots indicate the errors present in the box plot. (b) Mass concentrations and fractions of chemical species during different pollution periods. “Others” represents the combined sum of other measured ions.
The AWC was 11 ± 5 and 73 ± 68.4 μg m−3 in the sandy haze and haze periods accounted for 10 and 31% of the PM2.5, respectively. The RH condition was primarily responsible for the low AWC in the sandy haze period. Furthermore, we divided the periods into five groups according to the actual humidity range (Figure 3). When RH < 30% represented the dry conditions, each 5% rise in RH was taken as a group to represent the gradual transformation of dry conditions into humid conditions. It can be found that the average concentration of PM2.5 in the sandy haze period (67 ± 9 μg m−3) was twice that in the haze period (36 ± 3 μg m−3) under dry conditions, but the average concentration of PM2.5 in the haze period under humid conditions was about twice as high as in the sandy haze period and has quadrupled, and except for CM elements, the proportions of components are similar. Meanwhile, with an increasing RH, the AWC increased significantly during different periods. With the increase in RH, AWC increased from 8 to 15% in the sandy haze period and from 11 to 19% in the haze period. The differences between 8% and 15% (differed by 7%) and between 11% and 19% (differed by 8%) are limited, showing that the AWC increase in sandy haze and polluted haze is similar. Thus, high RH might determine the AWC concentrations under both haze and sandy haze periods and lead to an increase in PM2.5, which is consistent with previous studies [34,35].

Figure 3.
Comparison of the mass concentration and fraction of AWC and chemical species under different ranges of relative humidity (RH) during the sandy haze (a) and haze periods (b).
3.3. Driving Factors of AWC
Sensitivity tests were conducted to assess the influence of chemical components and meteorological parameters on AWC. As shown in Figure 4, the most important factor for AWC was RH, followed by SO42− and TNO3. The AWC concentrations rose slowly over the RH range from 30 to 80% (Figure 4e). Significant explosive growth in AWC occurred when RH exceeded 80%, which was higher than the DRH of most chemical components in PM2.5 and reached the point of dampness to form a liquid state [36,37]. The temperature had little effect on AWC in the range of −3–13 °C. However, when the temperature increases above 20 °C, the AWC will exhibit an obvious downward trend [38,39].
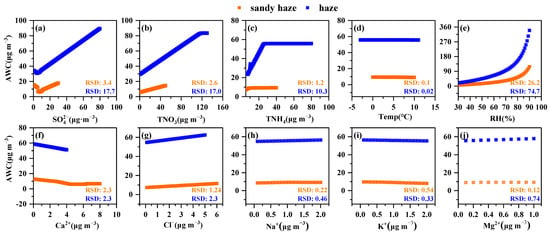
Figure 4.
Sensitivity tests of AWC to SO42−(a), TNO3(b), TNH4(c), Ca2+(f), Cl−(g), Na+(h), K+(i), Mg2+(j), and meteorological parameters (Temp(d) and RH(e)) during different pollution periods. The relative standard deviation (% RSD) represents the sensitivity degree of pH to this variable.
It was found that the RSD of AWC variations (Figure 4e) in the haze period (74.7%) was higher than that in the sandy haze period (26.2%), which may be due to the difference in components. Figure S4 suggests that AWC was well correlated with SNA (R2 > 0.5), but poorly correlated with other components (R2 < 0.5), indicating a strong interplay between inorganic composition and AWC. Figure 4a reveals that elevated SO42− was crucial in the increase in AWC and had a larger influence on AWC concentration in the haze period (RSD = 17.7%) than in the sandy haze period (RSD = 3.4%). The effect of TNO3 and TNH4 on AWC was similar to that of SO42−, but the effect flattens out after a typical value, which is consistent with previous studies [38]. This phenomenon may be because there is not enough TNO3 to react with the excessive levels of TNH4. In the process of increasing the concentration of NH3 in the ammonia−nitrite−sulfate−water system, NH3 reacts with sulfuric acid first and then with HNO3 to form ammonium nitrate. After most of the nitric acid is converted to ammonium nitrate, it is difficult to dissolve more ammonia into aerosol droplets [39]. In contrast, the increase in Ca2+ ions led to a decrease in AWC. Therefore, the low AWC concentration in the sandy haze period may be affected by low RH, low SNA concentration, and high Ca2+ concentration.
To reduce the complexity of the aerosol system, two important parameters (sulfate ratio: RSO42 = [Na++NH4+]/[SO42−], sodium salt ratio: RNa+ = [Na+]/[SO42−]) are introduced to divide different sub-systems [40]: (i) if RSO42− < 1, excessed sulfate existed as the chemical forms of NaHSO4 and NH4HSO4; (ii) 1 ≤ RSO42− < 2, sulfate existed as NaHSO4, NH4HSO4, Na2SO4, (NH4)2SO4, and (NH4)3H(SO4)2; (iii) if RSO42− ≥ 2 and RNa+ < 2, then insufficient sulfate and sodium existed as Na2SO4, (NH4)2SO4, NH4NO3, and NH4Cl; and (iv) if RSO42− ≥ 2 and RNa+ ≥ 2, insufficient sulfate and excess sodium, the constituent forms include Na2SO4, NaNO3, NaCl, NH4NO3, and NH4Cl. Figure 5 shows the contribution of the chemical composition to the AWC quantified by changing the RH value using the method of ZSR [41]. CaCl2 and (NH4)2SO4 were the major contributors to the AWC, accounting for 39% and 49%, when RH was between 30 and 46% in the sandy haze period and when RH was between 30 and 57% in the haze period, respectively. With the increase in RH, the contribution of (NH4)2SO4 and Na2SO4 decreased, and NH4NO3 gradually became the main contributor to the sandy haze period (RH range: 47–90%) and haze period (RH range: 58–90%), contributing 45% and 56%, respectively. It is evident that (NH4)2SO4 has higher water uptake than NH4NO3 at an RH lower than 50%, which is consistent with the previous study in India [42].
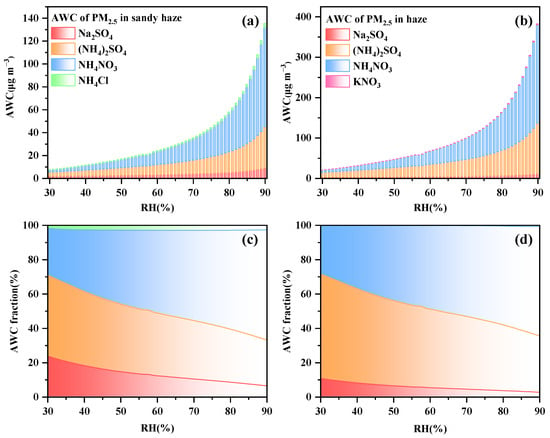
Figure 5.
The estimated AWC concentrations and fractions attributed to different compositions in sandy haze (a,c) and haze (b,d), respectively.
3.4. Impact of the AWC on Secondary Aerosol Formation
The sulfur oxidation ratio (SOR) and nitrogen oxidation ratio (NOR) were defined as the molar ratio of sulfate to total sulfur and nitrate to total nitric acid [43,44]:
where n represents the molar concentration (mol m−3).
To understand the impacts of AWC on nitrate and sulfate, Figure 6 plotted the correlation of AWC with sulfate and nitrate at day and night mapped SOR and NOR, respectively. The results show higher AWC concentrations and correlation coefficients between SO42− and NO3− with AWC at night than those during the day. Therefore, to analyze the effect of AWC on SNA generation, nighttime data were selected for further analysis. Research on the formation of sulfate has determined that the formation of sulfate during haze was mainly from the aqueous-phase reactions [26].

Figure 6.
Relationship between SO42− and AWC (a,b), and NO3− and AWC (c,d) in sandy haze and haze periods, respectively. The circles were colored according to the SOR and NOR.
The sulfate production rates of aqueous-phase reactions, such as S(IV) oxidation by O3, TMI, and NO2, are calculated to identify further the AWC effect on sulfate formation in different pollution periods, as seen in Figure 7a. The effect of AWC on the oxidation rate of TMI was negligible, but the oxidation rates of NO2 and O3 increased with the increase in AWC. However, the oxidation pathways of NO2 and O3 calculated in the datasets of the sandy haze and haze periods were lower than the S(IV) pathway of TMI oxidation shown in Figure 7b. In addition, there were significant differences in the rates of TMI oxidation between the sandy haze and haze periods, which cannot be explained by AWC. The pH, another factor affecting the sulfur oxidation rate, is analyzed in Figure 7c. The aqueous oxidation rates of S(IV) by NO2, O3, and TMI were strongly pH-dependent. The NO2 and O3 oxidation rates increased with increasing pH, and TMI-catalyzed oxidation decreased with pH. The trend is consistent with previous research on pH sensitivity [45,46]. Therefore, the rate of TMI-catalyzed (0.9 μg m−3 h−1) in the haze period was more than two orders of magnitude higher than that of the sandy haze period (7.6 × 10−3 μg m−3 h−1), which may be attributed to the pH (3.7–6) in the haze period. The high pH value in the sandy haze period might be due to the high Ca2+, Mg2+, and K+ concentrations since the low volatility of these cations allows them to preferentially neutralize sulfates and nitrates over NH3 [47]. In summary, AWC provided a medium for the aqueous-phase reaction of sulfate, while high pH suppressed the dominant reactions (i.e., TMI oxidation) during the process of sandy haze.
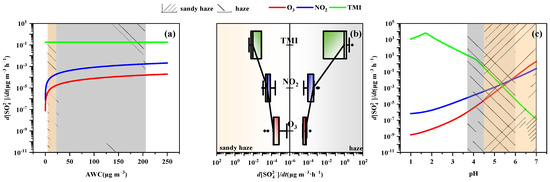
Figure 7.
Sensitivity tests of sulfate production rates by altering inputs of AWC (a) and pH (c), comparison of the aqueous-phase sulfate production rates by different oxidation routes between the sandy haze and haze periods during the nighttime (18:00–07:00 UTC + 8). (b) Light brown and gray shaded areas indicate characteristic AWC and pH range under sandy haze and haze periods in Zhengzhou, respectively.
The formation pathway of nitrate in the atmosphere is different during the day and night due to the light conditions. During the daytime, nitrate mainly comes from the oxidation of NO2 by OH radicals in the gas phase, and at night, it mainly comes from the heterogeneous hydrolysis of N2O5 [48,49]. Considering that NO3 radicals in the atmosphere are mainly produced by the oxidation of NO2 by O3, [50], the correlation between [NO2]2 × [O3] and NOR can roughly represent the contribution of N2O5 to the heterogeneous hydrolysis of atmospheric nitrate at night. As shown in Figure 8, the variations in [NO2]2 × [O3] in the nighttime were all positively correlated with NOR during both the haze (R2 = 0.6) and sandy haze periods (R2 = 0.11). Therefore, the heterogeneous hydrolysis of N2O5 made a remarkable contribution to atmospheric nitrate even during the sandy haze episodes.
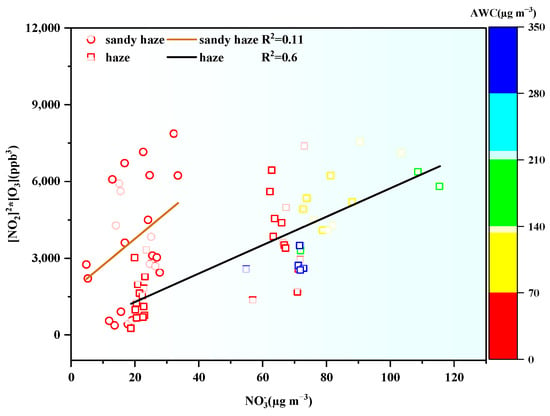
Figure 8.
The correlation between NO3− and [NO2]2 × [O3] in the nighttime (18:00–07:00 UTC + 8), the color scale represents the concentration of AWC.
4. Conclusions
A comprehensive analysis was performed using a series of high-time-resolution instruments in Zhengzhou during sandy haze and haze periods. The ISORROPIA-II model was used to investigate the AWC value and its driving factors. The AWC was 11 ± 5 and 73 ± 68 μg m−3 in the sandy haze and haze periods and accounted for 10% and 31% of the PM2.5, respectively. The predicted AWC values of PM2.5 were significantly affected by the particle components and meteorological parameters. AWC increased slowly at lower RH and evolved to a sharp rise above 80% RH. Elevated SO42−, TNO3, and TNH4 were crucial in the increase in AWC and had a larger influence on AWC concentration in the haze period than in the sandy haze period. (NH4)2SO4 was the major contributor to the AWC when the RH was between 30 and 46% in the sandy haze period and when the RH was between 30 and 57% in the haze period, accounting for 28% and 49%, respectively. With the increase in RH, NH4NO3 gradually become the main contributor to the sandy haze period (RH range: 47–90%) and haze period (RH range: 58–90%), contributing 45% and 56%, respectively. The increase in Ca2+ ions led to a decrease in AWC. Therefore, the low AWC concentration in the sandy haze period may be affected by RH, and in turn, it will subsequently exhibit high Ca2+ concentration.
The effect of AWC on the oxidation rate of TMI was negligible, but the rates of NO2 and O3 oxidation increased with the increase in AWC. The low rate of TMI-catalyzed in the sandy haze period may be attributed to the high pH as a result of the high Ca2+, Mg2+, and K+ concentrations. In addition, AWC also promoted the formation of nitrate by affecting the heterogeneous hydrolysis of N2O5. Therefore, the AWC in PM and its impact on secondary particulate matter components during the sandy haze periods should not be ignored.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/atmos15070850/s1, Figure S1. Location of the sampling site in Zhengzhou; Figure S2. (a) hourly concentration of NO3−/EC plotted against SO42−/EC (b) CM concentration correlated with SNA; Figure S3. HYSPLIT backward trajectories for 6 January 2021 in Zhengzhou; Figure S4. Hourly concentrations of individual species plotted against AWC concentration. The crustal matter (CM) and organic matter (OM) were calculated as in Text S5; Table S1. Concentrations (μg·m−3) of Inorganic Ions in PM2.5, Gaseous Pollutants, and Meteorological Parameters during 2021 in Zhengzhou, China; Table S2. Constants for calculating the apparent Henry’s constant (H*); Table S3. Constants for calculating the ionization constants (K); Table S4. Concentrations (μg·m−3) of Inorganic Ions in PM2.5, Gaseous Pollutants, and Meteorological Parameters during 2021 in Zhengzhou, China [51,52,53,54,55].
Author Contributions
R.Z. and S.W. designed and led this study. S.W. and S.Z. were responsible for all observations and data collection. S.Z. wrote the paper. P.K. interpreted the data and discussed the results. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the China Postdoctoral Science Foundation (2023M733220), the Zhengzhou PM2.5 and O3 Collabo-rative Control and Monitoring Project (20220347A), and the National Key Research and Development Program of China (No. 2017YFC0212403).
Data Availability Statement
All data in this work are available by contacting the corresponding author Shenbo Wang (shbwang@zzu.edu.cn) and Ruiqin Zhang (rqzhang@zzu.edu.cn). The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Jerrett, M. The death toll from air-pollution sources. Nature 2015, 525, 330–331. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhao, P.S.; Ge, S.S.; Ding, J. Aerosol liquid water content of PM2.5 and its influencing factors in Beijing, China. Sci. Total Environ. 2022, 839, 156342. [Google Scholar] [CrossRef] [PubMed]
- Won, W.S.; Oh, R.; Lee, W.; Kim, K.Y.; Ku, S.; Su, P.C.; Yoon, Y.J. Impact of fine particulate matter on visibility at incheon international airport, South Korea. Aerosol Air Qual. Res. 2020, 20, 1048–1061. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Tantoh, D.M.; Lu, W.Y.; Chen, C.-Y.; Liaw, Y.-P. Cigarette smoking and PM2.5 might jointly exacerbate the risk of metabolic syndrome. Front. Public Health 2024, 11, 1234799. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Lowenthal, D.H.; Chen, L.W.; Wang, X.L.; Watson, J.G. Mass reconstruction methods for PM2.5: A review. Air Qual. Atmos. Health 2015, 8, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.J.; Wang, Y.; Tan, T.E.; Zhu, Y.S.; Li, M.R.; Shang, D.J.; Wang, H.C.; Lu, K.D.; Guo, S.; Zeng, L.M.; et al. Aerosol liquid water driven by anthropogenic inorganic salts: Implying its key role in haze formation over the North China Plain. Environ. Sci. Technol. Lett. 2018, 3, 160–166. [Google Scholar] [CrossRef]
- Boreddy, S.K.R.; Nair, V.S.; Babu, S.S. Assessment of submicron aerosol liquid water content and mass-based growth factors in South Asian outflow over the Indian Ocean. Sci. Total Environ. 2023, 901, 166461. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.V.; Petters, M.D.; Suda, S.R.; Guo, H.; Weber, R.J.; Carlton, A.G. Trends in particle-phase liquid water during the southern oxidant and aerosol study. Atmos. Chem. Phys. 2014, 14, 10911–10930. [Google Scholar] [CrossRef]
- Jin, X.A.; Wang, Y.Y.; Li, Z.Q.; Zhang, F.; Xu, W.Q.; Sun, Y.L.; Fan, X.X.; Chen, G.Y.; Wu, H.; Ren, J.Y.; et al. Significant contribution of organics to aerosol liquid water content in winter in Beijing, China. Atmos. Chem. Phys. 2020, 20, 901–914. [Google Scholar] [CrossRef]
- Herrmann, H.; Schaefer, T.; Tilgner, A.; Styler, S.A.; Weller, C.; Teich, M.; Otto, T. Tropospheric aqueous-phase chemistry: Kinetics, mechanisms, and its coupling to a changing gas phase. Chem. Rev. 2015, 115, 4259–4334. [Google Scholar] [CrossRef]
- Tan, W.; Yu, Y.; Li, C.; Li, J.; Kang, L.; Dong, H.; Zeng, L.; Zhu, T. Profiling Aerosol Liquid Water Content Using a Polarization Lidar. Environ. Sci. Technol. 2020, 54, 3129–3137. [Google Scholar] [CrossRef] [PubMed]
- Pilinis, C.; Seinfeld, J.H.; Grosjean, D. Water content of atmospheric aerosols. Atmos. Environ. 1989, 23, 1601–1606. [Google Scholar] [CrossRef]
- Wang, H.C.; Lu, K.D.; Chen, X.R.; Zhu, Q.D.; Wu, Z.J.; Wu, Y.S.; Sun, K. Fast particulate nitrate formation via N2O5 uptake aloft in winter in Beijing. Atmos. Chem. Phys. 2018, 18, 10483–10495. [Google Scholar] [CrossRef]
- Wu, J.R.; Bei, N.F.; Hu, B.; Liu, S.X.; Zhou, M.; Wang, Q.Y.; Li, X.; Liu, L.; Feng, T.; Liu, Z.R. Is water vapor a key player of the wintertime haze in North China Plain? Atmos. Chem. Phys. 2019, 19, 8721–8739. [Google Scholar] [CrossRef]
- Speer, R.E.; Edney, E.O.; Kleindienst, T.E. Impact of organic compounds on the concentrations of liquid water in ambient PM2.5. J. Aerosol Sci. 2003, 34, 63–77. [Google Scholar] [CrossRef]
- Ansari, A.S.; Pandis, S.N. Water absorption by secondary organic aerosol and its effect on inorganic aerosol behavior. Environ. Sci. Technol. 2000, 34, 71–77. [Google Scholar] [CrossRef]
- Usher, C.R.; Michel, A.E.; Grassian, V.H. Reactions on mineral dust. Chem. Rev. 2003, 103, 4883–4940. [Google Scholar] [CrossRef] [PubMed]
- Vlasenko, A.; Sjogren, S.; Weingartner, E.; Stemmler, K.; Gaggeler, H.W.; Ammann, M. Effect of humidity on nitric acid uptake to mineral dust aerosol particles. Atmos. Chem. Phys. 2006, 6, 2147–2160. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Zheng, G.J.; Wei, C.; Mu, Q.; Zheng, B.; Wang, Z.B.; Gao, M.; Zhang, Q.; He, K.B.; Carmichael, G.; et al. Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China. Sci. Adv. 2016, 2, 1601530. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.; Sinha, B.; van Pinxteren, D.; Tilgner, A.; Fomba, K.W.; Schneider, J.; Roth, A.; Gnauk, T.; Fahlbusch, B.; Mertes, S.; et al. Enhanced role of transition metal ion catalysis during in-cloud oxidation of SO2. Science 2013, 340, 727–730. [Google Scholar] [CrossRef]
- Liu, M.X.; Song, Y.; Zhou, T.; Xu, Z.Y.; Yan, C.Q.; Zheng, M.; Wu, Z.J.; Hu, M.; Wu, Y.S.; Zhu, T. Fine particle pH during severe haze episodes in northern China. Geophys. Res. Lett. 2017, 44, 5213–5221. [Google Scholar] [CrossRef]
- Sullivan, R.C.; Guazzotti, S.A.; Sodeman, D.A.; Prather, K.A. Direct observations of the atmospheric processing of Asian mineral dust. Atmos. Chem. Phys. 2007, 7, 1213–1236. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.M.; Shen, X.L.; Huang, D. Importance of atmospheric aging in reactivity of mineral dust aerosol: A case study of heterogeneous reaction of gaseous hydrogen peroxide on processed mineral particles. Atmos. Chem. Phys. 2011, 11, 28563–28586. [Google Scholar] [CrossRef]
- Dong, Z.; Su, F.; Zhang, Z.; Wang, S. Observation of chemical components of PM2.5 and secondary inorganic aerosol formation during haze and sandy haze days in Zhengzhou, China. J. Environ. Sci. 2020, 88, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.Y.; Kuang, Y.; Liang, L.L.; He, Y.; Cheng, H.B.; Bian, Y.X.; Tao, J.C.; Zhang, G.; Zhao, P.S.; Ma, N. Dust-Dominated coarse particles as a medium for rapid secondary organic and inorganic aerosol formation in highly polluted air. Environ. Sci. Technol. 2020, 54, 15710–15721. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Yin, S.S.; Zhang, R.Q.; Yang, L.M.; Zhao, Q.Y.; Zhang, L.S.; Yan, Q.S.; Jiang, N.; Tang, X.Y. Insight into the formation of secondary inorganic aerosol based on high-time-resolution data during haze episodes and snowfall periods in Zhengzhou, China. Sci. Total Environ. 2018, 660, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Fountoukis, C.; Nenes, A. ISORROPIA II: A computationally efficient thermodynamic equilibrium model for K+–Ca2+–Mg2+–Na+–Cl−–H2O aerosols. Atmos. Chem. Phys. 2007, 7, 4639–4659. [Google Scholar] [CrossRef]
- Guo, H.; Xu, L.; Bougiatioti, A.; Cerully, K.M.; Capps, S.L.; Hite, J.R.; Carlton, A.G.; Lee, S.H.; Bergin, M.H.; Ng, N.L.; et al. Fine-particle water and pH in the southeastern United States. Atmos. Chem. Phys. 2015, 15, 5211–5228. [Google Scholar] [CrossRef]
- Song, S.J.; Gao, M.; Xu, W.Q.; Shao, J.Y.; Shi, G.L.; Wang, S.X.; Wang, Y.X.; Sun, Y.L.; Michael, B.M. Fine-particle pH for Beijing winter haze as inferred from different thermodynamic equilibrium models. Atmos. Chem. Phys. 2018, 18, 7423–7438. [Google Scholar] [CrossRef]
- Fang, X.Z.; Liu, Y.Y.; Li, K.J.; Wang, T.; Deng, Y.; Feng, Y.Q.; Yang, Y.; Cheng, H.Y.; Chen, J.M.; Zhang, L.W. Atmospheric nitrate formation through oxidation by carbonate radical. ACS Earth Space Chem. 2021, 5, 1801–1811. [Google Scholar] [CrossRef]
- Chen, C.R.; Zhang, H.X.; Yan, W.J.; Wu, N.A.; Zhang, Q.; He, K.B. Aerosol water content enhancement leads to changes in the major formation mechanisms of nitrate and secondary organic aerosols in winter over the North China Plain. Environ. Pollut. 2021, 287, 117625. [Google Scholar] [CrossRef]
- Cheng, Y.; Cao, X.B.; Yu, Q.Q.; Liu, J.M.; Ma, W.L.; Qi, H.; Zhang, Q.; He, K.B. Synergy of multiple drivers leading to severe winter haze pollution in a megacity in Northeast China. Atmos. Res. 2022, 270, 106075. [Google Scholar] [CrossRef]
- Feng, Z.M.; Liu, Y.C.; Zheng, F.X.; Yan, C.; Fu, P.; Zhang, Y.S.; Lian, C.F.; Wang, W.G.; Cai, J.; Du, W.; et al. Highly oxidized organic aerosols in Beijing: Possible contribution of aqueous-phase chemistry. Atmos. Environ. 2022, 273, 118971. [Google Scholar] [CrossRef]
- Bian, Y.X.; Zhao, C.S.; Ma, N.; Chen, J.; Xu, W.Y. A study of aerosol liquid water content based on hygroscopicity measurements at high relative humidity in the North China Plain. Atmos. Chem. Phys. 2014, 14, 6417–6426. [Google Scholar] [CrossRef]
- Shen, X.J.; Sun, J.Y.; Zhang, X.Y.; Zhang, Y.M.; Zhong, J.T.; Wang, X.; Wang, Y.Q.; Xia, C. Variations in submicron aerosol liquid water content and the contribution of chemical components during heavy aerosol pollution episodes in winter in Beijing. Sci. Total Environ. 2019, 693, 133521. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.R.; Beyer, K.D. Deliquescence Relative Humidities of Organic and Inorganic Salts Important in the Atmosphere. J. Phys. Chem. A 2016, 120, 9948–9957. [Google Scholar] [CrossRef]
- Sun, S.E.; Chang, S.Y.; Lee, C.T. The development and evaluation of a sequential aerosol-water measurement system. Atmos. Environ. 2021, 264, 118671. [Google Scholar] [CrossRef]
- Su, J.; Zhao, P.S.; Ding, J.; Du, X.; Dou, Y.J. Insights into measurements of water soluble ions in PM2.5 and their gaseous precursors in Beijing. J. Environ. Sci. 2021, 102, 123–127. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, P.; Su, J.; Dong, Q.; Du, X.; Zhang, Y. Aerosol pH and its driving factors in Beijing. Atmos. Chem. Phys. 2019, 19, 7939–7954. [Google Scholar] [CrossRef]
- Nenes, A.; Pandis, S.N.; Pilinis, C. ISORROPIA: A New Thermodynamic Equilibrium Model for Multiphase Multicomponent Inorganic Aerosols. Aquat. Geochem. 1998, 4, 123–152. [Google Scholar] [CrossRef]
- Wang, Z.C. Relationship Among the Raoult Law, Zdanovskii-Stokes-Robinson rule, and two extended zdanovskii-stokes-robinson rules of wang. J. Chem. Eng. Data 2009, 54, 187–192. [Google Scholar] [CrossRef]
- Gopinath, A.K.; Raj, S.S.; Kommula, S.M.; Jose, C.; Panda, U.; Bishambu, Y.; Ojha, N.; Ravikrishna, R.; Liu, P.F.; Gunthe, S.S. Complex interplay between organic and secondary inorganic aerosols with ambient relative humidity implicates the aerosol liquid water content over india during wintertime. J. Geophys. Res. 2022, 127, e2021JD036430. [Google Scholar] [CrossRef]
- Liu, H.B.; Talifu, D.; Ding, X.; Wang, X.M.; Abulizi, A.; Tursun, Y.; An, J.Q.; Wang, W.; Zhang, X.X.; Zhang, Y.Y. Particles liquid water and acidity determine formation of secondary inorganic ions in Urumqi, NW China. Atmos. Res. 2021, 260, 105622. [Google Scholar] [CrossRef]
- Zhong, J.T.; Zhang, X.Y.; Dong, Y.S.; Wang, Y.Q.; Liu, C.; Wang, J.Z.; Zhang, Y.M.; Che, H.C. Feedback effects of boundary-layer meteorological factors on cumulative explosive growth of PM2.5 during winter heavy pollution episodes in Beijing from 2013 to 2016. Atmos. Chem. Phys. 2018, 18, 247–258. [Google Scholar] [CrossRef]
- Guo, H.Y.; Nenes, A.; Weber, R.J. The underappreciated role of nonvolatile cations in aerosol ammonium-sulfate molar ratios. Atmos. Chem. Phys. 2018, 18, 17307–17323. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, W.X.; Yang, L.X.; Gao, X.M.; Nie, W.; Yu, Y.C.; Xu, P.J.; Zhou, Y.; Wang, Z. The secondary formation of inorganic aerosols in the droplet mode through heterogeneous aqueous reactions under haze conditions. Atmos. Environ. 2012, 63, 68–76. [Google Scholar] [CrossRef]
- Guo, H.Y.; Weber, R.J.; Nenes, A. High levels of ammonia do not raise fine particle pH sufficiently to yield nitrogen oxide-dominated sulfate production. Sci. Rep. 2017, 7, 12109. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, Y.S.; Ma, Q.X.; Ma, J.Z.; Chu, B.W.; Ji, D.S.; Tang, G.Q.; Liu, C.; Zhang, H.X.; Hao, J.M. Mineral dust and NOx promote the conversion of SO2 to sulfate in heavy pollution days. Sci. Rep. 2014, 4, 4172. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.X.; Wang, T.; Liu, C.; He, H.; Wang, Z.; Wang, W.H.; Liang, Y.T. SO2 Initiates the efficient conversion of NO2 to HONO on MgO surface. Environ. Sci. Technol. 2017, 51, 3767–3775. [Google Scholar] [CrossRef]
- Wang, J.; Pan, Y.P.; Tian, S.L.; Chen, X.; Wang, L.; Wang, Y.S. Size distributions and health risks of particulate trace elements in rural areas in northeastern China. Atmos. Res. 2016, 168, 191–204. [Google Scholar] [CrossRef]
- Clegg, S.L.; Brimblecombe, P.; Wexler, A.S. Thermodynamic model of the system H+-NH4+-SO42−-NO3−-H2O at tropospheric temperatures. J. Phys. Chem. A 1998, 102, 2137–2154. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Li, Y.; Wang, C.; Wang, W.; Yin, S.; Zhang, R. Effect of ammonia on fine-particle pH in agricultural regions of China: Comparison between urban and rural sites. Atmos. Chem. Phys. 2020, 20, 2719–2734. [Google Scholar] [CrossRef]
- Pye, H.O.T.; Nenes, A.; Alexander, B.; Ault, A.P.; Barth, M.C.; Clegg, S.L.; Collett, J.L.; Fahey, K.M.; Hennigan, C.J.; Herrmann, H. The acidity of atmospheric particles and clouds. Atmos. Chem. Phys. 2020, 20, 4809–4888. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 2nd ed.; Welly: Hoboken, NJ, USA, 2006. [Google Scholar]
- Xing, L.; Fu, T.; Cao, J.; Lee, S.C.; Wang, G.; Ho, K.; Cheng, M.C.; You, C.; Wang, T. Seasonal and spatial variability of the OM/OC mass ratios and high regional correlation between oxalic acid and zinc in Chinese urban organic aerosols. Atmos. Chem. Phys. 2013, 13, 4307–4318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).